Survey of Sustainable Wearable Strain Sensors Enabled by Biopolymers and Conductive Organic Polymers
Abstract
1. Introduction
2. Biopolymers for Sustainable Substrates
2.1. Chitosan
2.2. Cellulose
2.3. Silk Fibroin
2.4. Challenges of Sustainable Substrates for Use in Strain Sensors
3. Organic Conductive Polymers in Sustainable Wearable Sensors
3.1. Polythiophene Sustainable Strain Sensors
| Sensor Composition 1 | Integration of PEDOT | Sustainable Component/Substrate | Conductivity | Applications Studied | Ref. |
|---|---|---|---|---|---|
| PAVK-PEDOT:PSS-PANS bilayer hydrogel | Blending and Spray-Coating | Carboxylated cellulose nano whiskers/carboxymethyl chitosan | 1.76 S/m | - | [83] |
| CNC-PEDOT:PSS/PVA hydrogel | Blending | Cellulose nanocrystals | 4.73 S/m | Human motion (wrist, finger, knee, and neck motion) | [84] |
| CMC-PEDOT:PSS composite film | Blending | Carboxymethyl cellulose | 5.5 × 102 Ω/sq. | Human motion (finger, throat, skin-wrinkling) and speech | [51] |
| PEDOT/PSS/CNF aerogel | Blending | Cellulose nanofibrils | 140 ± 30 S/m | - | [85] |
| PVA/Gly-CNC/PVP/PEDOT films | Blending | Cellulose nanocrystals | 0.017 ± 0.001 S/m | Human motion (finger, palm, elbow, neck, pulse, and smile) | [86] |
| PEDOT/GNP Fabric | Spray-coating | Cotton fabric | 25 Ω/sq. | Human motion (finger) | [87] |
| PEDOT/MWCNT Fabric | Spray-coating and in situ polymerization (adsorption) | Polyester–latex mesh fabric | 121 S/m | Human motion (knee, finger and wrist) | [88] |
| PEDOT:PSS/HEC film | Spin coating | Hydroxyethyl cellulose film | 581 Ω/sq. | Human motion (skin wrinkling, touch, finger, wrist, breathing, and walking) | [89] |
3.2. Mechanical Properties of PEDOT Sustainable Sensors
3.3. Sustainable vs. PDMS Sensors
4. Sustainable PEDOT Strain Sensors
4.1. Sensor Performance Criteria
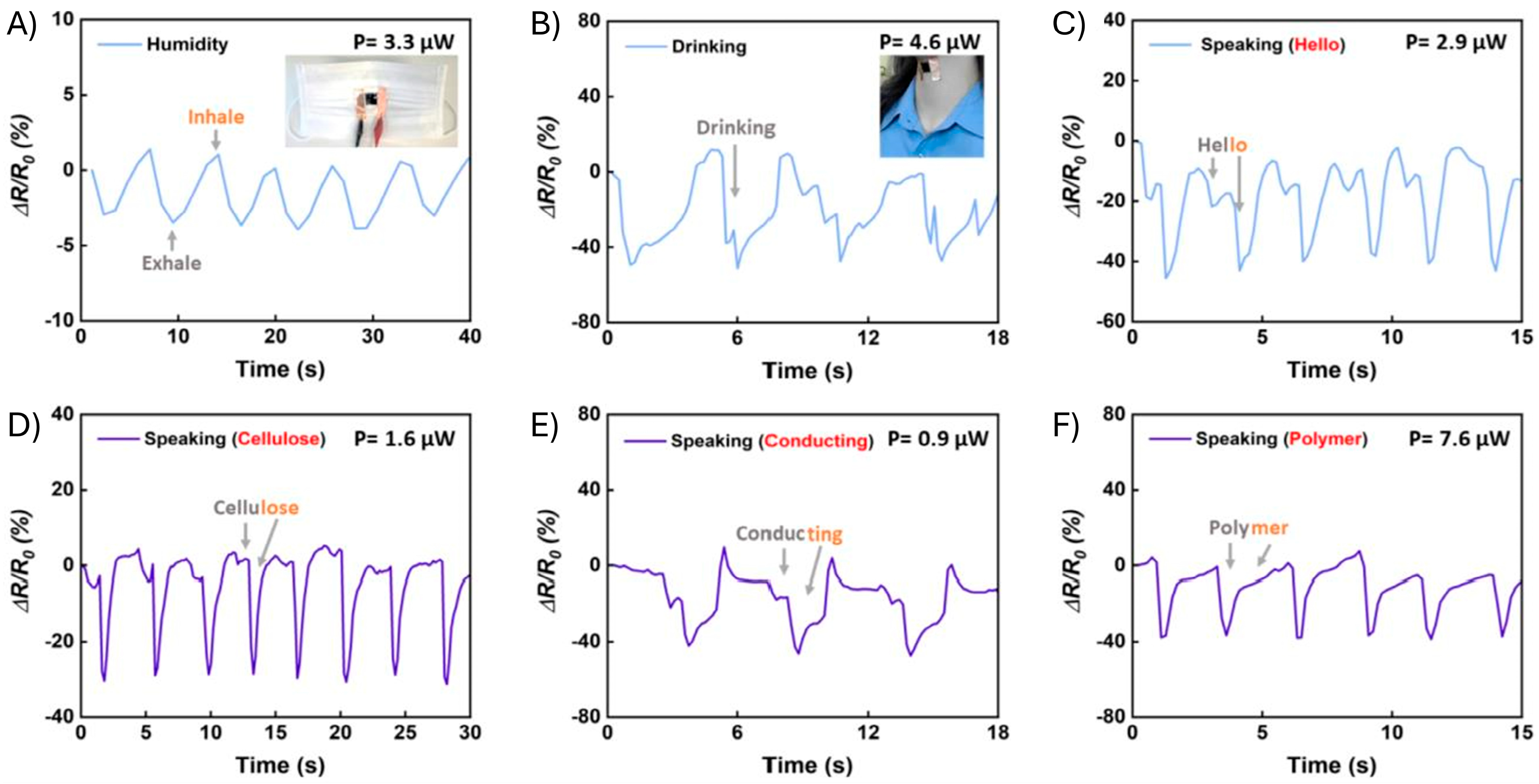
4.2. Macromovement Sensors
4.3. Micromovement/Vibration Sensors
5. Perspectives and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Wei, Y.; Qiu, Y. Advanced Flexible Skin-like Pressure and Strain Sensors for Human Health Monitoring. Micromachines 2021, 12, 695. [Google Scholar] [CrossRef]
- Verma, R.P.; Sahu, P.S.; Rathod, M.; Mohapatra, S.S.; Lee, J.; Saha, B. Ultra-Sensitive and Highly Stretchable Strain Sensors for Monitoring of Human Physiology. Macromol. Mater. Eng. 2022, 307, 2100666. [Google Scholar] [CrossRef]
- Liza, L.; Kabir, M.H.; Jiang, L.; Jerrams, S.; Chen, S. The Technology of Wearable Flexible Textile-Based Strain Sensors for Monitoring Multiple Human Motions: Construction, Patterning and Performance. Sens. Diagn. 2023, 2, 1414–1436. [Google Scholar] [CrossRef]
- Sun, S.; Liu, Y.; Chang, X.; Jiang, Y.; Wang, D.; Tang, C.; He, S.; Wang, M.; Guo, L.; Gao, Y. A Wearable, Waterproof, and Highly Sensitive Strain Sensor Based on Three-Dimensional Graphene/Carbon Black/Ni Sponge for Wirelessly Monitoring Human Motions. J. Mater. Chem. C 2020, 8, 2074–2085. [Google Scholar] [CrossRef]
- Lozoya-Santos, J.D.-J.; Félix-Herrán, L.C.; Tudón-Martínez, J.C.; Vargas-Martinez, A.; Ramirez-Mendoza, R.A. Design and Implementation of an Iot-Oriented Strain Smart Sensor with Exploratory Capabilities on Energy Harvesting and Magnetorheological Elastomer Transducers. Appl. Sci. 2020, 10, 4387. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Guan, J.; Wang, D.; Tang, M.; Ma, Y.; Xia, H. A Flexible Wearable Strain Sensor for Human-Motion Detection and a Human–Machine Interface. J. Mater. Chem. C 2022, 10, 15554–15564. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, N.; Wu, Y.; RamaKrishna, S.; Hongyu, Z. Wearable Strain Sensors: State-of-the-Art and Future Applications. Mater. Adv. 2023, 4, 1444–1459. [Google Scholar] [CrossRef]
