Eco-Friendly Conductive Hydrogels: Towards Green Wearable Electronics
Abstract
1. Introduction
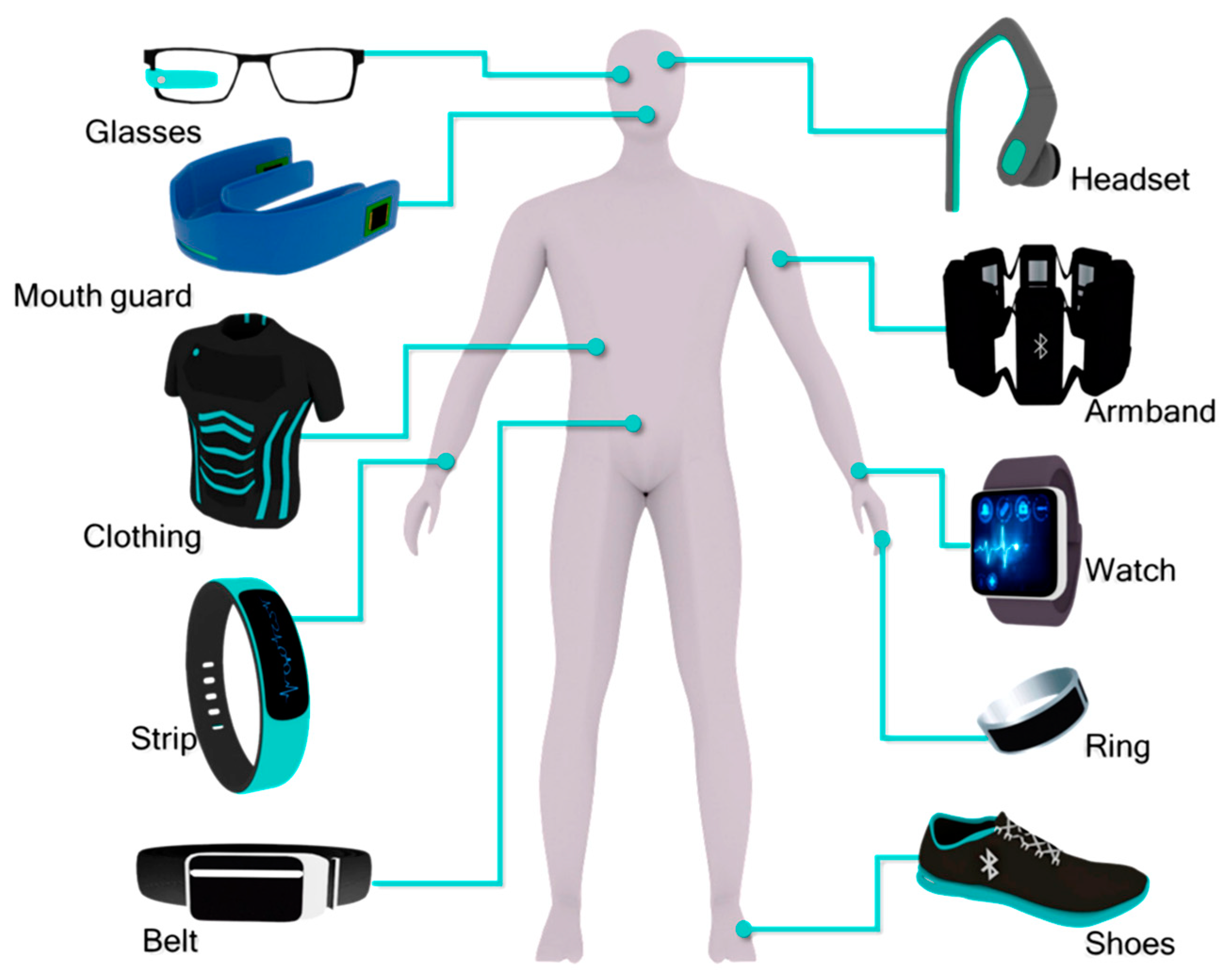
1.1. Overview of Wearable Electronics (WE)
1.2. Importance of Conductive Hydrogels (CHG)
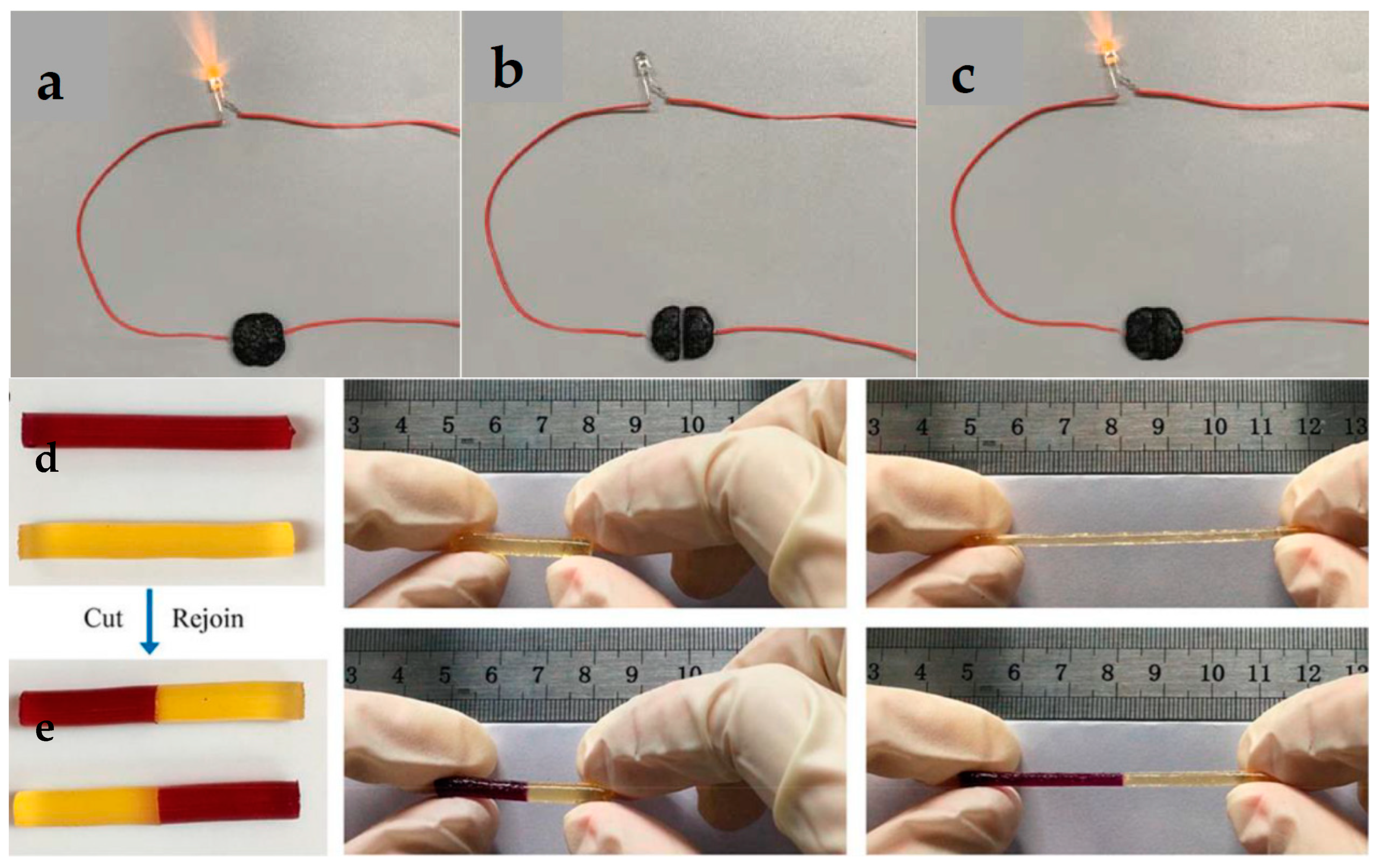
- (I)
- Stability against dehydration: dehydration reduces hydrogel conductivity and flexibility, affecting long-term performance. Solutions include water retention strategies: incorporating hydrophilic polymers and humectants, such as glycerol, polyethylene glycol, or sorbitol, to retain moisture by forming hydrogen bonds with water molecules or ionic liquids (e.g., choline chloride-based) that prevent water evaporation while maintaining ionic conductivity; crosslinking strategies for water binding, as double-network hydrogels that combine covalent and physical crosslinking (e.g., polyacrylamide-alginate) to trap water within a stable matrix, e.g., polyacrylamide-clay hydrogels prevent water loss while maintaining flexibility [65]; introduction of hydrophobic domains (e.g., fluorinated or silicone-based groups) to reduce evaporation; surface coatings or encapsulation by protective layers (e.g., mussel-inspired polydopamine or zwitterionic coatings) to minimize water loss; elastomer encapsulation by thin layers of silicone allow flexibility while limiting dehydration [66].
