The First Example of a Model Amphiphilic Polymer Conetwork Containing a Hydrophobic Oligopeptide: The Case of End-Linked Tetra[Poly(ethylene glycol)-b-oligo(L-alanine)]
Abstract
1. Introduction
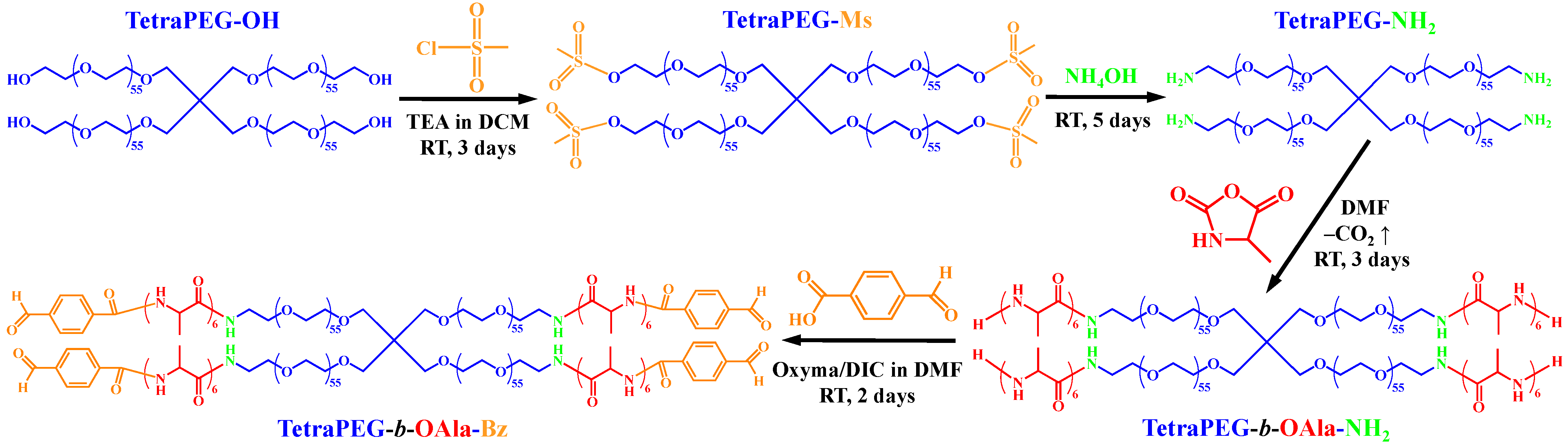
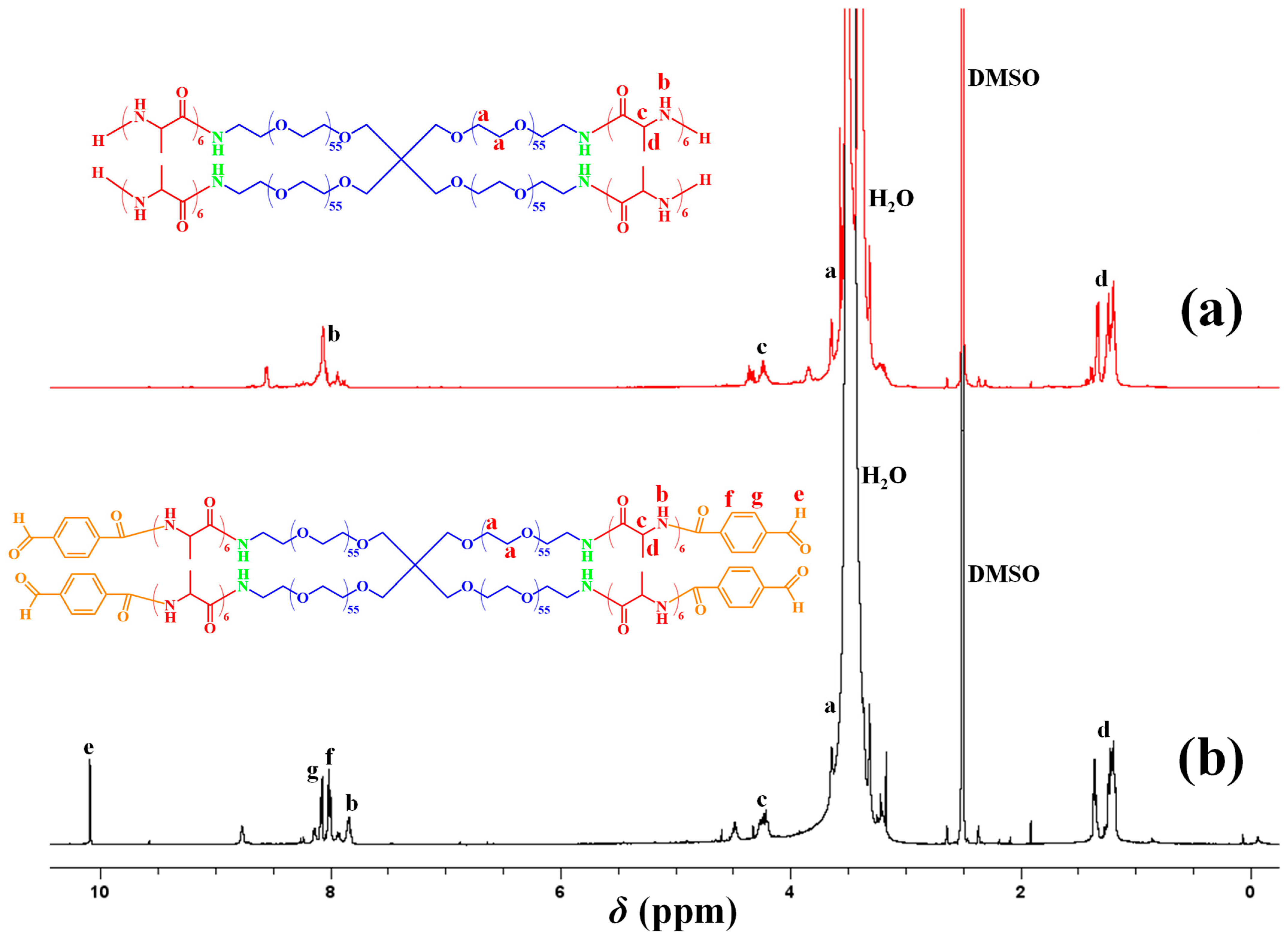
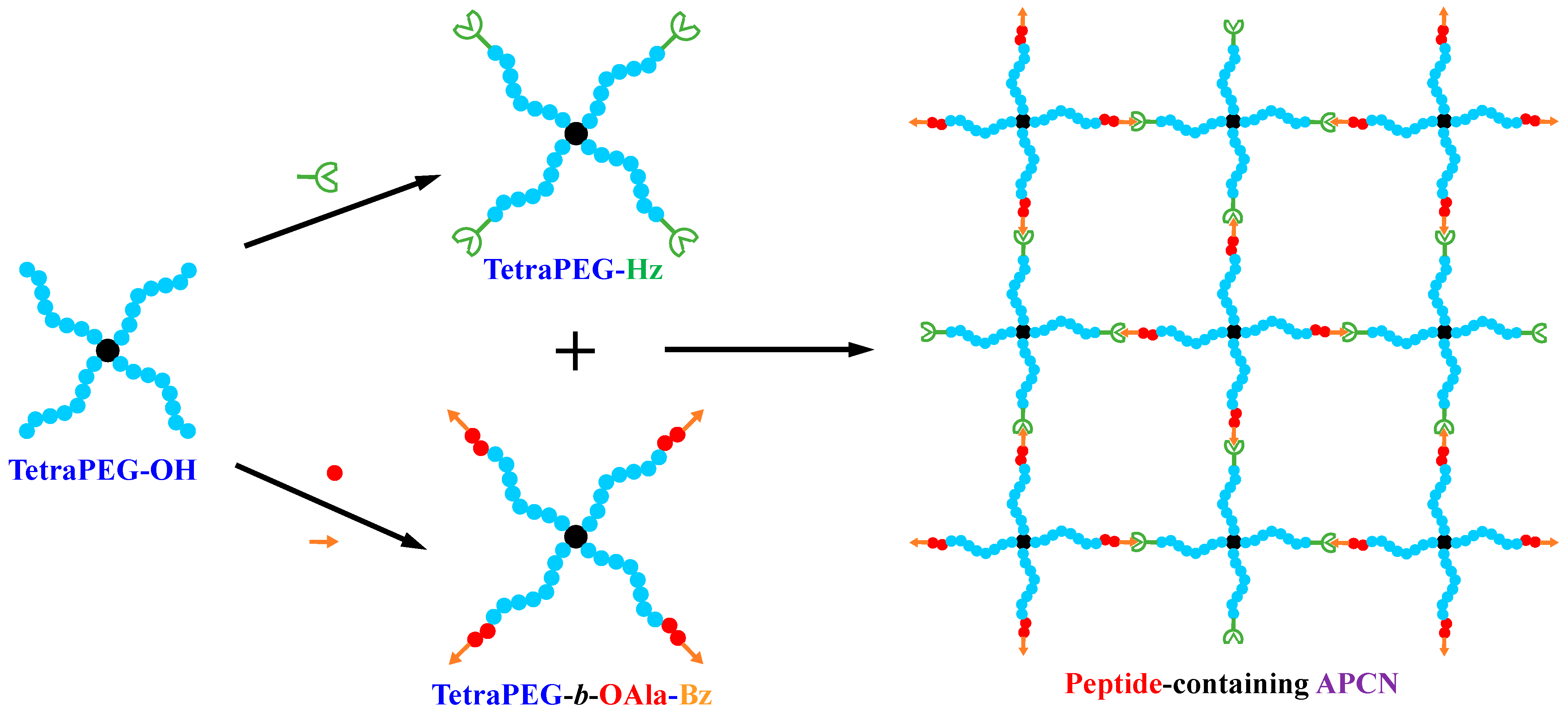
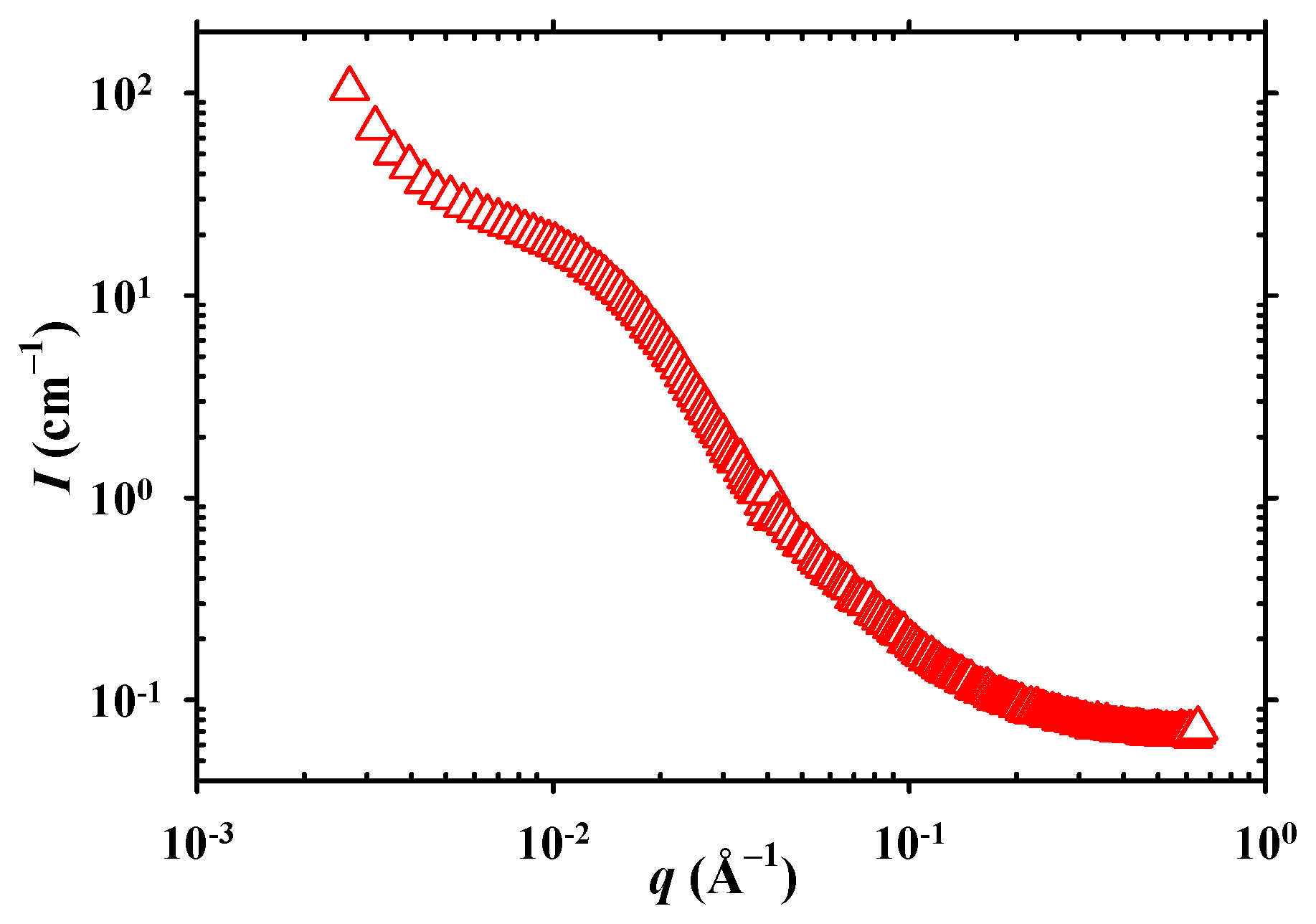
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Synthetic Methods
4.3. Characterization Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varnava, C.K.; Patrickios, C.S. Polymer Networks One Hundred Years after the Macromolecular Hypothesis: A Tutorial Review. Polymer 2021, 215, 123322. [Google Scholar] [CrossRef]
- American Chemistry Council. Resin Review: The Annual Statistical Report of the North American Plastics Industry; American Chemistry Council: Washington, DC, USA, 2019. [Google Scholar]
- Chen, D.; Kennedy, J.P.; Allen, A.J. Amphiphilic Networks. I. Network Synthesis by Copolymerization of Methacryloyl-Capped Polyisobutylene with 2-(Dimethylamino) Ethyl Methacrylate and Characterization of the Networks. J. Macromol. Sci. Chem. 1988, 25, 389–401. [Google Scholar] [CrossRef]
- Weber, M.; Stadler, R. Hydrophilic-Hydrophobic Two-Component Polymer Networks: 1. Synthesis of Reactive Poly(ethylene oxide) Telechelics. Polymer 1988, 29, 1064–1070. [Google Scholar] [CrossRef]
- Weber, M.; Stadler, R. Hydrophilic-Hydrophobic Two-Component Polymer Networks: 2. Synthesis and Characterization of Poly(ethylene oxide)-linked-Polybutadiene. Polymer 1988, 29, 1071–1078. [Google Scholar] [CrossRef]