- Souri, H.; Banerjee, H.; Jusufi, A.; Radacsi, N.; Stokes, A.A.; Park, I.; Sitti, M.; Amjadi, M. Wearable and Stretchable Strain Sensors: Materials, Sensing Mechanisms, and Applications. Adv. Intell. Syst. 2020, 2, 2000039. [Google Scholar] [CrossRef]
- Shekh, M.I.; Zhu, G.; Xiong, W.; Wu, W.; Stadler, F.J.; Patel, D.; Zhu, C. Dynamically Bonded, Tough, and Conductive MXene@oxidized Sodium Alginate: Chitosan Based Multi-Networked Elastomeric Hydrogels for Physical Motion Detection. Int. J. Biol. Macromol. 2023, 224, 604–620. [Google Scholar] [CrossRef]
- Tang, C.; Xu, M.; Yi, W.; Zhang, Z.; Occhipinti, E.; Dong, C.; Ravenscroft, D.; Jung, S.M.; Lee, S.; Gao, S.; et al. Ultrasensitive Textile Strain Sensors Redefine Wearable Silent Speech Interfaces with High Machine Learning Efficiency. npj Flex. Electron. 2024, 8, 27. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Zhang, M.; Hao, H.; Yan, S. Resistance-Type Strain Sensor Based on Carbon Nanofiber/Polypyrrole Composite Membrane with High Sensitivity. Polym. Compos. 2024, 45, 8876–8888. [Google Scholar] [CrossRef]
- Glisic, B. Concise Historic Overview of Strain Sensors Used in the Monitoring of Civil Structures: The First One Hundred Years. Sensors 2022, 22, 2397. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable Sensors for Monitoring the Physiological and Biochemical Profile of the Athlete. npj Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, E.; Chung, J.W.; Cho, H.; Jeong, S.; Kim, H.; Jang, D.; Kim, H.; Yoon, J.; Lee, G.H.; et al. Silent Speech Recognition with Strain Sensors and Deep Learning Analysis of Directional Facial Muscle Movement. ACS Appl. Mater. Interfaces 2022, 14, 54157–54169. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Montree, R.J.H.; Que, S.; Peri, E.; Vullings, R. An Overview of the Sensors for Heart Rate Monitoring Used in Extramural Applications. Sensors 2022, 22, 4035. [Google Scholar] [CrossRef]
- Yi, H.; Wang, S.; Mei, S.; Li, Z. Conductive Polymer Composites for Resistive Flexible Strain Sensors. Polymer 2024, 307, 127286. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, J.; Gao, Q.; Zhang, J.; Zhang, J.; Omisore, O.M.; Wang, L.; Li, H. Polydimethylsiloxane (PDMS)-Based Flexible Resistive Strain Sensors for Wearable Applications. Appl. Sci. 2018, 8, 345. [Google Scholar] [CrossRef]
- Ozek, E.A.; Tasdelen, M.C.; Tanyeli, S.; Yapici, M.K. Strain Sensing Graphene Functionalized PET Films Based on a Facile Dip Coating Approach. In Proceedings of the 2021 IEEE International Flexible Electronics Technology Conference (IFETC), Columbus, OH, USA, 8–11 August 2021; pp. 1–3. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, W.; Xu, T.; Zhao, W.; Zhang, J.; Bai, C. Flexible Strain Sensors Based on Thermoplastic Polyurethane Fabricated by Electrospinning: A Review. Sensors 2024, 24, 4793. [Google Scholar] [CrossRef]
- Lei, C.; Li, Q.; Chen, W.; Yu, G. Biopolymeric Gels: Advancements in Sustainable Multifunctional Materials. Adv. Mater. 2025, 2419906. [Google Scholar] [CrossRef]
- Vohra, A.; Filiatrault, H.L.; Amyotte, S.D.; Carmichael, R.S.; Suhan, N.D.; Siegers, C.; Ferrari, L.; Davidson, G.J.E.; Carmichael, T.B. Reinventing Butyl Rubber for Stretchable Electronics. Adv. Funct. Mater. 2016, 26, 5222–5229. [Google Scholar] [CrossRef]