- (II)
- Scalability for mass production: large-scale production must be cost-effective, energy-efficient, and repeatable to meet mass-market demands. Use of natural biopolymers, cellulose, chitosan, gelatin, and alginate-based conductive hydrogels reduce costs compared to synthetic polymers, as well as reduced reliance on costly carbon nanomaterials (CNTs, graphene) by optimizing MXenes, PEDOT:PSS, or metal nanowires. PEDOT:PSS with crosslinked biopolymers provides affordable conductivity. Use of high-throughput fabrication methods, such as 3D printing and direct ink writing; extrusion-based printing of hydrogel networks ensures precision while reducing material waste. Scalable crosslinking and polymerization by UV rapid photopolymerization and sustainable methods using enzyme-catalyzed gelation (e.g., horseradish peroxidase for silk-based hydrogels) reduce reaction time compared to traditional thermal curing [67].
- (III)
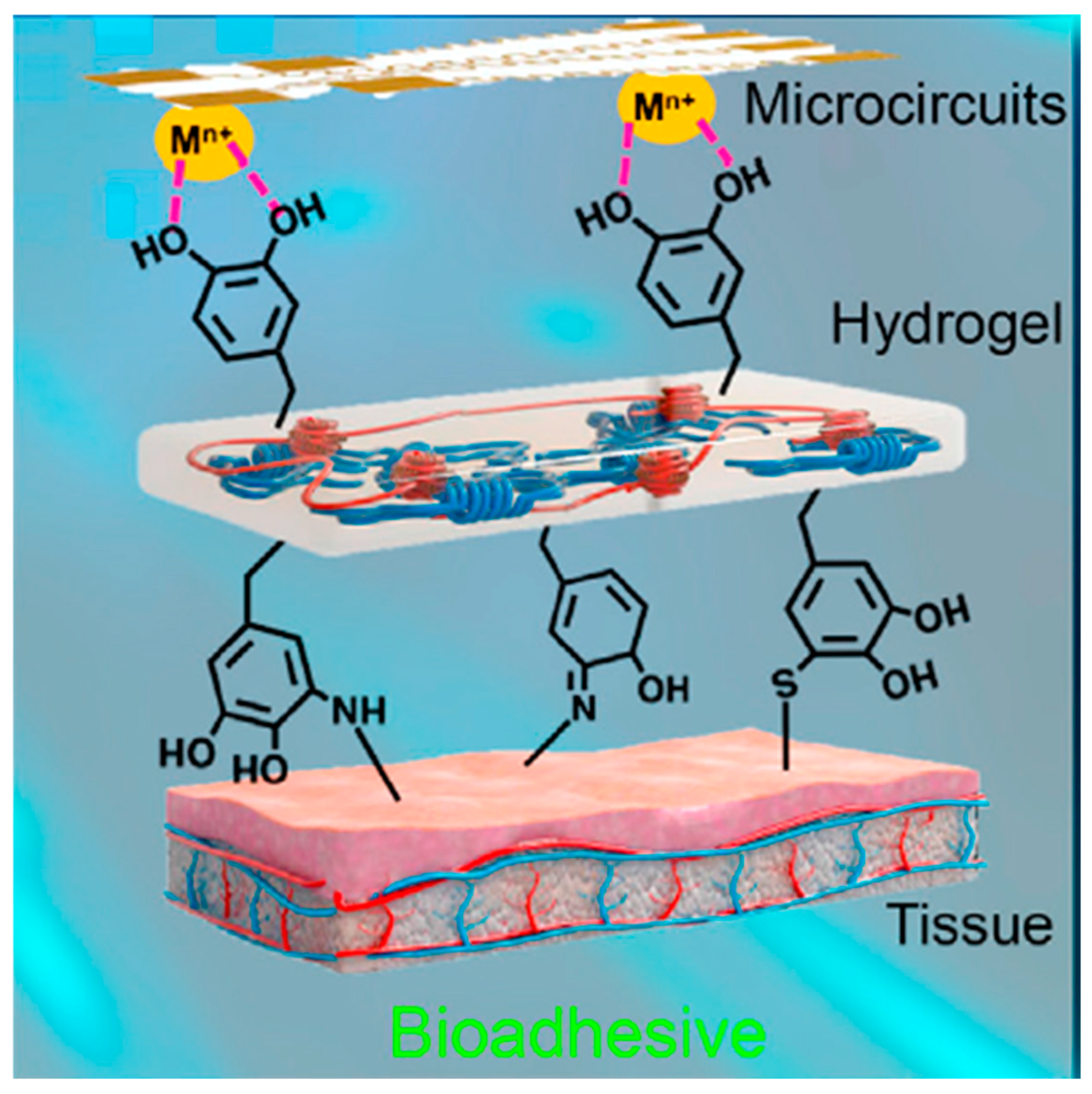