- Patrickios, C.S.; Georgiou, T.K. Covalent Amphiphilic Polymer Networks. Curr. Opin. Colloid Interface Sci. 2003, 8, 76–85. [Google Scholar] [CrossRef]
- Erdodi, G.; Kennedy, J.P. Amphiphilic Conetworks: Definition, Synthesis, Applications. Prog. Polym. Sci. 2006, 31, 1–18. [Google Scholar] [CrossRef]
- Mespouille, L.; Hedrick, J.L.; Dubois, P. Expanding the Role of Chemistry to Produce New Amphiphilic Polymer (Co)Networks. Soft Matter 2009, 5, 4878–4892. [Google Scholar] [CrossRef]
- Tsalikis, D.G.; Ciobanu, M.; Patrickios, C.S.; Higuchi, Y. Model Amphiphilic Polymer Conetworks in the Bulk: Dissipative Particle Dynamics Simulations of Their Self-assembly and Mechanical Properties. Macromolecules 2023, 56, 9299–9311. [Google Scholar] [CrossRef]
- Guzman, G.; Es-Haghi, S.S.; Nugay, T.; Cakmak, M. Zero-Order Antibiotic Release from Multilayer Contact Lenses: Nonuniform Drug and Diffusivity Distributions Produce Constant-Rate Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1600775. [Google Scholar] [CrossRef]
- Biswas, A.; Chandel, A.K.S.; Anuradha, N.; Vadadoriya, N.; Mamtani, V.; Jewrajka, S.K. Structurally Heterogeneous Amphiphilic Conetworks of Poly(vinyl imidazole) Derivatives with Potent Antimicrobial Properties and Cytocompatibility. ACS Appl. Mater. Interfaces 2023, 15, 46333–46346. [Google Scholar] [CrossRef]
- McLeod, K.R.; Tew, G.N. Microphase-Separated Thiol−ene Conetworks from Telechelic Macromonomers with Asymmetric Molecular Weights. Macromolecules 2017, 50, 8042–8047. [Google Scholar] [CrossRef]