- Dauzon, E.; Lin, Y.; Faber, H.; Yengel, E.; Sallenave, X.; Plesse, C.; Goubard, F.; Amassian, A.; Anthopoulos, T.D. Stretchable and Transparent Conductive PEDOT:PSS-Based Electrodes for Organic Photovoltaics and Strain Sensors Applications. Adv. Funct. Mater. 2020, 30, 2001251. [Google Scholar] [CrossRef]
- Raman, S.; Ravi Sankar, A. Intrinsically Conducting Polymers in Flexible and Stretchable Resistive Strain Sensors: A Review. J. Mater. Sci. 2022, 57, 13152–13178. [Google Scholar] [CrossRef]
- Gullapalli, H.; Vemuru, V.S.M.; Kumar, A.; Botello-Mendez, A.; Vajtai, R.; Terrones, M.; Nagarajaiah, S.; Ajayan, P.M. Flexible Piezoelectric Zno-Paper Nanocomposite Strain Sensor. Small 2010, 6, 1641–1646. [Google Scholar] [CrossRef]
- Malik, M.S.; Zulfiqar, M.H.; Khan, M.A.; Mehmood, M.Q.; Massoud, Y. Facile Pressure-Sensitive Capacitive Touch Keypad for a Green Intelligent Human–Machine Interface. Sensors 2022, 22, 8113. [Google Scholar] [CrossRef]
- Tian, Y.; He, P.; Yang, B.; Yi, Z.; Lu, L.; Liu, J. A Flexible Piezoelectric Strain Sensor Array with Laser-Patterned Serpentine Interconnects. IEEE Sens. J. 2020, 20, 8463–8468. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Spanswick, J. Copper-Mediated Atom Transfer Radical Polymerization. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1–10, pp. 377–428. [Google Scholar] [CrossRef]
- Han, F.; Chen, S.; Wang, F.; Liu, M.; Li, J.; Liu, H.; Yang, Y.; Zhang, H.; Liu, D.; He, R.; et al. High-Conductivity, Self-Healing, and Adhesive Ionic Hydrogels for Health Monitoring and Human-Machine Interactions Under Extreme Cold Conditions. Adv. Sci. 2025, 2412726. [Google Scholar] [CrossRef]
- Piro, B.; Tran, H.V.; Thu, V.T. Sensors Made of Natural Renewable Materials: Efficiency, Recyclability or Biodegradability—The Green Electronics. Sensors 2020, 20, 5898. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, Y.; Zhang, Y.; Yu, C.; Cao, S. Physicochemical, Mechanical and Thermal Properties of Chitosan Films with and without Sorbitol. Int. J. Biol. Macromol. 2014, 70, 340–346. [Google Scholar] [CrossRef]
- Cohen, E. Chapter 2—Chitin Biochemistry: Synthesis, Hydrolysis and Inhibition. In Advances in Insect Physiology: Insect Integument and Colour; Casas, J., Simpson, S.J., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 38, pp. 5–74. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan Composite Films: Thermal, Structural, Mechanical and Antifungal Properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Effects of Drying Methods and Plasticizer Concentration on Some Physical and Mechanical Properties of Edible Chitosan Films. J. Food Eng. 2010, 99, 216–224. [Google Scholar] [CrossRef]
- Bharti, B.M.; Bhuvana, T.; Chandraprakash, C. Burst and Physicochemical Characteristics of Glycerol-Added Chitosan Films for Food Packaging. ACS Food Sci. Technol. 2023, 3, 772–780. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Xiao, X.; Duan, Q.; Bai, H.; Cao, Y.; Zhang, Y.; Alee, M.; Yu, L. Improved Hydrophobicity, Antibacterial and Mechanical Properties of Polyvinyl Alcohol/Quaternary Chitosan Composite Films for Antibacterial Packaging. Carbohydr. Polym. 2023, 312, 120755. [Google Scholar] [CrossRef] [PubMed]
