Radiation Characterization of Smart Aerogels Based on Hollow VO2 Particles
Abstract
1. Introduction
2. Results and Discussion
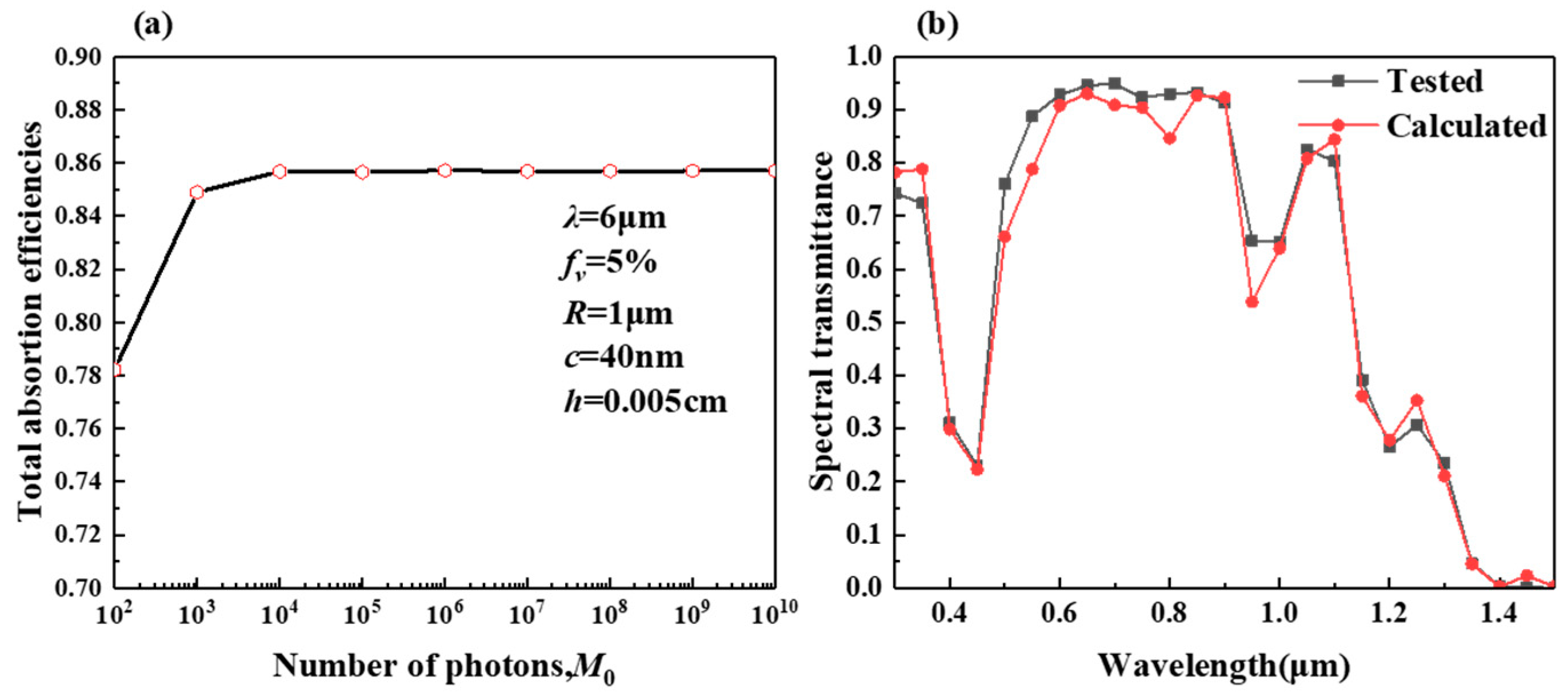
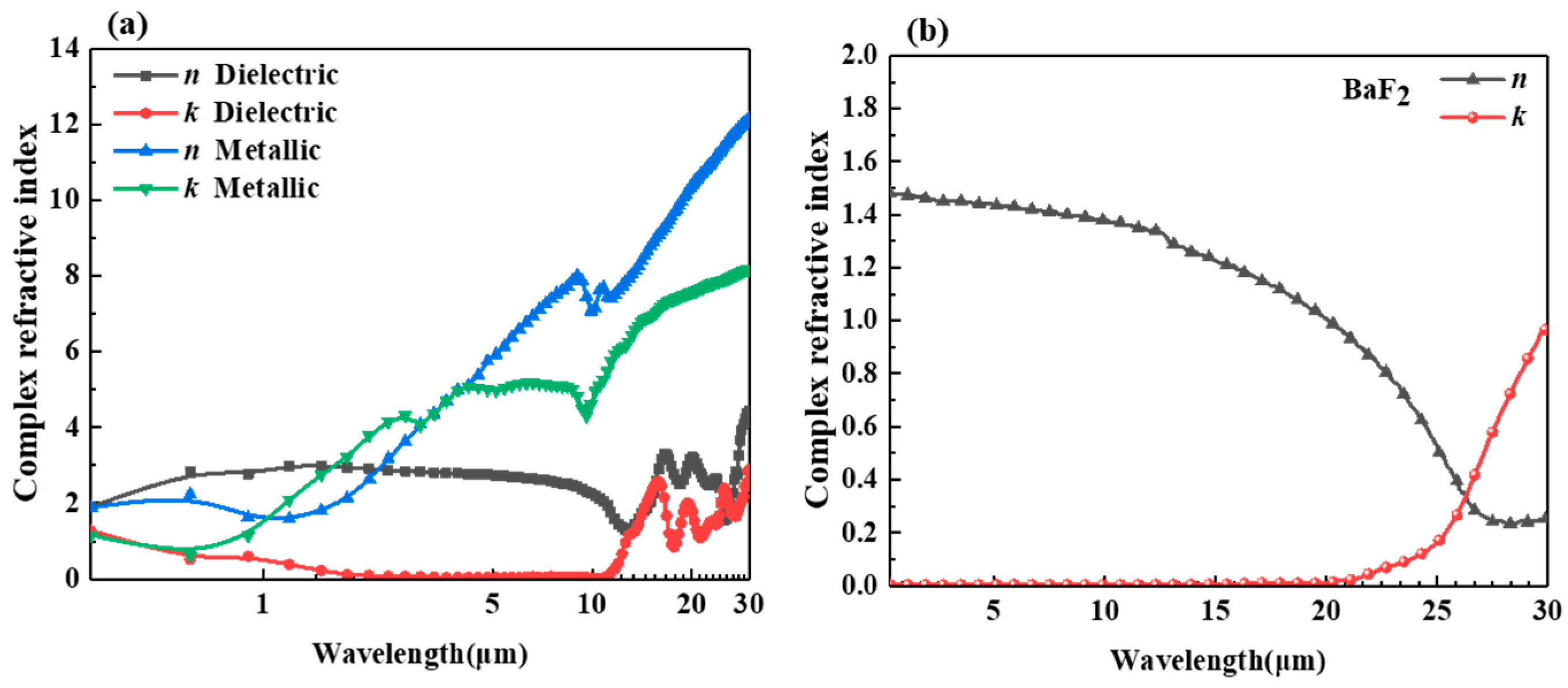
2.1. Validation of the Method
2.2. Radiation Properties of the Hollow VO2 Particles
2.2.1. Effect of Different Layer Thicknesses c on the Particle Absorption Factor for a Particle Size of r2 = 1 μm
2.2.2. Effect of Different Radii on the Absorption Factor of Nucleoshell Particles at VO2 Shell Thickness of 40 nm
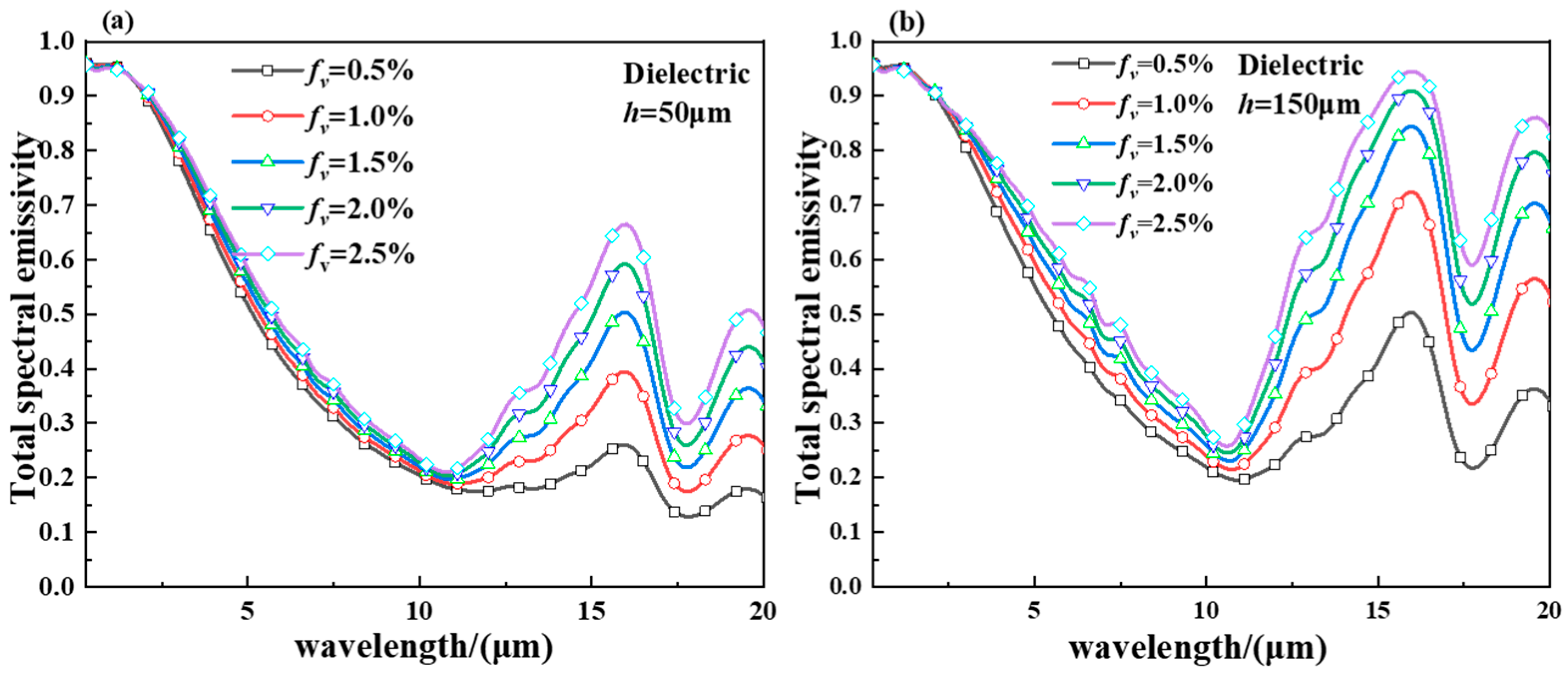
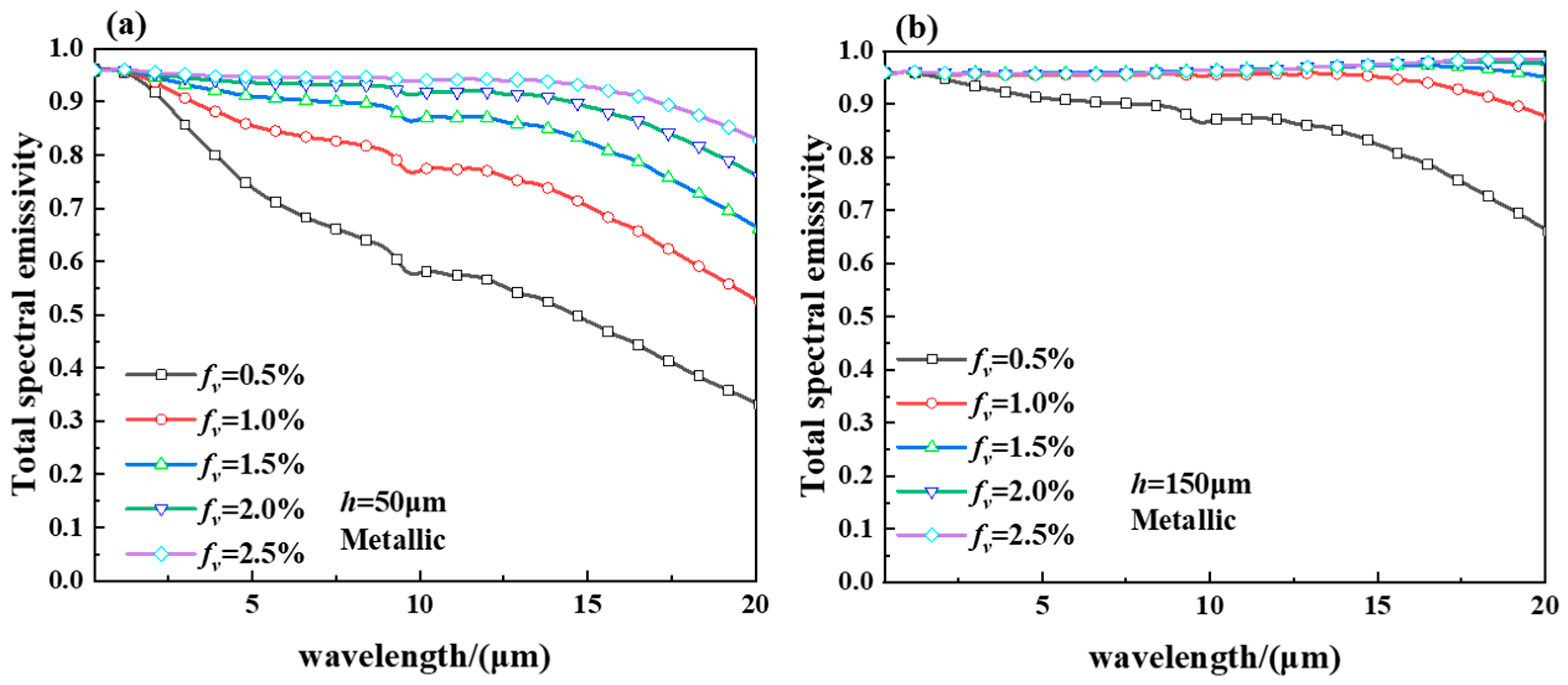
2.3. Spectral Emittance of the Hollow VO2 Particles-Based Smart Aerogel
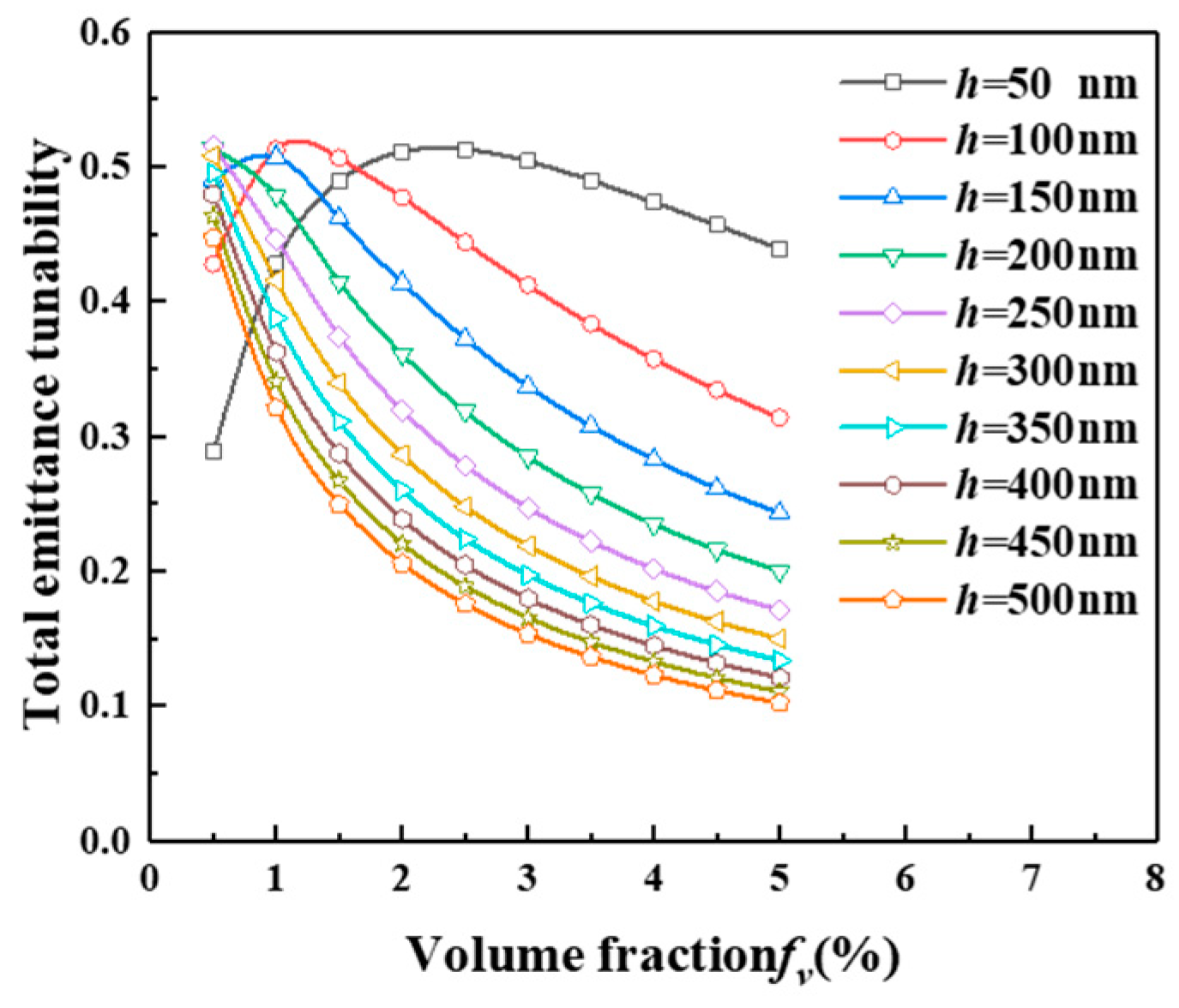
2.4. Total Emittance of the Hollow VO2 Particles-Based Smart Aerogel
3. Conclusions
4. Materials and Methods
4.1. The Models of the Hollow VO2 Particle-Based Smart Aerogel
4.2. Theoretical Calculations for the Smart Aerogels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, Q.; Yu, W.; Xie, G.; Li, Y.; Wu, C.; Jiang, G.; Zhou, Y.; Xie, H. Novel Photothermochromic Smart Window Based on PNIPAm-glass-MXene/PAM with High Shield, Fast Response, and Excellent Stability. Sol. RRL 2023, 7, 2200990. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.; Wang, Q.; Alelyani, S.M.; Qu, J. Investigation of CoSO4-Based Ag Nanofluids as Spectral Beam Splitters for Hybrid PV/T Applications. Sol. Energy 2019, 177, 387–394. [Google Scholar] [CrossRef]
