Development of Polyampholyte Cellulose-Based Hydrogels for Diapers with Improved Biocompatibility
Abstract
1. Introduction
2. Results and Discussion
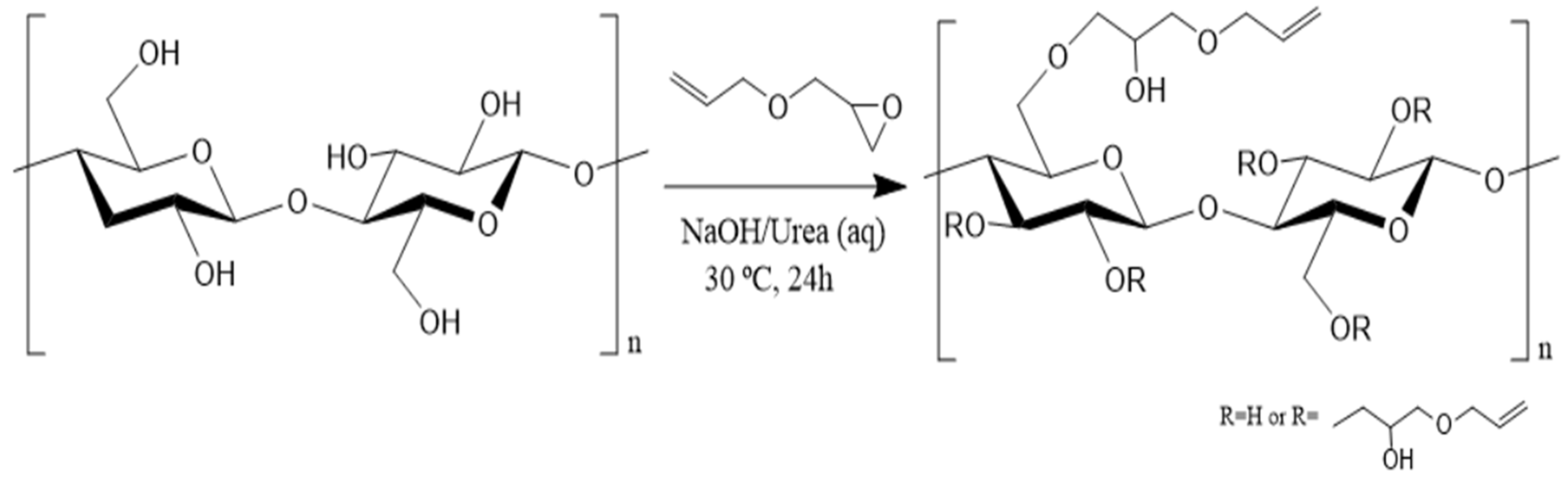
2.1. Synthesis and Characterization of AC Derivative
2.1.1. NMR Spectroscopy of AC Derivative
2.1.2. FTIR Spectroscopy of AC Derivative
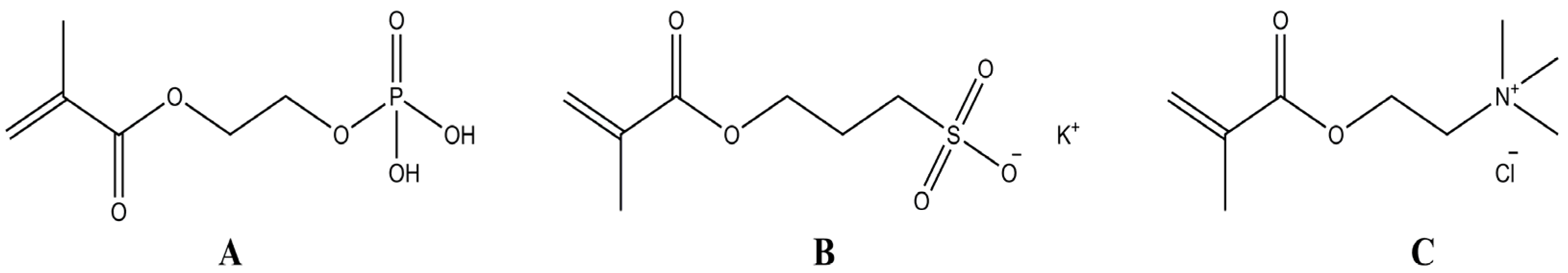
2.2. Preparation and Optimization of Cellulose-Based Hydrogels
2.2.1. Effect of Monomers Ratio
2.2.2. Effect of Crosslinker Quantity
2.2.3. Effect of Initiator Amount
2.2.4. AC: Monomers Ratio Influence
2.3. Evaluation of the Most Promising Cellulose-Based Hydrogels for Practical Applications
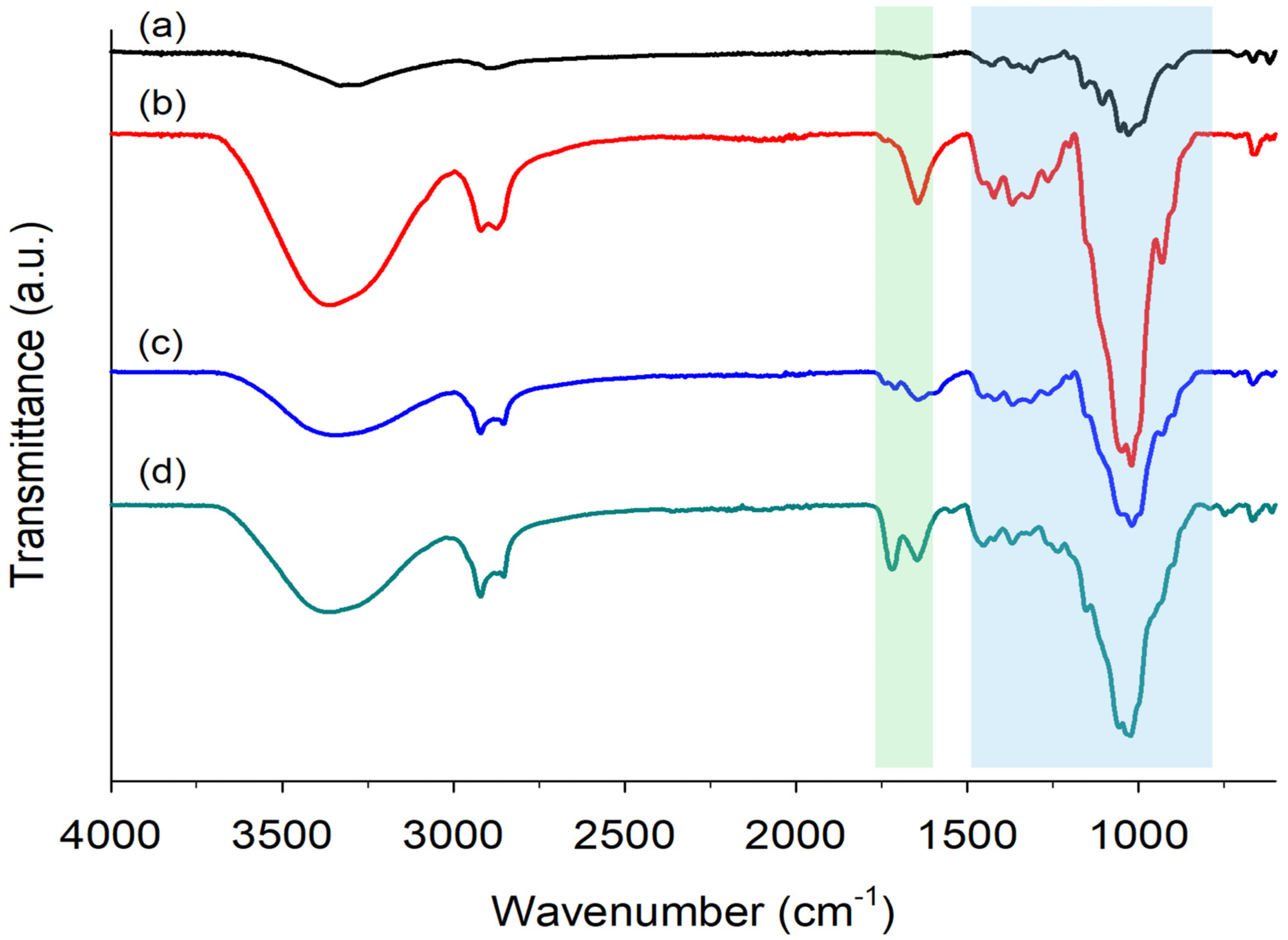
2.3.1. FTIR Spectroscopy of Hydrogels
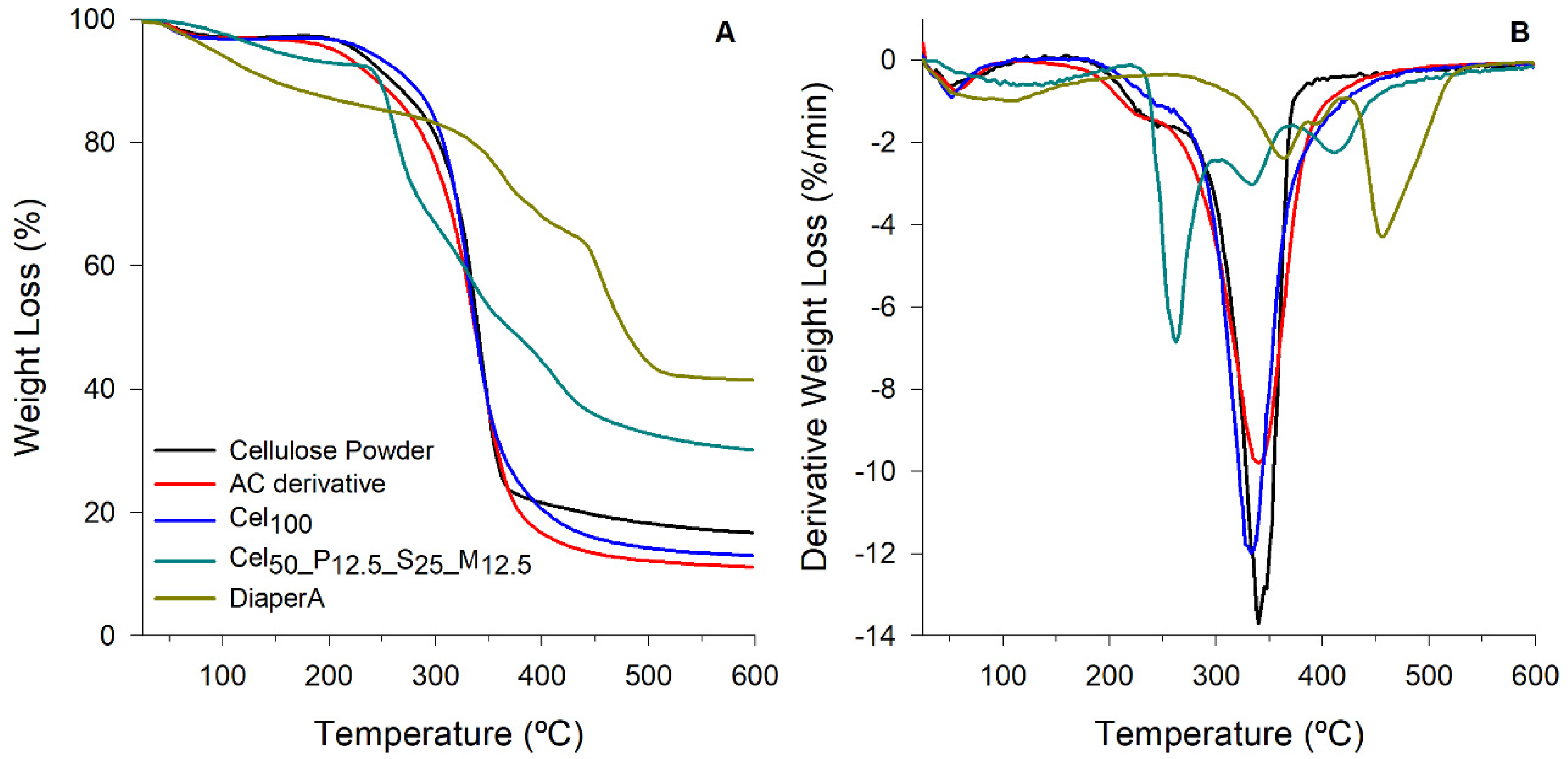
2.3.2. Thermal Properties
2.3.3. Swelling Capacity
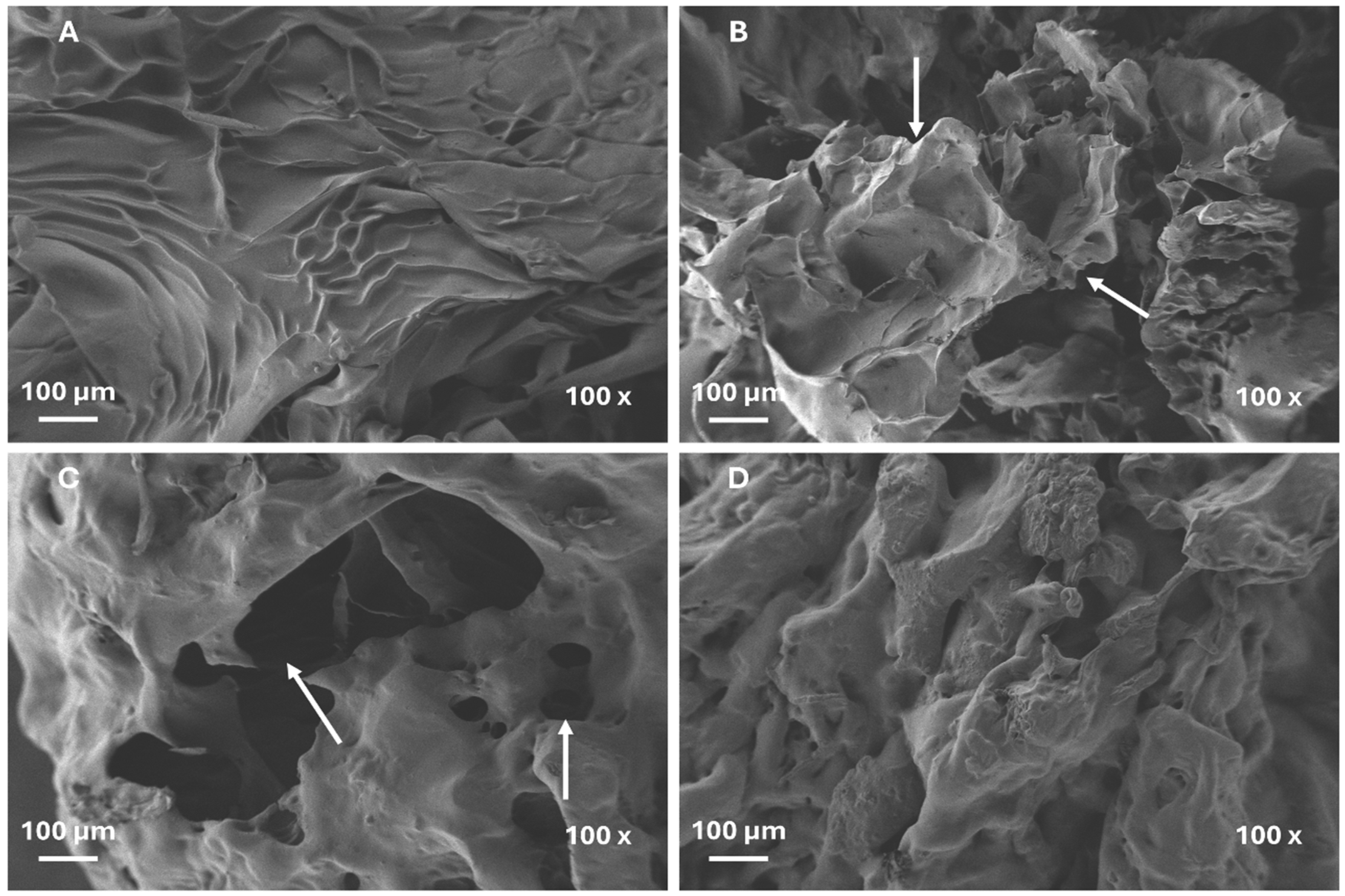
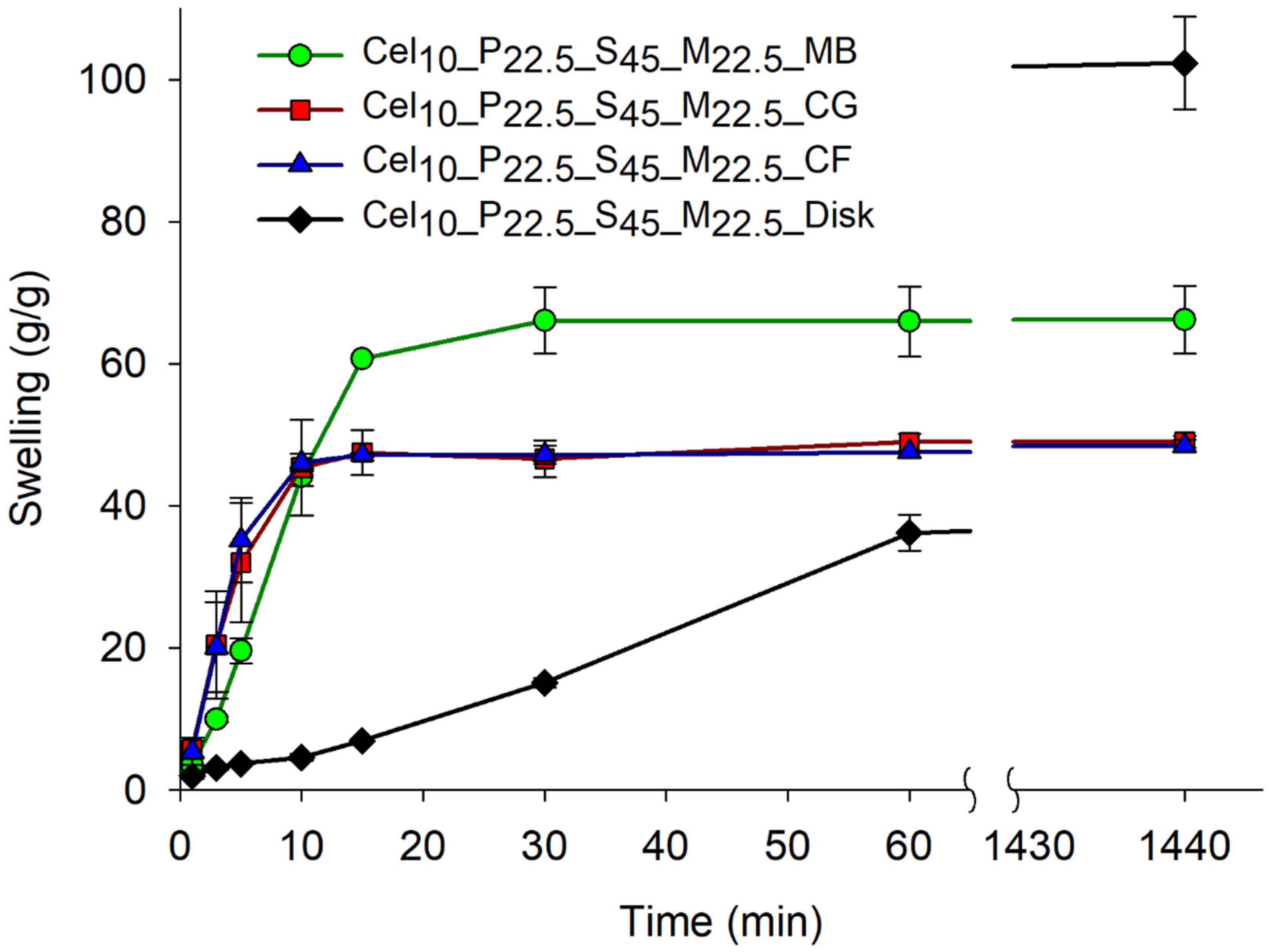
- Particle-size and morphology impact on the swelling capacity
- Fluids salinity influence
- Fluids pH effect
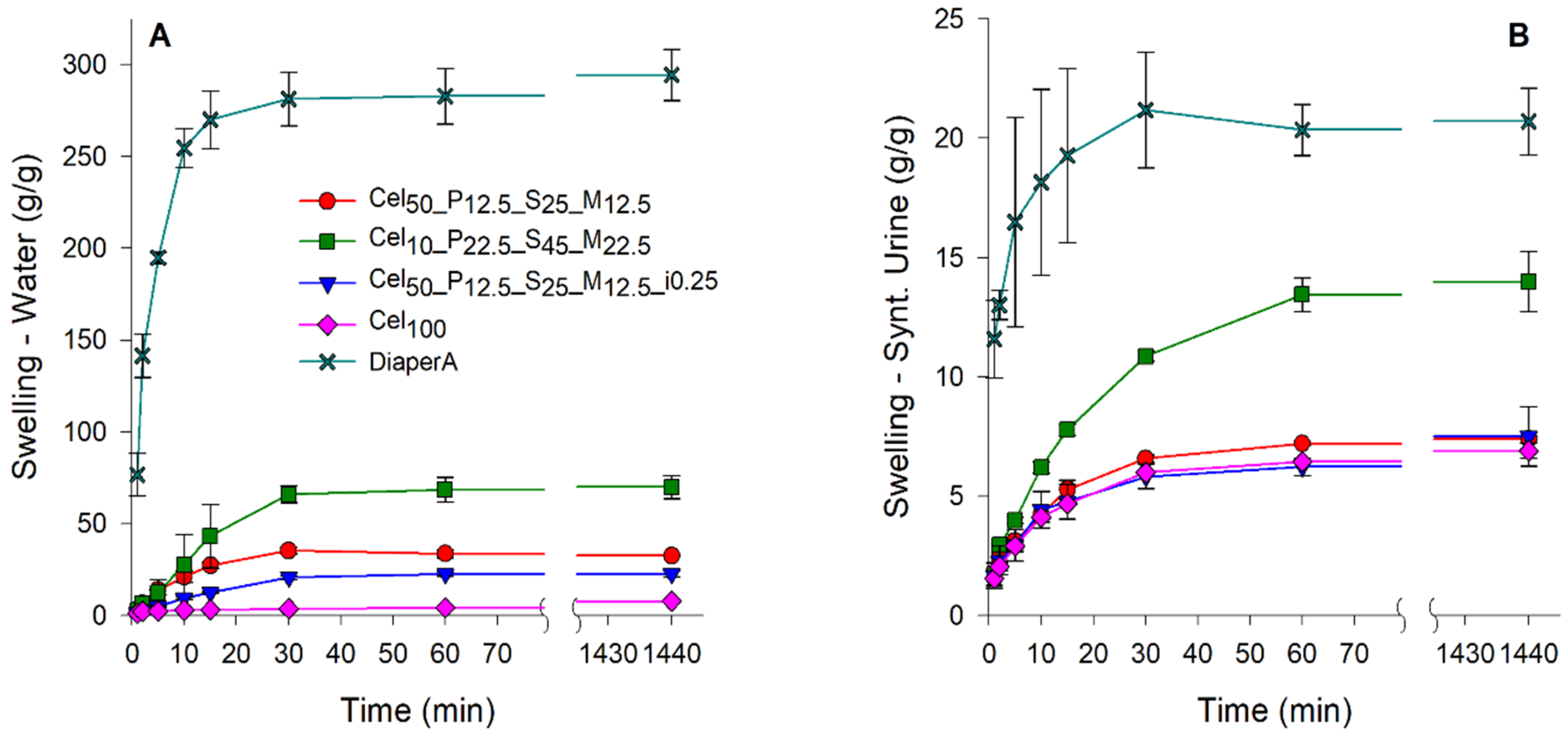
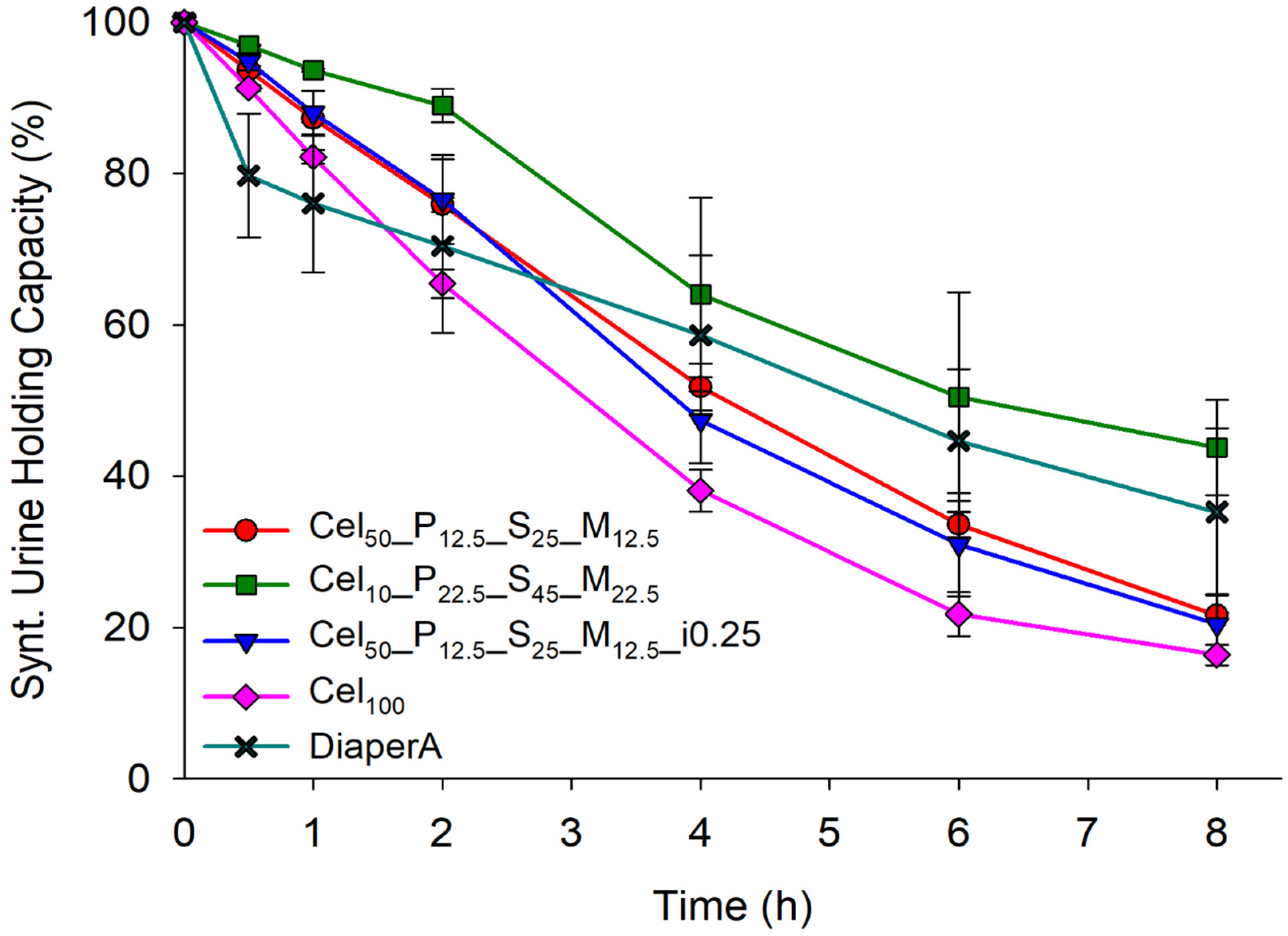
2.3.4. Fluids Retention Capacity of Hydrogels
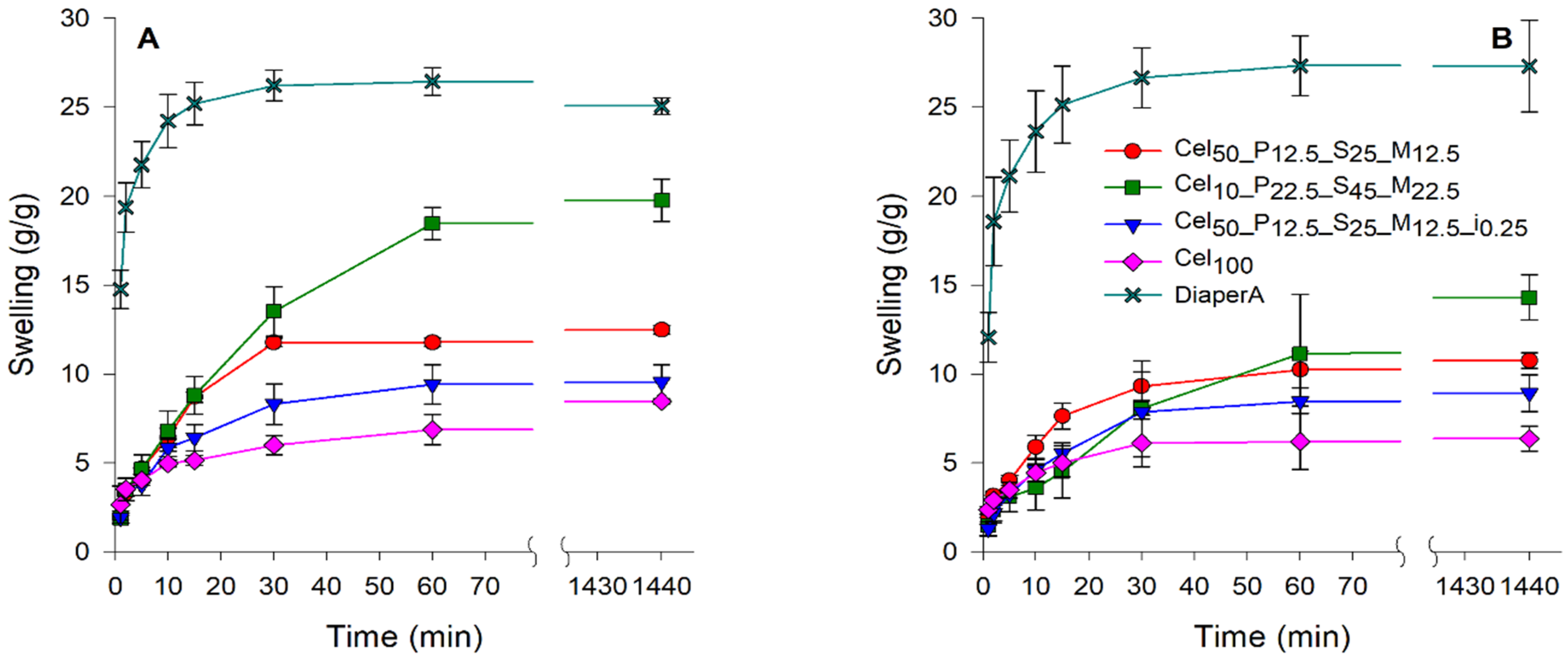
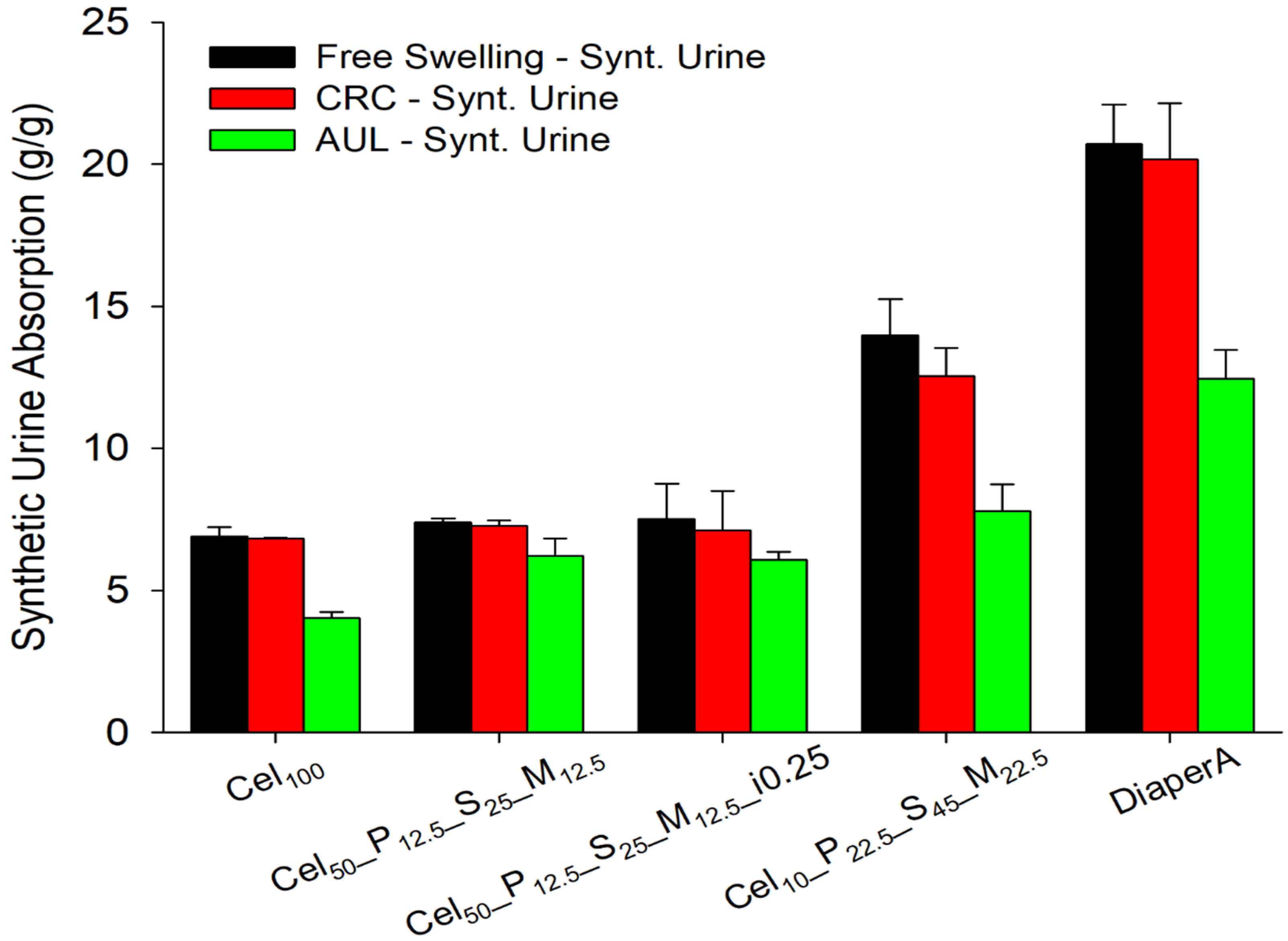
2.3.5. Centrifuge Retention Capacity (CRC) of Hydrogels
2.3.6. Absorbency Underload (AUL) of Hydrogels
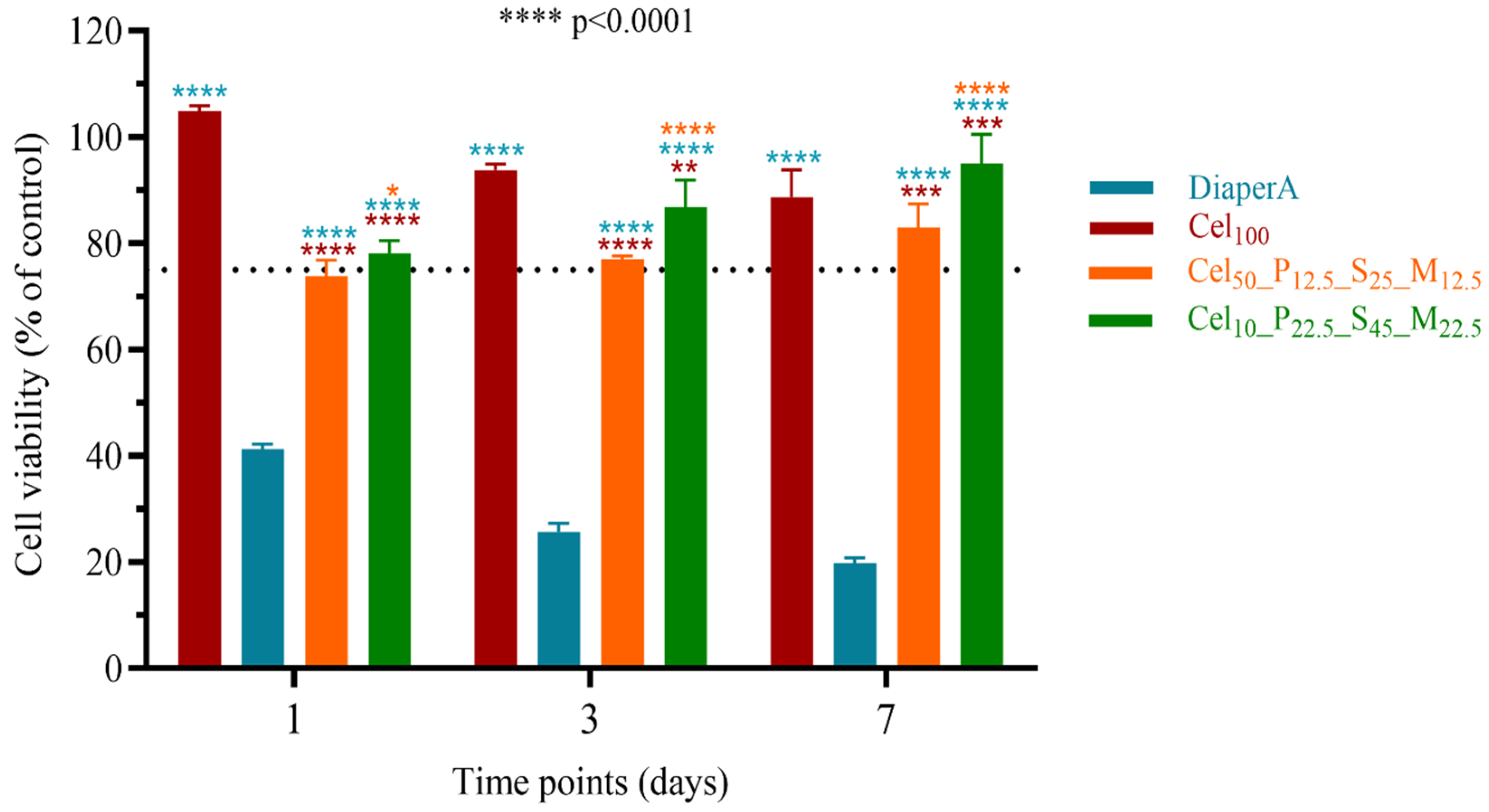
2.3.7. Cytotoxicity Evaluation of Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Cellulose Pulp Pre-Treatment
4.2.2. Allyl Cellulose (AC) Derivative Preparation
4.2.3. Cellulose Hydrogels Preparation
4.2.4. Isolation of SAP from Commercial Diaper
4.3. Characterizations
4.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
4.3.2. Nuclear Magnetic Resonance Spectroscopy (NMR)
4.3.3. Morphology
4.3.4. Thermal Studies
4.3.5. Gel Fraction
4.3.6. Free-Swelling Capacity
4.3.7. Swelling—Synthetic Urine and pH Buffers
4.3.8. Synthetic Urine Retention Capacity
4.3.9. Absorbency Under Load
4.3.10. Centrifuge Retention Capacity
4.3.11. Cytotoxicity Evaluation
4.3.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashari, A.; Rouhani Shirvan, A.; Shakeri, M. Cellulose-Based Hydrogels for Personal Care Products. Polym. Adv. Technol. 2018, 29, 2853–2867. [Google Scholar] [CrossRef]
- Perez, M.V.; Navarro, P.X.S.; Morillas, A.V.; Valdemar, R.M.E.; Araiza, J.P.H.L. Waste Management and Environmental Impact of Absorbent Hygiene Products: A Review. Waste Manag. Res. 2021, 39, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kwon, H.; Kim, J. Safety Evaluation of Absorbent Hygiene Pads: A Review on Assessment Framework and Test Methods. Sustainability 2018, 10, 4146. [Google Scholar] [CrossRef]
- Jyoti, D.; Sinha, R. Physiological Impact of Personal Care Product Constituents on Non-Target Aquatic Organisms. Sci. Total Environ. 2023, 905, 167229. [Google Scholar] [CrossRef]
- Ntekpe, M.; Mbong, E.; Mbong, O.; Edem, E.; Edem, N.; Hussain, S. Disposable Diapers: Impact of Disposal Methods on Public Health and the Environment. Am. J. Med. Public Health 2020, 1, 1009. [Google Scholar]
- Płotka-Wasylka, J.; Makoś-Chełstowska, P.; Kurowska-Susdorf, A.; Treviño, M.J.S.; Guzmán, S.Z.; Mostafa, H.; Cordella, M. End-of-Life Management of Single-Use Baby Diapers: Analysis of Technical, Health and Environment Aspects. Sci. Total Environ. 2022, 836, 155339. [Google Scholar] [CrossRef] [PubMed]
- Ismaeilimoghadam, S.; Jonoobi, M.; Hamzeh, Y.; Azimi, B.; Mezzetta, A.; Guazzelli, L.; Cinelli, P.; Seggiani, M.; Danti, S. Development and Characterization of Sodium Alginate-Based Bio-Hybrid Super Absorbent Polymer with High Retention Capacity Suitable for Baby Diapers. J. Polym. Environ. 2024, 32, 5212–5230. [Google Scholar] [CrossRef]
- Demichelis, F.; Martina, C.; Fino, D.; Tommasi, T.; Deorsola, F.A. Life Cycle Assessment of Absorbent Hygiene Product Waste: Evaluation and Comparison of Different End-of-Life Scenarios. Sustain. Prod. Consum. 2023, 38, 356–371. [Google Scholar] [CrossRef]
