Preparation and Characterization of Dual-Network Multifunctional Hydrogels Based on Peach Gum Polysaccharides: Ultrafast Self-Healing Ability, Favorable Mechanical Tunability, and Controlled Release Properties
Abstract
1. Introduction
2. Results and Discussion
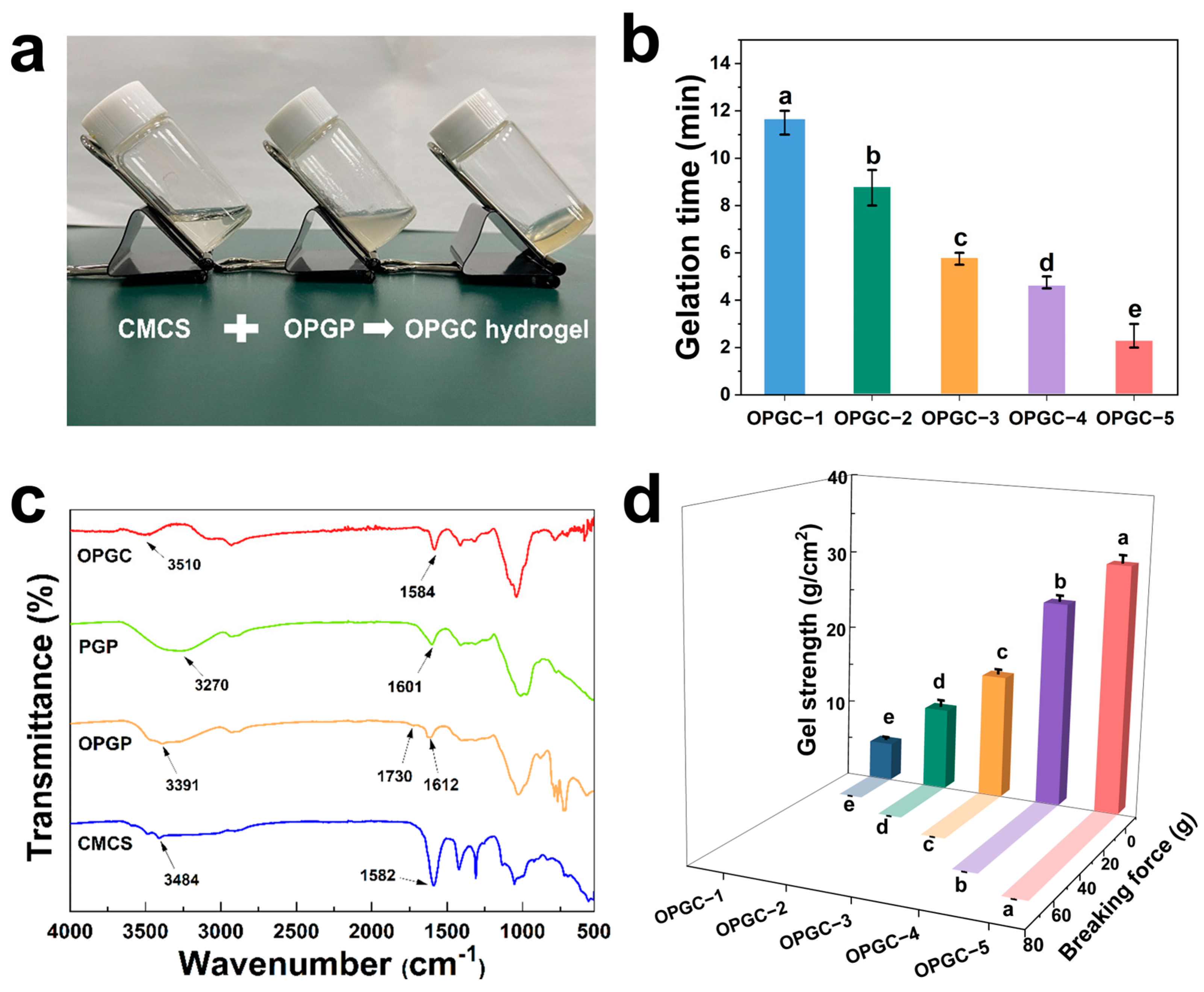
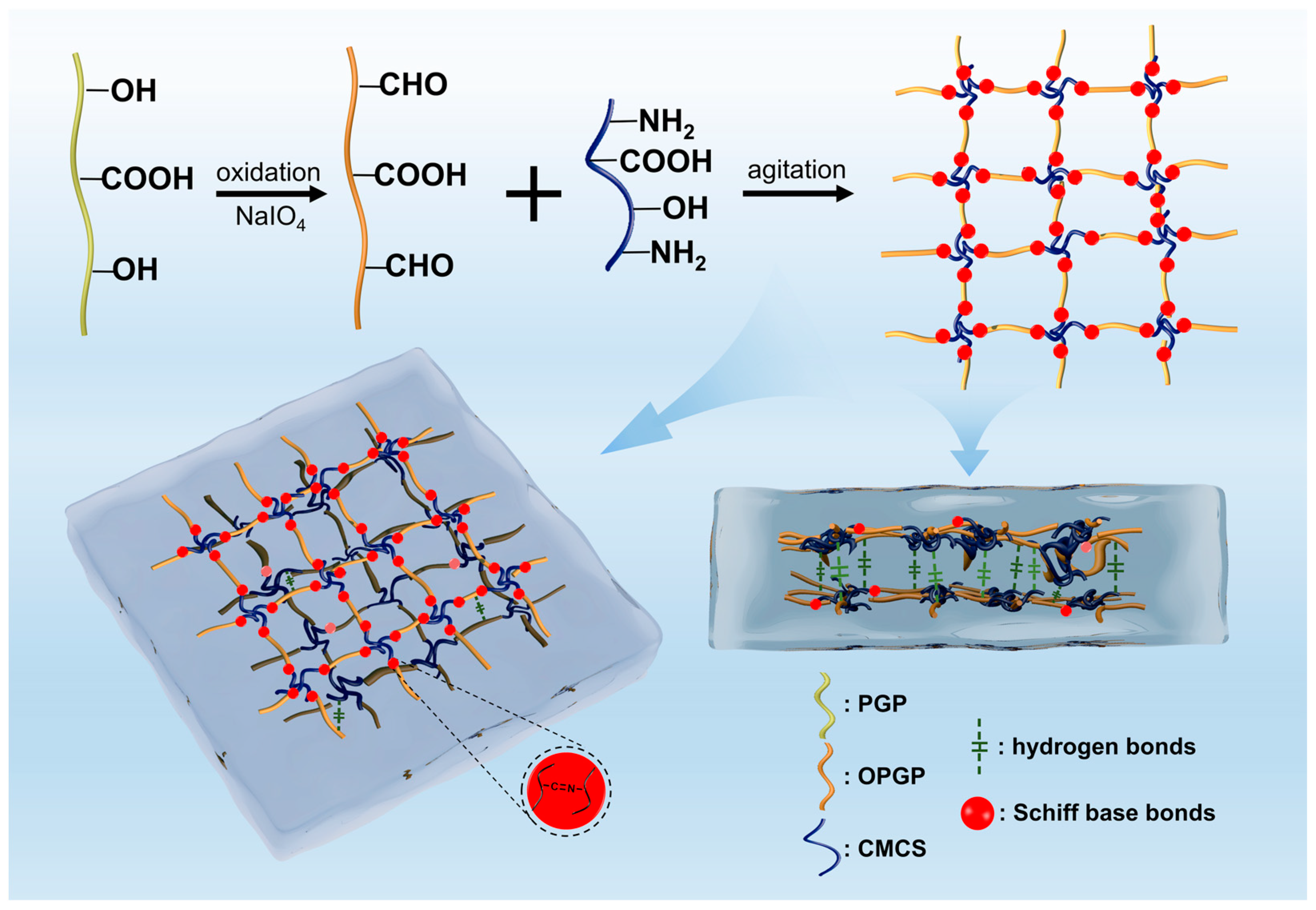
2.1. Synthesis and Characterization of OPGC Hydrogels
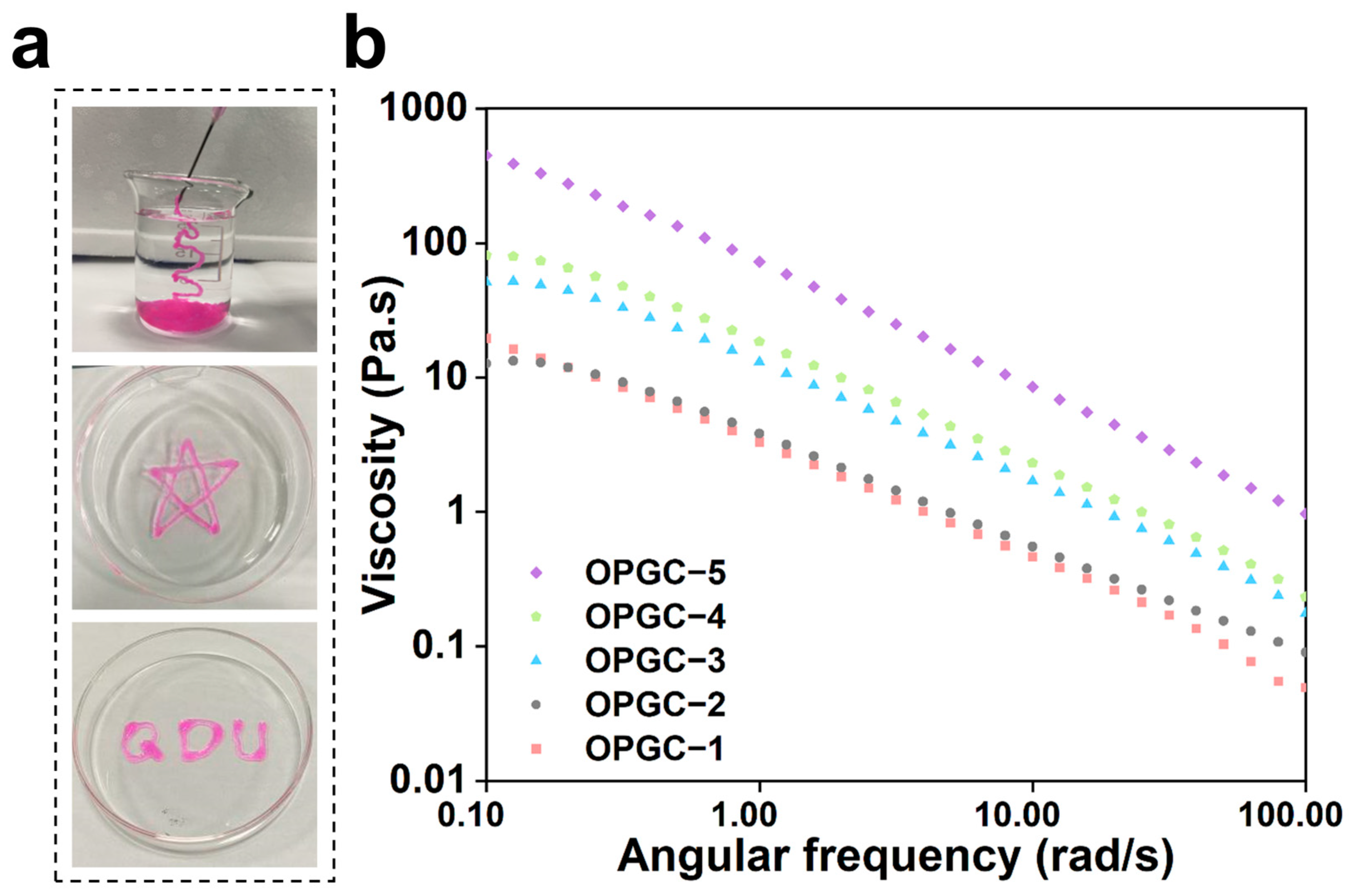
2.2. Mechanical Properties of OPGC Hydrogels
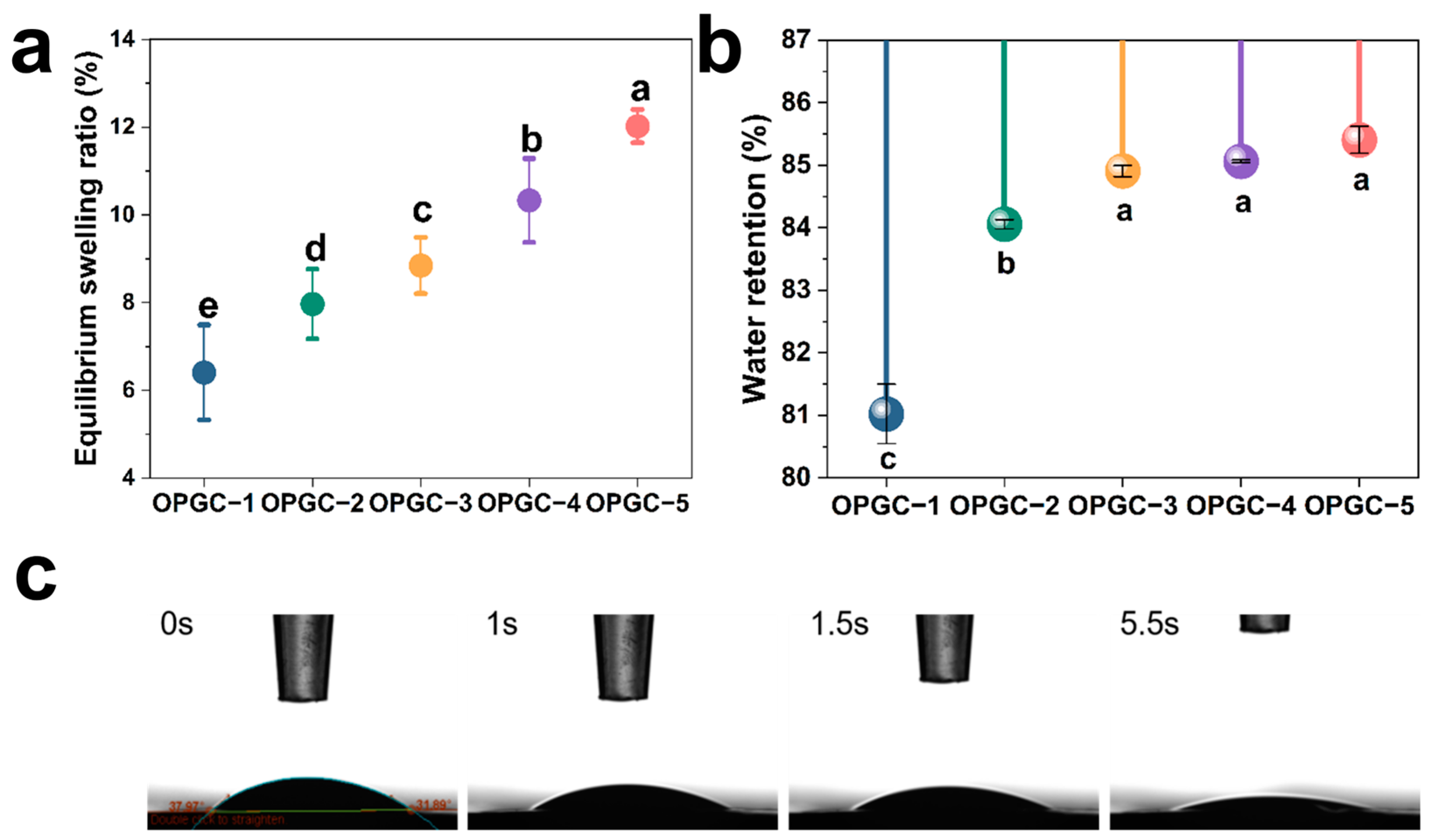
2.3. Hydration Properties of OPGC Hydrogels
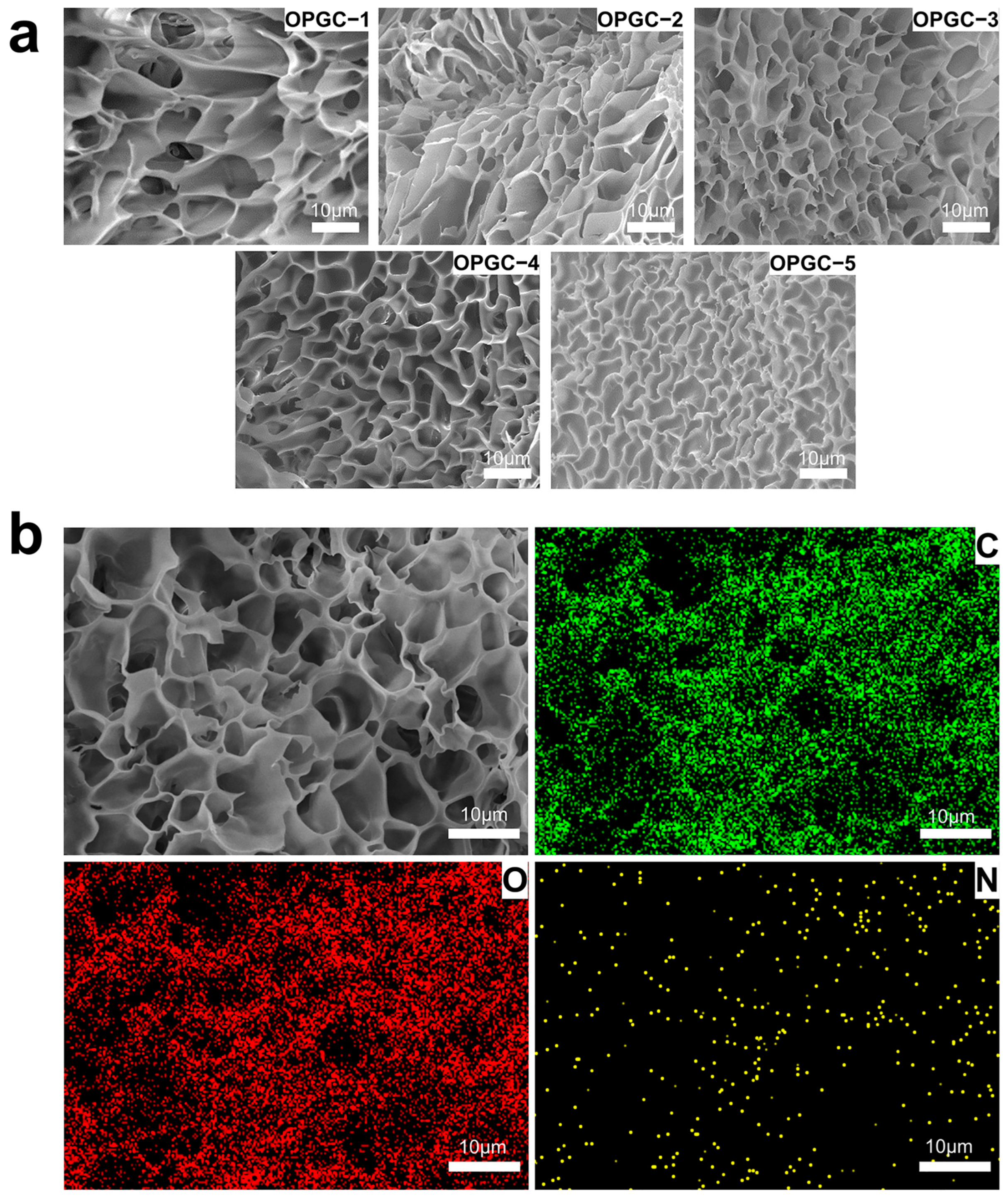
2.4. Morphology Analysis of OPGC Hydrogels
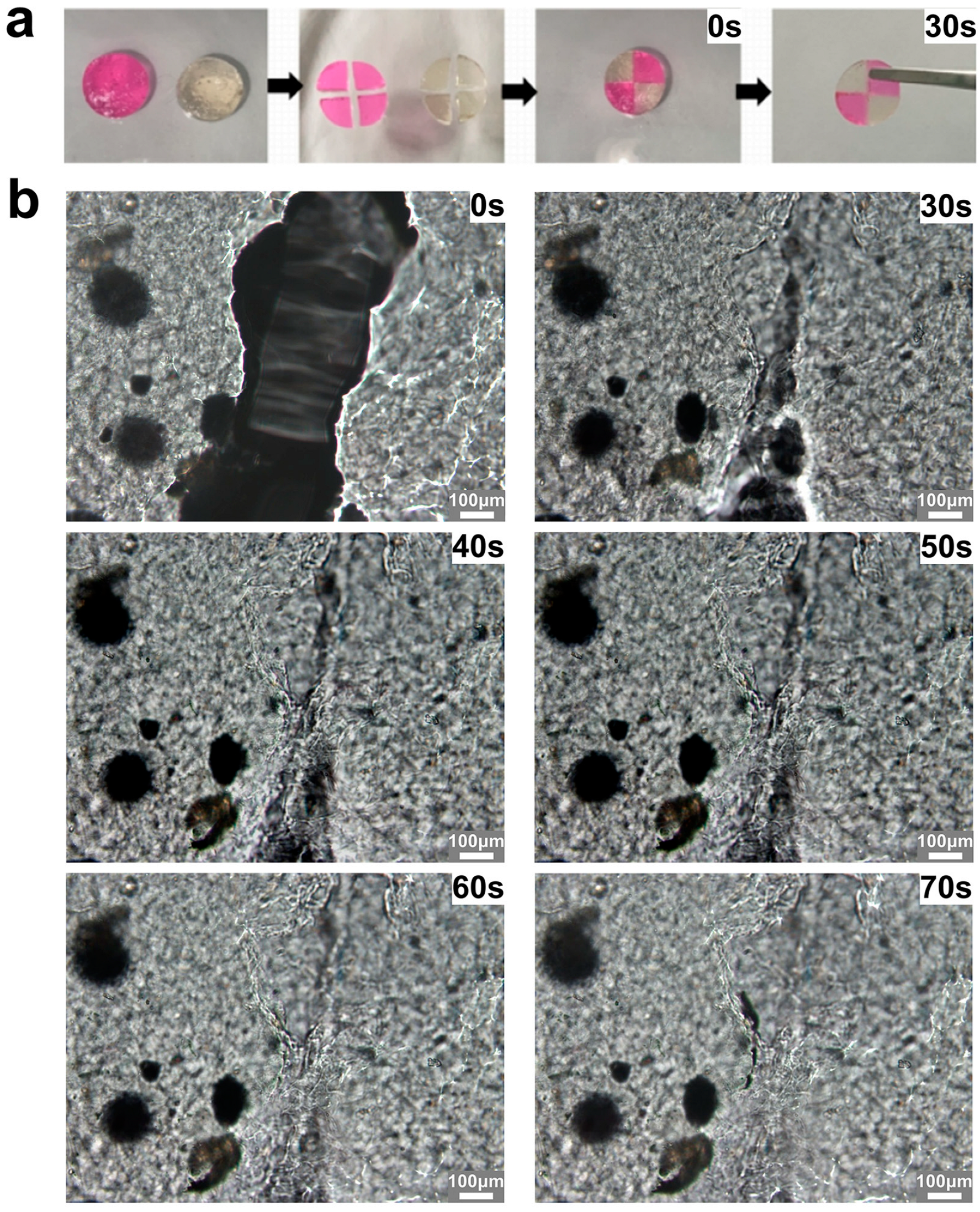
2.5. Self-Healing Ability and Injectability of OPGC Hydrogels
2.6. Physiological Functional Activity of OPGC Hydrogels
2.7. In Vivo Wound-Healing Evaluation
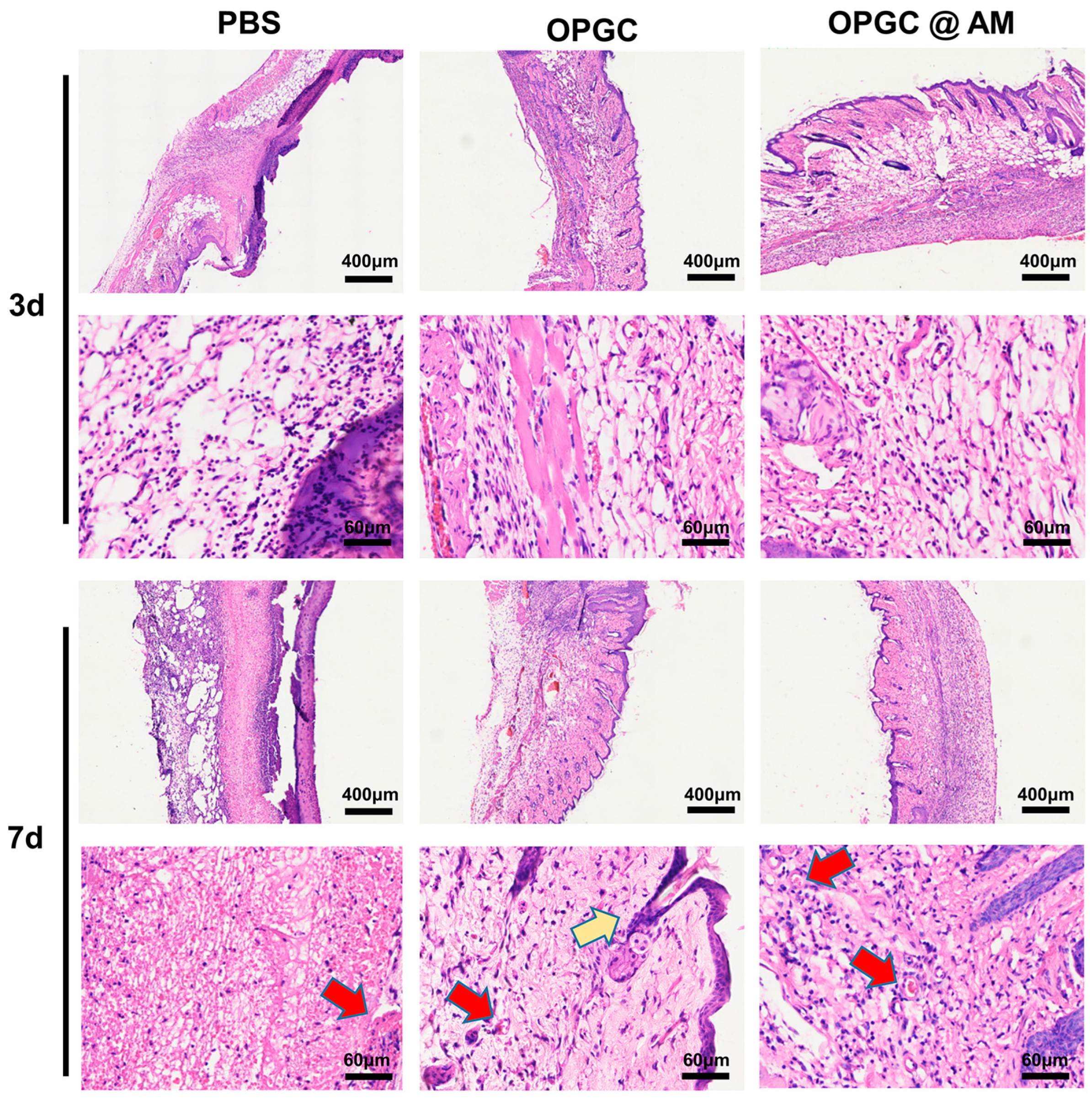
2.8. Analysis of HE Staining in Wounded Skin
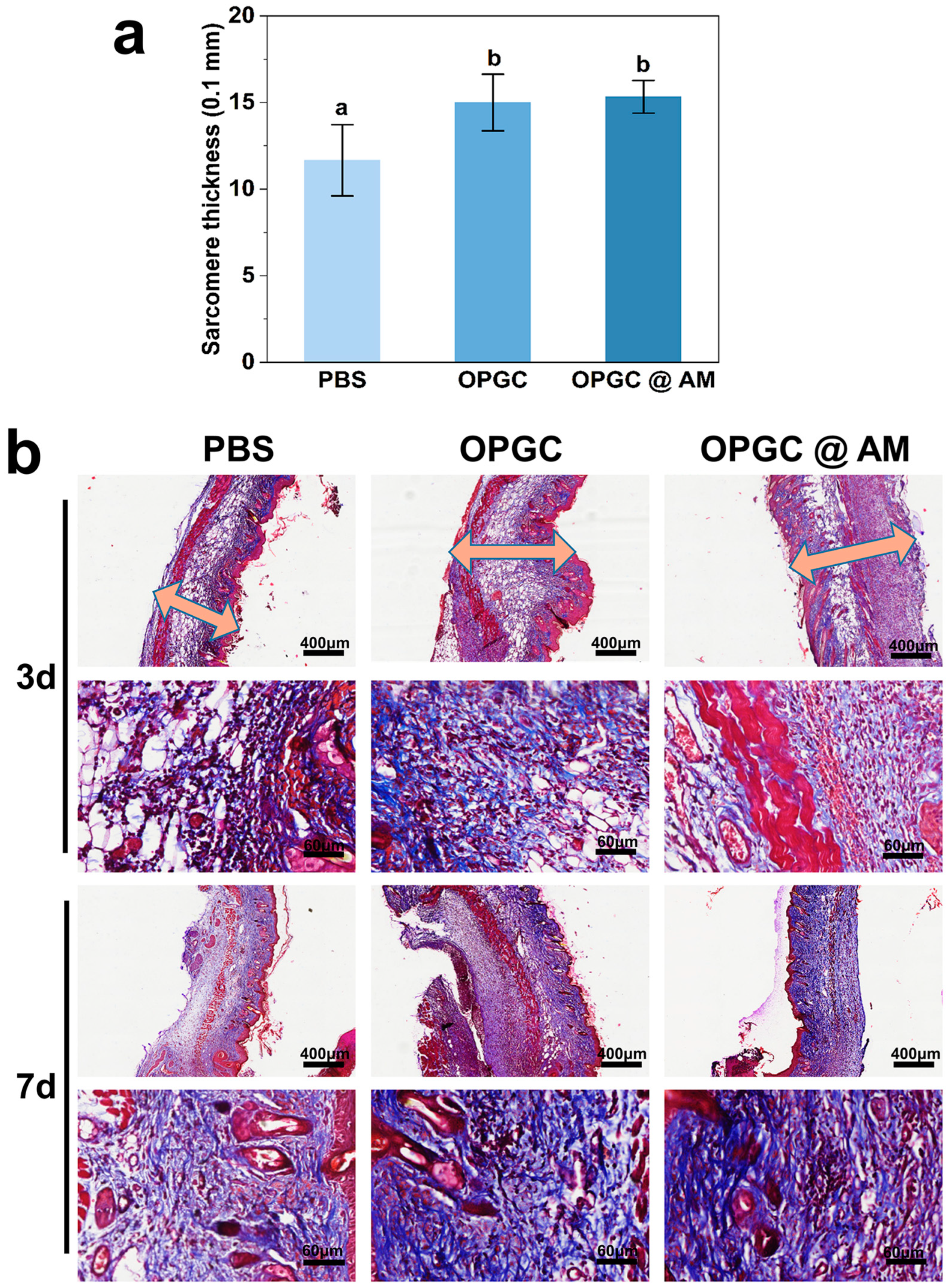
2.9. Masson Staining Analysis of Wounded Skin
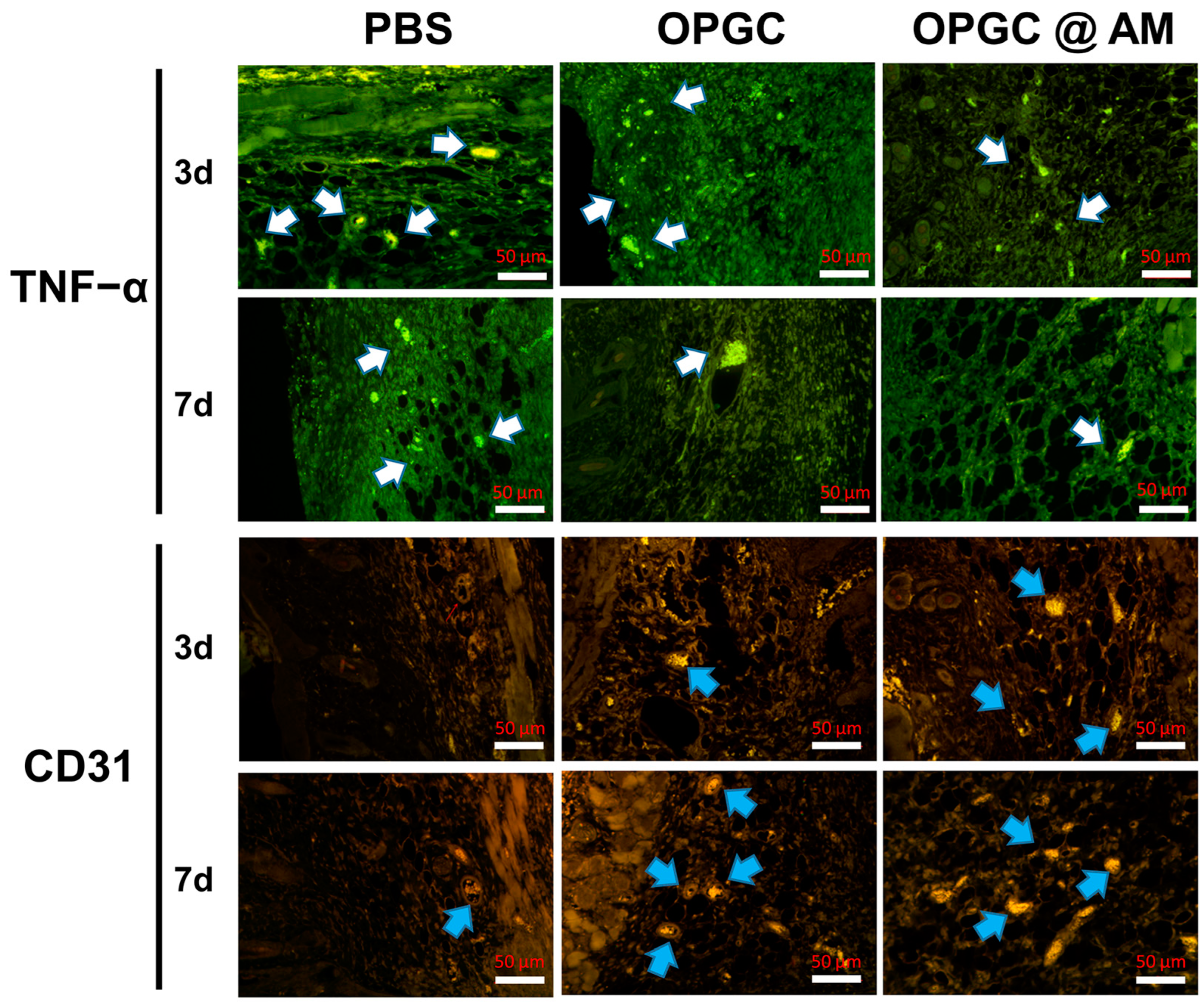
2.10. Immunofluorescence Staining Analysis of Wound Skin
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of OPGP
4.3. Determination of Aldehyde Groups Content in OPGP
4.4. Synthesis of OPGC Hydrogels
4.5. Gelation Time of OPGC Hydrogels
4.6. Fourier Transform Infrared (FTIR) of OPGC Hydrogels
4.7. Determination of Breaking Force and Gel Strength of OPGC Hydrogels
4.8. Rheological Analysis of OPGC Hydrogels
4.9. Physical Properties of OPGC Hydrogels
4.10. Scanning Electron Microscopy Characterization of OPGC Hydrogels
4.11. Measuring the Self-Healing Ability of OPGC Hydrogels