- Bao, Y.; Xie, M.; Guo, R. Research progress of VO2 intelligent thermoregulation coating. Mater. Rep. 2025, 39, 24030036. Available online: http://kns.cnki.net/kcms/detail/50.1078.TB.20240429.1744.008.html (accessed on 4 February 2025). [CrossRef]
- Gu, J.; Wei, H.; Zhao, T.; Ren, F.; Geng, C.; Guan, H.; Liang, S.; Chen, X.; Shi, Y.; Zhao, J.; et al. Unprecedented Spatial Manipulation and Transformation of Dynamic Thermal Radiation Based on Vanadium Dioxide. ACS Appl. Mater. Interfaces 2024, 16, 10352–10360. [Google Scholar] [CrossRef]
- Wang, S.; Liu, M.; Kong, L.; Long, Y.; Jiang, X.; Yu, A. Recent Progress in VO2 Smart Coatings: Strategies to Improve the Thermochromic Properties. Prog. Mater. Sci. 2016, 81, 1–54. [Google Scholar] [CrossRef]
- Chang, T.; Cao, X.; Dedon, L.R.; Long, S.; Huang, A.; Shao, Z.; Li, N.; Luo, H.; Jin, P. Optical Design and Stability Study for Ultrahigh-Performance and Long-Lived Vanadium Dioxide-Based Thermochromic Coatings. Nano Energy 2018, 44, 256–264. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, J.; Dou, S.; Li, Y. Principles and progress of photo-thermal regulation of vanadium dioxide smart windows. J. Opt. 2024, 44, 1925004. