Alginate–Gelatin Hydrogel Scaffold Model for Hypoxia Induction in Glioblastoma Embedded Spheroids
Abstract
1. Introduction
2. Results and Discussion
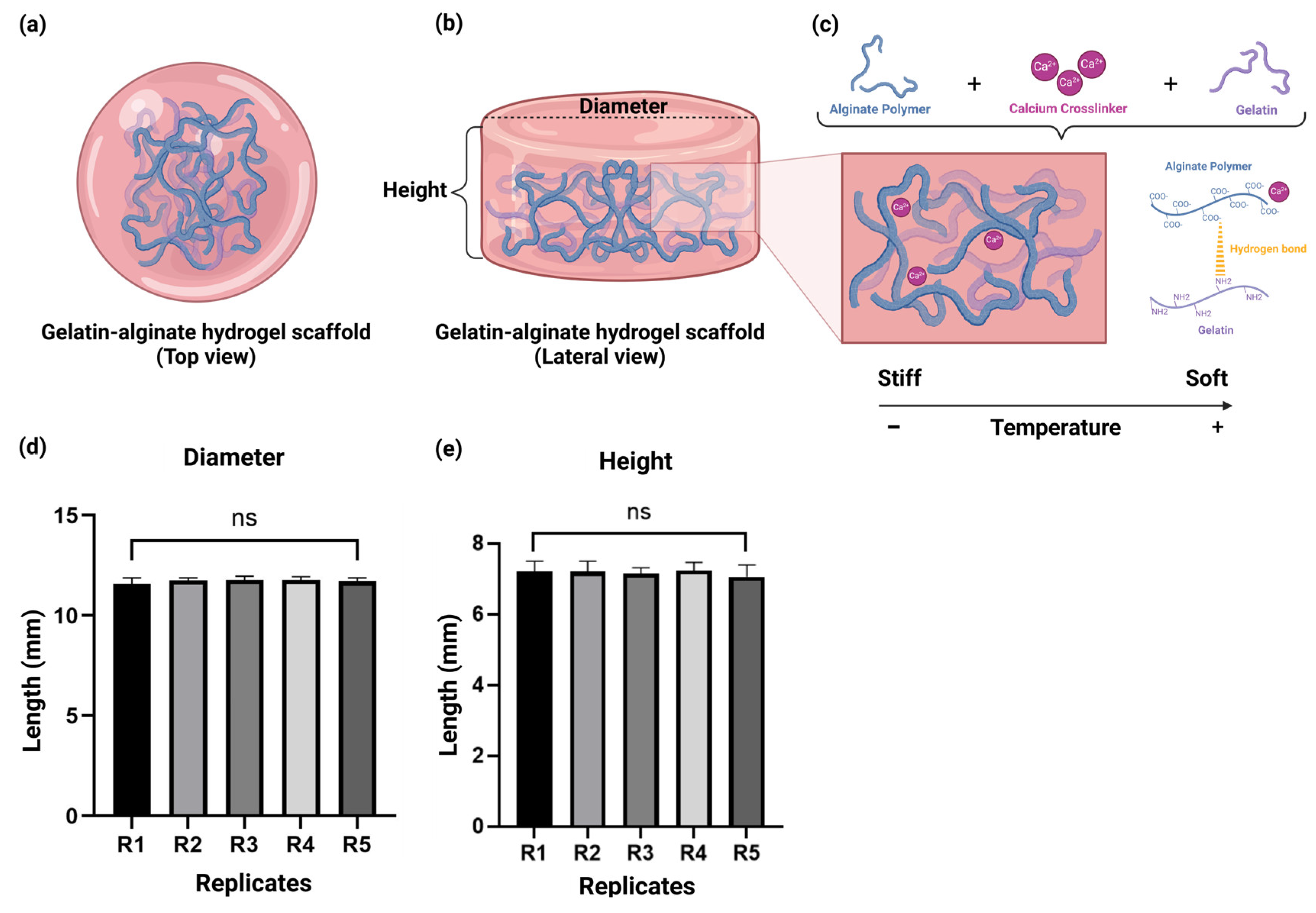
2.1. The Alginate–Gelatin Hydrogel Scaffold Demonstrated Suitable Reproducibility
2.2. ATR-FTIR Spectroscopy Suggest That the Negative Group of the Alginate Could Be Associated with the Positive Charge of the Gelatin
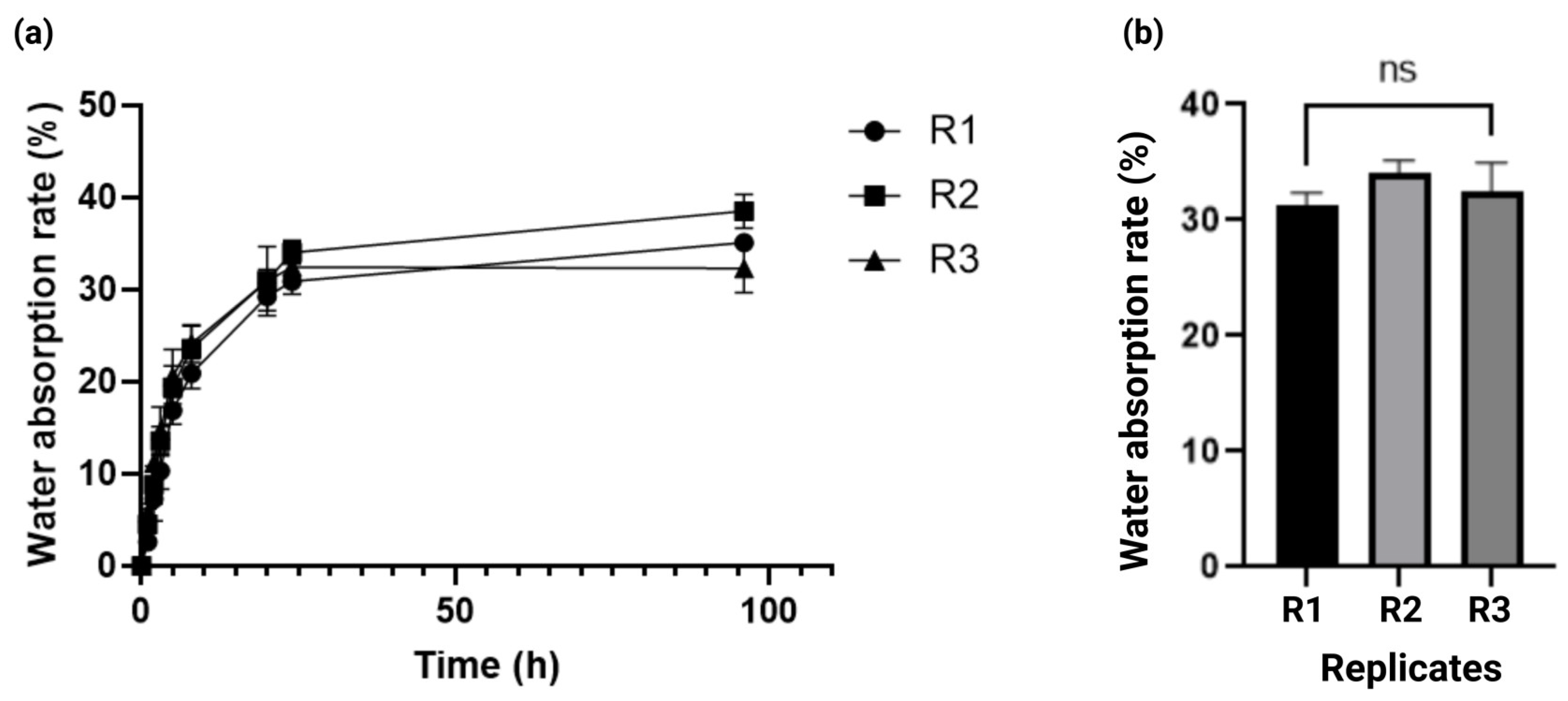
2.3. Kinetics of Solvent Absorption in the Alginate–Gelatin Hydrogel Scaffold
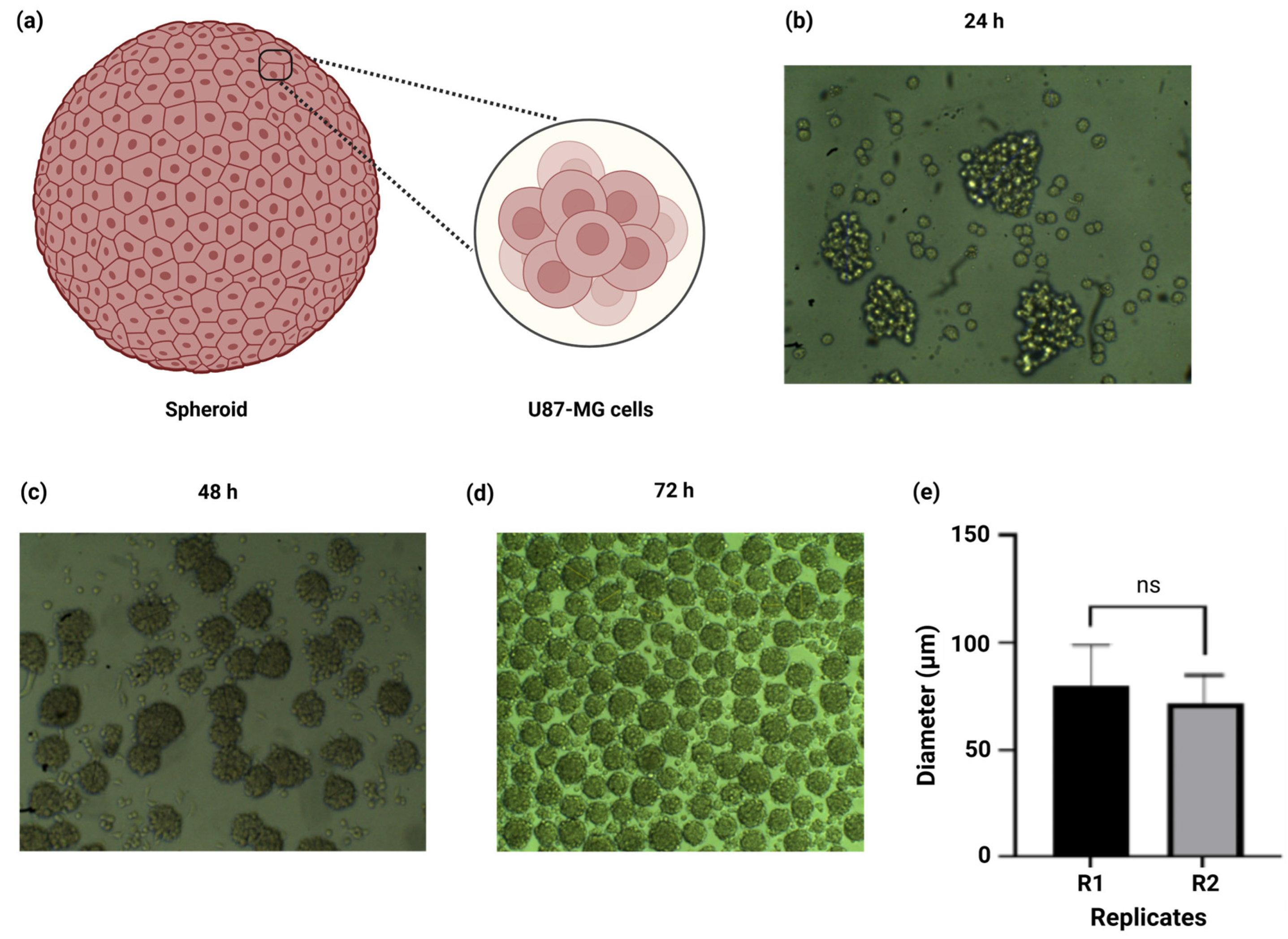
2.4. Spheroid Size Demonstrated Reproducible and Homogenous Size Amongst Replicates
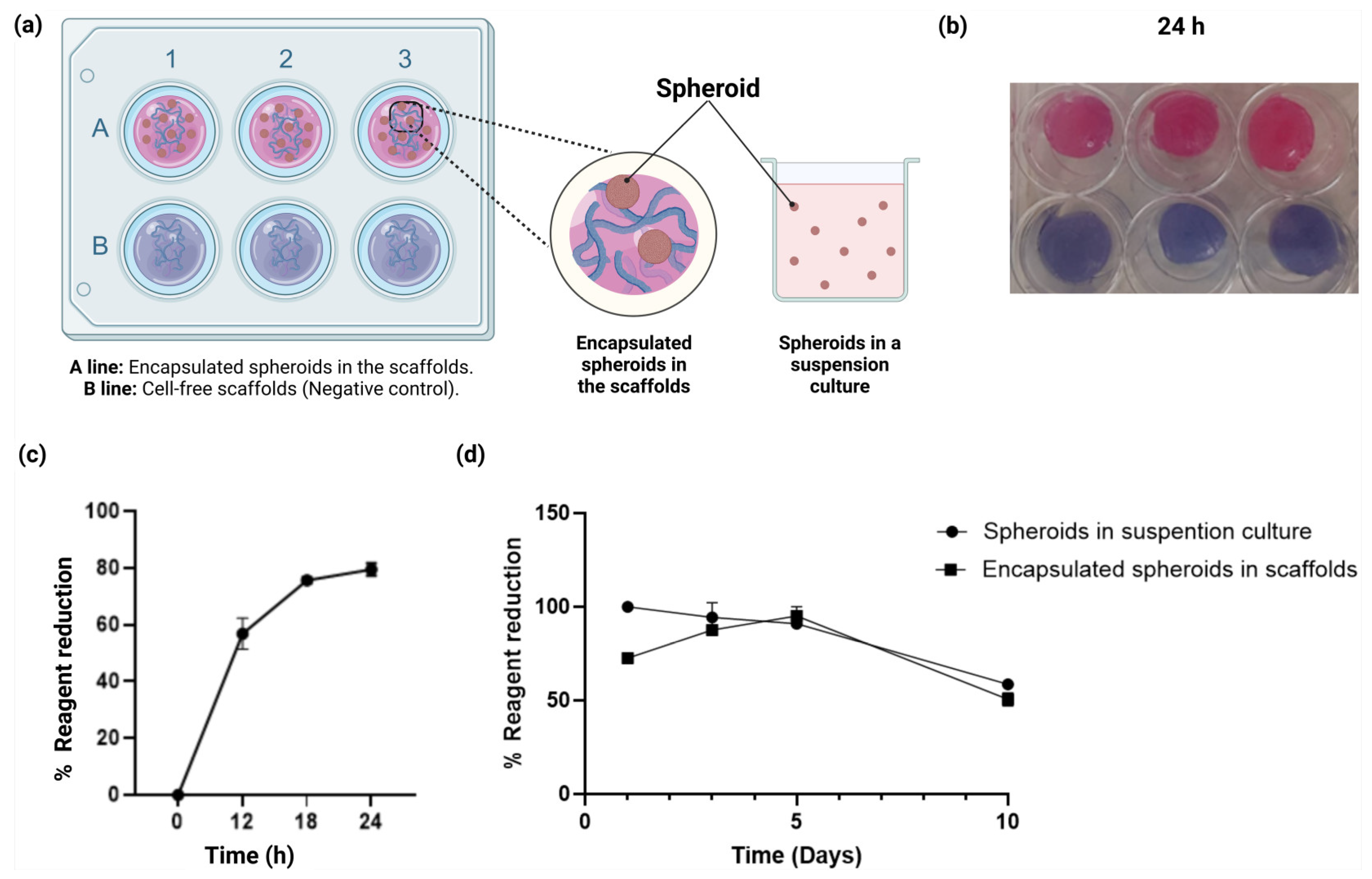
2.5. Cell Viability Assays of the Spheroids Within the Scaffolds
2.6. Microstructural Analyses with SEM and Cell Adhesion of U87-MG Cells to the Scaffold Structure
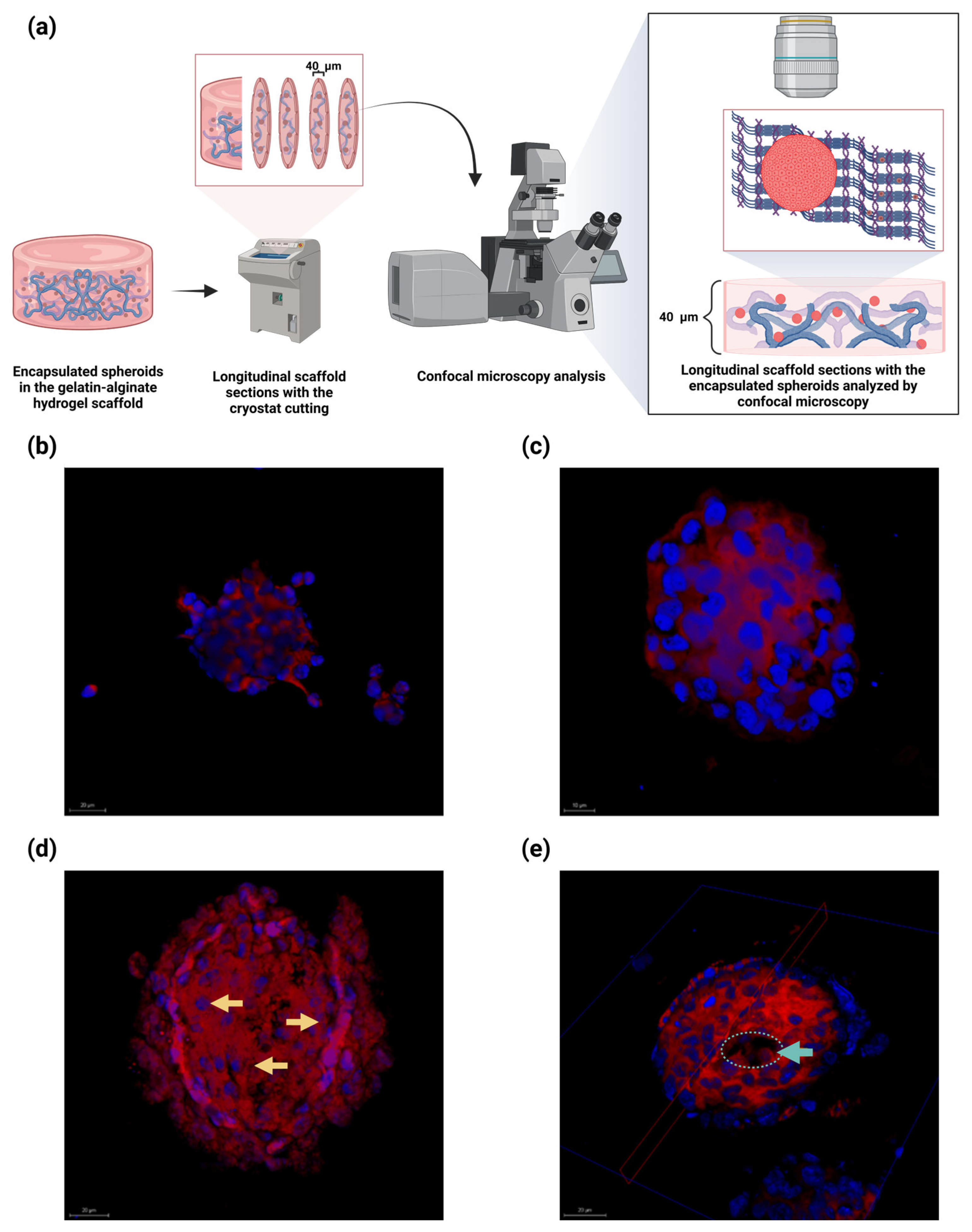
2.7. Embedded Spheroids Within the Alginate–Gelatin Hydrogel Scaffold Exhibited Heterogenous Proliferating Cells, Neighboring Pseudopalisading Cells near the Center and a Necrotic Central Core
2.8. Embedded Spheroids in the Alginate–Gelatin Hydrogel Scaffold Expressed HIF-1α in a Time-Dependent Manner
3. Conclusions
4. Materials and Methods
4.1. Generation of the Alginate–Gelatin Scaffold
4.1.1. Hydrogel Scaffold Composition and Crosslinking
4.1.2. Hydrogel Scaffold Weight Evaluation
4.1.3. Fourier Transform Infrared (ATR-FTIR) Characterization
4.1.4. Water Absorption Rate Determination
4.2. Cell Culture in Monolayer and Spheroid with or Without Hydrogel Scaffold
4.2.1. Cell Culture in Monolayer
4.2.2. Spheroid Generation
4.2.3. Spheroid Cell Counting
4.2.4. Cell Culture Within Scaffolds
4.3. Embedded Spheroids in Alginate–Gelatin Scaffolds Microscopic Analysis
4.3.1. Scaffold SEM Analysis with and Without Cells
4.3.2. Cell Staining and Confocal Microscopy
4.4. HIF1-α Analysis of Spheroids in the Alginate–Gelatin Scaffolds
HIF1-α Immunofluorescence Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, A.; Faujdar, C.; Sharma, R.; Sharma, S.; Malik, B.; Neplai, K.; Liou, J.P. Glioblastoma: Current status, emerging targets, and recent advances. J. Med. Chem. 2022, 65, 8596–8685. [Google Scholar] [CrossRef]