- Tsigkou, K.; Zagklis, D.; Vasileiadi, A.; Kostagiannakopoulou, C.; Sotiriadis, G.; Anastopoulos, I.; Kostopoulos, V.; Zafiri, C.; Kornaros, M. Used Disposable Nappies: Environmental Burden or Resource for Biofuel Production and Material Recovery? Resour. Conserv. Recycl. 2022, 185, 106493. [Google Scholar] [CrossRef]
- Carlucci, G. New Technologies for Feminine Hygiene Products with Reduced Environmental Impact. In Proceedings of the WIT Transactions on Ecology and the Environment; WIT Press: Sri Ganganagar, India, 2012; Volume 155, pp. 597–606. [Google Scholar]
- Yang, Y.; Liang, Z.; Zhang, R.; Zhou, S.; Yang, H.; Chen, Y.; Zhang, J.; Yin, H.; Yu, D. Research Advances in Superabsorbent Polymers. Polymers 2024, 16, 501. [Google Scholar] [CrossRef]
- Haque, M.O.; Mondal, M.I.H. Cellulose-Based Hydrogel for Personal Hygiene Applications. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–21. ISBN 978-3-319-76573-0. [Google Scholar]
- Zhang, W.; Wang, P.; Liu, S.; Chen, J.; Chen, R.; He, X.; Ma, G.; Lei, Z. Factors Affecting the Properties of Superabsorbent Polymer Hydrogels and Methods to Improve Their Performance: A Review. J. Mater. Sci. 2021, 56, 16223–16242. [Google Scholar] [CrossRef]
- Ma, X.; Wen, G. Development History and Synthesis of Super-Absorbent Polymers: A Review. J. Polym. Res. 2020, 27, 136. [Google Scholar] [CrossRef]
- Buchholz, F.L. Superabsorbent Polymers: An Idea Whose Time Has Come. J. Chem. Educ. 1996, 73, 512. [Google Scholar] [CrossRef]
- Darbre, P.D. (Ed.) Chapter 1-Introduction to Personal Care Products. In Personal Care Products and Human Health; Academic Press: London, UK, 2023; pp. 3–31. ISBN 978-0-323-99684-6. [Google Scholar]
- Patiño-Masó, J.; Serra-Parareda, F.; Tarrés, Q.; Mutjé, P.; Espinach, F.X.; Delgado-Aguilar, M. TEMPO-Oxidized Cellulose Nanofibers: A Potential Bio-Based Superabsorbent for Diaper Production. Nanomaterials 2019, 9, 1271. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Bhaladhare, S.; Das, D. Cellulose: A Fascinating Biopolymer for Hydrogel Synthesis. J. Mater. Chem. B 2022, 10, 1923–1945. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Nishat, N.; Jadoun, S.; Ansari, M.Z. Polysaccharide Based Superabsorbent Hydrogels and Their Methods of Synthesis: A Review. Carbohydr. Polym. Technol. Appl. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Zou, P.; Yao, J.; Cui, Y.-N.; Zhao, T.; Che, J.; Yang, M.; Li, Z.; Gao, C. Advances in Cellulose-Based Hydrogels for Biomedical Engineering: A Review Summary. Gels 2022, 8, 364. [Google Scholar] [CrossRef]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose Hydrogels: Green and Sustainable Soft Biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Berradi, A.; Aziz, F.; Achaby, M.E.; Ouazzani, N.; Mandi, L. A Comprehensive Review of Polysaccharide-Based Hydrogels as Promising Biomaterials. Polymers 2023, 15, 2908. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-Based Hydrogels: Present Status and Application Prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Liu, T.; Chen, W.; Li, K.; Long, S.; Li, X.; Huang, Y. Toughening Weak Polyampholyte Hydrogels with Weak Chain Entanglements via a Secondary Equilibrium Approach. Polymers 2023, 15, 2644. [Google Scholar] [CrossRef]
- Toleutay, G.; Su, E.; Yelemessova, G. Equimolar Polyampholyte Hydrogel Synthesis Strategies with Adaptable Properties. Polymers 2023, 15, 3131. [Google Scholar] [CrossRef]
- Haag, S.L.; Bernards, M.T. Polyampholyte Hydrogels in Biomedical Applications. Gels 2017, 3, 41. [Google Scholar] [CrossRef]
- Arredondo, R.; Yuan, Z.; Sosa, D.; Johnson, A.; Beims, R.F.; Li, H.; Wei, Q.; Xu, C.C. Performance of a Novel, Eco-Friendly, Cellulose-Based Superabsorbent Polymer (Cellulo-SAP): Absorbency, Stability, Reusability, and Biodegradability. Can. J. Chem. Eng. 2023, 101, 1762–1771. [Google Scholar] [CrossRef]
- Omidian, H.; Akhzarmehr, A.; Chowdhury, S.D. Advancements in Cellulose-Based Superabsorbent Hydrogels: Sustainable Solutions across Industries. Gels 2024, 10, 174. [Google Scholar] [CrossRef]