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, C.; Chen, S.; Luo, H.; Gao, Y. Crystallised Mesoporous TiO2 (A)–VO2 (M/R) Nanocomposite Films with Self-Cleaning and Excellent Thermochromic Properties. J. Mater. Chem. A 2014, 2, 11874–11884. [Google Scholar] [CrossRef]
- Wan, J.; Ren, Q.; Wu, N.; Gao, Y. Density Functional Theory Study of M-Doped (M = B, C, N, Mg, Al) VO2 Nanoparticles for Thermochromic Energy-Saving Foils. J. Alloys Compd. 2016, 662, 621–627. [Google Scholar] [CrossRef]
- Zhou, X.; Ping, Y.; Gao, J.; Gu, D.; Zhou, H.; Yang, M.; Jiang, Y. Facile Fabrication of HfO2/Nanocomposite Vanadium Oxide Bilayer Film with Enhanced Thermochromic Properties and Excellent Durability. Appl. Surf. Sci. 2022, 597, 153729. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Yu, X.; Tang, G.H.; Wang, X.; Du, M. Scalable Self-Adaptive Radiative Cooling Film through VO2-Based Switchable Core–Shell Particles. Renew. Energy 2024, 224, 120208. [Google Scholar] [CrossRef]
- Li, Y.; Ji, S.; Gao, Y.; Luo, H.; Jin, P. Modification of Mott Phase Transition Characteristics in VO2 @TiO2 Core/Shell Nanostructures by Misfit-Strained Heteroepitaxy. ACS Appl. Mater. Interfaces 2013, 5, 6603–6614. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Niklasson, G.A.; Granqvist, C.G. Nanothermochromics: Calculations for VO2 Nanoparticles in Dielectric Hosts Show Much Improved Luminous Transmittance and Solar Energy Transmittance Modulation. J. Appl. Phys. 2010, 108, 063525. [Google Scholar] [CrossRef]
- Cao, C.; Gao, Y.; Luo, H. Pure Single-Crystal Rutile Vanadium Dioxide Powders: Synthesis, Mechanism and Phase-Transformation Property. J. Phys. Chem. C 2008, 112, 18810–18814. [Google Scholar] [CrossRef]
- Xu, F.; Cao, X.; Shao, Z.; Sun, G.; Long, S.; Luo, H.; Jin, P. Highly Enhanced Thermochromic Performance of VO2 Film Using “Movable” Antireflective Coatings. ACS Appl. Mater. Interfaces 2019, 11, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Song, Y.; Chen, S.; Liu, L. Wavelength-Selective Emitter Compatible with Multiband Stealth and Dual-Band Heat Dissipation. Appl. Therm. Eng. 2025, 267, 125805. [Google Scholar] [CrossRef]
- Zheng, J.; Bao, S.; Jin, P. TiO2(R)/VO2(M)/TiO2(A) Multilayer Film as Smart Window: Combination of Energy-Saving, Antifogging and Self-Cleaning Functions. Nano Energy 2015, 11, 136–145. [Google Scholar] [CrossRef]
- Xie, B.; Yang, Z.; Liu, L. All-Season Smart Film with Multimode Modulation of Solar-Thermal Radiation Based on Phase Change Materials VO2/IST. Appl. Therm. Eng. 2025, 266, 125690. [Google Scholar] [CrossRef]