- Wardhono, E.Y.; Pinem, M.P.; Susilo, S.; Siom, B.J.; Sudrajad, A.; Pramono, A.; Meliana, Y.; Guénin, E. Modification of Physio-Mechanical Properties of Chitosan-Based Films via Physical Treatment Approach. Polymers 2022, 14, 5216. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kamil, J.Y.V.A.; Shahidi, F. Chitosan as an Edible Invisible Film for Quality Preservation of Herring and Atlantic Cod. J. Agric. Food Chem. 2002, 50, 5167–5178. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for Food Packaging: Recent Advances in Active and Intelligent Films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Gan, P.G.; Sam, S.T.; Abdullah, M.F.; Omar, M.F.; Tan, W.K. Water Resistance and Biodegradation Properties of Conventionally-Heated and Microwave-Cured Cross-Linked Cellulose Nanocrystal/Chitosan Composite Films. Polym. Degrad. Stab. 2021, 188, 109563. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Ramesh, M.N.; Tharanathan, R.N. Effect of Plasticizers and Fatty Acids on Mechanical and Permeability Characteristics of Chitosan Films. Food Hydrocoll. 2007, 21, 1113–1122. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-Mechanical Properties of Chitosan Films with Carvacrol and Grape Seed Extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Yan, M.; Wang, Q. Chitosan-Based Transparent and Conductive Hydrogel with Highly Stretchable, Adhesive and Self-Healing as Skin-like Sensor. Int. J. Biol. Macromol. 2023, 242, 124746. [Google Scholar] [CrossRef]
- Sahoo, S.D.; Vasudha, T.K.; Muthuvijayan, V.; Prasad, E. Chitosan-Based Self-Healable and Adhesive Hydrogels for Flexible Strain Sensor Application. ACS Appl. Polym. Mater. 2022, 4, 9176–9185. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; Fabi, J.P. Chapter 2—Polysaccharides as Natural Nanoencapsulants for Controlled Release of Compounds. In Micro and Nano Technologies; Castro, G., Nadda, A., Nguyen, T., Sharma, S., Gupta, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–39. [Google Scholar] [CrossRef]
- Orlando, I.; Roy, I. Cellulose-Based Hydrogels for Wound Healing; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Z.; Li, A.; Chen, N.; Rao, J.; Zeng, Q. Nanocellulose Composite Films in Food Packaging Materials: A Review. Polymers 2024, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Esua, O.J.; Nickhil, C.; Whiteside, W.S. Microcrystalline Cellulose for Active Food Packaging Applications: A Review. Food Packag. Shelf Life 2023, 36, 101048. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, S.; Qian, L.; Wei, N.; Nica, V.; Coseri, S.; Han, F. Super Stretchable, Self-Healing, Adhesive Ionic Conductive Hydrogels Based on Tailor-Made Ionic Liquid for High-Performance Strain Sensors. Adv. Funct. Mater. 2022, 32, 2204565. [Google Scholar] [CrossRef]
- Yang, B.; Yuan, W. Highly Stretchable, Adhesive, and Mechanical Zwitterionic Nanocomposite Hydrogel Biomimetic Skin. ACS Appl. Mater. Interfaces 2019, 11, 40620–40628. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; Zhang, L.; He, C.; Mo, X.; Wang, H. Polyvinyl Alcohol/Hydroxyethylcellulose Containing Ethosomes as a Scaffold for Transdermal Drug Delivery Applications. Appl. Biochem. Biotechnol. 2020, 191, 1624–1637. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.; Kim, J.H.; Entifar, S.A.N.; Prameswati, A.; Wibowo, A.F.; Kim, S.; Lim, D.C.; Lee, J.; Moon, M.W.; et al. Stretchable and Conductive Cellulose/Conductive Polymer Composite Films for On-Skin Strain Sensors. Materials 2022, 15, 5009. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Cui, G.; Liu, L.; Zhou, C.; Wu, G. Hydroxyethyl Cellulose-Based Stretchable, Antifreeze, Ion-Conductive Hydrogel Sensor. Eur. Polym. J. 2024, 202, 112603. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A.; Dickey, M.D. A Highly Conductive and Ultra-Stretchable Polyaniline/Cellulose Nanocrystal/Polyacrylamide Hydrogel with Hydrophobic Associations for Wearable Strain Sensors. J. Mater. Chem. A 2024, 12, 9552–9562. [Google Scholar] [CrossRef]
