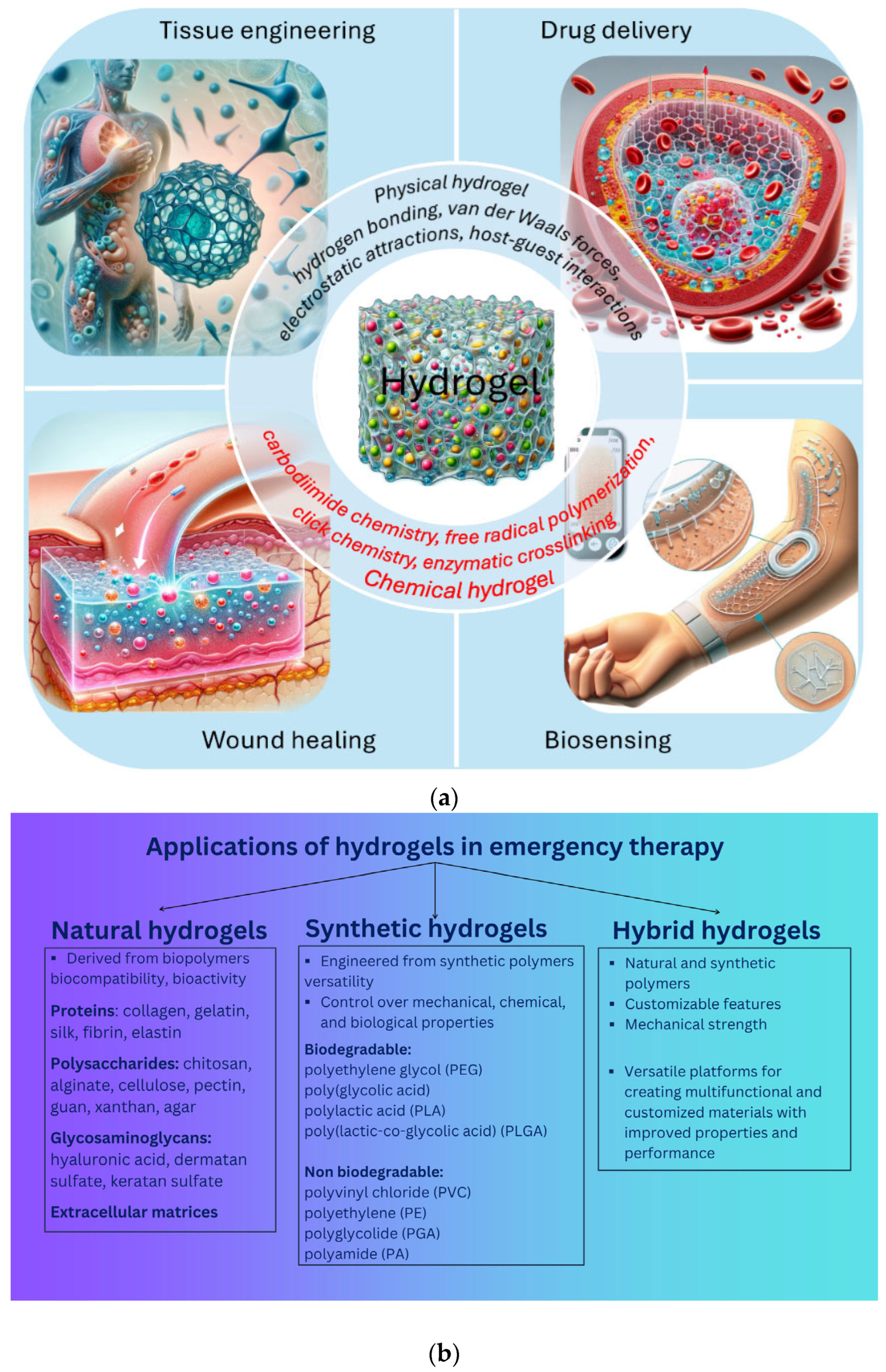
Applications of Hydrogels in Emergency Therapy
Abstract
1. Introduction
2. Characteristics of Hydrogels for Emergency Trauma Management
- Swelling Behavior—Determines the hydrogel’s ability to absorb water, influencing cell viability, nutrient diffusion, and mechanical stability.
- Mechanical Properties—Include elasticity, stiffness, and strength, which should match the native tissue for proper integration and function.
- Biocompatibility—Ensures that the hydrogel does not trigger an immune response or toxicity, promoting cell attachment and proliferation.
- Biodegradability—Allows for controlled degradation at a rate compatible with tissue regeneration while avoiding harmful byproducts.
- Porosity and Permeability—Affect cell migration, nutrient transport, and waste removal, crucial for sustaining cell activity.
- Crosslinking Density—Impacts mechanical strength, swelling capacity, and degradation rate, influencing overall hydrogel performance.
- Gelation Mechanism and Kinetics—Should allow for easy handling, in situ gelation, and adaptability to different applications (e.g., injectable hydrogels).
- Surface Chemistry—Can be modified to enhance cell adhesion, drug loading, and interaction with biological molecules.
- Hydrophilicity/Hydrophobicity Balance—Regulates water retention, protein adsorption, and cellular response.
- Drug and Growth Factor Delivery Capability—Supports the controlled and sustained release of bioactive molecules for tissue regeneration.
- -
- Dressings with the release of bioactive substances for wound healing, encompassing areas such as infection prevention, rapid hemostasis and adhesion adaptation, inflammation control and immune regulation, granulation tissue formation, re-epithelialization, and scar prevention and treatment;
- -
- Tissue engineering (3D scaffold, tissue development, growth factor, implantation);
- -
- Controlled drug delivery (in the form of nanocapsules, nanospheres, nanoshells, micelles, niosomes, nanoparticles, dendrimers, liposomes);
- -
- Contact lenses;
- -
- Disposable diapers;
- -
- Cosmetics;
- -
- Biodetection.
- (i)
- Strong adhesion in wet conditions, high mechanical strength, and suitable swelling properties to quickly halt bleeding and maintain biological pressure such as blood flow and tissue compression;
- (ii)
- Potent antibacterial and wound-healing properties to prevent infections and support tissue regeneration;
- (iii)
- Excellent biocompatibility and biodegradability, ensuring no inflammatory or toxic effects;
- (iv)
- The ability to be quickly deployed and applied.
- (i)
- Rapid in situ gelation;
- (ii)
- The ability to be injected for soft filling and in an adaptable manner to irregular wounds, with a ready-to-use format (e.g., prepacked in a syringe without requiring additional preparation);
- (iii)
- Ease of application, allowing use by injured soldiers or untrained civilians.
- (i)
- Provide a hydrated environment that safeguards cells and sensitive biomolecules (such as peptides, proteins, DNA, and oligonucleotides).
- (ii)
- Enable efficient nutrient exchange and waste removal, supporting cell viability.
- (iii)
- Can be functionalized with cell adhesion ligands to enhance biocompatibility and interaction with tissues.
- (iv)
- Injectable formulations allow for in situ gelation at body temperature, facilitating minimally invasive applications.
- (v)
- Generally biocompatible, reducing the risk of immune response or toxicity.
- (i)
- Can be difficult to manipulate and apply in clinical settings.
- (ii)
- Often exhibit weak mechanical properties, limiting their structural integrity.
- (iii)
- Loading drugs and cells while achieving effective crosslinking can be challenging.
- (iv)
- May be unsuitable as prefabricated matrices for in vitro applications.
- (v)
- Sterilization processes can be complex and may alter hydrogel properties.
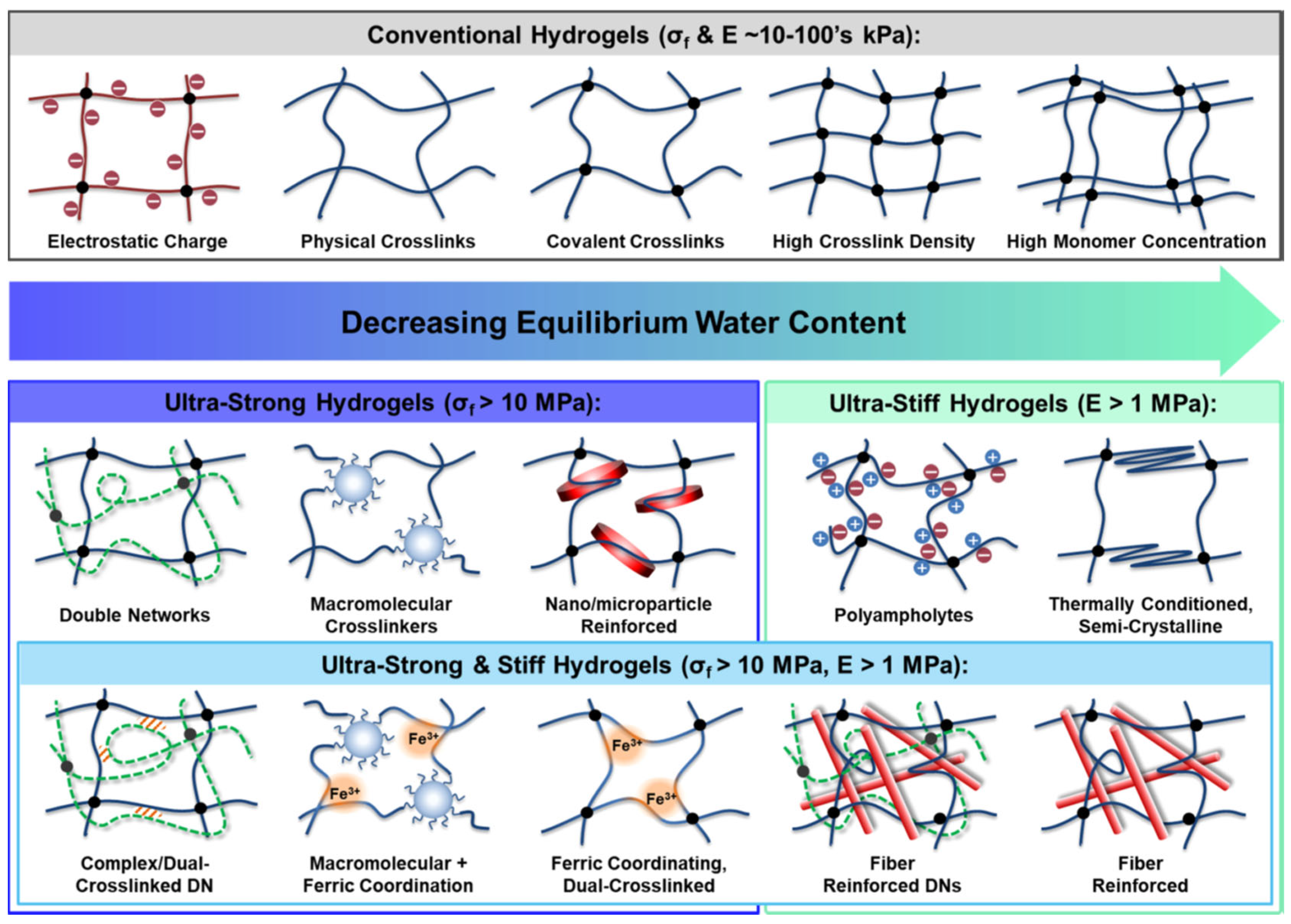
2.1. Robust Mechanical Strength
2.2. Adhesive Properties
- High swelling capacity and water content, maintaining a moist environment while absorbing exudate.
- Full wound coverage, ensuring perfect sealing of tissue defects.
- Tunable mechanical properties suitable for subcutaneous tissue, reducing stress concentration at the interface.
- A porous, bioactive structure resembling the native extracellular matrix, fostering a favorable healing environment.
- Functioning as a carrier for cells, drugs, and biological factors.
- Serving as a natural mechanical barrier against infection.
- Biodegradability, eliminating the need for removal.
- Adaptability to various wound types and shapes.
- Being simple and convenient to apply.
- Excellent biocompatibility.
- High customizability for different tissue conditions.