- Liu, C.; Cao, X.; Kamyshny, A.; Law, J.Y.; Magdassi, S.; Long, Y. VO2/Si–Al Gel Nanocomposite Thermochromic Smart Foils: Largely Enhanced Luminous Transmittance and Solar Modulation. J. Colloid Interface Sci. 2014, 427, 49–53. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, W.; Zhao, J.; Zheng, C.; Liu, L. Design of VO2-Based Spacecraft Smart Radiator with Low Solar Absorptance. Appl. Therm. Eng. 2024, 236, 121751. [Google Scholar] [CrossRef]
- Zhao, X.; Mofid, S.A.; Jelle, B.P.; Tan, G.; Yin, X.; Yang, R. Optically-Switchable Thermally-Insulating VO2-Aerogel Hybrid Film for Window Retrofits. Appl. Energy 2020, 278, 115663. [Google Scholar] [CrossRef]
- Chen, X.; Guo, S.; Tan, S.; Ma, J.; Xu, T. An environmentally friendly chitosan-derived VO2/carbon aerogel for radar infrared compatible stealth. Carbon 2023, 213, 118313. [Google Scholar] [CrossRef]
- Klemmed, B.; Besteiro, L.V.; Benad, A.; Georgi, M.; Wang, Z.; Govorov, A. Hybrid Plasmonic–Aerogel Materials as Optical Superheaters with Engineered Resonances. Angew. Chem. 2020, 132, 1713–1719. [Google Scholar] [CrossRef]