- Uversky, V.N. The Intrinsic Disorder Alphabet. III. Dual Personality of Serine. Intrinsically Disord. Proteins 2015, 3, e1027032. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Murugesan, B.; Zhang, Y.; Chen, Z.; Yang, X.; Cai, Y. An Oriented MXene/Silk Fibroin Nanofiber Hydrogel with High Strength and Strain Response Ability. New J. Chem. 2024, 48, 4570–4579. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, M.; Sun, Y.; Zuo, B. Self-Healing, Wet-Adhesion Silk Fibroin Conductive Hydrogel as a Wearable Strain Sensor for Underwater Applications. Chem. Eng. J. 2022, 446, 136931. [Google Scholar] [CrossRef]
- Shi, L.; Wang, F.; Zhu, W.; Xu, Z.; Fuchs, S.; Hilborn, J.; Zhu, L.; Ma, Q.; Wang, Y.; Weng, X.; et al. Self-Healing Silk Fibroin-Based Hydrogel for Bone Regeneration: Dynamic Metal-Ligand Self-Assembly Approach. Adv. Funct. Mater. 2017, 27, 1700591. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Haq, F.; Farid, A.; Kiran, M.; Faisal, S.; Ullah, A.; Ullah, N.; Bokhari, A.; Mubashir, M.; Chuah, L.F.; et al. Challenges Associated with Cellulose Composite Material: Facet Engineering and Prospective. Environ. Res. 2023, 223, 115429. [Google Scholar] [CrossRef]
- Zuppolini, S.; Salama, A.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Cellulose Amphiphilic Materials: Chemistry, Process and Applications. Pharmaceutics 2022, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Oberlintner, A.; Bajić, M.; Kalčíková, G.; Likozar, B.; Novak, U. Biodegradability Study of Active Chitosan Biopolymer Films Enriched with Quercus Polyphenol Extract in Different Soil Types. Environ. Technol. Innov. 2021, 21, 101318. [Google Scholar] [CrossRef]
- Paudel, S.; Regmi, S.; Janaswamy, S. Effect of Glycerol and Sorbitol on Cellulose-Based Biodegradable Films. Food Packag. Shelf Life 2023, 37, 101090. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured Metal Oxide-Based Biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, Z.; Wang, X.; Xia, H. A High-Performance Strain Sensor for the Detection of Human Motion and Subtle Strain Based on Liquid Metal Microwire. Nanomaterials 2024, 14, 231. [Google Scholar] [CrossRef]
- Chen, H.; Zhuo, F.; Zhou, J.; Liu, Y.; Zhang, J.; Dong, S.; Liu, X.; Elmarakbi, A.; Duan, H.; Fu, Y. Advances in Graphene-Based Flexible and Wearable Strain Sensors. Chem. Eng. J. 2023, 464, 142576. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Liu, X.; Xia, S.; Gao, Y.; Gao, G. Flexible and Wearable Strain Sensors Based on Conductive Hydrogels. J. Polym. Sci. 2022, 60, 2663–2678. [Google Scholar] [CrossRef]
- Han, P.; Liang, S.; Zou, H.; Wang, X. Structure, Principle and Performance of Flexible Conductive Polymer Strain Sensors: A Review. J. Mater. Sci. Mater. Electron. 2024, 35, 775. [Google Scholar] [CrossRef]
- Groenendaal, L.; Zotti, G.; Aubert, P.H.; Waybright, S.M.; Reynolds, J.R. Electrochemistry of Poly(3,4-Alkylenedioxythiophene) Derivatives. Adv. Mater. 2003, 15, 855–879. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, K.; Chen, R.; Zhou, Y.; Chen, S.; Zheng, Y.; Li, M.; Xu, C.; Tang, X.; Zang, Z.; et al. The Role of Mineral Acid Doping of PEDOT:PSS and Its Application in Organic Photovoltaics. Adv. Electron. Mater. 2020, 6, 29–33. [Google Scholar] [CrossRef]
- Welsh, D.M.; Kumar, A.; Morvant, M.C.; Reynolds, J.R. Fast Electrochromic Polymers Based on New Poly(3,4-Alkylenedioxythiophene) Derivatives. Synth. Met. 1999, 102, 967–968. [Google Scholar] [CrossRef]