- Paw, I.; Carpenter, R.C.; Watabe, K.; Debinski, W.; Lo, H.W. Mechanisms regulating glioma invasion. Cancer Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef]
- Domènech, M.; Hernández, A.; Plaja, A.; Martínez-Balibrea, E.; Balañà, C. Hypoxia: The Cornerstone of Glioblastoma. Int. J. Mol. Sci. 2021, 22, 12608. [Google Scholar] [CrossRef]
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; Van Meir, E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005, 7, 134–153. [Google Scholar] [CrossRef]
- Grimes, D.R.; Jansen, M.; Macauley, R.J.; Scott, J.G.; Basanta, D. Evidence for hypoxia increasing the tempo of evolution in glioblastoma. Br. J. Cancer 2020, 123, 1562–1569. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Semenza, G. Signal transduction to hypoxia-inducible factor 1. Biochem. Pharmacol. 2002, 64, 993–998. [Google Scholar] [CrossRef]
- Mayer, A.; Schneider, F.; Vaupel, P.; Sommer, C.; Schmidberger, H. Differential expression of HIF-1 in glioblastoma multiforme and anaplastic astrocytoma. Int. J. Oncol. 2012, 41, 1260–1270. [Google Scholar] [CrossRef]
- Semenza, G.L. Hydroxylation of HIF-1: Oxygen sensing at the molecular level. Physiology 2004, 19, 176–182. [Google Scholar] [CrossRef]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef]
- Schmidt, M.C.; Antweiler, S.; Urban, N.; Mueller, W.; Kuklik, A.; Meyer-Puttlitz, B.; Wiestler, O.D.; Louis, D.N.; Fimmers, R.; von Deimling, A. Impact of genotype and morphology on the prognosis of glioblastoma. J. Neuropathol. Exp. Neurol. 2002, 61, 321–328. [Google Scholar] [CrossRef]
- Søndergaard, K.L.; Hilton, D.A.; Penney, M.; Ollerenshaw, M.; Demaine, A.G. Expression of hypoxia-inducible factor 1alpha in tumours of patients with glioblastoma. Neuropathol. Appl. Neurobiol. 2002, 28, 210–217. [Google Scholar] [CrossRef]
- Said, H.M.; Hagemann, C.; Staab, A.; Stojic, J.; Kühnel, S.; Vince, G.H.; Flentje, M.; Roosen, K.; Vordermark, D. Expression patterns of the hypoxia-related genes osteopontin, CA9, erythropoietin, VEGF and HIF-1alpha in human glioma in vitro and in vivo. Radiother. Oncol. 2007, 83, 398–405. [Google Scholar] [CrossRef]