- Dech, S.; Cramer, T.; Ladisch, R.; Bruns, N.; Tiller, J.C. Solid–Solid Interface Adsorption of Proteins and Enzymes in Nanophase-Separated Amphiphilic Conetworks. Biomacromolecules 2011, 12, 1594–1601. [Google Scholar] [CrossRef]
- Schoenfeld, I.; Dech, S.; Ryabenky, B.; Daniel, B.; Glowacki, B.; Ladisch, R.; Tiller, J.C. Investigations on Diffusion Limitations of Biocatalyzed Reactions in Amphiphilic Polymer Conetworks in Organic Solvents. Biotechnol. Bioeng. 2013, 110, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Sittko, I.; Kremser, K.; Roth, M.; Kuehne, S.; Stuhr, S.; Tiller, J.C. Amphiphilic Polymer Conetworks with Defined Nanostructure and Tailored Swelling Behavior for Exploring the Activation of an Entrapped Lipase in Organic Solvents. Polymer 2015, 64, 122–129. [Google Scholar] [CrossRef]
- Nicolson, P.C.; Vogt, J. Soft Contact Lens Polymers: An Evolution. Biomaterials 2001, 22, 3273–3283. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, Z.; Es-Haghi, S.S.; Cakmak, M. Recent Trends in Advanced Contact Lenses. Adv. Healthc. Mater. 2019, 8, 1801390. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K. Controlled/“Living” Radical Polymerization. Atom Transfer Radical Polymerization in the Presence of Transition-Metal Complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of Methyl Methacrylate with the Carbon Tetrachloride/Dichlorotris-(triphenylphosphine)ruthenium(II)/Methylaluminum Bis(2,6-di-tert-butylphenoxide) Initiating System: Possibility of Living Radical Polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Kamata, H.; Akagi, Y.; Kayasuga-Kariya, Y.; Chung, U.I.; Sakai, T. “Nonswellable” Hydrogel without Mechanical Hysteresis. Science 2014, 343, 873–875. [Google Scholar] [CrossRef]