- Berquist, Z.J.; Turaczy, K.K.; Lenert, A. Plasmon-enhanced greenhouse selectivity for high-temperature solar thermal energy conversion. ACS Nano 2020, 14, 12605–12613. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, Z.; Pang, H.Q.; Pan, N. Design and optimization of core/shell structures as highly efficient opacifiers for silica aerogels as high-temperature thermal insulation. Int. J. Therm. Sci. 2018, 133, 206–215. [Google Scholar] [CrossRef]
- Li, M.; Cheng, Y.; Fang, C.; Zhang, X.; Han, H. W/Al Co-doping VO2 nanoparticles for high performance passive infrared stealth films with enhanced durability. Ceram. Int. 2024, 50, 1443–1451. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, L.; Weng, X.; Qi, L.; Wei, B. Passive smart thermal control coatings incorporating CaF2/VO2 core–shell microsphere structures. Nano Lett. 2021, 21, 3908–3914. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Chen, K.; Li, W.; Fan, S. Self-adaptive radiative cooling based on phase change materials. Opt. Express 2018, 26, A777–A787. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Zheng, C.; Hong, Y.; Xu, Z.; Wang, H. High-temperature infrared camouflage with efficient thermal management. Light Sci. Appl. 2020, 9, 60. [Google Scholar] [CrossRef]
- Yu, Q. Principle of Radiant Heat Transfer; Harbin Institute of Technology Publishing: Harbin, China, 2000; pp. 10–15. [Google Scholar]