- Integration with other materials: wearable electronics require hydrogels to integrate with metals, polymers, and textiles while maintaining flexibility and conductivity. Hydrogel–elastomer blends combine hydrogels with silicone or polyurethane elastomers to improve mechanical strength [68]. Electrospun nanofiber reinforcement of hydrogels improves mechanical stability. MXenes, silver nanowires, CNTs, and graphene enhance conductivity while maintaining stretchability. Ionic liquids (e.g., LiCl, NaCl solutions) for ionically conductive hydrogels or carbon-based fillers for electronic conductivity enhance the conductivity. For better skin adhesion, mussel-inspired polydopamine coatings enhance adhesion between hydrogel and stretchable electronics [69]. Interpenetrating polymer networks enhance compatibility with soft substrates like textiles and silicone.
2. Material Components of Eco-Friendly CHG
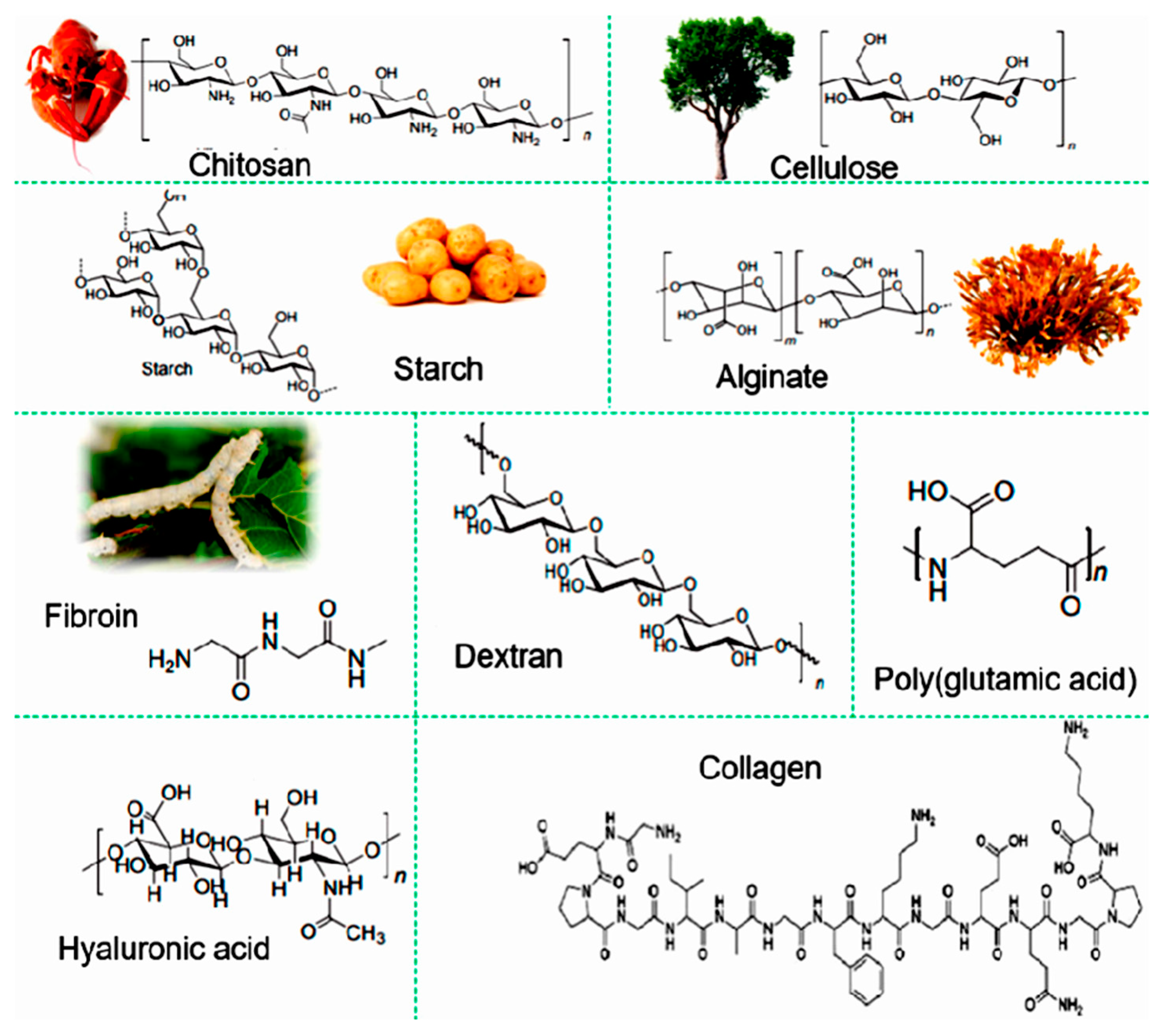
2.1. Natural Polymers