- Bunk, C.; Löser, L.; Fribiczer, N.; Komber, H.; Jakisch, L.; Scholz, R.; Voit, B.; Seiffert, S.; Saalwächter, K.; Lang, M.; et al. Amphiphilic Model Networks based on PEG and PCL Tetra-arm Star Polymers with Complementary Reactivity. Macromolecules 2022, 55, 6573–6589. [Google Scholar] [CrossRef]
- Apostolides, D.E.; Patrickios, C.S.; Simon, M.; Gradzielski, M.; Blanazs, A.; Mussault, C.; Marcellan, A.; Alexander, N.; Wesdemiotis, C. Model Dynamic Covalent Thermoresponsive Amphiphilic Polymer Co-networks Based on Acylhydrazone End-linked Tetronic T904 Star Block Copolymers. Polym. Chem. 2023, 14, 201–211. [Google Scholar] [CrossRef]
- Löser, L.; Bunk, C.; Scholz, R.; Lang, M.; Böhme, F.; Saalwächter, K. Structural Characterization of Amphiphilic CoNetworks in Selective and Nonselective Solvents Using 1H NMR and SAXS. Macromolecules 2024, 57, 940–954. [Google Scholar] [CrossRef]
- Apostolides, D.E.; Michael, G.; Patrickios, C.S.; Notredame, B.; Zhang, Y.; Gohy, J.-F.; Prévost, S.; Gradzielski, M.; Jung, F.A.; Papadakis, C.M. Dynamic Covalent Amphiphilic Polymer Conetworks Based on End-linked Pluronic F108: Preparation, Characterization and Evaluation as Matrices for Gel Polymer Electrolytes. ACS Appl. Mater. Interfaces 2024, 16, 23813–23825. [Google Scholar] [CrossRef]
- Wang, P.; Deng, G.; Zhou, L.; Li, Z.; Chen, Y. Ultrastretchable, Self-Healable Hydrogels Based on Dynamic Covalent Bonding and Triblock Copolymer Micellization. ACS Macro Lett. 2017, 6, 881–886. [Google Scholar] [CrossRef]