- Persano, L.; Pistollato, F.; Rampazzo, E.; Della Puppa, A.; Abbadi, S.; Frasson, C.; Volpin, F.; Indraccolo, S.; Scienza, R.; Basso, G. BMP2 sensitizes glioblastoma stem-like cells to Temozolomide by affecting HIF-1α stability and MGMT expression. Cell Death Dis. 2012, 3, e412. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, P. Clinical and prognostic significance of HIF-1α in glioma patients: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 22073–22083. [Google Scholar] [PubMed]
- Sharpe, M.A.; Baskin, D.S. Monoamine oxidase B levels are highly expressed in human gliomas and are correlated with the expression of HiF-1α and with transcription factors Sp1 and Sp3. Oncotarget 2016, 7, 3379–3393. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Wang, Z.; Yang, Y.; Tian, J.; Liu, G.; Guan, D.; Cao, X.; Zhang, Y.; Hao, A. GRIM-19 opposes reprogramming of glioblastoma cell metabolism via HIF1α destabilization. Carcinogenesis 2013, 34, 1728–1736. [Google Scholar] [CrossRef][Green Version]
- Huang, S.; Qi, P.; Zhang, T.; Li, F.; He, X. The HIF-1α/miR-224-3p/ATG5 axis affects cell mobility and chemosensitivity by regulating hypoxia-induced protective autophagy in glioblastoma and astrocytoma. Oncol. Rep. 2019, 41, 1759–1768. [Google Scholar] [CrossRef]
- Wang, P.; Yan, Q.; Liao, B.; Zhao, L.; Xiong, S.; Wang, J.; Zou, D.; Pan, J.; Wu, L.; Deng, Y.; et al. The HIF1α/HIF2α-miR210-3p network regulates glioblastoma cell proliferation, dedifferentiation and chemoresistance through EGF under hypoxic conditions. Cell Death Dis. 2020, 11, 992. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Mo, F.; Patel, G.; Butterworth, K.; Shao, C.; Prise, K.M. The Roles of HIF-1α in Radiosensitivity and Radiation-Induced Bystander Effects Under Hypoxia. Front. Cell Dev. Biol. 2021, 9, 637454. [Google Scholar] [CrossRef]
- Tang, J.H.; Ma, Z.X.; Huang, G.H.; Xu, Q.F.; Xiang, Y.; Li, N.; Sidlauskas, K.; Zhang, E.E.; Lv, S.Q. Downregulation of HIF-1a sensitizes U251 glioma cells to the temozolomide (TMZ) treatment. Exp. Cell Res. 2016, 343, 148–158. [Google Scholar] [CrossRef]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Wanigasekara, J.; Cullen, P.J.; Bourke, P.; Tiwari, B.; Curtin, J.F. Advances in 3D culture systems for therapeutic discovery and development in brain cancer. Drug Discov. Today 2023, 28, 103426. [Google Scholar] [CrossRef]