- Qi, H.; Chang, C.; Zhang, L. Effects of Temperature and Molecular Weight on Dissolution of Cellulose in NaOH/Urea Aqueous Solution. Cellulose 2008, 15, 779–787. [Google Scholar] [CrossRef]
- Paula, C.T.B.; Rebelo, R.C.; Coelho, J.; Serra, A.C. The Impact of the Introduction of Hydrolyzed Cellulose on the Thermal and Mechanical Properties of LDPE Composites. Eur. J. Wood Wood Prod. 2019, 77, 1095–1106. [Google Scholar] [CrossRef]
- Setu, M.N.I.; Mia, M.Y.; Lubna, N.J.; Chowdhury, A.A. Preparation of Microcrystalline Cellulose from Cotton and Its Evaluation as Direct Compressible Excipient in the Formulation of Naproxen Tablets. Dhaka Univ. J. Pharm. Sci. 2015, 13, 187–192. [Google Scholar] [CrossRef]
- Qi, H.; Liebert, T.; Heinze, T. Homogenous Synthesis of 3-Allyloxy-2-Hydroxypropyl-Cellulose in NaOH/Urea Aqueous System. Cellulose 2012, 19, 925–932. [Google Scholar] [CrossRef]
- Tong, R.; Chen, G.; Tian, J.; He, M. Highly Transparent, Weakly Hydrophilic and Biodegradable Cellulose Film for Flexible Electroluminescent Devices. Carbohydr. Polym. 2020, 227, 115366. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Chen, G.; Pan, D.; Qi, H.; Li, R.; Tian, J.; Lu, F.; He, M. Highly Stretchable and Compressible Cellulose Ionic Hydrogels for Flexible Strain Sensors. Biomacromolecules 2019, 20, 2096–2104. [Google Scholar] [CrossRef]
- Silva, R.; Rebelo, R.C.; Paula, C.T.B.; Pereira, P.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J. All-Cellulose Resin for 3D Printing Hydrogels via Digital Light Processing (DLP). Int. J. Biol. Macromol. 2025, 306, 141389. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Rebelo, R.C.; Coelho, J.F.J.; Serra, A.C. Novel Thermally Regenerated Flexible Cellulose-Based Films. Eur. J. Wood Wood Prod. 2024, 82, 1813–1826. [Google Scholar] [CrossRef]
- Ribeiro, D.C.M.; Rebelo, R.C.; De Bon, F.; Coelho, J.F.J.; Serra, A.C. Process Development for Flexible Films of Industrial Cellulose Pulp Using Superbase Ionic Liquids. Polymers 2021, 13, 1767. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.C.; Ribeiro, D.C.M.; Pereira, P.; De Bon, F.; Coelho, J.F.J.; Serra, A.C. Cellulose-Based Films with Internal Plasticization with Epoxidized Soybean Oil. Cellulose 2023, 30, 1823–1840. [Google Scholar] [CrossRef]
- Tong, R.; Chen, G.; Tian, J.; He, M. Highly Stretchable, Strain-Sensitive, and Ionic-Conductive Cellulose-Based Hydrogels for Wearable Sensors. Polymers 2019, 11, 2067. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, D.H.; Lee, Y.S. The Influence of Monomer Composition and Surface-CrossLinking Condition on Biodegradation and Gel Strength of Super Absorbent Polymer. Polymers 2021, 13, 663. [Google Scholar] [CrossRef]
- Mallik, A.K.; Shahruzzaman, M.; Sakib, M.N.; Zaman, A.; Rahman, M.S.; Islam, M.M.; Islam, M.S.; Haque, P.; Rahman, M.M. Benefits of Renewable Hydrogels over Acrylate and Acrylamide-Based Hydrogels BT. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 197–243. ISBN 978-3-319-77830-3. [Google Scholar]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic Acid/Acrylamide Based Hydrogels and Its Properties—A Review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Klaunig, J.E. Acrylamide Carcinogenicity. J. Agric. Food Chem. 2008, 56, 5984–5988. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Pfeifer, G.P. A Review of Mechanisms of Acrylamide Carcinogenicity. Carcinogenesis 2007, 28, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Beland, F.A.; Mellick, P.W.; Olson, G.R.; Mendoza, M.C.B.; Marques, M.M.; Doerge, D.R. Carcinogenicity of Acrylamide in B6C3F1 Mice and F344/N Rats from a 2-Year Drinking Water Exposure. Food Chem. Toxicol. 2013, 51, 149–159. [Google Scholar] [CrossRef]
- McLaughlin, J.E.; Parno, J.; Garner, F.M.; Clary, J.J.; Thomas, W.C.; Murphy, S.R. Comparison of the Maximum Tolerated Dose (MTD) Dermal Response in Three Strains of Mice Following Repeated Exposure to Acrylic Acid. Food Chem. Toxicol. 1995, 33, 507–513. [Google Scholar] [CrossRef]