- Mortensen, K.; Annaka, M. Structural Study of Four-Armed Amphiphilic Star-Block Copolymers: Pristine and End-Linked Tetronic T1307. ACS Macro Lett. 2016, 5, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, M.; Ito, M.; Mortensen, K.; Yashima, S.; Tokita, M.; Annaka, M. Friction Coefficient of Well-Defined Hydrogel Networks. Macromolecules 2016, 49, 634–642. [Google Scholar] [CrossRef]
- Mortensen, K.; Annaka, M. Stretching PEO−PPO Type of Star Block Copolymer Gels: Rheology and Small-Angle Scattering. ACS Macro Lett. 2018, 7, 1438–1442. [Google Scholar] [CrossRef]
- Walker, C.N.; Bryson, K.C.; Hayward, R.C.; Tew, G.N. Wide Bicontinuous Compositional Windows from Co-Networks Made with Telechelic Macromonomers. ACS Nano 2014, 8, 12376–12385. [Google Scholar] [CrossRef]
- Kondo, S.; Hiroi, T.; Han, Y.S.; Kim, T.H.; Shibayama, M.; Chung, U.I.; Sakai, T. Reliable Hydrogel with Mechanical “Fuse Link” in an Aqueous Environment. Adv. Mater. 2015, 27, 7407–7411. [Google Scholar] [CrossRef]
- Clarke, B.R.; Tew, G.N. Bottlebrush Amphiphilic Polymer Co-Networks. Macromolecules 2022, 55, 5131–5139. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, S.T.R.; Belluati, A.; Tervoort, E.; Mattich, I.; Hertel, B.; Russell, S.; Gouveia, M.G.; Grysan, P.; Mugemana, C.; Studart, A.R.; et al. Microfluidically Produced Microcapsules with Amphiphilic Polymer Conetwork Shells. Adv. Mater. Technol. 2024, 9, 2400109. [Google Scholar] [CrossRef]