2.3. Antibacterial Properties
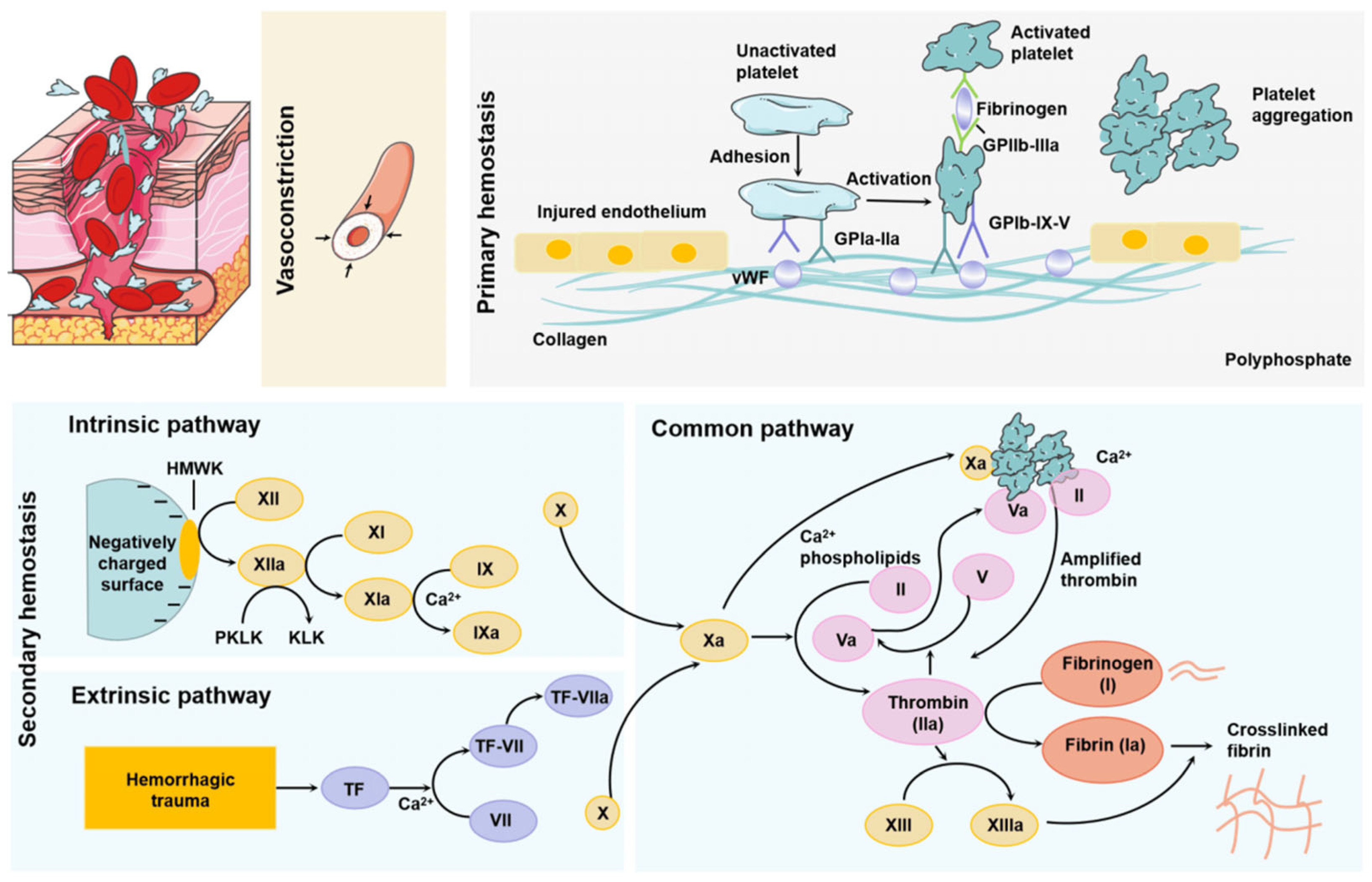
2.4. Hemorrhage Control and/or Emergency Hemostasis
2.5. In Situ Gelation Properties
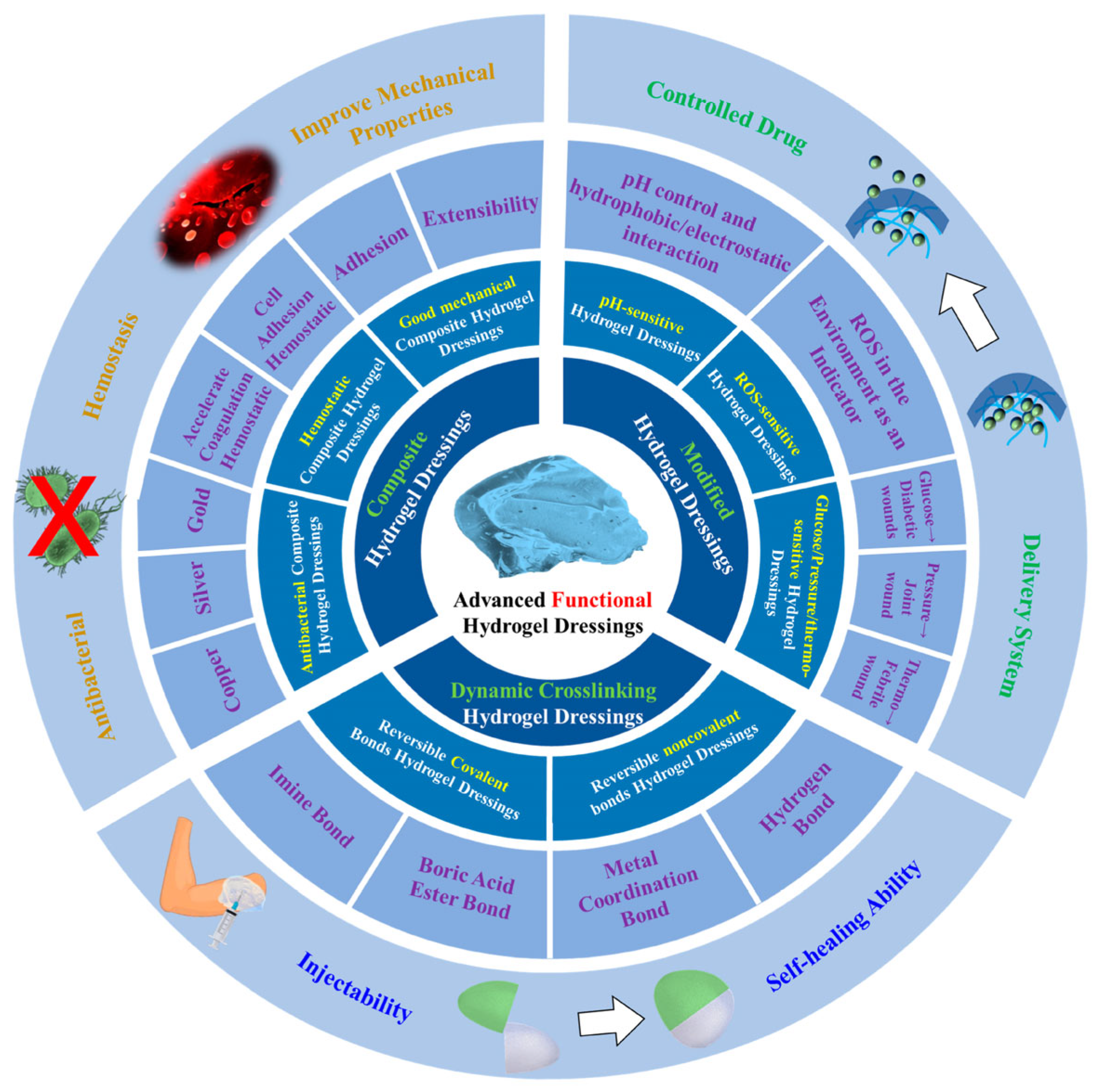
3. Applications of Hydrogels in Emergency Therapy
3.1. Hydrogels for Ophthalmic Injuries
3.2. Hydrogels for Organ Injuries
3.3. Hydrogels for Brain Injuries
3.4. Hydrogels for Spinal Cord Injuries
3.5. Hydrogels for Acute Colitis
3.6. Hydrogels for Localized Drug Delivery
3.7. Hydrogels for Breast Cancer Therapy
4. Challenges and Limitations
4.1. Mechanical Strength and Durability Issues
4.2. Risk of Infection and Biodegradation Control in Hydrogel-Based Emergency Therapy
- (i)
- Hydrolytic degradation: This occurs by breaking the polymer chains due to reactions with water molecules. For hydrogels made from natural or synthetic polymers with hydrolysable bonds, water can trigger the gradual cleavage of these bonds, leading to degradation of the material. Hydrolytic degradation is generally specific to biocompatible hydrogels, such as those made from polyesters or polysaccharides.
- (ii)
- Enzymatic degradation: In hydrogels derived from natural polymers such as collagen, chitosan, or hyaluronic acid, enzymes present in the body (such as proteases, lipases or cellulases) can break down the polymer network. This degradation process is often slower and more specific, providing a controlled decomposition of the material according to the body’s natural enzymatic environment.
- (iii)
- Oxidative degradation: Some hydrogels, especially those containing certain synthetic polymers or crosslinkers, are susceptible to oxidation when exposed to reactive oxygen species (ROS), which are generated during inflammation or oxidative stress. This can lead to the breakdown of the polymer matrix and damage the structural integrity of the hydrogel.
- (iv)
- Physical degradation: In some cases, hydrogel degradation can occur due to physical processes such as swelling, compression, or mechanical stress. Over time, the hydrogel can lose its shape or structure, leading to a gradual degradation of the material.
- (i)
- Polymer composition: Natural polymers, such as alginate or collagen, tend to degrade faster than synthetic polymers, such as polyethylene glycol (PEG) or polyvinyl alcohol (PVA), which may be more resistant to degradation.
- (ii)
- Crosslinking density: The degree of crosslinking in a hydrogel influences its stability. Highly crosslinked hydrogels generally degrade more slowly because the interconnected polymer chains are more resistant to breakage. Conversely, hydrogels with low crosslinking densities degrade more rapidly.
- (iii)
- Environmental conditions: The pH, temperature, and ionic strength of the environment can affect the rate of degradation. For example, acidic conditions can accelerate hydrolytic degradation, while high temperatures or oxidative conditions can increase the rate of decomposition of certain materials.
- (iv)
- Body fluids and enzymes: The presence of specific enzymes or body fluids (e.g., blood, lymph) can influence the rate of degradation, especially for hydrogels designed to degrade in response to specific biochemical cues. Enzyme-mediated biodegradation is often slower but more controlled, offering the potential for prolonged function in the body.
- (i)
- Infection risk: The high water content of hydrogels creates a favorable environment for bacterial and fungal growth. Hydrogels used for chronic wounds, burns, or implants may become contaminated over time. Many hydrogels lack intrinsic antimicrobial activity, increasing the risk of biofilm formation. Cross-contamination by improper handling or storage can introduce pathogenic bacteria, leading to secondary infections. Infection risks can be reduced by incorporating antimicrobial agents like silver nanoparticles, chitosan, or iodine to inhibit bacterial growth. Smart hydrogels for infection monitoring and pH-sensitive hydrogels can detect early signs of infection and release antimicrobial agents when infection is detected. Pre-sterilized, single-use hydrogel dressings for emergency wound care and improved storage solutions can prevent microbial contamination before application.
- (ii)
- Inflammation and immune response: Certain degradation products, especially those from synthetic hydrogels, can induce localized inflammation or an immune response. This can cause pain, redness, and swelling at the site of application and could complicate the healing process, especially if the hydrogel degradation products are perceived as foreign by the immune system.
- (iii)
- Toxicity induced by degradation products: The breakdown of certain hydrogels, especially synthetic ones, can produce toxic byproducts that can harm surrounding tissues. For example, the degradation of polyesters or polyurethanes can release acidic byproducts, which can lead to tissue irritation, necrosis, or delayed healing.
- (iv)
- Mechanical failure: Some hydrogels, especially those used for structural support or as scaffolds in emergency care, can degrade too quickly, compromising their mechanical properties and leading to failure in their function. For example, a hydrogel used in wound healing or burn care may lose its structural integrity too early, leading to reduced efficacy and potential complications.
- (v)
- Bioaccumulation: In cases where synthetic polymers are used, degradation products can accumulate in the body over time if not properly removed. This could lead to chronic toxicity or long-term adverse effects, especially if the degradation of the hydrogel is not well controlled.
- Controlled degradation—Tailoring the chemical composition and crosslinking of hydrogels can allow for better control over their degradation rates, for example, allowing them to degrade at specific rates in response to physiological conditions such as changes in pH or the presence of certain enzymes.
- Use of biodegradable polymers—The development of hydrogels based on fully biodegradable natural polymers (e.g., collagen, hyaluronic acid) or biocompatible synthetic materials (e.g., PLGA—poly(lactic-co-glycolic acid)) can lead to non-toxic byproducts that are safely absorbed or eliminated by the body.
- The addition of biocompatible additives such as anti-inflammatory agents, antimicrobial agents, or antioxidants to the hydrogel formulation can reduce the risk of infection, inflammation, or oxidative damage during the hydrogel degradation process.
4.3. Cost and Scalability of Hydrogel-Based Medical Applications in Emergency Medicine
4.4. The Gap Between Laboratory Research and Practical Deployments
5. Future Perspectives and Innovations
- Smart and stimuli-responsive hydrogels will be designed to respond dynamically to physiological and environmental changes, allowing for precision medicine in emergency care: pH-responsive hydrogels that release drugs in response to infection-induced acidity changes; temperature-sensitive hydrogels that solidify or liquefy based on body temperature, aiding in burn and trauma treatment; enzyme-activated hydrogels that trigger drug release only when specific enzymes indicate infection or injury; and light- and magnet-responsive hydrogels that allow remote-controlled drug delivery for non-invasive treatment. These innovations will enable real-time, site-specific therapy, reducing treatment time and side effects in emergencies.
- Injectable and self-healing hydrogels administered via syringes that solidify inside wounds or internal injuries, preventing excessive bleeding, repairing themselves after damage, and ensuring long-term stability in tissue engineering and wound closure. These properties will revolutionize minimally invasive treatments, especially in prehospital and battlefield care.
- Hydrogel-based wearable and implantable devices. The integration of hydrogels with wearable biosensors and implantable devices will enable continuous health monitoring and automated therapy: hydrogel biosensors that detect changes in glucose, lactate, or inflammation levels, enabling timely drug release and implantable drug-eluting hydrogels that offer long-term medication release, reducing the need for repeated dosing in critical conditions. These advancements will support automated, patient-specific treatment approaches in emergency medicine.
- Nanotechnology-enhanced hydrogels incorporating nanoparticles with hydrogels will enhance their strength, conductivity, and drug-carrying capacity, enabling controlled drug delivery and antimicrobial properties, beneficial for burns and chronic wounds. Such innovations will help create stronger, longer-lasting, and highly efficient medical hydrogels for emergency care.
- AI-integrated and 3D-printed hydrogels. Artificial intelligence (AI) and 3D printing will transform hydrogel-based emergency solutions, allowing for personalized and on-demand production. AI-designed hydrogels will optimize formulations for specific emergency applications. Moreover, 3D and 4D bioprinted hydrogels will enable customized wound dressings and tissue scaffolds for rapid organ repair. These developments will make hydrogels more adaptable, efficient, and widely accessible. AI could improve the functionality and properties of hydrogels in particular:
- -
- In the design and optimization of materials by using machine learning (ML), algorithms can optimize the synthesis of hydrogels and predict how different material compositions and processing conditions affect the final properties of hydrogels (e.g., mechanical strength, degradation rate, and bioactivity). In addition, the ability to rapidly analyze large amounts of data and predict optimal material combinations can significantly accelerate the development of next-generation hydrogels. AI can also help design hydrogels with controlled-release capabilities for drug delivery in cases where the polymer network needs to degrade at a specific rate or respond to environmental stimuli such as pH or temperature.
- -
- AI and computational models can simulate how hydrogels might perform in complex biological environments. This helps researchers predict how the material will behave over time, including how it interacts with cells, tissues, or drugs. This predictive capability reduces the need for expensive and time-consuming experiments.
- -
- Three-dimensionally printed hydrogels can also be used to produce patient-specific implants or prosthetics. These implants can be designed to integrate with biological tissues and provide targeted drug delivery or act as tissue substitutes. AI can further improve design by predicting the best material properties and design features based on the anatomy and clinical needs of the individual patient.
- Sustainable and cost-effective hydrogels. To increase scalability and affordability, future hydrogels will incorporate eco-friendly and biodegradable materials; plant-based hydrogels derived from cellulose, alginate, and chitosan, ensuring biocompatibility and sustainability; and low-cost crosslinking techniques that reduce manufacturing costs, making hydrogels more accessible for low-resource settings. These advancements will ensure that high-quality emergency treatments are available to a wider global population.
- Recent advances in hydrogels commercialization. Over the past decade, advances in hydrogel research have accelerated, leading to significant advances in commercialization and clinical trial development. These advances are driven by both improvements in hydrogel material properties, the development of 3D printing technologies, and the integration of AI. Despite these advances, the clinical use and commercialization of hydrogels on a large scale presents numerous challenges. The commercialization of hydrogels involves both the development of new hydrogel formulations and the expansion of manufacturing techniques to meet market demands. To heal wounds faster and reduce pain and scarring, several types of hydrogel-based wound care products are marketed:
- -
- Hydrocolloid dressings are widely useful in clinical settings especially for chronic wounds, such as diabetic ulcers or pressure ulcers, developed by companies such as Smith & Nephew and Convatec.
- -
- Bioactive hydrogels for tissue regeneration containing bioactive agents, such as growth factors or peptides that promote tissue regeneration, are marketed by the companies MIMETAS and CELLINK, for example.
- -
- Self-healing hydrogels for long-term wound management are developed by the company 3M.
- -
- Injectable hydrogels to deliver drugs or biological substances directly to a targeted site, reducing systemic side effects, are marketed by the companies Medytox and Evolus.