- Vodička, P.; Gut, I.; Frantík, E. Effects of Inhaled Acrylic Acid Derivatives in Rats. Toxicology 1990, 65, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Lugović-Mihić, L.; Filija, E.; Varga, V.; Premuž, L.; Parać, E.; Tomašević, R.; Barac, E.; Špiljak, B. Unwanted Skin Reactions to Acrylates: An Update. Cosmetics 2024, 11, 127. [Google Scholar] [CrossRef]
- Nikolić, L.B.; Zdravković, A.S.; Nikolić, V.D.; Ilić-Stojanović, S.S. Synthetic Hydrogels and Their Impact on Health and Environment. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1363–1391. ISBN 978-3-319-77830-3. [Google Scholar]
- Kemal, E.; Adesanya, K.O.; Deb, S. Phosphate Based 2-Hydroxyethyl Methacrylate Hydrogels for Biomedical Applications. J. Mater. Chem. 2011, 21, 2237–2245. [Google Scholar] [CrossRef]
- Guven, M.N.; Balaban, B.; Demirci, G.; Yagci Acar, H.; Okay, O.; Avci, D. Bisphosphonate-Functionalized Poly(Amido Amine) Crosslinked 2-Hydroxyethyl Methacrylate Hydrogel as Tissue Engineering Scaffold. Eur. Polym. J. 2021, 159, 110732. [Google Scholar] [CrossRef]
- Paripovic, D.; Hall-Bozic, H.; Klok, H.-A. Osteoconductive Surfaces Generated from Peptide Functionalized Poly(2-Hydroxyethyl Methacrylate-Co-2-(Methacryloyloxy)Ethyl Phosphate) Brushes. J. Mater. Chem. 2012, 22, 19570–19578. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Photopolymerization of Phosphoric Acid Ester-Based Self-Etch Dental Adhesives. Dent. Mater. J. 2013, 32, 10–18. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lambré, C.; et al. Safety Assessment of the Substance Phosphoric Acid, Mixed Esters with 2-Hydroxyethyl Methacrylate, for Use in Food Contact Materials. EFSA J. 2020, 18, e06120. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.A.L.; Fonseca, A.C.; Fabela, I.G.P.; Serra, A.C. Synthesis and Characterization of High Performance Superabsorbent Hydrogels Using Bis[2-(Methacryloyloxy)Ethyl] Phosphate as Crosslinker. Express Polym. Lett. 2016, 10, 248–258. [Google Scholar] [CrossRef]
- Romischke, J.; Eickner, T.; Grabow, N.; Kragl, U.; Oschatz, S. 3-Sulfopropyl Acrylate Potassium-Based Polyelectrolyte Hydrogels: Sterilizable Synthetic Material for Biomedical Application. RSC Adv. 2024, 14, 28881–28888. [Google Scholar] [CrossRef]
- Yu, Y.; Cirelli, M.; Li, P.; Ding, Z.; Yin, Y.; Yuan, Y.; de Beer, S.; Vancso, G.J.; Zhang, S. Enhanced Stability of Poly(3-Sulfopropyl Methacrylate Potassium) Brushes Coated on Artificial Implants in Combatting Bacterial Infections. Ind. Eng. Chem. Res. 2019, 58, 21459–21465. [Google Scholar] [CrossRef]
- Gallo, M.; Gámiz, F. Choline: An Essential Nutrient for Human Health. Nutrients 2023, 15, 2900. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: An Essential Nutrient for Humans. Nutrition 2000, 16, 669–671. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Xu, D.; Zhang, X.; Du, J.; Liu, R.; Zhao, R.; Chen, Q.; Volodine, A.; Dewil, R.; et al. Choline Chloride Modification of Nanofiltration Membranes for Improving Heavy Metal Ions Separation in Wastewater. J. Memb. Sci. 2024, 708, 123079. [Google Scholar] [CrossRef]
- Fanfoni, L.; Marsich, E.; Turco, G.; Breschi, L.; Cadenaro, M. Development of Di-Methacrylate Quaternary Ammonium Monomers with Antibacterial Activity. Acta Biomater. 2021, 129, 138–147. [Google Scholar] [CrossRef]
- Uka, D.; Kukrić, T.; Krstonošić, V.; Jović, B.; Kordić, B.; Pavlović, K.; Popović, B.M. NADES Systems Comprising Choline Chloride and Polyphenols: Physicochemical Characterization, Antioxidant and Antimicrobial Activities. J. Mol. Liq. 2024, 410, 125683. [Google Scholar] [CrossRef]
- Song, Y.; Sun, N.; Jiang, Y.; Zhu, H.; Yu, Y.; Lai, G.; Yang, X. High Hydrophilic and Antibacterial Efficient UV−Curable Silicone−Containing Choline Chloride Quaternary Ammonium Salts Functionalized Materials. Macromol. Rapid Commun. 2024, 45, 2400300. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Siryk, O.; Goncharuk, O.; Samchenko, Y.; Kernosenko, L.; Szewczuk-Karpisz, K. Comparison of Structural, Water-Retaining and Sorption Properties of Acrylamide-Based Hydrogels Cross-Linked by Physical and Chemical Methods. ChemPhysChem 2024, 25, e202300812. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Liu, Y.-T.; Liu, X.-Y.; Shi, F.-K.; Zhang, L.-Q.; Zhu, M.-F.; Xie, X.-M. Dually Cross-Linked Single Network Poly(Acrylic Acid) Hydrogels with Superior Mechanical Properties and Water Absorbency. Soft Matter 2016, 12, 5420–5428. [Google Scholar] [CrossRef]