- Wanigasekara, J.; Barcia, C.; Cullen, P.J.; Tiwari, B.; Curtin, J.F. Plasma induced reactive oxygen species-dependent cytotoxicity in glioblastoma 3D tumourspheres. Plasma Processes Polym. 2022, 19, e2100157. [Google Scholar] [CrossRef]
- Chhetri, A.; Rispoli, J.V.; Lelièvre, S.A. 3D Cell Culture for the Study of Microenvironment-Mediated Mechanostimuli to the Cell Nucleus: An Important Step for Cancer Research. Front. Mol. Biosci. 2021, 8, 628386. [Google Scholar] [CrossRef]
- Carter, E.P.; Roozitalab, R.; Gibson, S.V.; Grose, R.P. Tumour microenvironment 3D-modelling: Simplicity to complexity and back again. Trends Cancer 2021, 7, 1033–1046. [Google Scholar] [CrossRef]
- Foglietta, F.; Canaparo, R.; Muccioli, G.; Terreno, E.; Serpe, L. Methodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids. Life Sci. 2020, 254, 117784. [Google Scholar] [CrossRef]
- Laks, D.R.; Crisman, T.J.; Shih, M.Y.; Mottahedeh, J.; Gao, F.; Sperry, J.; Garrett, M.C.; Yong, W.H.; Cloughesy, T.F.; Liau, L.M.; et al. Large-scale assessment of the gliomasphere model system. Neuro Oncol. 2016, 18, 1367–1378. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Colombo, E.; Cattaneo, M.G. Multicellular 3D Models to Study Tumour-Stroma Interactions. Int. J. Mol. Sci. 2021, 22, 1633. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Bort, E.; Kieler, M.; Sharma, S.; Candido, J.B.; Loessner, D. 3D approaches to model the tumor microenvironment of pancreatic cancer. Theranostics 2020, 10, 5074–5089. [Google Scholar] [CrossRef] [PubMed]
- Alzeeb, G.; Metges, J.P.; Corcos, L.; Le Jossic-Corcos, C. Three-Dimensional Culture Systems in Gastric Cancer Research. Cancers 2020, 12, 2800. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef]
- Fisher, M.F.; Rao, S.S. Three-dimensional culture models to study drug resistance in breast cancer. Biotechnol. Bioeng. 2020, 117, 2262–2278. [Google Scholar] [CrossRef]
- Ruiz-Garcia, H.; Alvarado-Estrada, K.; Schiapparelli, P.; Quinones-Hinojosa, A.; Trifiletti, D.M. Engineering Three-Dimensional Tumor Models to Study Glioma Cancer Stem Cells and Tumor Microenvironment. Front. Cell Neurosci. 2020, 14, 558381. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e16. [Google Scholar] [CrossRef]
- Darrigues, E.; Nima, Z.A.; Griffin, R.J.; Anderson, J.M.; Biris, A.S.; Rodriguez, A. 3D cultures for modeling nanomaterial-based photothermal therapy. Nanoscale Horiz. 2020, 5, 400–430. [Google Scholar]