- -
- Hydrogel microneedles, an atraumatic microtechnique to deliver vaccines or drugs, are marketed by Microneedle Technologies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spahn, D.R.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernandez-Mondejar, E.; Gordini, G.; Stahel, P.F.; Hunt, B.J.; Komadina, R.; Neugebauer, E.; et al. Management of bleeding following major trauma: A European guideline. Crit. Care 2007, 11, R17. [Google Scholar] [CrossRef]
- Curry, N.; Hopewell, S.; Dorée, C.; Hyde, C.; Brohi, K.; Stanworth, S. The acute management of trauma hemorrhage: A systematic review of randomized controlled trials. Crit. Care 2011, 15, R92. [Google Scholar] [CrossRef]
- Cirocchi, R.; Prigorschi, D.; Properzi, L.; Matteucci, M.; Duro, F.; Tebala, G.D.; Cirillo, B.; Sapienza, P.; Brachini, G.; Lauricella, S.; et al. Is the Use of Tourniquets More Advantageous than Other Bleeding Control Techniques in Patients with Limb Hemorrhage? A Systematic Review and Meta-Analysis. Medicina 2025, 61, 93. [Google Scholar] [CrossRef]
- Cappellini, I.; Baldini, A.; Baraghini, M.; Bartolucci, M.; Cantafio, S.; Crocco, A.; Zini, M.; Magazzini, S.; Menici, F.; Pavoni, V.; et al. The Use of REBOA in a Zone Trauma Center Emergency Department for the Management of Massive Hemorrhages Secondary to Major Trauma, with Subsequent Transfer to a Level 1 Trauma Center for Surgery After Hemodynamic Stabilization. Emerg. Care Med. 2025, 2, 1. [Google Scholar] [CrossRef]
- Muirhead, B.; Weiss, A.D.H. Massive hemorrhage and transfusion in the operating room. Can. J. Anesth./J. Can. Anesth. 2017, 64, 962–978. [Google Scholar] [CrossRef]
- Weymouth, W.; Long, B.; Koyfman, A.; Winckler, C. Whole Blood in Trauma: A Review for Emergency Clinicians. J. Emerg. Med. 2019, 56, 491–498. [Google Scholar] [CrossRef]
- Saviano, A.; Perotti, C.; Zanza, C.; Longhitano, Y.; Ojetti, V.; Franceschi, F.; Bellou, A.; Piccioni, A.; Jannelli, E.; Ceresa, I.F.; et al. Blood Transfusion for Major Trauma in Emergency Department. Diagnostics 2024, 14, 708. [Google Scholar] [CrossRef]
- Petrosoniak, A.; Pavenski, K.; da Luz, L.T.; Callum, J. Massive Hemorrhage Protocol: A Practical Approach to the Bleeding Trauma Patient. Emerg. Med. Clin. N. Am. 2023, 41, 51–69. [Google Scholar] [CrossRef]
- Trentzsch, H.; Goossen, K.; Prediger, B.; Schweigkofler, U.; Hilbert-Carius, P.; Hanken, H.; Bieler, D. Stop the bleed “–Prehospital bleeding control in patients with multiple and/or severe injuries—A systematic review and clinical practice guideline–A systematic review and clinical practice guideline. Eur. J. Trauma Emerg. Surg. 2025, 51, 92. [Google Scholar] [CrossRef]
- Xiao, X.; Wu, Z. A Narrative Review of Different Hemostatic Materials in Emergency Treatment of Trauma. Emerg. Med. Int. 2022, 2022, 6023261. [Google Scholar] [CrossRef]
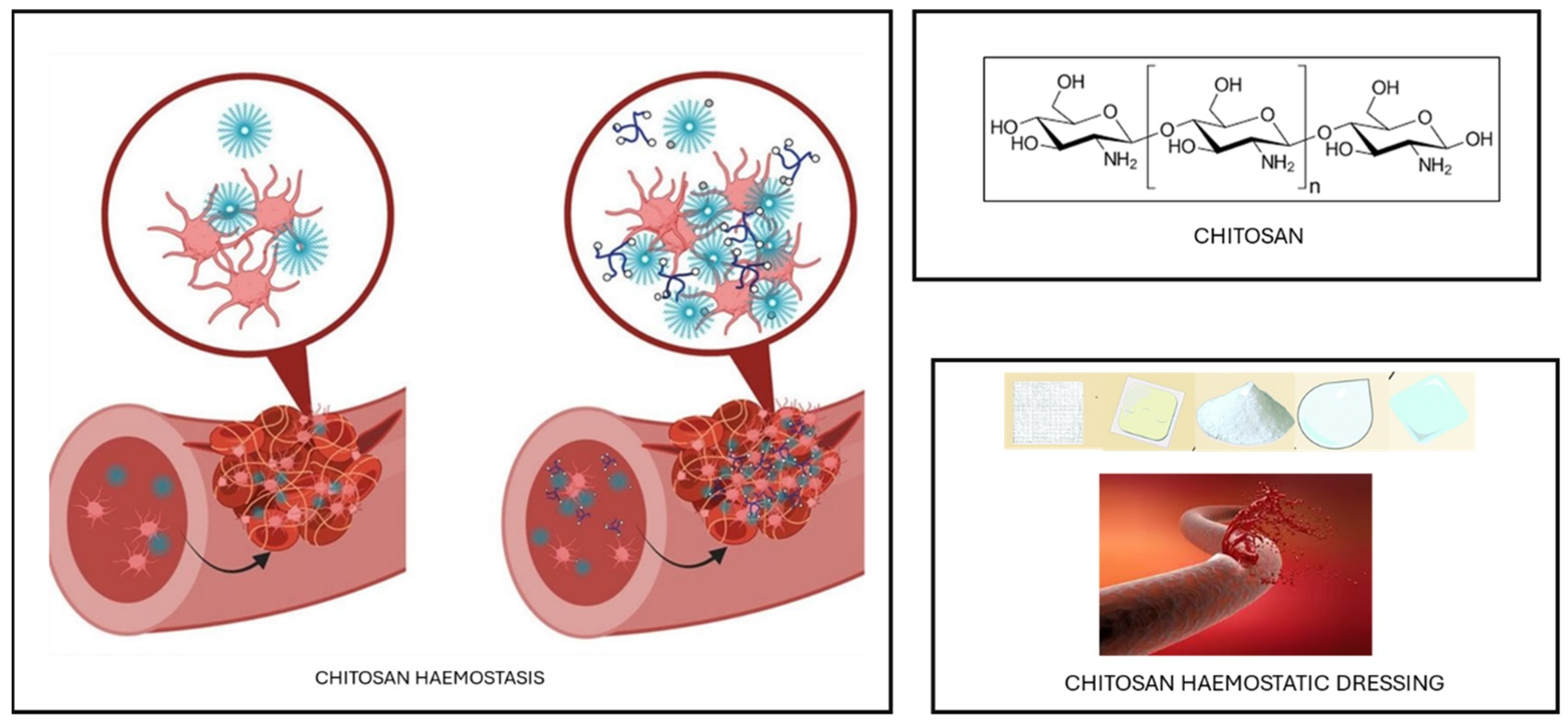
- Gheorghiță, D.; Moldovan, H.; Robu, A.; Bița, A.-I.; Grosu, E.; Antoniac, A.; Corneschi, I.; Antoniac, I.; Bodog, A.D.; Băcilă, C.I. Chitosan-Based Biomaterials for Hemostatic Applications: A Review of Recent Advances. Int. J. Mol. Sci. 2023, 24, 10540. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, L. Applications of injectable hemostatic materials in wound healing: Principles, strategies, performance requirements, and future perspectives. Theranostics 2023, 13, 4615–4635. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Romero, A.; Ostos, F.J.; Rafii-El-Idrissi Benhnia, M.; Perez-Puyana, V. Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan. Polymers 2022, 14, 272. [Google Scholar] [CrossRef]
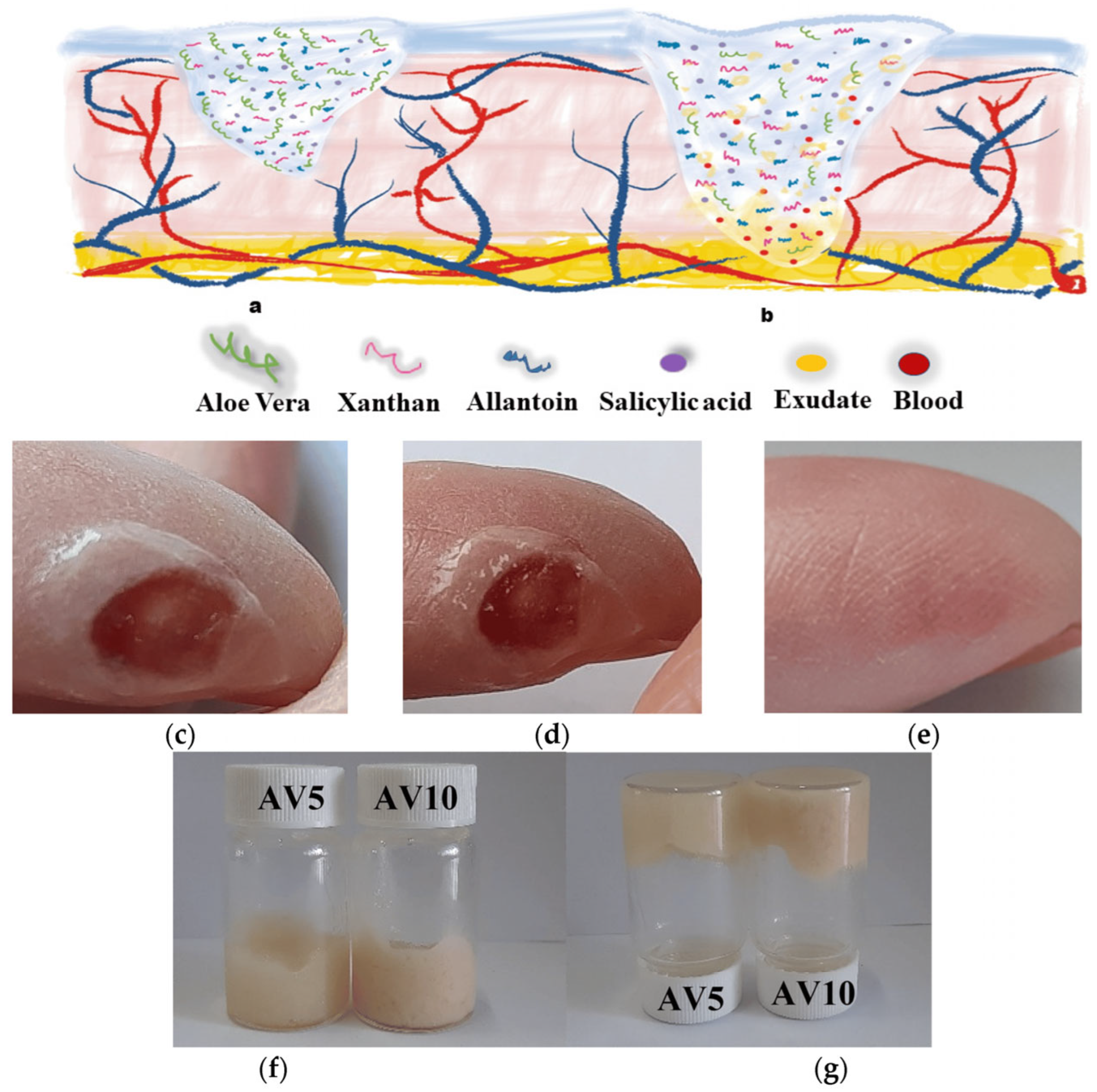
- Chelu, M.; Popa, M.; Ozon, E.A.; Pandele Cusu, J.; Anastasescu, M.; Surdu, V.A.; Calderon Moreno, J.; Musuc, A.M. High-Content Aloe vera Based Hydrogels: Physicochemical and Pharmaceutical Properties. Polymers 2023, 15, 1312. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M. Hydrogels with Essential Oils: Recent Advances in Designs and Applications. Gels 2024, 10, 636. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Portnov, T.; Shulimzon, T.R.; Zilberman, M. Injectable hydrogel-based scaffolds for tissue engineering applications. Rev. Chem. Eng. 2017, 33, 91–107. [Google Scholar] [CrossRef]
- Chelu, M.; Moreno, J.C.; Atkinson, I.; Cusu, J.P.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.M.; Musuc, A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel func-tional natural biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424. [Google Scholar] [CrossRef]
- Rezakhani, L.; Gharibshahian, M.; Salehi, M.; Zamani, S.; Abpeikar, Z.; Ghaderzadeh, O.; Cheraghali, D. Recent advances in hydrogels applications for tissue engineering and clinical trials. Regen. Ther. 2024, 26, 635–645. [Google Scholar] [CrossRef]
- Park, H.-I.; Sung, A.-Y. Establishment of Optimal Drug Delivery System and Evaluation of Utilization of Hydrogel Contact Lens According to the Addition Method of Tretinoin and Bovine Serum Albumin. Polymers 2025, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Mei, H.; Zhang, Z.; Song, F.; Jiang, L.; Huang, T.; Xiao, J. Corneal first aid lens: Collagen-based hydrogels loading aFGF as contact lens for treating corneal injuries. J. Control Release 2025, 379, 251–265. [Google Scholar] [CrossRef]
- Michalicha, A.; Belcarz, A.; Giannakoudakis, D.A.; Staniszewska, M.; Barczak, M. Designing Composite Stimuli-Responsive Hydrogels for Wound Healing Applications: The State-of-the-Art and Recent Discoveries. Materials 2024, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Orhan, B.; Karadeniz, D.; Kalaycıoğlu, Z.; Kaygusuz, H.; Torlak, E.; Erim, F.B. Foam-based antibacterial hydrogel composed of carboxymethyl cellulose/polyvinyl alcohol/cerium oxide nanoparticles for potential wound dressing. Int. J. Biol. Macromol. 2025, 291, 138924. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Jabbari, P.; Martins-Green, M.; Noshadi, I. A choline-based bio-ionic liquid functionalized gelatin methacryloyl hydrogel for chronic wound healing. Eur. Polym. J. 2025, 228, 113787. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M.; Aricov, L.; Ozon, E.A.; Iosageanu, A.; Stefan, L.M.; Prelipcean, A.-M.; Popa, M.; Moreno, J.C. Antibacterial Aloe vera Based Biocompatible Hydrogel for Use in Dermatological Applications. Int. J. Mol. Sci. 2023, 24, 3893. [Google Scholar] [CrossRef]
- Li, X.F.; Lu, P.; Jia, H.R.; Li, G.; Zhu, B.; Wang, X.; Wu, F.G. Emerging materials for hemostasis. Coord. Chem. Rev. 2023, 475, 214823. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M. Advanced Biomedical Applications of Multifunctional Natural and Synthetic Biomaterials. Processes 2023, 11, 2696. [Google Scholar] [CrossRef]
- Zhou, M.; Lin, X.; Wang, L.; Yang, C.; Yu, Y.; Zhang, Q. Preparation and application of hemostatic hydrogels. Small 2024, 20, 2309485. [Google Scholar] [CrossRef]
- James, D.; Pennardt, A.M. Trauma Care Principles. In StatPearls [Internet]; Stat Pearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Simon, L.V.; Lopez, R.A.; King, K.C. Blunt Force Trauma. In StatPearls [Internet]; Stat Pearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470338/ (accessed on 7 August 2023).
- Asensio, J.A.; Verde, J.M. Penetrating Wounds. In Encyclopedia of Intensive Care Medicine; Vincent, J.L., Hall, J.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- EL-Andari, R.; O’Brien, D.; Bozso, S.J.; Nagendran, J. Blunt cardiac trauma: A narrative review. Mediastinum 2021, 5, 28. [Google Scholar] [CrossRef]
- Varma, D.; Brown, P.; Clements, W. Importance of the Mechanism of Injury in Trauma Radiology Decision-Making. Korean J. Radiol. 2023, 24, 522–528. [Google Scholar] [CrossRef]
- Schaefer, T.J.; Tannan, S.C. Thermal Burns. In StatPearls [Internet]; Stat Pearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430773/ (accessed on 7 August 2023).