- Österholm, A.M.; Ponder, J.F.; Kerszulis, J.A.; Reynolds, J.R. Solution Processed PEDOT Analogues in Electrochemical Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 13492–13498. [Google Scholar] [CrossRef] [PubMed]
- Ponder, J.F.; Österholm, A.M.; Reynolds, J.R. Designing a Soluble PEDOT Analogue without Surfactants or Dispersants. Macromolecules 2016, 49, 2106–2111. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Yuan, Y.; Shu, J.; Deng, X.; Li, L. Vapor-Phase Polymerization of PEDOT for Wearable Fabric Pressure Sensors. J. Electron. Mater. 2022, 51, 1128–1136. [Google Scholar] [CrossRef]
- Amirabad, R.; Saadatabadi, A.R.; Siadati, M.H. Preparation of Polyaniline/Graphene Coated Wearable Thermoelectric Fabric Using Ultrasonic-Assisted Dip-Coating Method. Mater. Renew. Sustain. Energy 2020, 9, 21. [Google Scholar] [CrossRef]
- Amoah, C.; Mahmood, U.; Skene, W.G. Sustainable Sensors Prepared by Environmentally Benign Means for Improving the Environmental Footprint of Wearable Electronics. Adv. Mater. Technol. 2025, 2401600. [Google Scholar] [CrossRef]
- Rudd, S.; Mahjoub, R.; Switalska, E.; Bassell, C.; Schmerl, N.; Cavallaro, A.A.; Stanford, N.; Evans, D.R. Surface Doping in Poly(3,4-Ethylenedioxythiophene)-Based Nanoscale Films: Insights for Polymer Electronics. ACS Appl. Nano Mater. 2022, 5, 12143–12153. [Google Scholar] [CrossRef]
- Gupta, S.; Datt, R.; Mishra, A.; Tsoi, W.C.; Patra, A.; Bober, P. Poly(3,4-Ethylenedioxythiophene):Poly(Styrene Sulfonate) in Antibacterial, Tissue Engineering and Biosensors Applications: Progress, Challenges and Perspectives. J. Appl. Polym. Sci. 2022, 139, e52663. [Google Scholar] [CrossRef]
- Yano, H.; Kudo, K.; Marumo, K.; Okuzaki, H. Fully Soluble Self-Doped Poly(3,4-Ethylenedioxythiophene) with an Electrical Conductivity Greater than 1000 S cm−1. Sci. Adv. 2019, 5, eaav9492. [Google Scholar] [CrossRef] [PubMed]
- Amoah, C.; Terán Morales, J.F.; Mahmood, U.; Skene, W. Nanowires with Conductivities Comparable to Their Bulk Films from an Electrospun Self-Doped Water-Soluble Conductive Polymer. ACS Appl. Electron. Mater. 2024, 7, 1745–1755. [Google Scholar] [CrossRef]
- Cha, S.; Choi, B.; Lee, E.; Cho, G. Improved Sheet Resistance of Nanofiber-Based Transparent Conducting Electrodes Using Silver Nanowires. Polymers 2021, 13, 3856. [Google Scholar] [CrossRef]
- Khasim, S.; Pasha, A.; Lakshmi, M.; Chellasamy, P.; Kadarkarai, M.; Darwish, A.A.A.; Hamdalla, T.A.; Al-Ghamdi, S.A.; Alfadhli, S. Post Treated PEDOT-PSS Films with Excellent Conductivity and Optical Properties as Multifunctional Flexible Electrodes for Possible Optoelectronic and Energy Storage Applications. Opt. Mater. 2022, 125, 112109. [Google Scholar] [CrossRef]
- Cao, G.; Cai, S.; Chen, Y.; Zhou, D.; Zhang, H.; Tian, Y. Facile Synthesis of Highly Conductive and Dispersible PEDOT Particles. Polymer 2022, 252, 124952. [Google Scholar] [CrossRef]
- He, C.; Xu, X.; Lin, Y.; Cui, Y.; Peng, Z. A Bilayer Skin-Inspired Hydrogel with Strong Bonding Interface. Nanomaterials 2022, 12, 1137. [Google Scholar] [CrossRef]
- Chai, X.; Tang, J.; Li, Y.; Cao, Y.; Chen, X.; Chen, T.; Zhang, Z. Highly Stretchable and Stimulus-Free Self-Healing Hydrogels with Multiple Signal Detection Performance for Self-Powered Wearable Temperature Sensors. ACS Appl. Mater. Interfaces 2023, 15, 18262–18271. [Google Scholar] [CrossRef]
- Zhou, J.; Hsieh, Y.-L. Conductive Polymer Protonated Nanocellulose Aerogels for Tunable and Linearly Responsive Strain Sensors. ACS Appl. Mater. Interfaces 2018, 10, 27902–27910. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, H.; Zhang, H.; Wu, Y.; Lee, D.; Wong, W.S.; Tang, X.S.; Park, J.; Yu, H.; Tam, K.C. Sensitive, Stretchable, and Sustainable Conductive Cellulose Nanocrystal Composite for Human Motion Detection. ACS Sustain. Chem. Eng. 2021, 9, 17351–17361. [Google Scholar] [CrossRef]