- Bunk, C.; Fribiczer, N.; Lucas, L.; Geisler, M.; Brigitte, V.; Seiffert, S.; Saalwächter, K.; Lang, M.; Böhme, F. Amphiphilic Polymer Co-Networks based on Cross-linked Tetra-PEG-b-PCL Star Block Copolymers. Polymer 2024, 304, 127149. [Google Scholar] [CrossRef]
- Benski, L.; Viran, I.; Katzenberg, F.; Tiller, J.C. Small-Angle X-Ray Scattering Measurements on Amphiphilic Polymer Conetworks Swollen in Orthogonal Solvents. Macromol. Chem. Phys. 2021, 222, 2000292. [Google Scholar] [CrossRef]
- Krumm, C.; Konieczny, S.; Dropalla, G.J.; Milbradt, M.; Tiller, J.C. Amphiphilic Polymer Conetworks Based on End Group Cross-Linked Poly(2-oxazoline) Homo- and Triblock Copolymers. Macromolecules 2013, 46, 3234–3245. [Google Scholar] [CrossRef]
- Erdödi, G.; Iván, B. Novel Amphiphilic Conetworks Composed of Telechelic Poly(ethylene oxide) and Three-Arm Star Polyisobutylene. Chem. Mater. 2004, 16, 959–962. [Google Scholar] [CrossRef]
- Binder, W.H.; Petraru, L.; Roth, T.; Groh, P.W.; Pálfi, V.; Keki, S.; Ivan, B. Magnetic and Temperature-Sensitive Release Gels from Supramolecular Polymers. Adv. Funct. Mater. 2007, 17, 1317–1326. [Google Scholar] [CrossRef]
- Wilhelm, S.A.; Maricanov, M.; Brandt, V.; Katzenberg, F.; Tiller, J.C. Amphiphilic Polymer Conetworks with Ideal and Non-ideal Swelling Behavior Demonstrated by Small Angle X-ray Scattering. Polymer 2022, 242, 124582. [Google Scholar] [CrossRef]
- Velasquez, S.T.R.; Jang, D.; Jenkins, P.; Liu, P.; Yang, L.; Korley, L.T.J.; Bruns, N. Peptide-Reinforced Amphiphilic Polymer Conetworks. Adv. Funct. Mater. 2022, 32, 2207317. [Google Scholar] [CrossRef]