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Dobson, G.P.; Morris, J.L.; Letson, H.L. Why are bleeding trauma patients still dying? Towards a systems hypothesis of trauma. Front. Physiol. 2022, 13, 990903. [Google Scholar] [CrossRef]
- Latif, R.K.; Clifford, S.P.; Baker, J.A.; Lenhardt, R.; Haq, M.Z.; Huang, J.; Farah, I.; Businger, J.R. Traumatic hemorrhage and chain of survival. Scand. J. Trauma Resusc. Emerg. Med. 2023, 31, 25. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.A.L.; Santos, M.R.; Santos, E.J.F.; Figueiredo, A.; Melo, F.; Albuquerque, S.; Cunha, M. Management of bleeding in trauma victims by Portuguese nurses in prehospital setting. Aust. J. Adv. Nurs. 2022, 39, 4–11. [Google Scholar] [CrossRef]
- Berry, C.; Gallagher, J.M.; Goodloe, J.M.; Dorlac, W.C.; Dodd, J.; Fischer, P.E. Prehospital Hemorrhage Control and Treatment by Clinicians: A Joint Position Statement. Prehospital Emerg. Care 2023, 27, 544–551. [Google Scholar] [CrossRef]
- Wohlgemut, J.M.; Pisirir, E.; Stoner, R.S.; Kyrimi, E.; Christian, M.; Hurst, T.; Marsh, W.; Perkins, Z.B.; Tai, N.R.M. Identification of major hemorrhage in trauma patients in the prehospital setting: Diagnostic accuracy and impact on outcome. Trauma Surg. Acute Care Open 2024, 9, e001214. [Google Scholar] [CrossRef] [PubMed]
- Carne, B.; Raina, A.; Bothara, R.; McCombie, A.; Fleischer, D.; Joyce, L.R. Factors contributing to death of major trauma victims with haemorrhage: A retrospective case-control study. Emerg. Med. Australas. 2023, 35, 968–975. [Google Scholar] [CrossRef]
- Shah, A.; Kerner, V.; Stanworth, S.J.; Agarwal, S. Major haemorrhage: Past, present and future. Anaesthesia 2023, 78, 93–104. [Google Scholar] [CrossRef]
- Meza Monge, K.; Rosa, C.; Sublette, C.; Pratap, A.; Kovacs, E.J.; Idrovo, J.-P. Navigating Hemorrhagic Shock: Biomarkers, Therapies, and Challenges in Clinical Care. Biomedicines 2024, 12, 2864. [Google Scholar] [CrossRef]
- Evans, K.M. Circulatory System. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T.K., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Lucas, B.P.; Misra, S.; Donnelly, W.T.; Daubenspeck, J.A.; Leiter, J.C. Relative Blood Volume Profiles Hours After Loop Diuretic Administration: A Systematic Review and Meta-analysis. CJC Open 2023, 5, 641–649. [Google Scholar] [CrossRef]
- Ng, K.T.; Yap, J.L.L.; Kwok, P.E. The effect of fibrinogen concentrate on postoperative blood loss: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Anesth. 2020, 63, 109782. [Google Scholar] [CrossRef]
- Nanashima, A.; Wada, T.; Kawano, F.; Hamada, K.; Taniguchi, T.; Furukawa, K. Managing uncontrolled bleeding in elective surgery: The role of damage control techniques. Int J Surg Case Rep. 2025, 128, 111040. [Google Scholar] [CrossRef] [PubMed]
- Hancock, A.; Weeks, A.D.; Tina, L.D. Assessing blood loss in clinical practice. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 61, 28–40. [Google Scholar] [CrossRef]
- Hinojosa-Laborde, C.; Hudson, I.L.; Ross, E.; Xiang, L.; Ryan, K.L. Pathophysiology of Hemorrhage as It Relates to the Warfighter. Physiology 2022, 37, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, F.G. Management of Hemorrhagic Shock: Physiology Approach, Timing and Strategies. J. Clin. Med. 2023, 12, 260. [Google Scholar] [CrossRef]
- Tegegne, S.S.; Gebregzi, A.H.; Arefayne, N.R. Deliberate hypotension as a mechanism to decrease intraoperative surgical site blood loss in resource limited setting: A systematic review and guideline. Int. J. Surg. Open 2021, 29, 55–65. [Google Scholar] [CrossRef]
- Kumar, R.; Kerbert, A.J.; Sheikh, M.F.; Roth, N.; Calvao, J.A.; Mesquita, M.D.; Jalan, R. Determinants of mortality in patients with cirrhosis and uncontrolled variceal bleeding. J. Hepatol. 2021, 74, 66–79. [Google Scholar] [CrossRef]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guidelines on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Pasalic, L.; Lippi, G. Getting smart with coagulation. J. Thromb. Haemost. 2022, 20, 1519–1522. [Google Scholar] [CrossRef]
- Barry, T.B.; Getzewich, K. CHAPTER 40—Hemostasis and coagulopathies. In Emergency Medicine Secrets, 5th ed.; Markovchick, V.J., Pons, P.T., Bakes, K.M., Eds.; Mosby: Maryland Heights, MO, USA, 2011; pp. 283–289. ISBN 9780323071673. [Google Scholar] [CrossRef]
- Sokou, R.; Parastatidou, S.; Konstantinidi, A.; Tsantes, A.G.; Iacovidou, N.; Piovani, D.; Bonovas, S.; Tsantes, A.E. Contemporary tools for evaluation of hemostasis in neonates. Where are we and where are we headed? Blood Rev. 2024, 64, 101157. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, J.; Xu, T.; Yao, H.; Yu, L.; Huang, D. Hemostasis Strategies and Recent Advances in Nanomaterials for Hemostasis. Molecules 2023, 28, 5264. [Google Scholar] [CrossRef]
- Scridon, A. Platelets and Their Role in Hemostasis and Thrombosis—From Physiology to Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef] [PubMed]
- Janus-Bell, E.; Mangin, P.H. The relative importance of platelet integrins in hemostasis, thrombosis and beyond. Haematologica 2023, 108, 1734. [Google Scholar] [CrossRef]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 1, 17–24. [Google Scholar] [CrossRef]
- Risman, R.A.; Kirby, N.C.; Bannish, B.E.; Hudson, N.E.; Tutwiler, V. Fibrinolysis: An illustrated review. Res. Pract. Thromb. Haemost. 2023, 7, 100081. [Google Scholar] [CrossRef] [PubMed]
- Chiara, O.; Cimbanassi, S.; Bellanova, G.; Chiarugi, M.; Mingoli, A.; Olivero, G.; Ribaldi, S.; Tugnoli, G.; Basilicò, S.; Bindi, F.; et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018, 18, 68. [Google Scholar] [CrossRef]
- Nisi, M.; Carli, E.; Gennai, S.; Gulia, F.; Izzetti, R. Hemostatic Agents for the Management of Bleeding Risk Associated with Oral Anticoagulant Therapy Following Tooth Extraction: A Systematic Review. Appl. Sci. 2022, 12, 11017. [Google Scholar] [CrossRef]
- Petricevic, M.; Goerlinger, K.; Milojevic, M.; Petricevic, M. Methodological Considerations for Studies Evaluating Bleeding Prediction Using Hemostatic Point-of-Care Tests in Cardiac Surgery. J. Clin. Med. 2024, 13, 6737. [Google Scholar] [CrossRef]
- Uba, Y.; Ogura, T.; Ueno, S.; Okuda, A.; Nishioka, N.; Miyano, A.; Yamamoto, Y.; Bessho, K.; Tomita, M.; Nakamura, J.; et al. Comparison of Endoscopic Hemostasis for Endoscopic Sphincterotomy Bleeding between a Novel Self-Assembling Peptide and Conventional Technique. J. Clin. Med. 2023, 12, 79. [Google Scholar] [CrossRef]
- Bezati, S.; Ventoulis, I.; Verras, C.; Boultadakis, A.; Bistola, V.; Sbyrakis, N.; Fraidakis, O.; Papadamou, G.; Fyntanidou, B.; Parissis, J.; et al. Major Bleeding in the Emergency Department: A Practical Guide for Optimal Management. J. Clin. Med. 2025, 14, 784. [Google Scholar] [CrossRef] [PubMed]
- Mock, C.; Lormand, J.D.; Goosen, J.; Joshipura, M.; Peden, M. Guidelines for Essential Trauma Care; World Health Organization: Geneva, Switzerland, 2004; Available online: https://www.who.int/publications/i/item/guidelines-for-essential-trauma-care (accessed on 7 August 2023).
- Sung, Y.K.; Lee, D.R.; Chung, D.J. Advances in the development of hemostatic biomaterials for medical application. Biomater. Res. 2021, 25, 37. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.P.; Rodrigues, J.M.; Mano, J.F. In situ generated hemostatic adhesives: From mechanisms of action to recent advances and applications. Biomater. Adv. 2023, 155, 213670. [Google Scholar] [CrossRef]
- Heitzer, M.; Winnand, P.; Bock, A.; Ooms, M.; Katz, M.S.; Kniha, K.; Grottke, O.; Hölzle, F.; Modabber, A. Evaluation of the Hemostatic Effect of an Innovative Tissue Adhesive during Extraction Therapy under Rivaroxaban in a Rodent Model. J. Funct. Biomater. 2023, 14, 333. [Google Scholar] [CrossRef]