4.12. Injectability Test of OPGC Hydrogels
4.13. In Vitro Antimicrobial Testing of Drug-Loaded OPGC Hydrogels
4.14. In Vitro Drug Release Assay of Drug-Loaded OPGC Hydrogels
4.15. Cytotoxicity Tests
4.16. Construction of Infected Wounds of Full-Thickness Skin Defects in Mice
4.17. Macro-Monitoring of Mouse Wounds and Calculation of Wound Closure Rates
4.18. Preparation and Staining of Mouse Skin Tissue Sections
4.19. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OPGC | Oxidized peach gum polysaccharide–carboxymethyl chitosan |
| OPGP | Oxidized peach gum polysaccharide |
| CMCS | Carboxymethyl chitosan |
| PGP | Peach gum polysaccharides |
References
- Pelloth, J.L.; Tran, P.A.; Walther, A.; Goldmann, A.S.; Frisch, H.; Truong, V.X.; Barner-Kowollik, C. Wavelength-selective softening of hydrogel networks. Adv. Mater. 2021, 33, 2102184. [Google Scholar] [CrossRef] [PubMed]
- Mohanto, S.; Narayana, S.; Merai, K.P.; Kumar, J.A.; Bhunia, A.; Hani, U.; Al Fatease, A.; Gowda, B.J.; Nag, S.; Ahmed, M.G. Advancements in gelatin-based hydrogel systems for biomedical applications: A state-of-the-art review. Int. J. Biol. Macromol. 2023, 253, 127143. [Google Scholar] [CrossRef] [PubMed]
- Abalymov, A.; Pinchasik, B.-E.; Akasov, R.A.; Lomova, M.; Parakhonskiy, B.V. Strategies for anisotropic fibrillar hydrogels: Design, cell alignment, and applications in tissue engineering. Biomacromolecules 2023, 24, 4532–4552. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Han, Y.; Teng, H.; Xu, Q. Crucial effect of ovomucin on alkali-induced egg white gel formation: Properties, structure and facilitation mechanism. Int. J. Biol. Macromol. 2024, 277, 134507. [Google Scholar]