- Zahid, M.; Papadopoulou, E.L.; Athanassiou, A.; Bayer, I.S. Strain-Responsive Mercerized Conductive Cotton Fabrics Based on PEDOT:PSS/Graphene. Mater. Des. 2017, 135, 213–222. [Google Scholar] [CrossRef]
- Cui, Y.; He, X.; Liu, W.; Zhu, S.; Zhou, M.; Wang, Q. Highly Stretchable, Sensitive, and Multifunctional Thermoelectric Fabric for Synergistic-Sensing Systems of Human Signal Monitoring. Adv. Fiber Mater. 2024, 6, 170–180. [Google Scholar] [CrossRef]
- Han, J.W.; Wibowo, A.F.; Park, J.; Kim, J.H.; Prameswati, A.; Entifar, S.A.N.; Lee, J.; Kim, S.; Chan Lim, D.; Moon, M.W.; et al. Highly Stretchable, Robust, and Conductive Lab-Synthesized PEDOT:PSS Conductive Polymer/Hydroxyethyl Cellulose Films for on-Skin Health-Monitoring Devices. Org. Electron. 2022, 105, 106499. [Google Scholar] [CrossRef]
- Kogje, M.; Satdive, A.; Mestry, S. Biopolymers: A Comprehensive Review of Sustainability, Environmental Impact, and Lifecycle Analysis; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Akinsemolu, A.A.; Idowu, A.M.; Onyeaka, H.N. Recycling Technologies for Biopolymers: Current Challenges and Future Directions. Polymers 2024, 16, 2770. [Google Scholar] [CrossRef]
- Yu, J.; Hou, X.; Cui, M.; Shi, S.; He, J.; Sun, Y.; Wang, C.; Chou, X. Flexible PDMS-Based Triboelectric Nanogenerator for Instantaneous Force Sensing and Human Joint Movement Monitoring. Sci. China Mater. 2019, 62, 1423–1432. [Google Scholar] [CrossRef]
- Guo, D.; Lei, X.; Chen, H.; Yi, L.; Li, Y.; Zhao, Y.; Liu, F.; Cheng, G.J. Highly Flexible and Sensitive Pressure Sensor: Fabrication of Porous PDMS/Graphene Composite via Laser Thermoforming. Adv. Sens. Res. 2024, 3, 2300165. [Google Scholar] [CrossRef]
- Farman, M.; Surendra; Prajesh, R.; Upadhyay, A.K.; Kumar, P.; Thouti, E. All-Polydimethylsiloxane-Based Highly Flexible and Stable Capacitive Pressure Sensors with Engineered Interfaces for Conformable Electronic Skin. ACS Appl. Mater. Interfaces 2023, 15, 34195–34205. [Google Scholar] [CrossRef] [PubMed]
- Masihi, S.; Panahi, M.; Maddipatla, D.; Hanson, A.J.; Bose, A.K.; Hajian, S.; Palaniappan, V.; Narakathu, B.B.; Bazuin, B.J.; Atashbar, M.Z. Highly Sensitive Porous PDMS-Based Capacitive Pressure Sensors Fabricated on Fabric Platform for Wearable Applications. ACS Sens. 2021, 6, 938–949. [Google Scholar] [CrossRef]
- Park, H.; Na, M.; Shin, D.; Kim, D.; Kim, E.; Kim, S.; Lee, D.; Sim, K. A Skin-Friendly Soft Strain Sensor with Direct Skin Adhesion Enabled by Using a Non-Toxic Surfactant. J. Mater. Chem. C 2023, 11, 9611–9619. [Google Scholar] [CrossRef]
- Luo, R.; Li, X.; Li, H.; Du, B.; Zhou, S. A Stretchable and Printable PEDOT:PSS/PDMS Composite Conductors and Its Application to Wearable Strain Sensor. Prog. Org. Coat. 2022, 162, 106593. [Google Scholar] [CrossRef]
- Baker, E.; Li, W.; Hodges, R.; Masso, S.; Jones, C.; Guo, Y.; Alt, M.; Antoniou, M.; Afshar, S.; Tosi, K.; et al. Harnessing Automatic Speech Recognition to Realise Sustainable Development Goals 3, 9, and 17 through Interdisciplinary Partnerships for Children with Communication Disability. Int. J. Speech Lang. Pathol. 2023, 25, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yi, B.; Zhou, Q.; Xu, R.; Liu, X.; Sung, H.-K.; Chernogor, L.; Cao, M.; Yao, Z.; Li, Y.; et al. Ecofriendly and High-Performance Flexible Pressure Sensor Derived from Natural Plant Materials for Intelligent Audible and Silent Speech Recognition. Nano Energy 2024, 126, 109701. [Google Scholar] [CrossRef]