- Song, H.; Xing, L.; Liu, W.; Wang, X.; Hou, Z.; Wang, Y.; Zhang, Z.; Li, Y.; Li, T.; Wang, X.; et al. Biomimetic and Multifunctional Hemostatic Hydrogel with Rapid Thermoresponsive Gelation and Robust Wet Adhesion for Emergency Hemostasis: A Rational Design Based on Photo-Cross- Linking Coordinated Hydrophilic−Hydrophobic Balance Strategies. Biomacromolecules 2023, 24, 3327–3344. [Google Scholar] [CrossRef]
- di Lena, F. Hemostatic polymers: The concept, state of the art and perspectives. J. Mater. Chem. B 2014, 2, 3567–3577. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef]
- Liu, L.; Jing, R.; Yao, L.; Wang, Y.; Mu, L.; Hu, Y. Hemostasis Strategies and Recent Advances in Hydrogels for Managing Uncontrolled Hemorrhage. ACS Appl. Bio Mater. 2025, 8, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Perri, P.; Scarcello, E.; Piro, P.; Sole, R.; Curcio, F.; Trombino, S. Chitosan Hemostatic Dressings: Properties and Surgical Applications. Polymers 2024, 16, 1770. [Google Scholar] [CrossRef]
- Chelu, M.; Magdalena Musuc, A. Biomaterials-Based Hydrogels for Therapeutic Applications; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, C.; Wang, T.; Xu, J. Advances in Functional Hydrogel Wound Dressings: A Review. Polymers 2023, 15, 2000. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M.; Popa, M.; Calderon Moreno, J.M. Chitosan Hydrogels for Water Purification Applications. Gels 2023, 9, 664. [Google Scholar] [CrossRef] [PubMed]
- Waqar, M.A.; Mubarak, N.; Khan, A.M.; Shaheen, F.; Mustafa, M.A.; Riaz, T. Recent advances in polymers, preparation techniques, applications and future perspectives of hydrogels. Int. J. Polym. Mater. Polym. Biomater. 2024, 74, 265–284. [Google Scholar] [CrossRef]
- Alsaka, L.; Alsaka, L.; Altaee, A.; Zaidi, S.J.; Zhou, J.; Kazwini, T. A Review of Hydrogel Application in Wastewater Purification. Separations 2025, 12, 51. [Google Scholar] [CrossRef]
- Chelu, M.; Popa, M.; Calderon Moreno, J.; Leonties, A.R.; Ozon, E.A.; Pandele Cusu, J.; Surdu, V.A.; Aricov, L.; Musuc, A.M. Green Synthesis of Hydrogel-Based Adsorbent Material for the Effective Removal of Diclofenac Sodium from Wastewater. Gels 2023, 9, 454. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Chelu, M.; Calderon Moreno, J.M.; Musuc, A.M.; Popa, M. Natural Regenerative Hydrogels for Wound Healing. Gels 2024, 10, 547. [Google Scholar] [CrossRef]
- Burdick, J.A.; Stevens, M.M. 11—Biomedical hydrogels. In Woodhead Publishing Series in Biomaterials, Biomaterials, Artificial Organs and Tissue Engineering; Hench, L.L., Jones, J.R., Eds.; Woodhead Publishing: Cambridge, UK, 2005; pp. 107–115. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Y.; Luo, D.; Li, P.; Chen, Y.; Fu, X.; Yue, Y.; Hou, R.; Liu, J.; Wang, X. Advantages and disadvantages of various hydrogel scaffold types: A research to improve the clinical conversion rate of loaded MSCs-Exos hydrogel scaffolds. Biomed. Pharmacother. 2024, 179, 117386. [Google Scholar] [CrossRef]
- Means, A.K.; Grunlan, M.A. Modern strategies to achieve tissue-mimetic, mechanically robust hydrogels. ACS Macro Lett. 2019, 8, 705–713. [Google Scholar] [CrossRef]
- Xu, D.; Harvey, T.; Begiristain, E.; Domínguez, C.; Sánchez-Abella, L.; Browne, M.; Cook, R.B. Measuring the elastic modulus of soft biomaterials using nanoindentation. J. Mech. Behav. Biomed. Mater. 2022, 133, 105329. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Section 11—Biocompatibility of hydrogels. In Gels Handbook; Osada, Y., Kajiwara, K., Fushimi, T., Irasa, O., Hirokawa, Y., Matsunaga, T., Shimomura, T., Wang, L., Ishida, H., Eds.; Academic Press: Cambridge, MA, USA, 2001; pp. 388–407. ISBN 9780123946904. [Google Scholar] [CrossRef]
- Azmir, M.S.N.A.; Moni, M.N.; Gobetti, A.; Ramorino, G.; Dey, K. Advances in modulating mechanical properties of gelatin-based hydrogel in tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2024, 74, 215–250. [Google Scholar] [CrossRef]
- Cabrera-Munguia, D.A.; Castañeda-Calzoncit, C.E.; Claudio-Rizo, J.A.; Caldera-Villalobos, M.; León-Campos, M.I.; Cano-Salazar, L.F. In vitro biocompatibility and drug release of collagen-mo-complexes hydrogels for tissue engineering. Macromol. Res. 2025. [Google Scholar] [CrossRef]
- Sousa, A.C.; Alvites, R.; Lopes, B.; Sousa, P.; Moreira, A.; Coelho, A.; Rêma, A.; Biscaia, S.; Cordeiro, R.; Faria, F.; et al. Hybrid scaffolds for bone tissue engineering: Integration of composites and bioactive hydrogels loaded with hDPSCs. Biomater. Adv. 2025, 166, 214042. [Google Scholar] [CrossRef]
- Huang, Y.; Jayathilaka, P.B.; Islam, M.S.; Tanaka, C.B.; Silberstein, M.N.; Kilian, K.A.; Kruzic, J.J. Structural aspects controlling the mechanical and biological properties of tough, double network hydrogels. Acta Biomater. 2022, 138, 301–312. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Rahman, M.S.; Akib, A.A.; Sohag, M.S.; Rakib, M.R.A.; Khan, M.A.R.; Yesmin, F.; Shakil, M.S.; Khan, M.M.R. Progress in hydrogel toughening: Addressing structural and crosslinking challenges for biomedical applications. Discov. Mater. 2025, 5, 5. [Google Scholar] [CrossRef]
- Kong, D.; Yang, B.; Yuan, H.; Du, C.; Tan, Y. 3D printing of glycerol-mediated alginate hydrogels with high strength and stiffness. J. Mater. Sci. Technol. 2025, 213, 268–275. [Google Scholar] [CrossRef]
- Kunkels, L.; Saldívar, M.C.; Putra, N.; Kruize, C.P.; Panahkhahi, S.; Leeflang, M.; Fratila-Apachitei, L.; Zadpoor, A.; Mirzaali, M. High-Performance 3D Printed Mechanically Interlocked Soft–Hard Interfaces of Hydrogels and Polylactide. Adv. Mater. Technol. 2025, 2401081. [Google Scholar] [CrossRef]
- Bryant, S.J.; Chowdhury, T.T.; Lee, D.A.; Bader, D.L.; Anseth, K.S. Crosslinking Density Influences Chondrocyte Metabolism in Dynamically Loaded Photocrosslinked Poly(ethylene glycol) Hydrogels. Ann. Biomed. Eng. 2004, 32, 407–417. [Google Scholar] [CrossRef]
- Panteli, P.A.; Patrickios, C.S. Complex Hydrogels Based on Multiply Interpenetrated Polymer Networks: Enhancement of Mechanical Properties via Network Multiplicity and Monomer Concentration. Macromolecules 2018, 51, 7533–7545. [Google Scholar] [CrossRef]
- Schoenmakers, D.C.; Rowan, A.E.; Kouwer, P.H.J. Crosslinking of fibrous hydrogels. Nat. Commun. 2018, 9, 2172. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Properties of Water Bound in Hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Crosby, C.O.; Stern, B.; Kalkunte, N.; Pedahzur, S.; Ramesh, S.; Zoldan, J. Interpenetrating polymer network hydrogels as bioactive scaffolds for tissue engineering. Rev. Chem. Eng. 2022, 38, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zheng, Z.; Zhu, D.; Wang, X. Hydrogels with high sacrifice efficiency of sacrificial bonds and with high strength and toughness due to dense entanglements of polymer chains. J. Colloid Interface Sci. 2025, 677, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Idumah, C.I.; Nwuzor, I.C.; Odera, R.S. Recent advances in polymer hydrogel nanoarchitectures and applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100143. [Google Scholar] [CrossRef]
- Estevez, A.T.; Abdallah, Y.K. Biomimetic Approach for Enhanced Mechanical Properties and Stability of Self-Mineralized Calcium Phosphate Dibasic–Sodium Alginate–Gelatine Hydrogel as Bone Replacement and Structural Building Material. Processes 2024, 12, 944. [Google Scholar] [CrossRef]
- Straksys, A.; Abouhagger, A.; Kirsnytė-Šniokė, M.; Kavleiskaja, T.; Stirke, A.; Melo, W.C.M.A. Development and Characterization of a Gelatin-Based Photoactive Hydrogel for Biomedical Application. J. Funct. Biomater. 2025, 16, 43. [Google Scholar] [CrossRef]
- Li, J.; Suo, Z.; Vlassak, J.J. Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef]
- Huang, M.; Shi, H.; Wei, N.; Luo, C.; Luo, F. An ultra-strong, ultra-stiff and anti-freezing hydrogel based on poly(vinyl alcohol). Mater. Lett. 2021, 300, 130172. [Google Scholar] [CrossRef]