- Asokan-Sheeja, H.; Awad, K.; Xu, J.; Le, M.; Nguyen, J.N.; Nguyen, N.; Nguyen, T.P.; Nguyen, K.T.; Hong, Y.; Varanasi, V.G. In Situ Synthesis and Self-Assembly of Peptide–PEG Conjugates: A Facile Method for the Construction of Fibrous Hydrogels. Biomacromolecules 2024, 25, 2814–2822. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, J.; Zhang, R.; Lin, J.; Zhou, M.; Qin, X.; Wang, K.; Zhou, Y.; Zhu, Q.; Jin, Y. Development of multi-cross-linking, rapid curing, and easy cleaning, edible hydrogels for meat preservation. Food Hydrocoll. 2024, 155, 110186. [Google Scholar] [CrossRef]
- Zheng, J.; Song, X.; Yang, Z.; Yin, C.; Luo, W.; Yin, C.; Ni, Y.; Wang, Y.; Zhang, Y. Self-assembly hydrogels of therapeutic agents for local drug delivery. J. Control. Release 2022, 350, 898–921. [Google Scholar]
- Yoneda, J.S.; de Araujo, D.R.; Sella, F.; Liguori, G.R.; Liguori, T.T.; Moreira, L.F.P.; Spinozzi, F.; Mariani, P.; Itri, R. Self-assembled guanosine-hydrogels for drug-delivery application: Structural and mechanical characterization, methylene blue loading and controlled release. Mater. Sci. Eng. C 2021, 121, 111834. [Google Scholar] [CrossRef]
- Ye, J.; Fu, S.; Zhou, S.; Li, M.; Li, K.; Sun, W.; Zhai, Y. Advances in hydrogels based on dynamic covalent bonding and prospects for its biomedical application. Eur. Polym. J. 2020, 139, 110024. [Google Scholar]
- Malik, U.S.; Niazi, M.B.K.; Jahan, Z.; Zafar, M.I.; Vo, D.-V.N.; Sher, F. Nano-structured dynamic Schiff base cues as robust self-healing polymers for biomedical and tissue engineering applications: A review. Environ. Chem. Lett. 2022, 20, 495–517. [Google Scholar] [CrossRef]