- Subirós-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An Efficient Additive for Peptide Synthesis to Replace the Benzotriazole-based HOBt and HOAt with a Lower Risk of Explosion. Chem. Eur. J. 2009, 15, 9394–9403. [Google Scholar] [CrossRef]
- El-Faham, A.; Al Marhoon, Z.; Abdel-Megeed, A.; Albericio, F. OxymaPure/DIC: An Efficient Reagent for the Synthesis of a Novel Series of 4-[2-(2-Acetylaminophenyl)-2-oxo-acetylamino]Benzoyl Amino Acid Ester Derivatives. Molecules 2013, 18, 14747–14759. [Google Scholar] [CrossRef] [PubMed]
- Apostolides, D.E.; Sakai, T.; Patrickios, C.S. Dynamic Covalent Star Poly(ethylene glycol) Model Hydrogels: A New Platform for Mechanically Robust, Multifunctional Materials. Macromolecules 2017, 50, 2155–2164. [Google Scholar] [CrossRef]
- Hamley, I.W.; Kirkham, S.; Dehsorkhi, A.; Castelletto, V.; Adamcik, J.; Mezzenga, R.; Ruokolainen, J.; Mazzuca, C.; Gatto, E.; Venanzi, M.; et al. Self-Assembly of a Model Peptide Incorporating a Hexa-Histidine Sequence Attached to an Oligo-Alanine Sequence, and Binding to Gold NTA/Nickel Nanoparticles. Biomacromolecules 2014, 15, 3412–3420. [Google Scholar] [CrossRef]
- Dewhurst, C.D. Graphical Reduction and Analysis Small-angle Neutron Scattering Program: GRASP. J. Appl. Cryst. 2023, 56, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
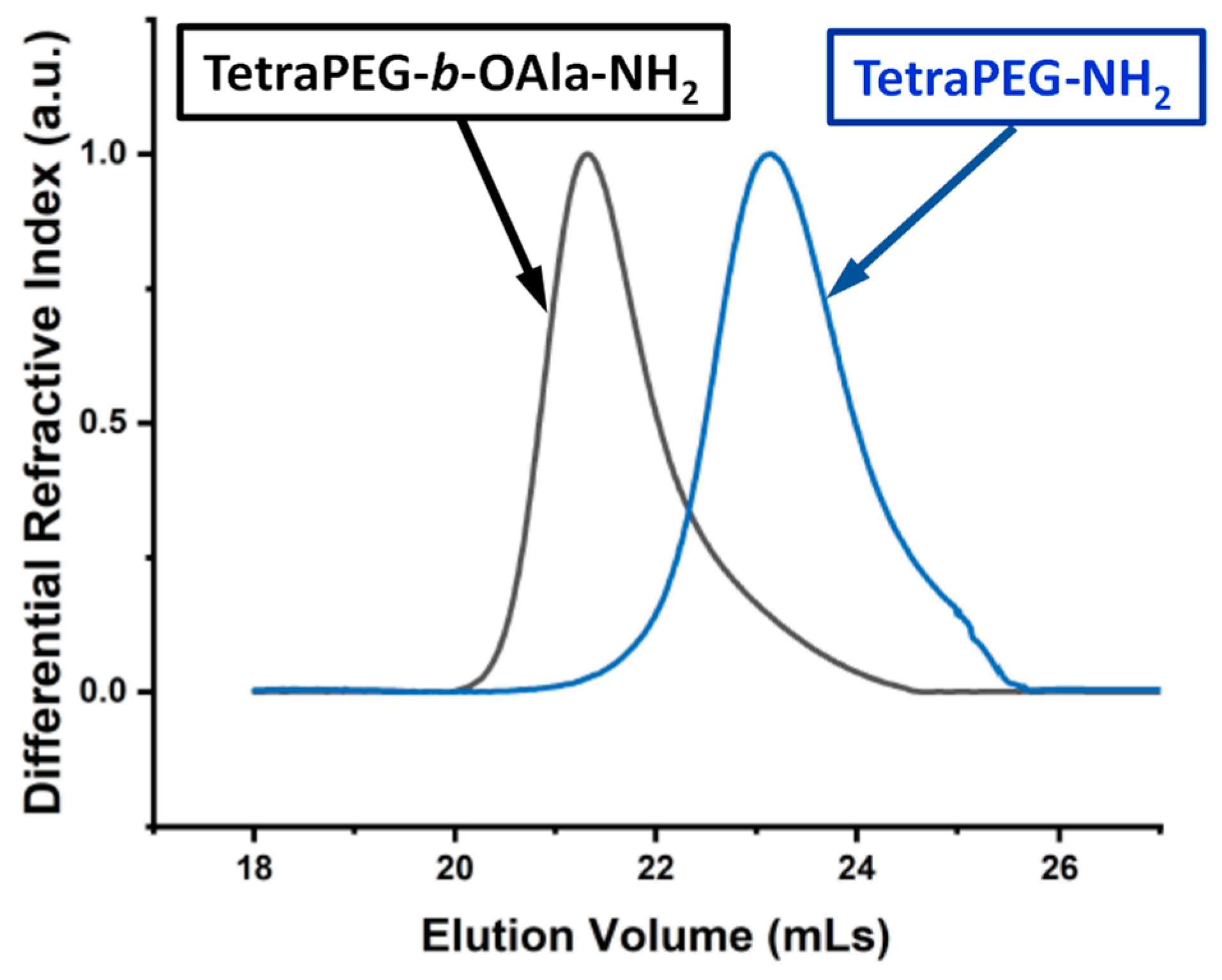
| No. | Sample Name | MMtheory (g mol–1) | Results from SEC | |
|---|---|---|---|---|
| Mn (g mol–1) | Ð | |||
| 1 | TetraPEG-NH2 | 9996 | 10,900 | 1.12 |