- Zhuo, H.; Dong, X.; Liu, Q.; Hong, L.; Zhang, Z.; Long, S.; Zhai, W. Bamboo-inspired ultra-strong nanofiber-reinforced composite hydrogels. Nat. Commun. 2025, 16, 980. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Lee, M.; Kwak, H.; Eom, Y.; Park, S.-A.; Sakai, T.; Jeon, H.; Koo, J.M.; Kim, D.; Cha, C.; Hwang, S.Y.; et al. Network of cyano-p-aramid nanofibres creates ultrastiff and water-rich hydrospongels. Nat. Mater. 2024, 23, 414–423. [Google Scholar] [CrossRef]
- Han, W.; Wang, S. Advances in Hemostatic Hydrogels That Can Adhere to Wet Surfaces. Gels 2023, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.X.; Zou, C.Y.; Hu, J.J.; Fan, M.H.; Jiang, Y.L.; Xiong, M.; Han, C.; Zhang, X.Z.; Li, Y.X.; Zhao, L.M.; et al. A Self-Assembly Pro-Coagulant Powder Capable of Rapid Gelling Transformation and Wet Adhesion for the Efficient Control of Non-Compressible Hemorrhage. Adv. Sci. 2024, 11, e2306289. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, Y.; Ming, J.; Ning, X.; Bai, S. Natural polymer-based bioadhesives as hemostatic platforms for wound healing. Int. J. Biol. Macromol. 2024, 256, 128275. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, W.D. Active and Mechanical Hemostatic Agents. Surgery 2007, 142 (Suppl. S4), S34–S38. [Google Scholar] [CrossRef]
- Huang, L.; Liu, G.L.; Kaye, A.D.; Liu, H. Advances in Topical Hemostatic Agent Therapies: A Comprehensive Update. Adv. Ther. 2020, 37, 4132–4148. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Zhang, Y.; Jiang, D.; Yang, L.; Lin, Q.; et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019, 10, 2060. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Hasany, M.; Kohestanian, M.; Dolatshahi-Pirouz, A.; Milani, M.; Mehrali, M. Recent advancements in bioadhesive self-healing hydrogels for effective chronic wound care. Adv. Colloid Interface Sci. 2024, 334, 103306. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Fan, Y.; Yu, S.; Liu, M.; Feng, L.; Sun, Q.; Pan, P. Principles and Design of Bionic Hydrogel Adhesives for Skin Wound Treatment. Polymers 2024, 16, 1937. [Google Scholar] [CrossRef] [PubMed]
- Karami, P.; Laurent, A.; Philippe, V.; Applegate, L.A.; Pioletti, D.P.; Martin, R. Cartilage Repair: Promise of Adhesive Orthopedic Hydrogels. Int. J. Mol. Sci. 2024, 25, 9984. [Google Scholar] [CrossRef]
- Shen, K.; Lv, Z.; Yang, Y.; Wang, H.; Liu, J.; Chen, Q.; Liu, Z.; Zhang, M.; Liu, J.; Cheng, Y. A Wet-Adhesion and Swelling-Resistant Hydrogel for Fast Hemostasis, Accelerated Tissue Injury Healing and Bioelectronics. Adv. Mater. 2025, 37, e2414092. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Grinstaff, M.W. Advances in Hydrogel Adhesives for Gastrointestinal Wound Closure and Repair. Gels 2023, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Guo, Y.; Qian, H.; Chen, Y.; Chen, Y.; Wang, J.; Wang, Y.; Martins, M.C.L.; Hu, X.; et al. Non-swelling polyelectrolyte complex hydrogels with tissue-matchable mechanical properties for versatile wet wound closure. Compos. Part B Eng. 2024, 279, 111456. [Google Scholar] [CrossRef]
- Cohen, N.S.; Bock, J.M.; May, A.K. Sepsis and postoperative surgical site infections. Surgery 2023, 174, 403–405. [Google Scholar] [CrossRef]
- Giano, M.; Ibrahim, Z.; Medina, S.; Sarhane, K.A.; Christensen, J.M.; Yamada, Y.; Brandacher, G.; Schneider, J.P. Injectable bioadhesive hydrogels with innate antibacterial properties. Nat. Commun. 2014, 5, 4095. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M.; Popa, M.; Calderon Moreno, J. Aloe vera-Based Hydrogels for Wound Healing: Properties and Therapeutic Effects. Gels 2023, 9, 539. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Razza, N.; Breloy, L.; Andaloussi, S.A.; Chiappone, A.; Sangermano, M.; Versace, D.L. Photoinduced chitosan–PEG hydrogels with long-term antibacterial properties. J. Mater. Chem. B 2019, 7, 6526–6538. [Google Scholar] [CrossRef]
- Fadakar Sarkandi, A.; Montazer, M.; Harifi, T.; Mahmoudi Rad, M. Innovative preparation of bacterial cellulose/silver nanocomposite hydrogels: In situ green synthesis, characterization, and antibacterial properties. J. Appl. Polym. Sci. 2021, 138, 49824. [Google Scholar] [CrossRef]
- Le Thi, P.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Park, K.M.; Park, K.D. Calcium peroxide-mediated in situ formation of multifunctional hydrogels with enhanced mesenchymal stem cell behaviors and antibacterial properties. J. Mater. Chem. B 2020, 8, 11033–11043. [Google Scholar] [CrossRef]
- Sanyal, A.; Roy, S.; Ghosh, A.; Chakraborty, M.; Ghosh, A.; Mandal, D. The next frontier in hemorrhagic management: A comprehensive review on development of natural polymer-based injectable hydrogels as promising hemostatic dressings. Chem. Eng. J. 2024, 497, 155033. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Fang, Y.; Li, P.; Zhou, W.; Wang, Z.; Chen, M.; Liu, H. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater. Sci. Eng. C 2020, 109, 110649. [Google Scholar] [CrossRef]
- Goder Orbach, D.; Roitman, I.; Coster Kimhi, G.; Zilberman, M. Formulation-Property Effects in Novel Injectable and Resilient Natural Polymer-Based Hydrogels for Soft Tissue Regeneration. Polymers 2024, 16, 2879. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Boffito, M.; Cassino, C.; Liberti, F.; Ciardelli, G.; Chiono, V. Design of Injectable Bioartificial Hydrogels by Green Chemistry for Mini-Invasive Applications in the Biomedical or Aesthetic Medicine Fields. Gels 2023, 9, 59. [Google Scholar] [CrossRef]
- Alves, P.; Simão, A.F.; Graça, M.F.P.; Mariz, M.J.; Correia, I.J.; Ferreira, P. Dextran-Based Injectable Hydrogel Composites for Bone Regeneration. Polymers 2023, 15, 4501. [Google Scholar] [CrossRef]
- Colby, R.H.; Boris, D.C.; Krause, W.E.; Dou, S. Shear thinning of unentangled flexible polymer liquids. Rheol. Acta 2007, 46, 569–575. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Pelletier, J.; Koyfman, A.; Long, B. High risk and low prevalence diseases: Open globe injury. Am. J. Emerg. Med. 2023, 64, 113–120. [Google Scholar] [CrossRef]
- Xu, P.; Chen, P.; Sun, Y.; Nuliqiman, M.; Zhou, Y.; Cao, J.; Yu, S.; Huang, J.; Ye, J. A novel injectable thermo/photo dual-crosslinking hydrogel based on modified chitosan for fast sealing open globe injury. Carbohydr. Polym. 2024, 331, 121854. [Google Scholar] [CrossRef]
- Weinstein, D.; Moran, V.; Culhane, J. Acute gastric perforation after leaving against medical advice: A case presentation. Trauma Case Rep. 2021, 37, 100598. [Google Scholar] [CrossRef] [PubMed]
- Amalia, R.; Vidyani, A.; I’tishom, R.; Efendi, W.I.; Danardono, E.; Wibowo, B.P.; Parewangi, M.L.; Miftahussurur, M.; Malaty, H.M. The Prevalence, Etiology and Treatment of Gastroduodenal Ulcers and Perforation: A Systematic Review. J. Clin. Med. 2024, 13, 1063. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Somma, C.; Sciuto, A.; Jutidamrongphan, W.; Pacella, D.; Esposito, F.; Puglia, M.; Mauriello, C.; Khanungwanitkul, K.; Pirozzi, F. MDCT Findings in Gastrointestinal Perforations and the Predictive Value according to the Site of Perforation. Tomography 2022, 8, 667–687. [Google Scholar] [CrossRef]
- Bašković, M.; Keretić, D.; Lacković, M.; Borić Krakar, M.; Pogorelić, Z. The Diagnosis and Management of Pediatric Blunt Abdominal Trauma—A Comprehensive Review. Diagnostics 2024, 14, 2257. [Google Scholar] [CrossRef]
- Oza, V.M.; Kothari, T.H. Endoscopic Management of Gastric Disruptions. Gastrointest. Disord. 2024, 6, 131–142. [Google Scholar] [CrossRef]
- Akashi, T.; Yamaguchi, N.; Shiota, J.; Tabuchi, M.; Kitayama, M.; Hashiguchi, K.; Matsushima, K.; Akazawa, Y.; Nakao, K. Characteristics and Risk Factors of Delayed Perforation in Endoscopic Submucosal Dissection for Early Gastric Cancer. J. Clin. Med. 2024, 13, 1317. [Google Scholar] [CrossRef]
- Hibbins, A.R.; Kumar, P.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Du Toit, L.C.; Pillay, V. Design of a Versatile pH-Responsive Hydrogel for Potential Oral Delivery of Gastric-Sensitive Bioactives. Polymers 2017, 9, 474. [Google Scholar] [CrossRef]