- Mo, C.; Xiang, L.; Chen, Y. Advances in Injectable and Self-healing Polysaccharide Hydrogel Based on the Schiff Base Reaction. Macromol. Rapid Commun. 2021, 42, 2100025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, L.; Ettoumi, F.-E.; Javed, M.; Li, L.; Lin, X.; Xu, Y.; Lu, Y.; Shao, X.; Luo, Z. Ultrasonic-assisted green extraction of peach gum polysaccharide for blue-emitting carbon dots synthesis. Sustain. Chem. Pharm. 2021, 24, 100555. [Google Scholar] [CrossRef]
- Juan-Polo, A.; Pavon, C.; de la Rosa-Ramírez, H.; López-Martínez, J. Use of raw peach gum as a sustainable additive for the development of water-sensitive and biodegradable thermoplastic starch films. Polymers 2023, 15, 3359. [Google Scholar] [CrossRef]
- Bouaziz, F.; Koubaa, M.; Ghorbel, R.E.; Chaabouni, S.E. Recent advances in Rosaceae gum exudates: From synthesis to food and non-food applications. Int. J. Biol. Macromol. 2016, 86, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, G.; Liu, D.; Li, M.; Liu, H.; Zhu, W.; Wang, K.; Ren, X. Peach gum polysaccharide protects intestinal barrier function, reduces inflammation and oxidative stress, and alleviates pulmonary inflammation induced by Enterococcus faecium E745. J. Funct. Foods 2024, 115, 106098. [Google Scholar] [CrossRef]
- Tenorio-Barajas, A.Y.; Olvera, M.d.l.L.; Romero-Paredes, G.; Altuzar, V.; Garrido-Guerrero, E.; Mendoza-Barrera, C. Chitosan, Chitosan/IgG-Loaded, and N-Trimethyl Chitosan Chloride Nanoparticles as Potential Adjuvant and Carrier-Delivery Systems. Molecules 2023, 28, 4107. [Google Scholar] [CrossRef] [PubMed]