- Saraydın, D.; Karadagˇ, E.; Işıkver, Y.; Şahiner, N.; Güven, O. The Influence of Preparation Methods on the Swelling and Network Properties of Acrylamide Hydrogels with Crosslinkers. J. Macromol. Sci. Part A 2004, 41, 419–431. [Google Scholar] [CrossRef]
- Pekcan, Ö.; Kara, S. Gelation Mechanisms. Mod. Phys. Lett. B 2012, 26, 1230019. [Google Scholar] [CrossRef]
- Matsumoto, A.; Kumagai, T.; Aota, H.; Kawasaki, H.; Arakawa, R. Reassessment of Free-Radical Polymerization Mechanism of Allyl Acetate Based on End-Group Determination of Resulting Oligomers by MALDI-TOF-MS Spectrometry. Polym. J. 2009, 41, 26–33. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, C.; Tang, W.; Chen, G.; Yin, S.-N.; Xie, W.; Wu, D. Crosslinking of Bacterial Cellulose toward Fabricating Ultrastretchable Hydrogels for Multiple Sensing with High Sensitivity. ACS Sustain. Chem. Eng. 2023, 11, 11548–11558. [Google Scholar] [CrossRef]
- Hwang, U.; Moon, H.; Park, J.; Jung, H.W. Crosslinking and Swelling Properties of PH-Responsive Poly(Ethylene Glycol)/Poly(Acrylic Acid) Interpenetrating Polymer Network Hydrogels. Polymers 2024, 16, 2149. [Google Scholar] [CrossRef]
- Pruksawan, S.; Lim, J.W.R.; Lee, Y.L.; Lin, Z.; Chee, H.L.; Chong, Y.T.; Chi, H.; Wang, F. Enhancing Hydrogel Toughness by Uniform Cross-Linking Using Modified Polyhedral Oligomeric Silsesquioxane. Commun. Mater. 2023, 4, 75. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Rogovina, S.; Aleksanyan, K.; Prut, E.; Gorenberg, A. Biodegradable Blends of Cellulose with Synthetic Polymers and Some Other Polysaccharides. Eur. Polym. J. 2013, 49, 194–202. [Google Scholar] [CrossRef]
- Li, Z.; Chen, W.; Zhang, L.; Cheng, Z.; Zhu, X. Fast RAFT Aqueous Polymerization in a Continuous Tubular Reactor: Consecutive Synthesis of a Double Hydrophilic Block Copolymer. Polym. Chem. 2015, 6, 5030–5035. [Google Scholar] [CrossRef]
- Lin, J.-T.; Lalevee, J.; Cheng, D.-C. A Critical Review for Synergic Kinetics and Strategies for Enhanced Photopolymerizations for 3D-Printing and Additive Manufacturing. Polymers 2021, 13, 2325. [Google Scholar] [CrossRef]
- Tong, R.; Chen, G.; Pan, D.; Tian, J.; Qi, H.; Li, R.; Lu, F.; He, M. Ultrastretchable and Antifreezing Double-Cross-Linked Cellulose Ionic Hydrogels with High Strain Sensitivity under a Broad Range of Temperature. ACS Sustain. Chem. Eng. 2019, 7, 14256–14265. [Google Scholar] [CrossRef]
- Altin, A.; Akgun, B.; Sarayli Bilgici, Z.; Begum Turker, S.; Avci, D. Synthesis, Photopolymerization, and Adhesive Properties of Hydrolytically Stable Phosphonic Acid-Containing (Meth)Acrylamides. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 511–522. [Google Scholar] [CrossRef]
- Ma, X.; Yun, H.; Wu, N.; Niu, F.; Yu, J. Superior Water-Resistant Poly(2-Hydroxyethyl Methacrylate Phosphate) Flame Retardant and a Transparent, Flame-Retardant, and Biodegradable Poly(Lactide) Blend Film. ACS Appl. Polym. Mater. 2021, 3, 1314–1323. [Google Scholar] [CrossRef]
- Mushtaq, S.; Ahmad, N.M.; Nasir, H.; Mahmood, A.; Janjua, H.A. Transpicuous-Cum-Fouling Resistant Copolymers of 3-Sulfopropyl Methacrylate and Methyl Methacrylate for Optronics Applications in Aquatic Medium and Healthcare. Adv. Polym. Technol. 2020, 2020, 5392074. [Google Scholar] [CrossRef]
- Onder, A.; Ilgin, P.; Ozay, H.; Ozay, O. Removal of Dye from Aqueous Medium with PH-Sensitive Poly[(2-(Acryloyloxy)Ethyl]Trimethylammonium Chloride-Co-1-Vinyl-2-Pyrrolidone] Cationic Hydrogel. J. Environ. Chem. Eng. 2020, 8, 104436. [Google Scholar] [CrossRef]
- Hussein, B.M.A.; Karkosh, Z.S.A.; Safi, I.N.; KarKosh, A.S. Impact of Phosphate Ester Addition on the Cytotoxicity of Heat Cured Denture Base Material. Mater. Today Proc. 2021, 42, 2025–2030. [Google Scholar] [CrossRef]