- Karageorgos, F.F.; Alexiou, M.; Tsoulfas, G.; Alexopoulos, A.H. Hydrogel-Based Vascularized Organ Tissue Engineering: A Systematized Review on Abdominal Organs. Gels 2024, 10, 653. [Google Scholar] [CrossRef] [PubMed]
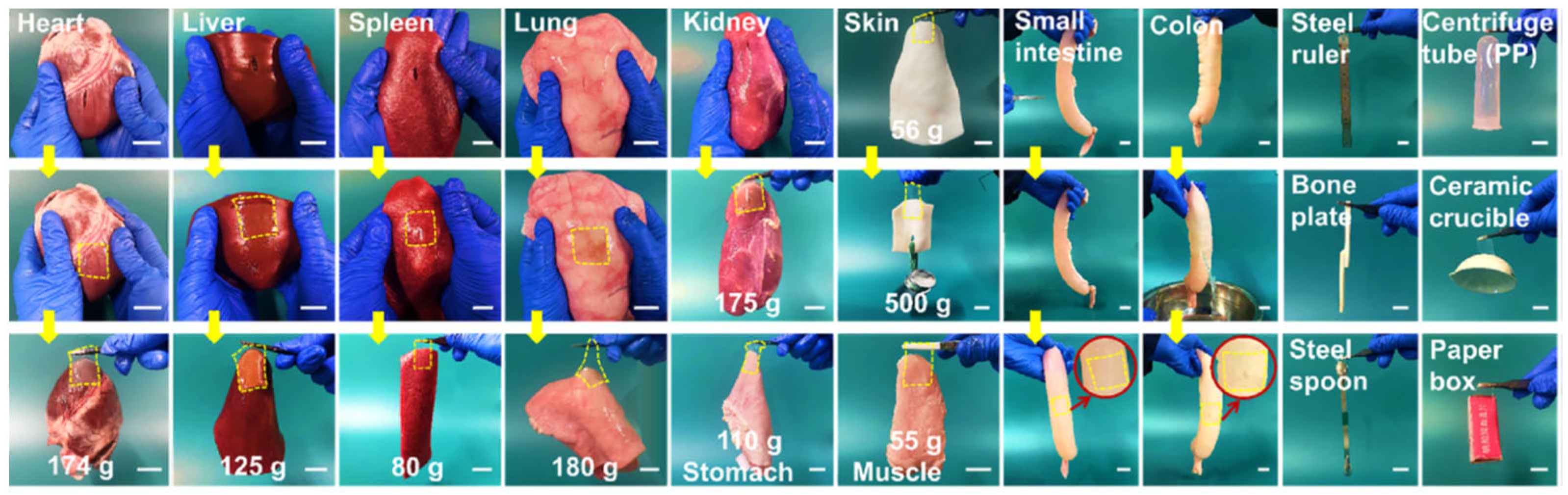
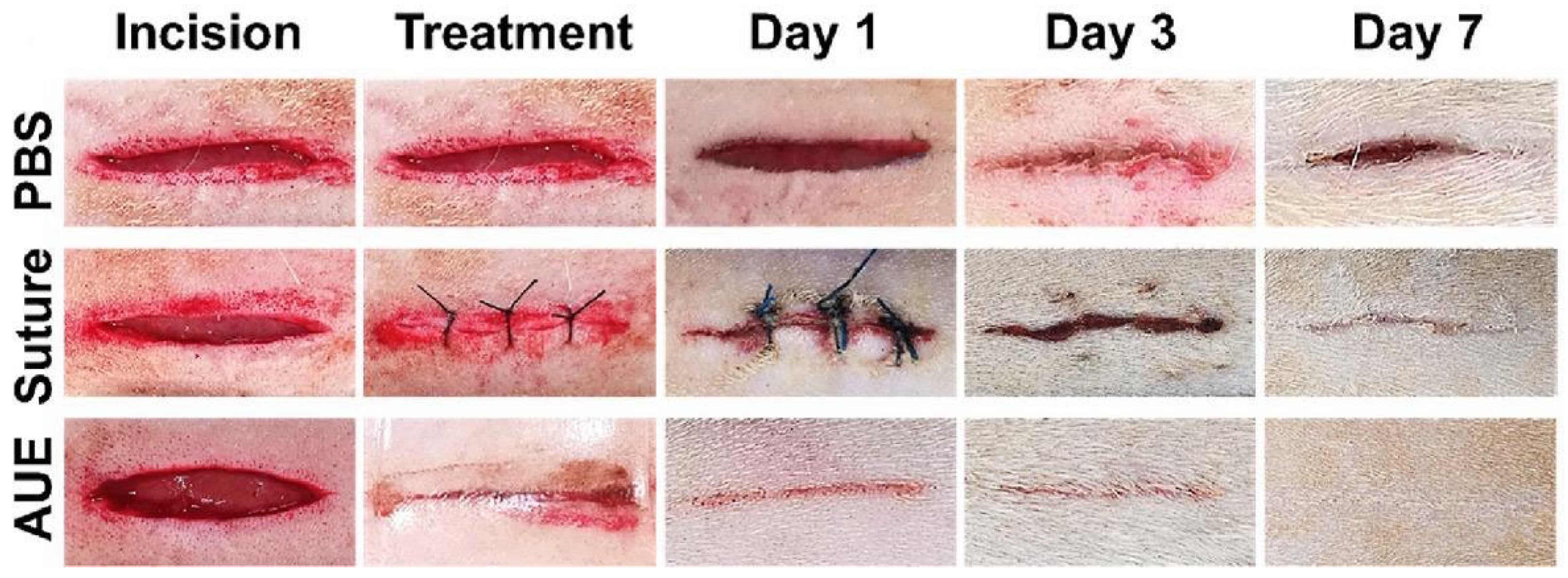
- Liang, L.; Wang, H.; Li, L.; Lin, D.; Guo, B.; Yao, M.; Li, J. Microparticle deposition induced asymmetric adhesive hydrogel for suture-less gastric trauma treatment. Chem. Eng. J. 2024, 485, 150086. [Google Scholar] [CrossRef]
- Talebian, S.; Shim, I.K.; Foroughi, J.; Orive, G.; Vine, K.L.; Kim, S.C.; Wallace, G.G. 3D-Printed Coaxial Hydrogel Patches with Mussel-Inspired Elements for Prolonged Release of Gemcitabine. Polymers 2021, 13, 4367. [Google Scholar] [CrossRef]
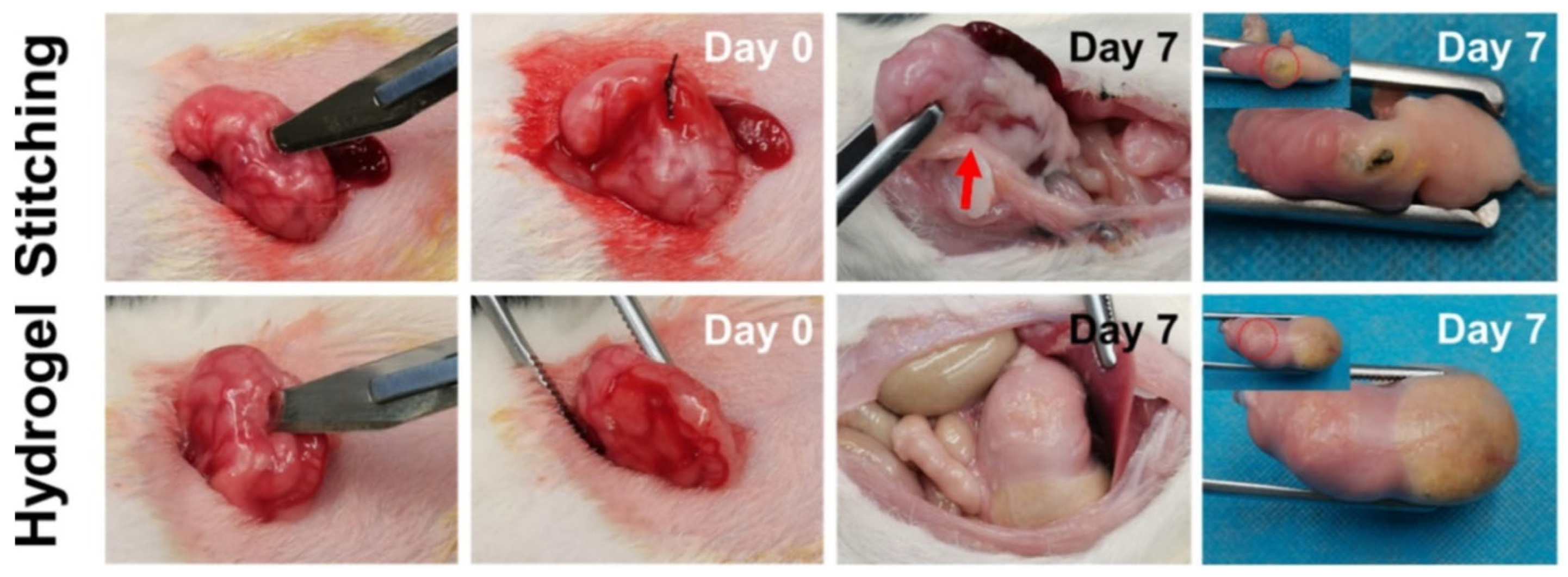
- Yu, J.; Qin, Y.; Yang, Y.; Zhao, X.; Zhang, Z.; Zhang, Q.; Su, Y.; Zhang, Y.; Cheng, Y. Robust hydrogel adhesives for emergency rescue and gastric perforation repair. Bioact. Mater. 2023, 19, 703–716. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Wang, W. A supramolecular hydrogel leveraging hierarchical multi-strength hydrogen-bonds hinged strategy achieving a striking adhesive-mechanical balance. Bioact. Mater. 2025, 43, 32–47. [Google Scholar] [CrossRef] [PubMed]
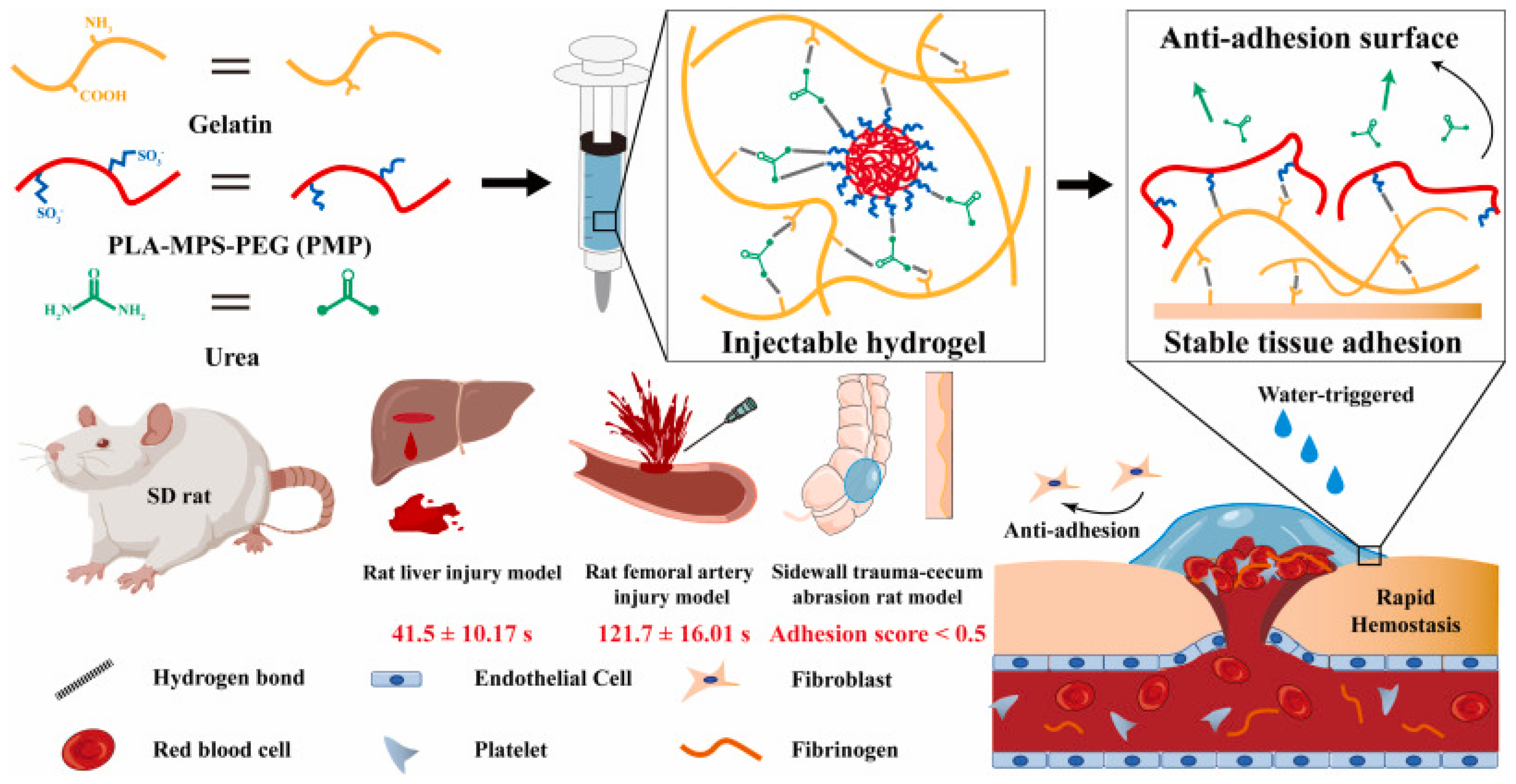
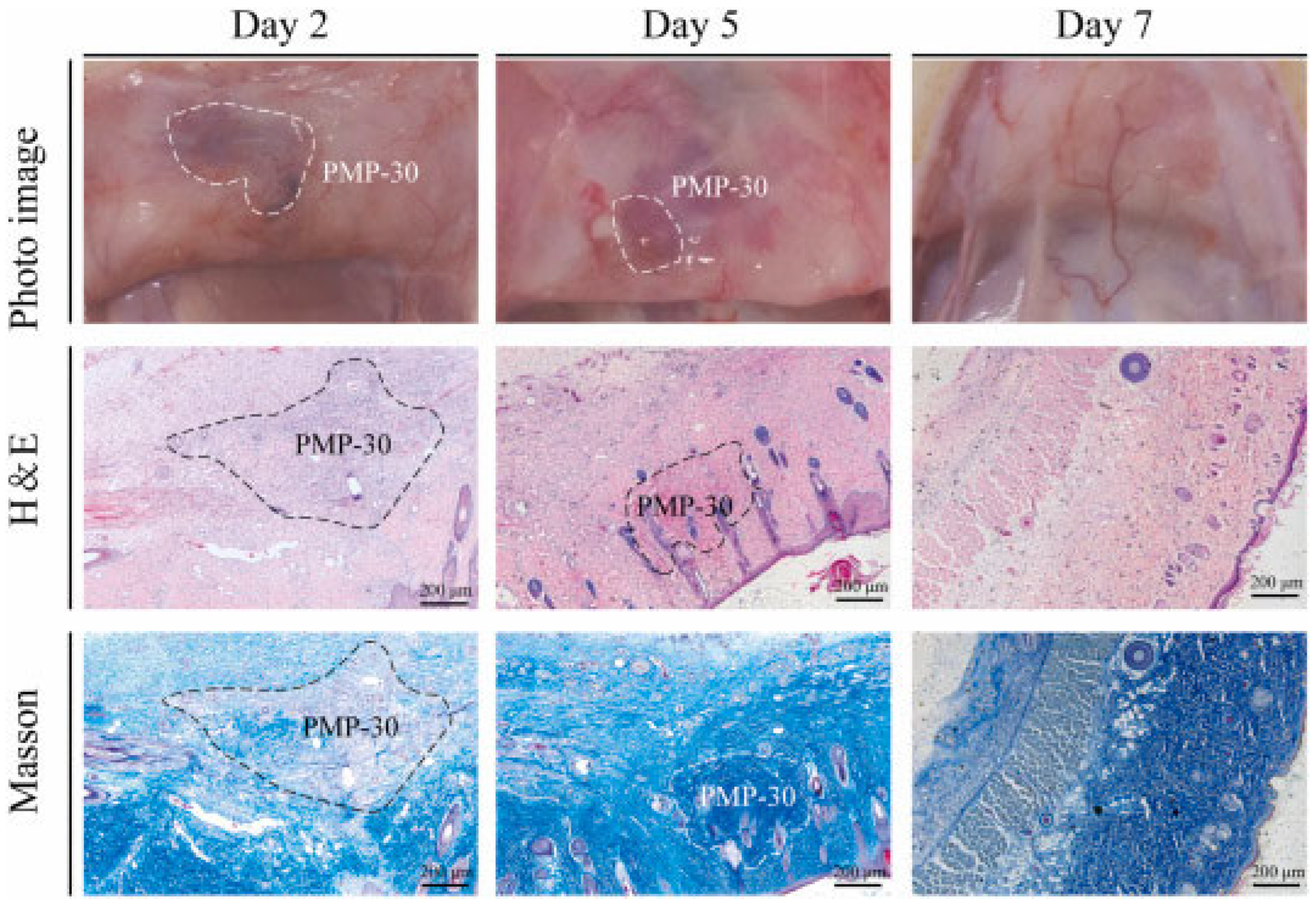
- Yang, X.; Wang, X.; Tang, L.; Sun, Z.; Gao, X.; Zhao, Y.; Hou, S.; Shi, J.; Lv, Q. Water triggered injectable polylactic acid hydrogel based on zwitterionic sulfobetaine modification for in-compressible bleeding and tissue anti-adhesion. Mater. Today Bio 2024, 30, 101431. [Google Scholar] [CrossRef]
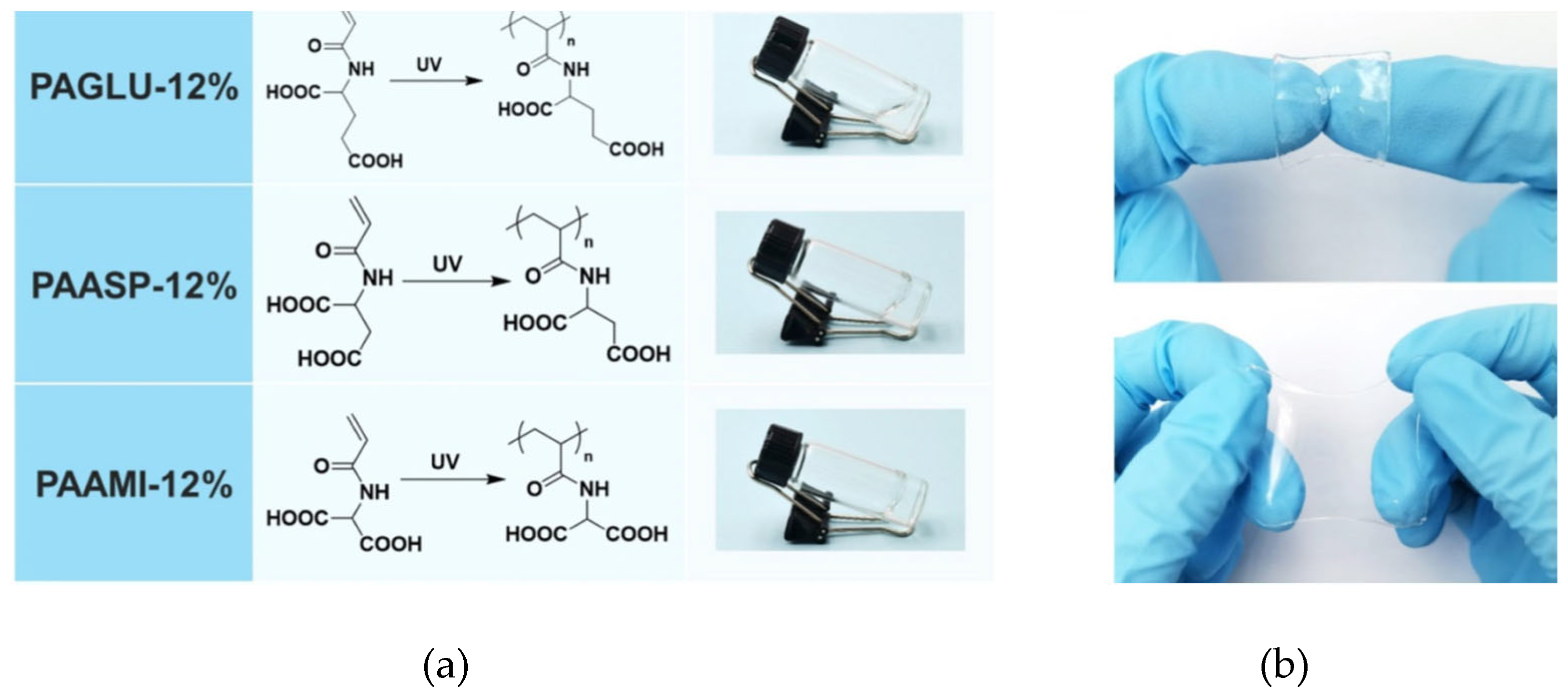
- Liu, T.; Sun, W.; Mu, C.; Zhang, X.; Xu, D.; Yan, Q.; Luan, S. Bionic double-crosslinked hydrogel of poly (γ-glutamic acid)/poly (N-(2-hydroxyethyl) acrylamide) with ultrafast gelling process and ultrahigh burst pressure for emergency rescue. Int. J. Biol. Macromol. 2024, 271, 132360. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Min, Y.; Ouyang, Q.; Fu, Y.; Mao, Y.; Xiang, S.; Hu, X.; Jiang, L. Study on an Injectable Chitosan–Lignin/Poloxamer Hydrogel Loaded with Platelet-Rich Plasma for Intrauterine Adhesion Treatment. Polymers 2025, 17, 474. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef]
- Obasa, A.A.; Olopade, F.E.; Juliano, S.L.; Olopade, J.O. Traumatic brain injury or traumatic brain disease: A scientific commentary. Brain Multiphysics 2024, 6, 100092. [Google Scholar] [CrossRef]
- Bankole, N.D.A.; Kuntz, C.; Planty-Bonjour, A.; Beaufort, Q.; Gaberel, T.; Cordonnier, C.; Pasi, M.; Schlunk, F.; Nawabi, J.; Zemmoura, I.; et al. Minimally Invasive Surgery for Spontaneous Intracerebral Hemorrhage: A Review. J. Clin. Med. 2025, 14, 1155. [Google Scholar] [CrossRef]
- Kılıç, G.; Engin, B.E.; Halabi, A.; Tuncer, C.; Sungur, M.A.; Alpay, M.; Kurtuluş, A.; Soylu, H.; Gök, A.; Polat, Ö. Mitigating Post-Subarachnoid Hemorrhage Complications: Anti-Inflammatory and Anti-Apoptotic Effects of Anakinra in an Experimental Study. J. Clin. Med. 2025, 14, 1253. [Google Scholar] [CrossRef]
- Han, Y.; Weng, W.; Zhang, Y.; Feng, Q.; Ma, Y.; Quan, A.; Fu, X.; Zhao, X.; Skudder-Hill, L.; Jiang, J.; et al. Intraoperative application of intelligent, responsive, self-assembling hydrogel rectifies oxygen and energy metabolism in traumatically injured brain. Biomaterials 2024, 306, 122495. [Google Scholar] [CrossRef]
- Phongpradist, R.; Chittasupho, C.; Singh, S.; Ontong, J.C.; Tadtong, S.; Akachaipaibul, P.; Punvittayagul, C.; Thongkorn, K.; Dejkriengkraikul, P.; Jiaranaikulwanitch, J.; et al. Investigation of a Thermoresponsive In Situ Hydrogel Loaded with Nanotriphala: Implications for Antioxidant, Anti-Inflammatory, and Antimicrobial Therapy in Nasal Disorders. Gels 2025, 11, 106. [Google Scholar] [CrossRef]
- da Silva Fiorin, F.; Godinho, D.B.; dos Santos, E.B.; Aguiar, A.S., Jr.; Schuch, F.B.; de Mello, M.T.; Radak, Z.; Fighera, M.R.; Royes, L.F.F. Relationship among depression, fatigue, and sleep after traumatic brain injury: The role of physical exercise as a non-pharmacological therapy. Exp. Neurol. 2025, 386, 115156. [Google Scholar] [CrossRef]
- Tabassum, S.; Wu, S.; Lee, C.-H.; Yang, B.S.K.; Gusdon, A.M.; Choi, H.A.; Ren, X.S. Mitochondrial-targeted therapies in traumatic brain injury: From bench to bedside. Neurotherapeutics 2025, 22, e00515. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.K.; Vemuganti, R. Antioxidant therapies in traumatic brain injury. Neurochem. Int. 2022, 152, 105255. [Google Scholar] [CrossRef]
- DeMaria, A.H.; Lee, J.S.; Webb, K. N-Oxalylglycine-Conjugated Hyaluronic Acid as a Macromolecular Prodrug for Therapeutic Angiogenesis. Gels 2025, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Bhargavi, K.M.; Gowthami, N.; Chetan, G.K.; Srinivas Bharath, M.M. Neuroprotective effects of nutraceuticals and natural products in traumatic brain injury. Neurochem. Int. 2025, 182, 105904. [Google Scholar] [CrossRef] [PubMed]
- Salagean, A.-A.; Moldovan, C.-A.-D.; Slevin, M. Utilisation of High Molecular Weight and Ultra-High Molecular Weight Hyaluronan in Management of Glioblastoma. Gels 2025, 11, 50. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and Advances in Stimuli-Responsive Hydrogels and Their Applications: A Review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Tsui, C.T.; MacGillivray, S.R.; Weber, S.M.; McAllister, L.; Churchward, M.A.; Dennison, C.R.; Todd, K.G. Applying a novel 3D hydrogel cell culture to investigate activation of microglia due to rotational kinematics associated with mild traumatic brain injury. J. Mech. Behav. Biomed. Mater. 2021, 114, 104176. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Gomez-Pinedo, U.; Hernández-Sapiéns, M.A.; Canales-Aguirre, A.A.; Espinosa-Andrews, H.; Matias-Guiu, J.; González-García, Y.; Mateos-Díaz, J.C. Biocompatibility of ferulic/succinic acid-grafted chitosan hydrogels for implantation after brain injury: A preliminary study. Mater. Sci. Eng. C 2021, 121, 111806. [Google Scholar] [CrossRef]
- Hao, Y.; Feng, L.; Liu, H.; Zhou, L.; Yu, X.; He, X.; Cheng, H.; Jin, L.; Wang, C.; Guo, R. Bioactive hydrogel synergizes neuroprotection, macrophage polarization, and angio-genesis to improve repair of traumatic brain injury. Mater. Today Bio 2024, 29, 101335. [Google Scholar] [CrossRef]
- Ma, X.; Agas, A.; Siddiqui, Z.; Kim, K.; Iglesias-Montoro, P.; Kalluru, J.; Kumar, V.; Haorah, J. Angiogenic peptide hydrogels for treatment of traumatic brain injury. Bioact. Mater. 2020, 5, 124–132. [Google Scholar] [CrossRef]
- Shi, J.; Tang, J.; Xu, J.; Jiang, N.; Yang, Y.; Chen, H.; Han, Y.; Fu, X. Applications of hy-drogels and nanoparticles in the treatment of traumatic brain injury. Front. Bioeng. Biotechnol. 2025, 12, 1515164. [Google Scholar] [CrossRef]
- Severs, L.J.; Katta, A.; Cates, L.N.; Dewees, D.M.; Hoagland, R.T.; Horner, P.J.; Hofstetter, C.P.; Khaing, Z.Z. Biomimetic 3D Hydrogels with Aligned Topography for Neural Tissue Engineering. Polymers 2024, 16, 3556. [Google Scholar] [CrossRef]
- Dell’Anno, M.T.; Strittmatter, S.M. Rewiring the spinal cord: Direct and indirect strategies. Neurosci. Lett. 2017, 652, 25–34. [Google Scholar] [CrossRef]
- M’koma, A.E. Inflammatory bowel disease: An expanding global health problem. Clin. Med. Insights Gastroenterol. 2013, 6, CGast-S12731. [Google Scholar] [CrossRef]
- Abbasi, M.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M. Novel biodegradable pH-sensitive hydrogels: An efficient controlled release system to manage ulcerative colitis. Int. J. Biol. Macromol. 2019, 136, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Sendon-Lago, J.; Rio, L.G.-d.; Eiro, N.; Diaz-Rodriguez, P.; Avila, L.; Gonzalez, L.O.; Vizoso, F.J.; Perez-Fernandez, R.; Landin, M. Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice. Pharmaceutics 2021, 13, 1127. [Google Scholar] [CrossRef] [PubMed]