- Taubner, T.; Marounek, M.; Synytsya, A. Preparation and characterization of hydrophobic and hydrophilic amidated derivatives of carboxymethyl chitosan and carboxymethyl β-glucan. Int. J. Biol. Macromol. 2020, 163, 1433–1443. [Google Scholar] [CrossRef]
- Li, D.; Dong, X.; Liu, X.; Lin, H.; Yang, D.; Shi, X.; Chen, C.; Tao, F.; Jiang, L.; Deng, H. Cellulose nanofibers embedded chitosan/tannin hydrogel with high antibacterial activity and hemostatic ability for drug-resistant bacterial infected wound healing. Carbohydr. Polym. 2024, 329, 121687. [Google Scholar] [CrossRef]
- Geng, Y.; Xue, H.; Zhang, Z.; Panayi, A.C.; Knoedler, S.; Zhou, W.; Mi, B.; Liu, G. Recent advances in carboxymethyl chitosan-based materials for biomedical applications. Carbohydr. Polym. 2023, 305, 120555. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Tian, W.; Peng, K.; Xue, Y.; Zhao, H.; Ma, X.; Shi, R.; Chen, Y. A sodium alginate/carboxymethyl chitosan dual-crosslinked injectable hydrogel scaffold with tunable softness/hardness for bone regeneration. Int. J. Biol. Macromol. 2024, 257, 128700. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, S.; Zhu, Q.; Cao, X.; Lv, K.; Feng, Y.; Yin, H. Insights into Polyacrylamide Hydrogels Used for Oil and Gas Exploration: Gelation Time, Gel Strength, and Adhesion Strength. Energy Fuels 2023, 37, 19548–19561. [Google Scholar]
- Zhu, K.; Yu, D.; Chen, X.; Song, G. Preparation, characterization and controlled-release property of Fe3+ cross-linked hydrogels based on peach gum polysaccharide. Food Hydrocoll. 2019, 87, 260–269. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Yu, F.; Zhao, Y.-x.; Mo, X.-m.; Pan, J.