| 2 | TetraPEG-b-OAla-NH2 | 11,700 | 12,700 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolides, D.E.; Michael, G.; Patrickios, C.S.; Sakai, T.; Kyroglou, I.; Kasimatis, M.; Iatrou, H.; Prévost, S.; Gradzielski, M. The First Example of a Model Amphiphilic Polymer Conetwork Containing a Hydrophobic Oligopeptide: The Case of End-Linked Tetra[Poly(ethylene glycol)-b-oligo(L-alanine)]. Gels 2025, 11, 331. https://doi.org/10.3390/gels11050331
Apostolides DE, Michael G, Patrickios CS, Sakai T, Kyroglou I, Kasimatis M, Iatrou H, Prévost S, Gradzielski M. The First Example of a Model Amphiphilic Polymer Conetwork Containing a Hydrophobic Oligopeptide: The Case of End-Linked Tetra[Poly(ethylene glycol)-b-oligo(L-alanine)]. Gels. 2025; 11(5):331. https://doi.org/10.3390/gels11050331
Chicago/Turabian StyleApostolides, Demetris E., George Michael, Costas S. Patrickios, Takamasa Sakai, Iro Kyroglou, Maria Kasimatis, Hermis Iatrou, Sylvain Prévost, and Michael Gradzielski. 2025. "The First Example of a Model Amphiphilic Polymer Conetwork Containing a Hydrophobic Oligopeptide: The Case of End-Linked Tetra[Poly(ethylene glycol)-b-oligo(L-alanine)]" Gels 11, no. 5: 331. https://doi.org/10.3390/gels11050331
APA StyleApostolides, D. E., Michael, G., Patrickios, C. S., Sakai, T., Kyroglou, I., Kasimatis, M., Iatrou, H., Prévost, S., & Gradzielski, M. (2025). The First Example of a Model Amphiphilic Polymer Conetwork Containing a Hydrophobic Oligopeptide: The Case of End-Linked Tetra[Poly(ethylene glycol)-b-oligo(L-alanine)]. Gels, 11(5), 331. https://doi.org/10.3390/gels11050331