- Poláková, L.; Raus, V.; Cuchalová, L.; Poręba, R.; Hrubý, M.; Kučka, J.; Větvička, D.; Trhlíková, O.; Sedláková, Z. SHARP hydrogel for the treatment of inflammatory bowel disease. Int. J. Pharm. 2022, 613, 121392. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, J.; Zhao, Q.; He, Z.; Tang, L.; Pu, Y.; He, B. Bio-adhesive and ROS-scavenging hydrogel microspheres for targeted ulcerative colitis therapy. Int. J. Pharm. 2023, 639, 122962. [Google Scholar] [CrossRef]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-Responsive Hydrogels for Local Post-Surgical Drug Delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Mohd Razak, R.; Harizal, N.A.A.; Azman, M.A.Z.; Mohd Redzuan, N.S.; Ogaili, R.H.; Kamarrudin, A.H.; Mohamad Azmi, M.F.; Kamaruddin, N.A.; Abdul Jamil, A.S.; Mokhtar, S.A.; et al. Deciphering the Effect of Hyaluronic Acid/Collagen Hydrogel for Pain Relief and Tissue Hydration in a Rat Model of Intervertebral Disc Degeneration. Polymers 2024, 16, 2574. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Caruso, B.R.; Li, W. Advanced Hydrogels in Breast Cancer Therapy. Gels 2024, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lewis, D.; Gerecht, S. Bioinspired hydrogels to engineer cancer microenvironments. Annu. Rev. Biomed. Eng. 2017, 19, 109–133. [Google Scholar] [CrossRef]
- Morales, X.; Cortés-Domínguez, I.; Ortiz-de-Solorzano, C. Modeling the Mechanobiology of Cancer Cell Migration Using 3D Biomimetic Hydrogels. Gels 2021, 7, 17. [Google Scholar] [CrossRef]
- Katti, P.D.; Jasuja, H. Current Advances in the Use of Tissue Engineering for Cancer Metastasis Therapeutics. Polymers 2024, 16, 617. [Google Scholar] [CrossRef]
- Hassan, G.; Özuğur Uysal, B. Effect of MoS2 on Simple and Novel PEG/κ-Carrageenan Hydrogels for TNBC Cancer Drug Delivery. J. Macromol. Sci. 2024, 64, 135–149. [Google Scholar] [CrossRef]
- Malek, S.; Jaafari, M.R.; Mahmoudi, A.; Mohammadi, M.; Malaekeh-Nikouei, B. Smart release injectable hydrogel co-loaded with liposomal combretastatin A4 and doxorubicin nanogel for local combinational drug delivery: A preclinical study. Int. J. Pharm. 2025, 671, 125213. [Google Scholar] [CrossRef]
- Almawash, S.; Mohammed, A.M.; El Hamd, M.A.; Osman, S.K. Injectable Hydrogels Based on Cyclodextrin/Cholesterol Inclusion Complexation and Loaded with 5-Fluorouracil/Methotrexate for Breast Cancer Treatment. Gels 2023, 9, 326. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, H.; Xu, S.; Zhao, J.; Wang, Y.; Pan, X.; Zhao, B.; Sun, Z.; Yin, Y.; Xu, L.; et al. Injectable doxorubicin-loaded hyaluronic acid-based hydrogel for locoregional therapy and inhibiting metastasis of breast cancer. Colloids Surf. B Biointerfaces 2025, 247, 114433. [Google Scholar] [CrossRef]
- Hou, X.; Guan, Y.; Lu, Y.; Wang, Y.; Xu, S.; Zhu, H.; Zhao, J.; Xiao, L.; He, S.; Shi, Y. Chitosan-based thermosensitive injectable hydrogel with hemostatic and antibacterial activity for preventing breast cancer postoperative recurrence and metastasis via chemo-photothermal therapy. Int. J. Biol. Macromol. 2025, 290, 138930. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Yuan, H.; Sun, K.; Zheng, Y.; Ju, S.; Xia, W. Clinical Experience in the Management of the Polyacrylamide Hydrogel (PAAG) Associated Complications Including Four Breast Cancer Cases: A Retrospective Study of 135 Cases. Aesth. Plast. Surg. 2024, 49, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cao, Y.; Yao, Y.; Zhang, D.; Wang, Y. Chitosan and hyaluronic acid in breast cancer treatment: Anticancer efficacy and nanoparticle and hydrogel development. Int. J. Biol. Macromol. 2025, 301, 140144. [Google Scholar] [CrossRef]
- Mondal, J.; Chakraborty, K.; Bunggulawa, E.J.; An, J.M.; Revuri, V.; Nurunnabi, M.; Lee, Y.K. Recent advancements of hydrogels in immunotherapy: Breast cancer treatment. J. Control Release 2024, 372, 1–30. [Google Scholar] [CrossRef]
- Sabino, I.J.; Lima-Sousa, R.; Alves, C.G.; Melo, B.L.; Moreira, A.F.; Correia, I.J.; de Melo-Diogo, D. Injectable in situ forming hydrogels incorporating dual-nanoparticles for chemo-photothermal therapy of breast cancer cells. Int. J. Pharm. 2021, 600, 120510. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, N.; Wu, S.; Lin, G.; Lu, W.; Shang, B.; Li, J. An injectable hydrogel for hemostasis and tumor suppression in intraoperative breast cancer. Biomater. Adv. 2025, 172, 214219. [Google Scholar] [CrossRef]
- Halder, S.; Das, T.; Kushwaha, R.; Misra, A.K.; Jana, K.; Das, D. Targeted and precise drug delivery using a glutathione-responsive ultra-short peptide-based injectable hydrogel as a breast cancer cure. Mater. Horiz. 2025, 12, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Kundrapu, D.B.; Rao, P.A.; Malla, R.R. Enhanced efficacy of quercetin and taxifolin encapsulated with pH-responsive injectable BSA hydrogel for targeting triple-negative breast cancer cells. Int. J. Biol. Macromol. 2025, 287, 138477. [Google Scholar] [CrossRef]
- Quarta, A.; Gallo, N.; Vergara, D.; Salvatore, L.; Nobile, C.; Ragusa, A.; Gaballo, A. Investigation on the Composition of Agarose–Collagen I Blended Hydrogels as Matrices for the Growth of Spheroids from Breast Cancer Cell Lines. Pharmaceutics 2021, 13, 963. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Jo, G.; Lee, B.Y.; Choi, J.W.; Chun, H.J.; Kim, H.S.; Yang, D.H. Injectable Glycol Chitosan Hydrogel Containing Folic Acid-Functionalized Cyclodextrin-Paclitaxel Complex for Breast Cancer Therapy. Nanomaterials 2021, 11, 317. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Yang, X.; Wang, Y.; Yu, L.; Sun, J.; Ding, J. Injectable hydrogels for the sustained delivery of a HER2-targeted antibody for preventing local relapse of HER2+ breast cancer after breast-conserving surgery. Theranostics 2019, 9, 6080–6098. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.E.; Shaw, A.K.; Strand, D.W.; Hayward, S.W. Cancer associated fibroblasts in cancer pathogenesis. Semin. Cell Dev. Biol. 2010, 21, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Shelkey, E.; Oommen, D.; Stirling, E.R.; Soto-Pantoja, D.R.; Cook, K.L.; Lu, Y.; Votanopoulos, K.I.; Soker, S. Immuno-reactive cancer organoid model to assess effects of the micro-biome on cancer immunotherapy. Sci. Rep. 2022, 12, 9983. [Google Scholar]
- Koch, M.K.; Ravichandran, A.; Murekatete, B.; Clegg, J.; Joseph, M.T.; Hampson, M.; Jenkinson, M.; Bauer, H.S.; Snell, C.; Liu, C.; et al. Exploring the potential of peg-heparin hydrogels to support long-term ex vivo culture of patient-derived breast explant tissues. Adv. Healthc. Mater. 2023, 12, 2202202. [Google Scholar] [CrossRef]
- Goder Orbach, D.; Zilberman, M. Formulation Effects on the Mechano-Physical Properties of In Situ-Forming Resilient Hydrogels for Breast Tissue Regeneration. J. Funct. Biomater. 2024, 15, 176. [Google Scholar] [CrossRef]


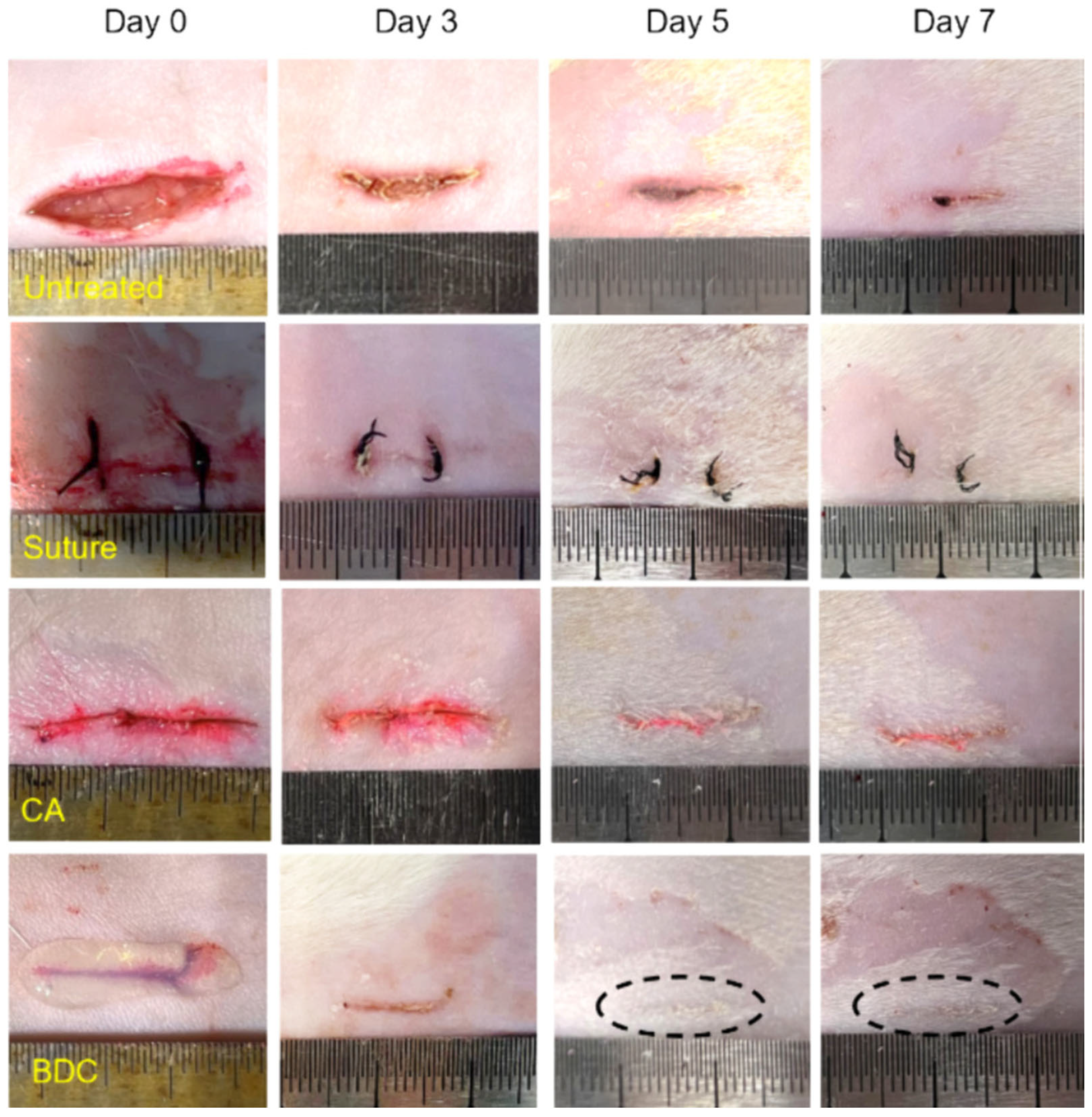
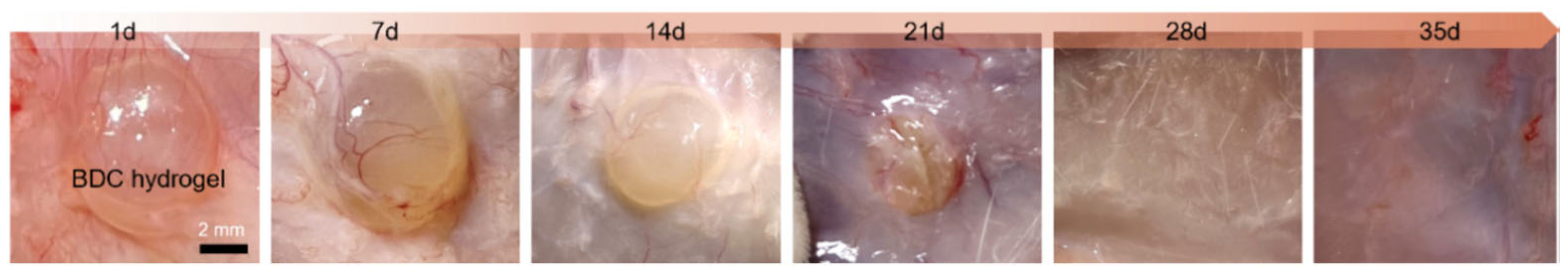
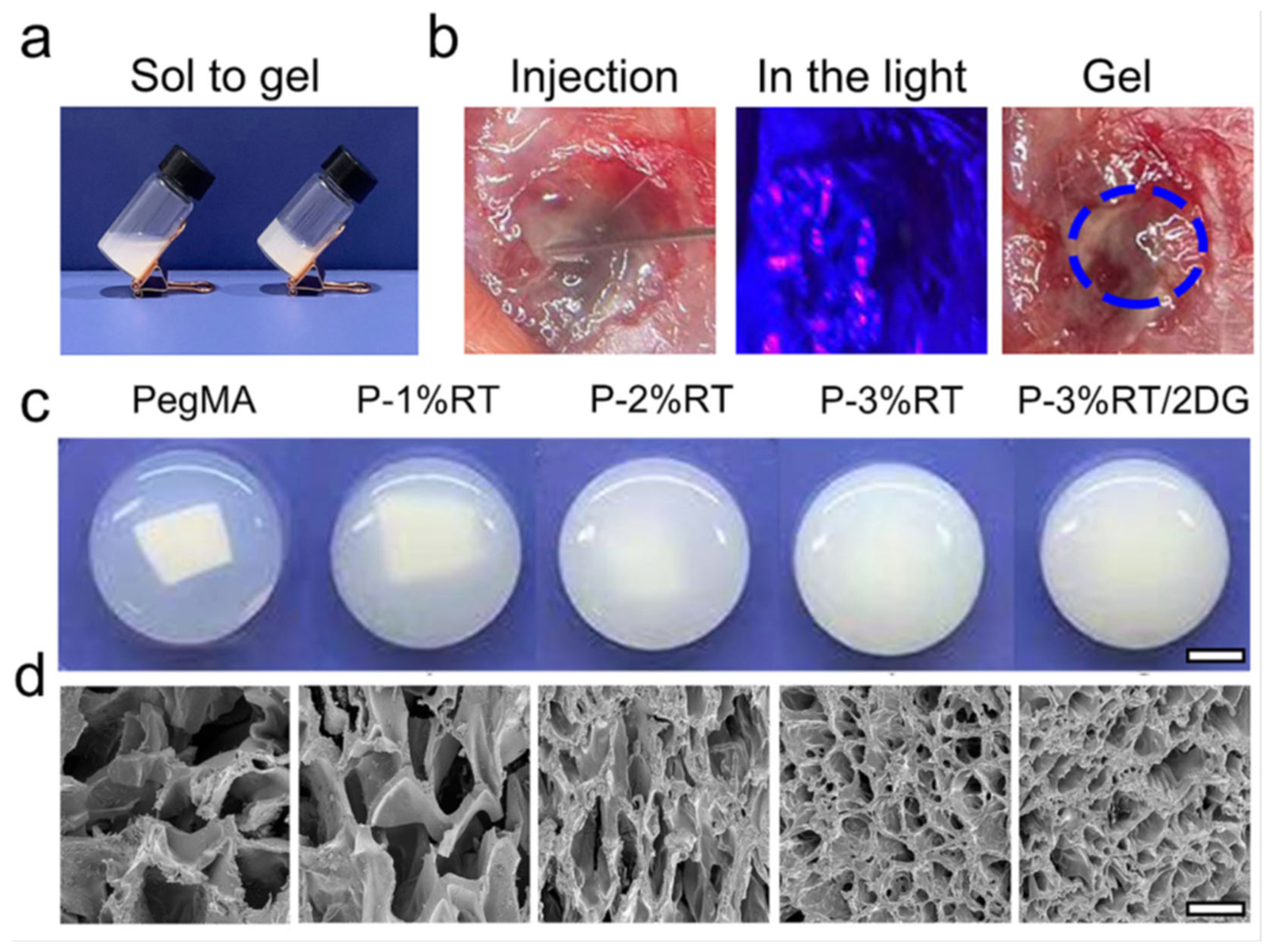
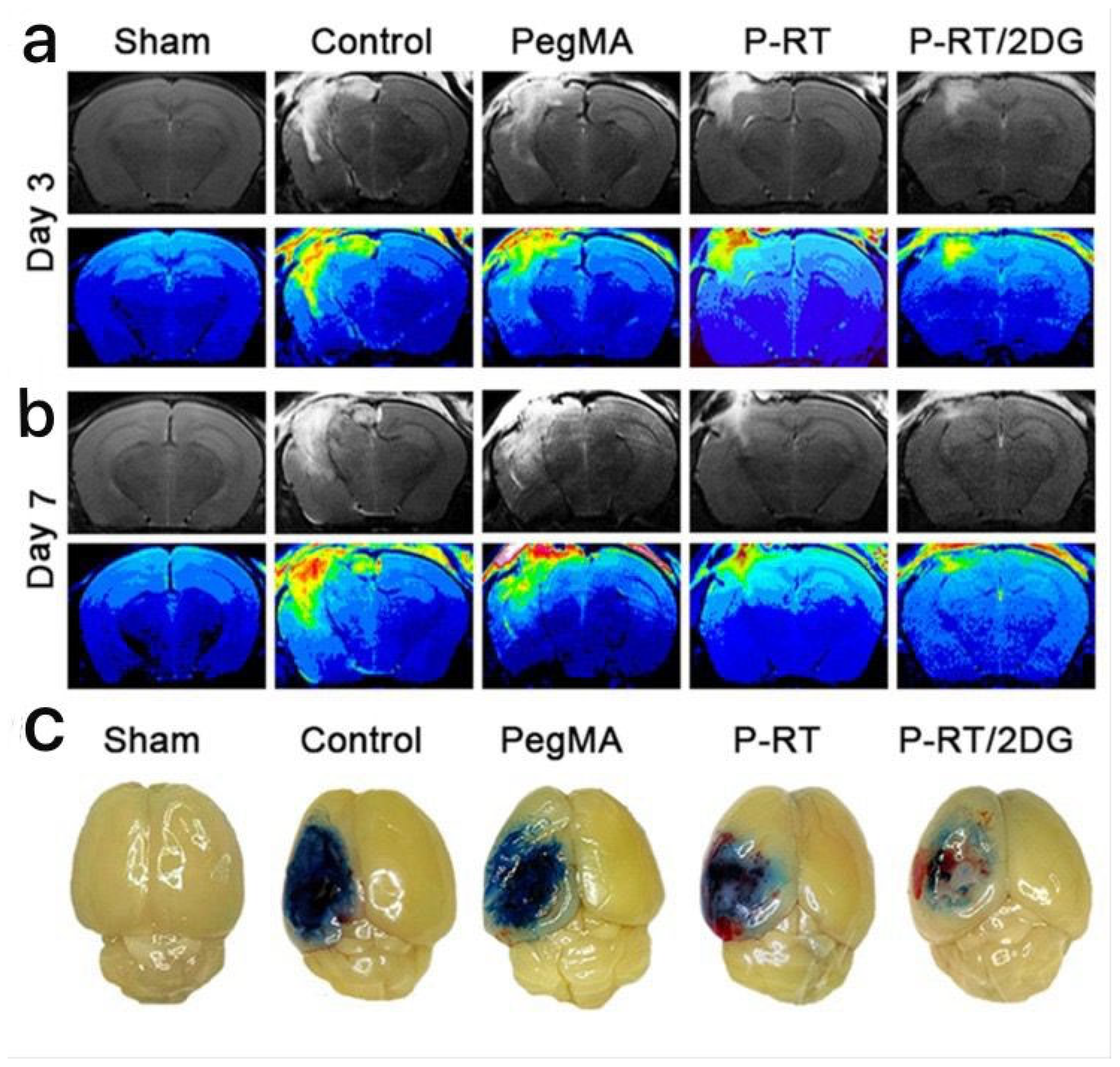
| Conventional Treatments | Common Benefits | Features of Hydrogel-Based Materials |
|---|---|---|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelu, M.; Popa, M.; Calderón Moreno, J.M. Applications of Hydrogels in Emergency Therapy. Gels 2025, 11, 234. https://doi.org/10.3390/gels11040234
Chelu M, Popa M, Calderón Moreno JM. Applications of Hydrogels in Emergency Therapy. Gels. 2025; 11(4):234. https://doi.org/10.3390/gels11040234
Chicago/Turabian StyleChelu, Mariana, Monica Popa, and José María Calderón Moreno. 2025. "Applications of Hydrogels in Emergency Therapy" Gels 11, no. 4: 234. https://doi.org/10.3390/gels11040234
APA StyleChelu, M., Popa, M., & Calderón Moreno, J. M. (2025). Applications of Hydrogels in Emergency Therapy. Gels, 11(4), 234. https://doi.org/10.3390/gels11040234