- Stanković, T.; Ranđelović, T.; Dragoj, M.; Stojković Burić, S.; Fernández, L.; Ochoa, I.; Pérez-García, V.M.; Pešić, M. In vitro biomimetic models for glioblastoma-a promising tool for drug response studies. Drug Resist. Updat. 2021, 55, 100753. [Google Scholar] [CrossRef]
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnight, T.R.; et al. Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol. 2016, 18, 1336–1345, Erratum in Nat. Cell Biol. 2023, 25, 787–788. https://doi.org/10.1038/s41556-023-01126-8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klein, E.; Hau, A.C.; Oudin, A.; Golebiewska, A.; Niclou, S.P. Glioblastoma Organoids: Pre-Clinical Applications and Challenges in the Context of Immunotherapy. Front. Oncol. 2020, 10, 604121. [Google Scholar] [CrossRef]
- Paolillo, M.; Comincini, S.; Schinelli, S. In Vitro Glioblastoma Models: A Journey into the Third Dimension. Cancers 2021, 13, 2449. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kievit, F.M.; Erickson, A.E.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Culture on 3D Chitosan-Hyaluronic Acid Scaffolds Enhances Stem Cell Marker Expression and Drug Resistance in Human Glioblastoma Cancer Stem Cells. Adv. Healthc. Mater. 2016, 5, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M.; Asgharzade, S.; Doostmohamadi, A.; Satari, A.; Hasannejad, F.; Arab, S. Development of a hydrogel-based three-dimensional (3D) glioblastoma cell lines culture as a model system for CD73 inhibitor response study. Biomed. Eng. Online 2024, 23, 127. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Law, A.M.K.; Rodriguez de la Fuente, L.; Grundy, T.J.; Fang, G.; Valdes-Mora, F.; Gallego-Ortega, D. Advancements in 3D Cell Culture Systems for Personalizing AntiCancer Therapies. Front. Oncol. 2021, 11, 782766. [Google Scholar] [CrossRef]
- Monteiro, M.V.; Gaspar, V.M.; Ferreira, L.P.; Mano, J.F. Hydrogel 3D in vitro tumor models for screening cell aggregation mediated drug response. Biomater. Sci. 2020, 8, 1855–1864. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8, Erratum in J. Biol. Eng. 2020, 14, 17. https://doi.org/10.1186/s13036-020-00239-0. [Google Scholar] [CrossRef]