- De Bon, F.; Azevedo, I.M.; Ribeiro, D.C.M.; Rebelo, R.C.; Coelho, J.F.J.; Serra, A.C. Scaling-Up an Aqueous Self-Degassing Electrochemically Mediated ATRP in Dispersion for the Preparation of Cellulose-Polymer Composites and Films. Polymers 2022, 14, 4981. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.B.; Bhagat, R.K.; Madras, G. Photo and Thermal Degradation of a Cationic Superabsorbent Polymer. Polym. Plast. Technol. Eng. 2013, 52, 58–65. [Google Scholar] [CrossRef]
- Pelras, T.; Hofman, A.H.; Germain, L.M.H.; Maan, A.M.C.; Loos, K.; Kamperman, M. Strong Anionic/Charge-Neutral Block Copolymers from Cu(0)-Mediated Reversible Deactivation Radical Polymerization. Macromolecules 2022, 55, 8795–8807. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of Acrylamide/Acrylic Acid Cellulose Hydrogels for the Adsorption of Heavy Metal Ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Su, Y.; Yue, Q.; Gao, B.; Wang, R. Synthesis of Wheat Straw Cellulose-g-Poly (Potassium Acrylate)/PVA Semi-IPNs Superabsorbent Resin. Carbohydr. Polym. 2013, 94, 539–546. [Google Scholar] [CrossRef]
- Hajiali, F.; Tajbakhsh, S.; Marić, M. Thermal Characteristics and Flame Retardance Behavior of Phosphoric Acid-Containing Poly(Methacrylates) Synthesized by RAFT Polymerization. Mater. Today Commun. 2020, 25, 101618. [Google Scholar] [CrossRef]
- Hofman, A.H.; Pedone, M.; Kamperman, M. Protected Poly(3-Sulfopropyl Methacrylate) Copolymers: Synthesis, Stability, and Orthogonal Deprotection. ACS Polym. Au 2022, 2, 169–180. [Google Scholar] [CrossRef]
- Alam, M.N.; Islam, M.S.; Christopher, L.P. Sustainable Production of Cellulose-Based Hydrogels with Superb Absorbing Potential in Physiological Saline. ACS Omega 2019, 4, 9419–9426. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Li, Z.; Hua, J.; Wu, M.; Gong, J.; Zhang, J.; Ban, L.; Huang, L. Novel Biological Hydrogel: Swelling Behaviors Study in Salt Solutions with Different Ionic Valence Number. Polymers 2018, 10, 112. [Google Scholar] [CrossRef]
- Simerville, J.A.; Maxted, W.C.; Pahira, J.J. Urinalysis: A Comprehensive Review. Am. Fam. Physician 2005, 71, 1153–1162. [Google Scholar] [PubMed]
- Bayat, M.R.; Baghani, M. A Review on Swelling Theories of PH-Sensitive Hydrogels. J. Intell. Mater. Syst. Struct. 2021, 32, 2349–2365. [Google Scholar] [CrossRef]
- Furukawa, N.; Fujihara, H. Acidity, Hydrogen Bonding and Metal Complexation of Sulfonic Acids and Derivatives. In Sulphonic Acids, Esters and Their Derivatives (1991); PATAI’S Chemistry of Functional Groups; John Wiley & Sons: Hoboken, NJ, USA, 1991; pp. 261–281. ISBN 9780470034392. [Google Scholar]
- Bastos, H.; Gallastegui, A.; de Lacalle, J.L.; Schaeffer, N.; Pringle, J.M.; Mecerreyes, D.; Pozo-Gonzalo, C. Ionic Polymer Absorbents Inspired by Deep Eutectic Solvents to Recover Cobalt and Nickel. New J. Chem. 2024, 48, 14672–14683. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, H. Crosslinked Carboxymethylchitosan-g-Poly(Acrylic Acid) Copolymer as a Novel Superabsorbent Polymer. Carbohydr. Res. 2006, 341, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Kurdtabar, M. Collagen-Based Highly Porous Hydrogel without Any Porogen: Synthesis and Characteristics. Eur. Polym. J. 2007, 43, 877–889. [Google Scholar] [CrossRef]
- Reshma, G.; Reshmi, C.R.; Shantikumar, V.N.; Menon, D. Superabsorbent Sodium Carboxymethyl Cellulose Membranes Based on a New Cross-Linker Combination for Female Sanitary Napkin Applications. Carbohydr. Polym. 2020, 248, 116763. [Google Scholar] [CrossRef]
- Bachra, Y.; Grouli, A.; Damiri, F.; Zhu, X.X.; Talbi, M.; Berrada, M. Synthesis, Characterization, and Swelling Properties of a New Highly Absorbent Hydrogel Based on Carboxymethyl Guar Gum Reinforced with Bentonite and Silica Particles for Disposable Hygiene Products. ACS Omega 2022, 7, 39002–39018. [Google Scholar] [CrossRef]