-f. In situ forming hydrogel of natural polysaccharides through Schiff base reaction for soft tissue adhesive and hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhen, M.; Wu, Y.; Ma, M.; Cheng, Y.; Jin, Y. Effect of catechin and tannins on the structural and functional properties of sodium alginate/gelatin/poly(vinylalcohol) blend films. Food Hydrocoll. 2023, 135, 108141. [Google Scholar]
- Ding, F.; Shi, X.; Wu, S.; Liu, X.; Deng, H.; Du, Y.; Li, H. Flexible polysaccharide hydrogel with pH-regulated recovery of self-healing and mechanical properties. Macromol. Mater. Eng. 2017, 302, 1700221. [Google Scholar]
- Chen, Y.; Gao, X.; Su, W.; Zhou, X.; Ding, Y.; Liu, S.; Ding, Y. Insight into transglutaminase cross-linking induced gel strength and thermal stability improving of gelatin-based emulsion-filled gels. Int. J. Food Sci. Technol. 2023, 58, 2909–2920. [Google Scholar]
- Krainer, S.; Hirn, U. Contact angle measurement on porous substrates: Effect of liquid absorption and drop size. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126503. [Google Scholar]
- Chen, Y.; Ye, Y.; Zhu, Z.; Xu, B.; Meng, L.; Yang, T.; Zhang, L.; Qian, J.; Liu, F. Preparation and characterization of peach gum/chitosan polyelectrolyte composite films with dual cross-linking networks for antibacterial packaging. Int. J. Biol. Macromol. 2024, 261, 129754. [Google Scholar]
- Wang, C.; Sun, J.; Long, Y.; Peng, L.; Li, Y.; Wang, R.; Qu, Y.; Yang, X. Rapid self-healing nanocomposite gel crosslinked by LDH for lost circulation control. Colloids Surf. A 2024, 695, 134207. [Google Scholar]