- Dai, X.; Ma, C.; Lan, Q.; Xu, T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 2016, 8, 045005. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; Leon-Rodriguez, A.; Kinsella, J.M. Directing the self-assembly of tumour spheroids by bioprinting cellular heterogeneous models within alginate/gelatin hydrogels. Sci. Rep. 2017, 7, 4575. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; De Leon-Rodriguez, A.; Kinsella, J.M. Bioprintable Alginate/Gelatin Hydrogel 3D In Vitro Model Systems Induce Cell Spheroid Formation. J. Vis. Exp. 2018, 137, 57826. [Google Scholar] [CrossRef]
- Bahraminasab, M.; Janmohammadi, M.; Arab, S.; Talebi, A.; Nooshabadi, V.T.; Koohsarian, P.; Nourbakhsh, M.S. Bone Scaffolds: An Incorporation of Biomaterials, Cells, and Biofactors. ACS Biomater. Sci. Eng. 2021, 7, 5397–5431. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Mũnoz, Z.; Shih, H.; Lin, C.C. Gelatin hydrogels formed by orthogonal thiol-norbornene photochemistry for cell encapsulation. Biomater. Sci. 2014, 2, 1063–1072. [Google Scholar]
- Khajavi, S.; Bahraminasab, M.; Arab, S.; Talebi, A.; Kokhaei, P.; Abdoos, H. Design and synthesis of berberine loaded nano-hydroxyapatite/gelatin scaffold for bone cancer treatment. New J. Chem. 2024, 48, 6977–6996. [Google Scholar]
- Cadamuro, F.; Ardenti, V.; Nicotra, F.; Russo, L. Alginate-Gelatin Self-Healing Hydrogel Produced via Static-Dynamic Crosslinking. Molecules 2023, 28, 2851. [Google Scholar] [CrossRef]
- Higuchi, A.; Ling, Q.D.; Hsu, S.T.; Umezawa, A. Biomimetic cell culture proteins as extracellular matrices for stem cell differentiation. Chem. Rev. 2012, 112, 4507–4540. [Google Scholar] [CrossRef]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J.; Liddington, R.C. Structural basis of collagen recognition by integrin alpha2beta1. Cell 2000, 101, 47–56. [Google Scholar] [CrossRef]
- Ulset, A.S.; Mori, H.; Dalheim, M.Ø.; Hara, M.; Christensen, B.E. Influence of amino acids, buffers, and ph on the γ-irradiation-induced degradation of alginates. Biomacromolecules 2014, 15, 4590–4597. [Google Scholar] [CrossRef]
- Teng, K.; An, Q.; Chen, Y.; Zhang, Y.; Zhao, Y. Recent Development of Alginate-Based Materials and Their Versatile Functions in Biomedicine, Flexible Electronics, and Environmental Uses. ACS Biomater. Sci. Eng. 2021, 7, 1302–1337. [Google Scholar] [CrossRef]