- Ha, S.-H.; Ha, S.-H.; Jeon, M.-B.; Cho, J.H.; Kim, J.-M. Highly Sensitive and Selective Multidimensional Resistive Strain Sensors Based on a Stiffness-Variant Stretchable Substrate. Nanoscale 2018, 10, 5105–5113. [Google Scholar] [CrossRef]
- Mo, F.; Huang, Y.; Li, Q.; Wang, Z.; Jiang, R.; Gai, W.; Zhi, C. A Highly Stable and Durable Capacitive Strain Sensor Based on Dynamically Super-Tough Hydro/Organo-Gels. Adv. Funct. Mater. 2021, 31, 2010830. [Google Scholar] [CrossRef]
- Gogurla, N.; Roy, B.; Park, J.-Y.; Kim, S. Skin-Contact Actuated Single-Electrode Protein Triboelectric Nanogenerator and Strain Sensor for Biomechanical Energy Harvesting and Motion Sensing. Nano Energy 2019, 62, 674–681. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Chang, X.; Xu, Y.; Zhao, G.; Zhu, Y.; Li, Y. A Highly Stretchable Strain Sensor with Both an Ultralow Detection Limit and an Ultrawide Sensing Range. J. Mater. Chem. A 2021, 9, 1795–1802. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; Asghar, W.; Liu, Y.; Li, F.; Sun, D.; Hu, C.; Wu, Z.; Shang, J.; Yu, Z.; et al. Liquid Metal-Based Strain Sensor with Ultralow Detection Limit for Human–Machine Interface Applications. Adv. Intell. Syst. 2021, 3, 2000235. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, G.; Wang, F.; Xu, Y.; Wang, C.; Zhu, Y.; Jiang, W. Design of Flexible Strain Sensor with Both Ultralow Detection Limit and Wide Sensing Range via the Multiple Sensing Mechanisms. Compos. Sci. Technol. 2021, 213, 108932. [Google Scholar] [CrossRef]
- Schmid, M.; Rath, D.; Diebold, U. Why and How Savitzky–Golay Filters Should Be Replaced. ACS Meas. Sci. Au 2022, 2, 185–196. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef] [PubMed]



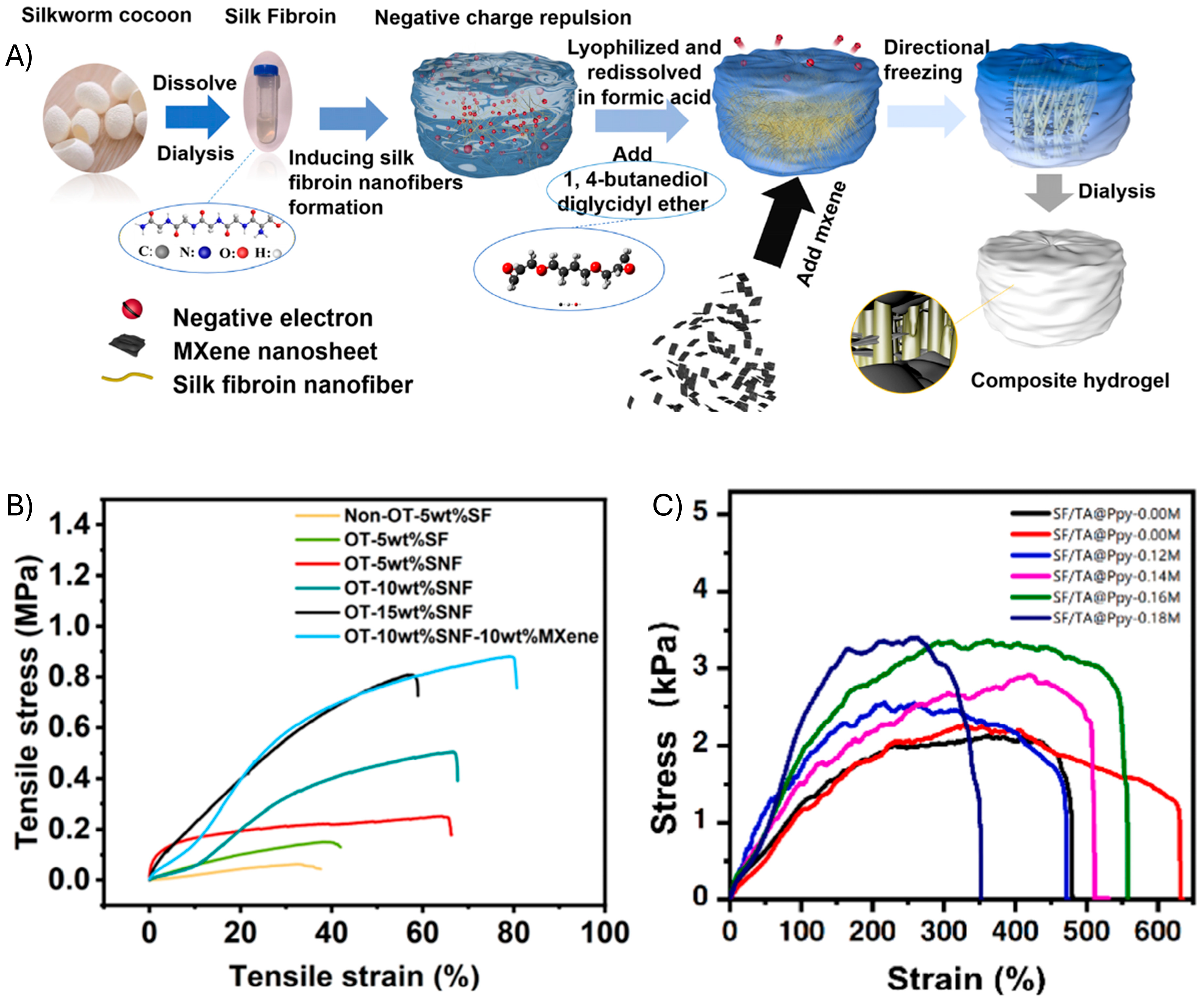
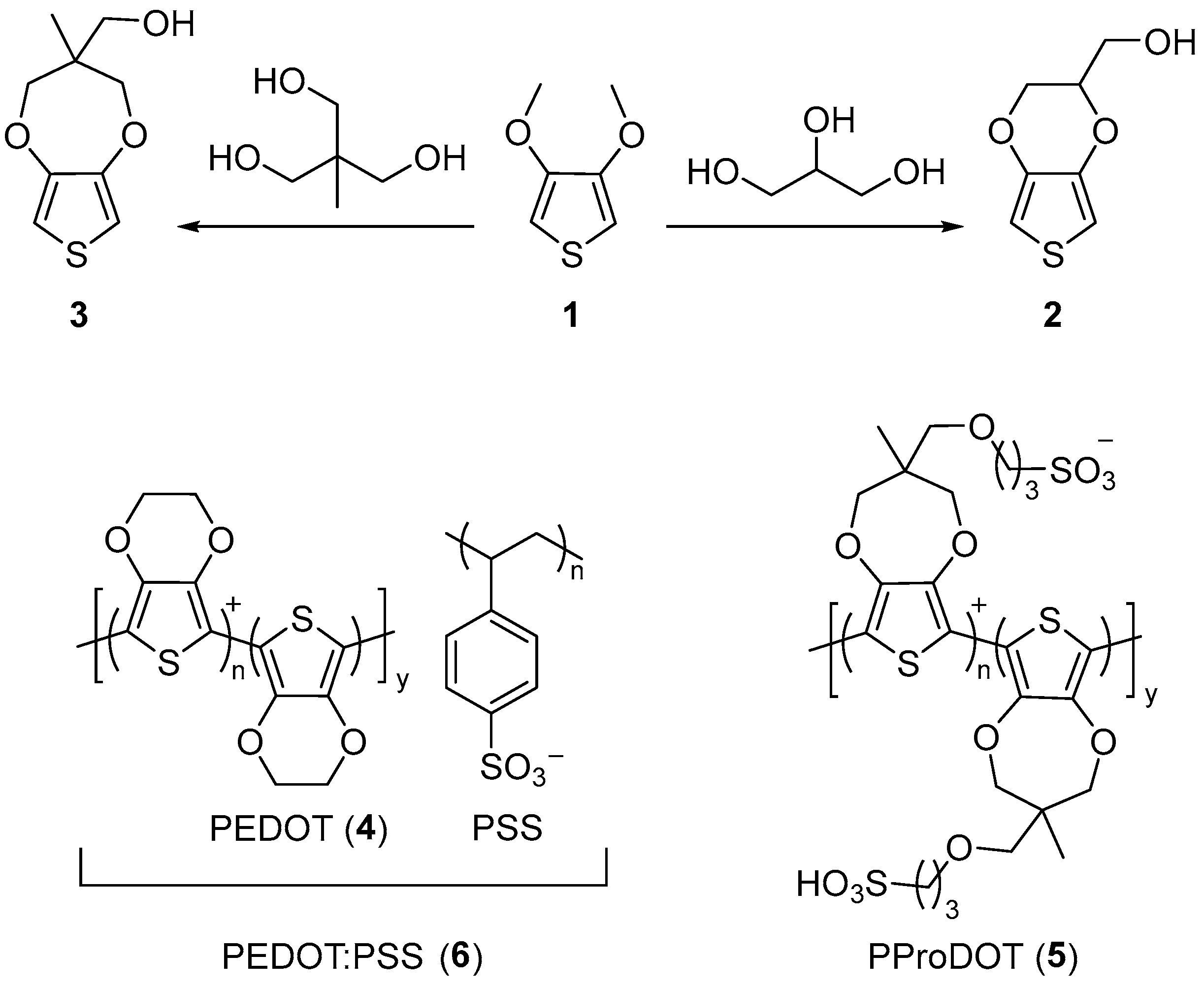
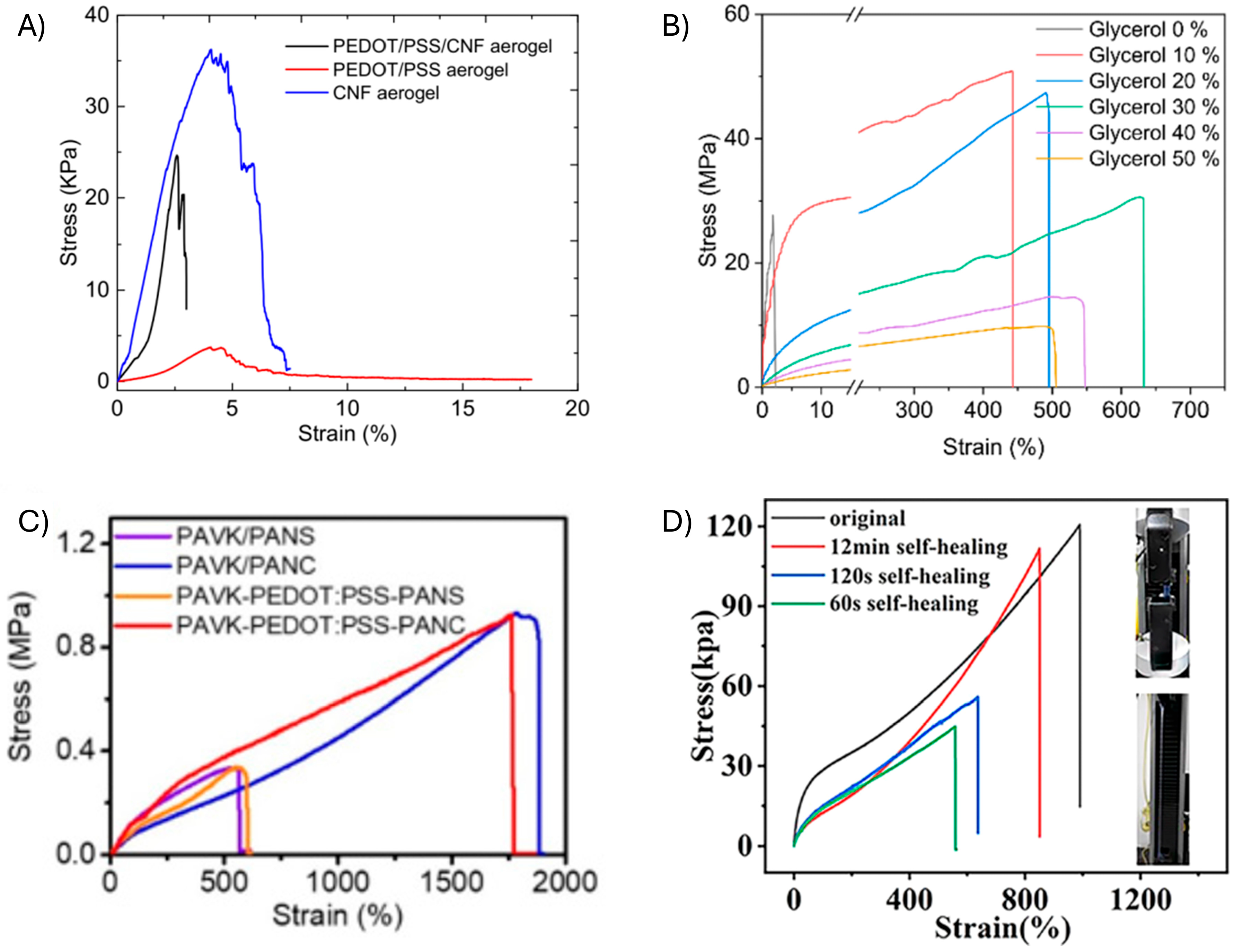
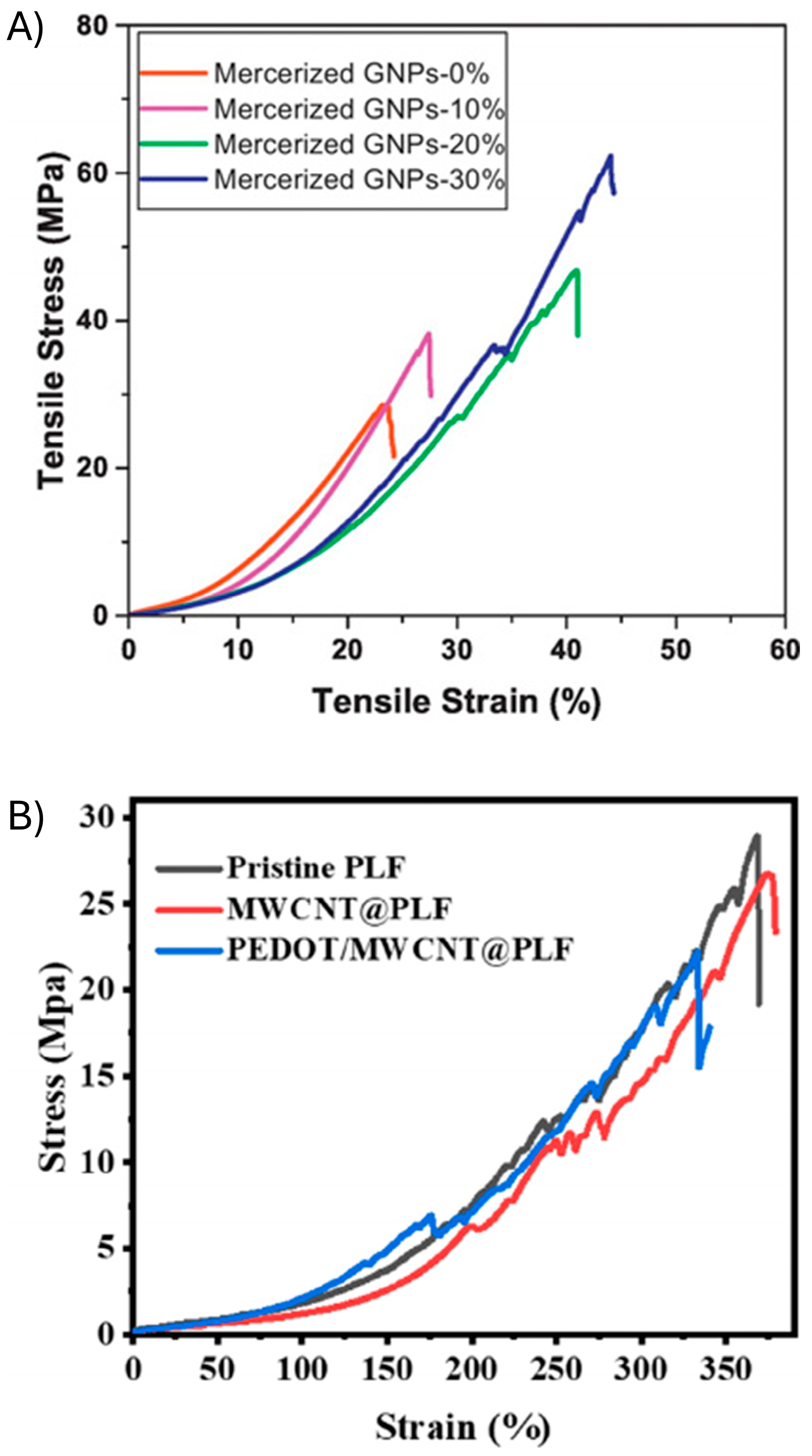

| Sensor Composition 1 | Elastomer/PEDOT Integration | Elastic Modulus | Elongation at Break (Eb) | Tensile Strength | Ref. |
|---|---|---|---|---|---|
| PEDOT:PSS/Zonyl/PDMS | PDMS sensor fabricated by blending | ~6.8 MPa | 5–15% | 5–25 MPa | [22] |
| PEDOT-PSS/PDMS | PDMS sensor fabricated by coating/printing | - | 150% | 1.43 ± 0.03% MPa | [97] |
| PEDOT:PSS/Tween/PDMS | PDMS sensor fabricated by blending | - | 75% | 0.8 MPa | [96] |
| PAVK-PEDOT:PSS-PANS/PANC bilayered hydrogel | Sustainable sensor fabricated by blending | 70 ± 8 kPa | 1800 ± 200% | 0.92 ± 0.08 MPa | [83] |
| PEDOT:PSS/HEC films | Sustainable sensor fabricated by coating | - | ~100% | - | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoah, C.; Skene, W.G. Survey of Sustainable Wearable Strain Sensors Enabled by Biopolymers and Conductive Organic Polymers. Gels 2025, 11, 235. https://doi.org/10.3390/gels11040235
Amoah C, Skene WG. Survey of Sustainable Wearable Strain Sensors Enabled by Biopolymers and Conductive Organic Polymers. Gels. 2025; 11(4):235. https://doi.org/10.3390/gels11040235
Chicago/Turabian StyleAmoah, Cephas, and W. G. Skene. 2025. "Survey of Sustainable Wearable Strain Sensors Enabled by Biopolymers and Conductive Organic Polymers" Gels 11, no. 4: 235. https://doi.org/10.3390/gels11040235
APA StyleAmoah, C., & Skene, W. G. (2025). Survey of Sustainable Wearable Strain Sensors Enabled by Biopolymers and Conductive Organic Polymers. Gels, 11(4), 235. https://doi.org/10.3390/gels11040235