- Jia, L.; Jiang, J.; Ren, A.; Wei, Z.; Xiang, T.; Zhou, S. Ultra-fast cryogenic self-healing ionic hydrogel for flexible wearable bioelectronics. Chem. Eng. J. 2024, 495, 153734. [Google Scholar]
- Chen, S.; Chen, L.; He, X.; Wu, R.; Peng, C. Photo-induced self-healing gel adsorbent for sustainable and efficient removal of Cr (VI) from water. Chem. Eng. J. 2025, 507, 160331. [Google Scholar] [CrossRef]
- Lei, Z.Q.; Xie, P.; Rong, M.Z.; Zhang, M.Q. Catalyst-free dynamic exchange of aromatic Schiff base bonds and its application to self-healing and remolding of crosslinked polymers. J. Mater. Chem. A 2015, 3, 19662–19668. [Google Scholar] [CrossRef]
- De France, K.J.; Cranston, E.D.; Hoare, T. Mechanically reinforced injectable hydrogels. ACS Appl. Polym. Mater. 2019, 2, 1016–1030. [Google Scholar]
- Lam, S.; Clairoux, A.; Udenigwe, C.C. Recent Advances on Antimicrobial Peptide and Polysaccharide Hydrogels. Food Hydrocoll. Health 2024, 6, 100180. [Google Scholar]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Fahimirad, S.; Fattahi, F.; Hatami, M.; Shabani, S.; Ghorbanpour, M. Nanotechnology-based biotherapeutics for physiological wound healing phases. Ind. Crops Prod. 2025, 226, 120608. [Google Scholar] [CrossRef]
- Tian, X.; Zheng, L.; Ma, J.; Xu, Y.; Zhang, Y.; Pi, Y. Inhibition of LAMP3 mediates the protective effect of vitamin D against hypoxia/reoxygenation in trophoblast cells. Braz. J. Med. Biol. Res. 2023, 56, e12816. [Google Scholar]
- Amer, H.; Nypelö, T.; Sulaeva, I.; Bacher, M.; Henniges, U.; Potthast, A.; Rosenau, T. Synthesis and characterization of periodate-oxidized polysaccharides: Dialdehyde xylan (DAX). Biomacromolecules 2016, 17, 2972–2980. [Google Scholar] [CrossRef]