- Mori, H.; Taketsuna, Y.; Shimogama, K.; Nishi, K.; Hara, M. Interpenetrating gelatin/alginate mixed hydrogel: The simplest method to prepare an autoclavable scaffold. J. Biosci. Bioeng. 2024, 137, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Dabkeviciute, G.; Maccioni, E.; Petrikaite, V. Effect of sunitinib derivatives on glioblastoma single-cell migration and 3D cell cultures. Am. J. Cancer Res. 2023, 13, 1377–1386. [Google Scholar]
- Jo, H.; Lee, S.; Kim, M.H.; Park, S.; Lee, S.Y. Recapitulating Glioma Stem Cell Niches Using 3D Spheroid Models for Glioblastoma Research. Biosensors 2024, 14, 539. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, S.J.; Wang, K.; Jana, S.; Wood, D.L.; Sytsma, S.K.; Sham, J.; Kievit, F.M.; Zhang, M. Porous chitosan-hyaluronic acid scaffolds as a mimic of glioblastoma microenvironment ECM. Biomaterials 2013, 34, 10143–10150. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xie, Q.; Gimple, R.C.; Zhong, Z.; Tam, T.; Tian, J.; Kidwell, R.L.; Wu, Q.; Prager, B.C.; Qiu, Z.; et al. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 2020, 30, 833–853. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, J.; Feng, X.; Chen, J. Fabrication and characterization of bioactive silk fibroin/wollastonite composite scaffolds. Mater. Sci. Eng. 2010, 30, 132–140. [Google Scholar] [CrossRef]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.J.; Mas-Moruno, C.; et al. A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148, Erratum in J. Mater. Sci. Mater. Med. 2018, 29, 39. https://doi.org/10.1007/s10856-018-6047-3. [Google Scholar] [CrossRef]
- Chang, M.W.; Lo, J.M.; Juan, H.F.; Chang, H.Y.; Chuang, C.Y. Combination of RGD compound and low-dose paclitaxel induces apoptosis in human glioblastoma cells. PLoS ONE 2012, 7, e37935. [Google Scholar] [CrossRef]
- Isogai, R.; Morio, H.; Okamoto, A.; Kitamura, K.; Furihata, T. Generation of a Human Conditionally Immortalized Cell-based Multicellular Spheroidal Blood-Brain Barrier Model for Permeability Evaluation of Macromolecules. Bio-Protocol 2022, 12, e4465. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Allard-Vannier, E.; Chourpa, I.; Hervé-Aubert, K. Nanomedicines functionalized with anti-EGFR ligands for active targeting in cancer therapy: Biological strategy, design and quality control. Int. J. Pharm. 