| Reference | Model | Emissivity Adjustment Rate ∆ε | Wavelength Range |
|---|---|---|---|
| Our work | Hollow particle | 51.295% | 0.3–30 μm |
| [26] | W/Al Co-doping VO2 nanoparticles | 48% | 8–14 μm |
| [27] | CaF2/VO2 | 36% | 4–12.5 μm |
| [28] | (Filter)/VO2/MgF2/W | 58.2% | 8–13 μm |
| [29] | (Sapphire)/VO2/PMMA/Au | 60% | 8–14 μm |
| [11] | VO2/CaF2; VO2/ZnS | 52.6%; 53.7% | 0.3–20 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Qin, S.; Xie, B.; Hou, T.; Wang, L.; Xie, Y.; Han, M. Radiation Characterization of Smart Aerogels Based on Hollow VO2 Particles. Gels 2025, 11, 273. https://doi.org/10.3390/gels11040273
Li X, Qin S, Xie B, Hou T, Wang L, Xie Y, Han M. Radiation Characterization of Smart Aerogels Based on Hollow VO2 Particles. Gels. 2025; 11(4):273. https://doi.org/10.3390/gels11040273
Chicago/Turabian StyleLi, Xingcan, Shengkai Qin, Bowei Xie, Tianbo Hou, Linkang Wang, Yinmo Xie, and Meiran Han. 2025. "Radiation Characterization of Smart Aerogels Based on Hollow VO2 Particles" Gels 11, no. 4: 273. https://doi.org/10.3390/gels11040273
APA StyleLi, X., Qin, S., Xie, B., Hou, T., Wang, L., Xie, Y., & Han, M. (2025). Radiation Characterization of Smart Aerogels Based on Hollow VO2 Particles. Gels, 11(4), 273. https://doi.org/10.3390/gels11040273