| NaIO4: Peach Gum Polysaccharides (PGP) | [-CHO] Content (mmol/g) |
|---|---|
| 0: 1 | - |
| 0.5: 1 | 0.78 ± 0.10 a |
| 1: 1 | 1.89 ± 0.13 b |
| 1.5: 1 | 2.13 ± 0.38 c |
| 2: 1 | 2.92 ± 0.14 d |
| 2.5: 1 | 3.30 ± 0.39 d |
| 3: 1 | 3.10 ± 0.15 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Han, Y.; Zhang, Z.; Hao, J.; Wan, H.; Jin, Y.; Xu, Q. Preparation and Characterization of Dual-Network Multifunctional Hydrogels Based on Peach Gum Polysaccharides: Ultrafast Self-Healing Ability, Favorable Mechanical Tunability, and Controlled Release Properties. Gels 2025, 11, 274. https://doi.org/10.3390/gels11040274
Liu B, Han Y, Zhang Z, Hao J, Wan H, Jin Y, Xu Q. Preparation and Characterization of Dual-Network Multifunctional Hydrogels Based on Peach Gum Polysaccharides: Ultrafast Self-Healing Ability, Favorable Mechanical Tunability, and Controlled Release Properties. Gels. 2025; 11(4):274. https://doi.org/10.3390/gels11040274
Chicago/Turabian StyleLiu, Boyu, Yumeng Han, Zhenqing Zhang, Jianing Hao, Hao Wan, Yongguo Jin, and Qi Xu. 2025. "Preparation and Characterization of Dual-Network Multifunctional Hydrogels Based on Peach Gum Polysaccharides: Ultrafast Self-Healing Ability, Favorable Mechanical Tunability, and Controlled Release Properties" Gels 11, no. 4: 274. https://doi.org/10.3390/gels11040274
APA StyleLiu, B., Han, Y., Zhang, Z., Hao, J., Wan, H., Jin, Y., & Xu, Q. (2025). Preparation and Characterization of Dual-Network Multifunctional Hydrogels Based on Peach Gum Polysaccharides: Ultrafast Self-Healing Ability, Favorable Mechanical Tunability, and Controlled Release Properties. Gels, 11(4), 274. https://doi.org/10.3390/gels11040274