- Yadav, S.; Illa, M.P.; Rastogi, T.; Sharma, C.S. High Absorbency Cellulose Acetate Electrospun Nanofibers for Feminine Hygiene Application. Appl. Mater. Today 2016, 4, 62–70. [Google Scholar] [CrossRef]
- Lacoste, C.; Lopez-Cuesta, J.-M.; Bergeret, A. Development of a Biobased Superabsorbent Polymer from Recycled Cellulose for Diapers Applications. Eur. Polym. J. 2019, 116, 38–44. [Google Scholar] [CrossRef]
- Bachra, Y.; Grouli, A.; Damiri, F.; Bennamara, A.; Berrada, M. A New Approach for Assessing the Absorption of Disposable Baby Diapers and Superabsorbent Polymers: A Comparative Study. Results Mater. 2020, 8, 100156. [Google Scholar] [CrossRef]
- Aizawa, M.; Suzuki, S. Properties of Water in Macromoleular Gels. III. Dilatometric Studies of the Properties of Water in Macromolecular Gels. Bull. Chem. Soc. Jpn. 1971, 44, 2967–2971. [Google Scholar] [CrossRef]
- Ramazani-Harandi, M.J.; Zohuriaan-Mehr, M.J.; Yousefi, A.A.; Ershad-Langroudi, A.; Kabiri, K. Rheological Determination of the Swollen Gel Strength of Superabsorbent Polymer Hydrogels. Polym. Test. 2006, 25, 470–474. [Google Scholar] [CrossRef]
- Rebelo, R.C.; Báguena, B.V.; Pereira, P.; Moreira, R.; Coelho, J.F.J.; Serra, A.C. Biocompatible Cellulose-Based Superabsorbents for Personal Care Products. J. Polym. Environ. 2024, 32, 5179–5194. [Google Scholar] [CrossRef]
- Saraiva, S.; Rénio, F.; Pereira, P.; Santos, P.; Paula, C.T.B.; Ramalho, A.; Serra, A.C.; Fonseca, A.C. Tackling the Problem of Tendon Adhesions: Physical Barriers Prepared from α-Amino Acid-Based Poly(Ester Amide)S. Polymers 2025, 17, 395. [Google Scholar] [CrossRef]
- Saraiva, S.; Pereira, P.; Paula, C.T.; Rebelo, R.C.; Coelho, J.F.J.; Serra, A.C.; Fonseca, A.C. Development of Electrospun Mats Based on Hydrophobic Hydroxypropyl Cellulose Derivatives. Mater. Sci. Eng. C 2021, 131, 112498. [Google Scholar] [CrossRef] [PubMed]
- Paula, C.T.B.; Leandro, A.; Pereira, P.; Coelho, J.F.J.; Fonseca, A.C.; Serra, A.C. Fast-Gelling Polyethylene Glycol/Polyethyleneimine Hydrogels Degradable by Visible-Light. Macromol. Biosci. 2024, 24, 2300289. [Google Scholar] [CrossRef]
- Almeida, R.O.; Ramos, A.; Alves, L.; Potsi, E.; Ferreira, P.J.T.; Carvalho, M.G.V.S.; Rasteiro, M.G.; Gamelas, J.A.F. Production of Nanocellulose Gels and Films from Invasive Tree Species. Int. J. Biol. Macromol. 2021, 188, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.O.; Ramos, A.; Kimiaei, E.; Österberg, M.; Maloney, T.C.; Gamelas, J.A.F. Improvement of the Properties of Nanocellulose Suspensions and Films by the Presence of Residual Lignin. Cellulose 2024, 31, 10951–10967. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L. Solubility of Cellulose in NaOH/Urea Aqueous Solution. Polym. J. 2000, 32, 866–870. [Google Scholar] [CrossRef]
- Naserifar, S.; Kuijpers, P.F.; Wojno, S.; Kádár, R.; Bernin, D.; Hasani, M. In Situ Monitoring of Cellulose Etherification in Solution: Probing the Impact of Solvent Composition on the Synthesis of 3-Allyloxy-2-Hydroxypropyl-Cellulose in Aqueous Hydroxide Systems. Polym. Chem. 2022, 13, 4111–4123. [Google Scholar] [CrossRef]
- Kleemann, C.; Zink, J.; Selmer, I.; Smirnova, I.; Kulozik, U. Effect of Ethanol on the Textural Properties of Whey Protein and Egg White Protein Hydrogels during Water-Ethanol Solvent Exchange. Molecules 2020, 25, 4417. [Google Scholar] [CrossRef] [PubMed]
- Paula, C.T.B.; Pereira, P.; Coelho, J.F.J.; Fonseca, A.C.; Serra, A.C. Development of Light-Degradable Poly(Urethane-Urea) Hydrogel Films. Mater. Sci. Eng. C 2021, 131, 112520. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, S.; Pereira, P.; Santos, P.; Ramalho, A.; Serra, A.C.; Fonseca, A.C. Electrospun Mats from α-Amino Acid Based Poly(Ester Amide)s: A Promising Material for the Prevention of Tendon Adhesions. React. Funct. Polym. 2024, 205, 106067. [Google Scholar] [CrossRef]
- Frazier, L.M. Superabsorbent Nanofiber Matrices. Ph.D. Thesis, University of Akron, Akron, OH, USA, 2006. [Google Scholar]
- Inc., AAT Bioquest Quest CalculateTM—Preparation and Recipe. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes (accessed on 25 August 2024).