2021, 605, 120795. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, K.; Reusch, U.; Fucek, I.; Knackmuss, S.; Weichel, M.; Gantke, T.; Molkenthin, V.; Zhukovsky, E.A.; Tesar, M.; Treder, M. Highly Specific and Effective Targeting of EGFRvIII-Positive Tumors with TandAb Antibodies. Front. Oncol. 2017, 7, 100. [Google Scholar] [CrossRef]
- Manzanares-Guzmán, A.; Alfonseca-Ladrón de Guevara, A.C.; Reza-Escobar, E.; Burciaga-Flores, M.; Canales-Aguirre, A.; Esquivel-Solís, H.; Lugo-Fabres, P.H.; Camacho-Villegas, T.A. Isolation and Characterization of the First Antigen-Specific EGFRvIII vNAR from Freshwater Stingray (Potamotrygon spp.) as a Drug Carrier in Glioblastoma Cancer Cells. Int. J. Mol. Sci. 2025, 26, 876. [Google Scholar] [CrossRef] [PubMed]
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Mechanical Properties of Alginate Hydrogels Cross-Linked with Multivalent Cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Rocío Aguilera-Marquez, J.; Manzanares-Guzmán, A.; García-Uriostegui, L.; Canales-Aguirre, A.A.; Camacho-Villegas, T.A.; Lugo-Fabres, P.H. Alginate–Gelatin Hydrogel Scaffold Model for Hypoxia Induction in Glioblastoma Embedded Spheroids. Gels 2025, 11, 263. https://doi.org/10.3390/gels11040263
del Rocío Aguilera-Marquez J, Manzanares-Guzmán A, García-Uriostegui L, Canales-Aguirre AA, Camacho-Villegas TA, Lugo-Fabres PH. Alginate–Gelatin Hydrogel Scaffold Model for Hypoxia Induction in Glioblastoma Embedded Spheroids. Gels. 2025; 11(4):263. https://doi.org/10.3390/gels11040263
Chicago/Turabian Styledel Rocío Aguilera-Marquez, Janette, Alejandro Manzanares-Guzmán, Lorena García-Uriostegui, Alejandro A. Canales-Aguirre, Tanya A. Camacho-Villegas, and Pavel H. Lugo-Fabres. 2025. "Alginate–Gelatin Hydrogel Scaffold Model for Hypoxia Induction in Glioblastoma Embedded Spheroids" Gels 11, no. 4: 263. https://doi.org/10.3390/gels11040263
APA Styledel Rocío Aguilera-Marquez, J., Manzanares-Guzmán, A., García-Uriostegui, L., Canales-Aguirre, A. A., Camacho-Villegas, T. A., & Lugo-Fabres, P. H. (2025). Alginate–Gelatin Hydrogel Scaffold Model for Hypoxia Induction in Glioblastoma Embedded Spheroids. Gels, 11(4), 263. https://doi.org/10.3390/gels11040263