- ISO 17190-6; 2020 Urine-Absorbing Aids for Incontinence—Polyacrylate Superabsorbent Powders Part 6: Test Method for Determination of the Fluid Retention Capacity in Saline Solution by Gravimetric Measurement Following Centrifugation. International Organization for Standardization: Geneva, Switzerland, 2020.
- Paula, C.T.B.; Madeira, A.B.; Pereira, P.; Branco, R.; Morais, P.V.; Coelho, J.F.J.; Fonseca, A.C.; Serra, A.C. ROS-Degradable PEG-Based Wound Dressing Films with Drug Release and Antibacterial Properties. Eur. Polym. J. 2022, 177, 111447. [Google Scholar] [CrossRef]
| Sample b | Monomer | Crosslinker c (% w/w) | Hydrogel Formation d | Maximum Swelling (g/g) | Gel Content (% w/w) |
|---|---|---|---|---|---|
| P_c1 | PAHEMA (P) | 1.0 | No | - | - |
| P_c3 | 3.0 | No | - | - | |
| P_c5 | 5.0 | Yes | 19.6 ± 1.5 | 31.1 ± 1.6 | |
| S_c1 | SPM (S) | 1.0 | No | - | - |
| S_c3 | 3.0 | Yes | 321.5 ± 20.8 | 52.0 ± 2.2 | |
| S_c5 | 5.0 | Yes | 272.9 ± 19.0 | 76.7 ± 2.7 | |
| M_c1 | METAC (M) | 1.0 | No | - | - |
| M_c3 | 3.0 | Yes | 242.6 ± 26.2 | 33.2 ± 2.6 | |
| M_c5 | 5.0 | Yes | 115.1 ± 7.0 | 74.3 ± 1.4 |
| Sample a | AC:Monomers Ratio b (% w/w) | Monomers Ratio c (%wmonomers/w) | Maximum Swelling (g/g) | Gel Content (% w/w) | ||
|---|---|---|---|---|---|---|
| PAHEMA | SPM | METAC | ||||
| Cel100 | 100 | - | - | - | 8.4 ± 0.4 | 97.7 ± 0.9 |
| Cel50_P50 | 50:50 | 50 | - | - | 13.2 ± 0.4 | 77.7 ± 3.0 |
| Cel50_S50 | - | 50 | - | 15.9 ± 1.4 | 87.6 ± 1.5 | |
| Cel50_M50 | - | - | 50 | 25.7 ± 0.9 | 89.1 ± 2.9 | |
| Cel50_P25_S25 | 25 | 25 | - | 16.2 ± 0.4 | 79.0 ± 1.5 | |
| Cel50_P25_M25 | 25 | - | 25 | 12.3 ± 0.7 | 79.0 ± 2.2 | |
| Cel50_S25_M25 | - | 25 | 25 | 14.2 ± 0.6 | 77.9 ± 1.3 | |
| Cel50_P16.7_S16.7_M16.7 | 16.7 | 16.7 | 16.7 | 29.1 ± 1.3 | 81.7 ± 0.7 | |
| Cel50_P25_S12.5_M12.5 | 25 | 12.5 | 12.5 | 19.0 ± 0.6 | 71.3 ± 1.0 | |
| Cel50_P12.5_S25_M12.5 | 12.5 | 25 | 12.5 | 22.2 ± 0.5 | 87.2 ± 4.8 | |
| Cel50_P12.5_S12.5_M25 | 12.5 | 12.5 | 25 | 22.2 ± 0.9 | 76.9 ± 1.5 | |
| Sample a | Monomers Ratio b (% w/wmonomers) | Crosslinker Amount (% w/w) | Maximum Swelling (g/g) | Gel Content (% w/w) | ||
|---|---|---|---|---|---|---|
| PAHEMA | SPM | METAC | ||||
| Cel50_P16.7_S16.7_M16.7 | 16.7 | 16.7 | 16.7 | 1.0 c | 29.1 ± 1.3 | 81.7 ± 0.7 |
| 0.5 | 29.4 ± 1.0 | 80.2 ± 1.3 | ||||
| 0.25 | 18.0 ± 1.9 | 56.2 ± 3.3 | ||||
| 0.1 | 9.1 ± 1.0 | 50.9 ± 1.4 | ||||
| Cel50_P12.5_S25_M12.5 | 12.5 | 25 | 12.5 | 1.0 c | 22.2 ± 0.5 | 87.2 ± 4.8 |
| 0.5 | 22.4 ± 0.8 | 86.0 ± 2.1 | ||||
| 0.25 | 17.5 ± 0.9 | 76.0 ± 2.8 | ||||
| 0.1 | 16.2 ± 0.8 | 57.4 ± 1.2 | ||||
| Sample a | Monomers Ratio b (% w/wmonomers) | Initiator Quantity (% w/w) | Maximum Swelling (g/g) | Gel Content (% w/w) | ||
|---|---|---|---|---|---|---|
| PAHEMA | SPM | METAC | ||||
| Cel50_P16.7_S16.7_M16.7 | 16.7 | 16.7 | 16.7 | 1.00 c | 29.4 ± 1.0 | 80.2 ± 1.3 |
| 0.75 | 50.0 ± 6.7 | 65.8 ± 2.7 | ||||
| 0.50 | 52.7 ± 10.5 | 67.1 ± 3.0 | ||||
| 0.25 | 60.5 ± 3.5 | 34.0 ± 3.6 | ||||
| Cel50_P12.5_S25_M12.5 | 12.5 | 25 | 12.5 | 1.00 c | 22.4 ± 0.8 | 86.0 ± 2.1 |
| 0.75 | 22.6 ± 2.3 | 80.7 ± 1.3 | ||||
| 0.50 | 38.2 ± 5.4 | 81.2 ± 2.2 | ||||
| 0.25 | 92.8 ± 8.4 | 42.5 ± 1.6 | ||||
| Sample a | AC:Monomers Ratio (% w/w) | Monomers Ratio b (% w/wmonomers) | Maximum Swelling (g/g) | Gel Content (% w/w) | ||
|---|---|---|---|---|---|---|
| PAHEMA | SPM | METAC | ||||
| Cel50_P16.7_S16.7_M16.7 | 50:50 c | 16.7 | 16.7 | 16.7 | 52.7 ± 10.5 | 67.1 ± 3.0 |
| Cel25_P25_S25_M25 | 25:75 | 25 | 25 | 25 | 53.2 ± 0.9 | 47.6 ± 1.4 |
| Cel10_P30_S30_M30 | 10:90 | 30 | 30 | 30 | 94.6 ± 2.9 | 38.5 ± 1.1 |
| Cel50_P12.5_S25_M12.5 | 50:50 c | 12.5 | 25 | 12.5 | 38.2 ± 5.4 | 81.2 ± 2.2 |
| Cel25_P18.8_S37.5_M18.8 | 25:75 | 18.75 | 37.5 | 18.75 | 37.8 ± 1.7 | 70.8 ± 2.2 |
| Cel10_P22.5_S45_M22.5 | 10:90 | 22.5 | 45 | 22.5 | 102.3 ± 6.5 | 49.2 ± 2.4 |
| Sample a | AC/Monomers Ratio b (% w/w) | Monomers Ratio c (% w/wmonomers) | Crosslinker Amount e (% w/w) | Initiator Quantity f (% w/w) | ||
|---|---|---|---|---|---|---|
| PAHEMA | SPM | METAC | ||||
| Cel100 | 100:0 | - | - | - | 0.5 | 0.5 |
| Cel50_P12.5_S25_M12.5 | 50:50 | 12.5 | 25 | 12.5 | 0.5 | |
| Cel50_P12.5_S25_M12.5_i0.25 d | 50:50 | 12.5 | 25 | 12.5 | 0.25 | |
| Cel10_P22.5_S45_M22.5 | 10:90 | 22.5 | 45 | 22.5 | 0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simões, B.; Rebelo, R.C.; Ledesma, S.; Pereira, P.; Moreira, R.; Ferreira, B.C.; Coelho, J.F.J.; Serra, A.C. Development of Polyampholyte Cellulose-Based Hydrogels for Diapers with Improved Biocompatibility. Gels 2025, 11, 282. https://doi.org/10.3390/gels11040282
Simões B, Rebelo RC, Ledesma S, Pereira P, Moreira R, Ferreira BC, Coelho JFJ, Serra AC. Development of Polyampholyte Cellulose-Based Hydrogels for Diapers with Improved Biocompatibility. Gels. 2025; 11(4):282. https://doi.org/10.3390/gels11040282
Chicago/Turabian StyleSimões, Beatriz, Rafael C. Rebelo, Sara Ledesma, Patrícia Pereira, Rui Moreira, Brígida C. Ferreira, Jorge F. J. Coelho, and Arménio C. Serra. 2025. "Development of Polyampholyte Cellulose-Based Hydrogels for Diapers with Improved Biocompatibility" Gels 11, no. 4: 282. https://doi.org/10.3390/gels11040282
APA StyleSimões, B., Rebelo, R. C., Ledesma, S., Pereira, P., Moreira, R., Ferreira, B. C., Coelho, J. F. J., & Serra, A. C. (2025). Development of Polyampholyte Cellulose-Based Hydrogels for Diapers with Improved Biocompatibility. Gels, 11(4), 282. https://doi.org/10.3390/gels11040282