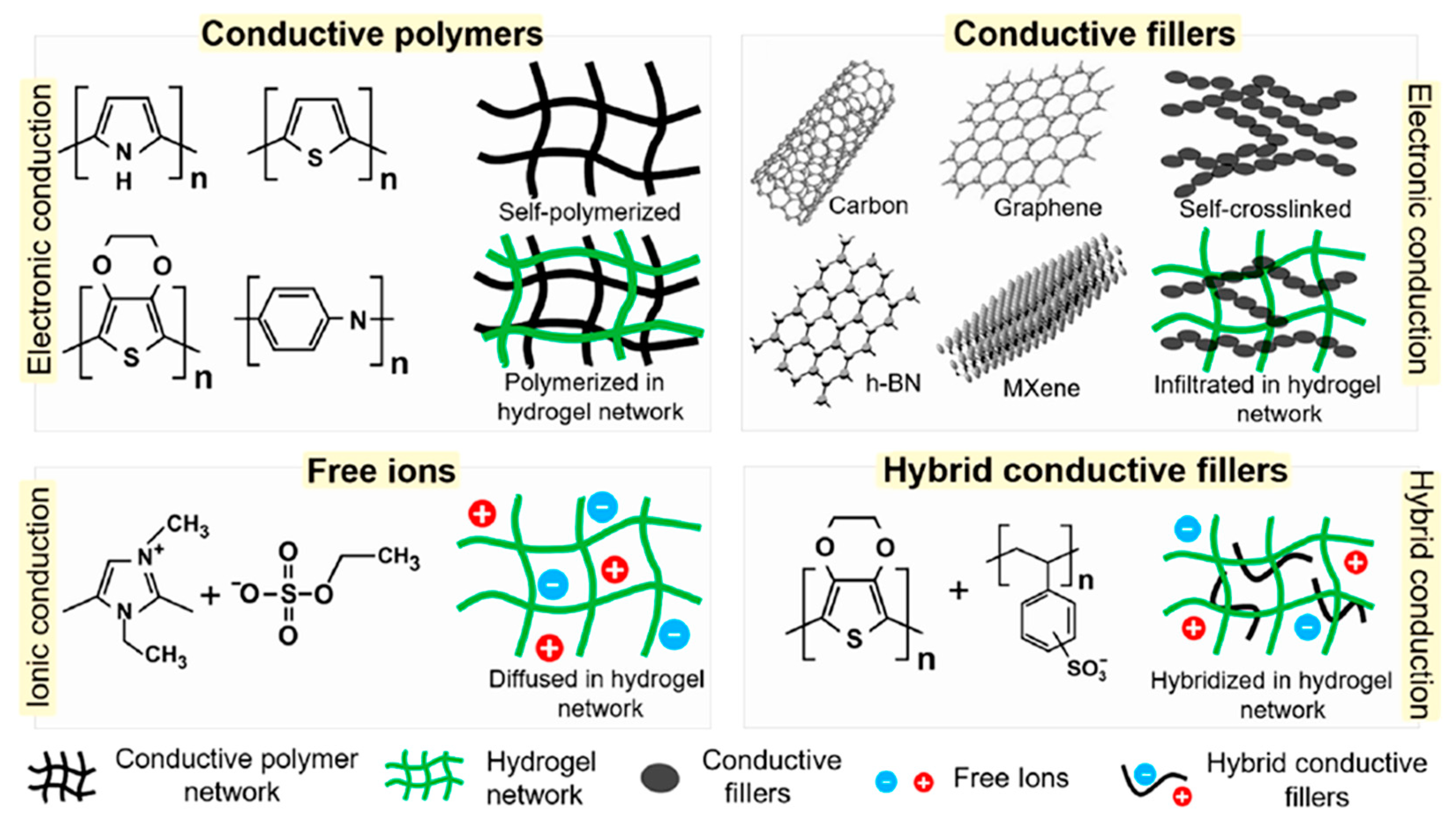
2.2. Conductive Materials for Natural Polymer Hydrogels
2.3. Green Crosslinkers and Additives
3. Green Synthesis Approaches
3.1. Solvent-Free and Aqueous Synthesis Methods
3.2. Biocompatible Crosslinking Techniques
| Method | Material/ Agent Used | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Chemical crosslinking | Genipin Tannic acid Citric acid Caffeic acid | Non-toxicity, sustainability, bioavailability. | Requires high concentrations of agents. Insufficient mechanical strength. | [146] |
| Ionic crosslinking | Ca2+, Cu2+, Fe3+, Ag+ | Forms stable 3D interconnected hydrogel structures and extremely robust. High mechanical strength and stability, antibacterial properties drug delivery purposes. | CaCl2 leads to rapid and difficult to control gelation. CaSO4 and CaCO3 reduce the gelation rate and increase the working time. Limited long-term stability under physiological conditions. | [147] |
| Thermal crosslinking | Polyvinyl alcohol, agarose, gelatin | Formation of the 3D network in a single step, in situ. Thermal and chemical stabilities of network structure. | Weak viscoelasticity. Easily degradable Poor mechanical properties. | [153] |
| Photo- polymerization | UV light | Rapid in situ crosslinking. Elimination of toxic agents. | Intricate alteration procedure. Storage conditions impact variability. Prone to bacterial contamination. | [150,151] |
| Freeze–thaw crosslinking | Polysaccharides, polyvinyl alcohol | No organic solvents and toxically crosslinking agents. Tunable structural, mechanical, biological properties. | Challenging uniform mixing of the initial polymer solution. Deterioration of mechanical properties. Uneven pore formation. Weak spots, fractures, or cracks. | [152] |
| Enzymatic crosslinking | Transglutaminase, Laccase, Peroxidase, Tyrosinase | Decreases food allergenicity by eliminating organic solvents and toxic crosslinking agents. | Limited broad-spectrum substrate. | [154,155,156] |
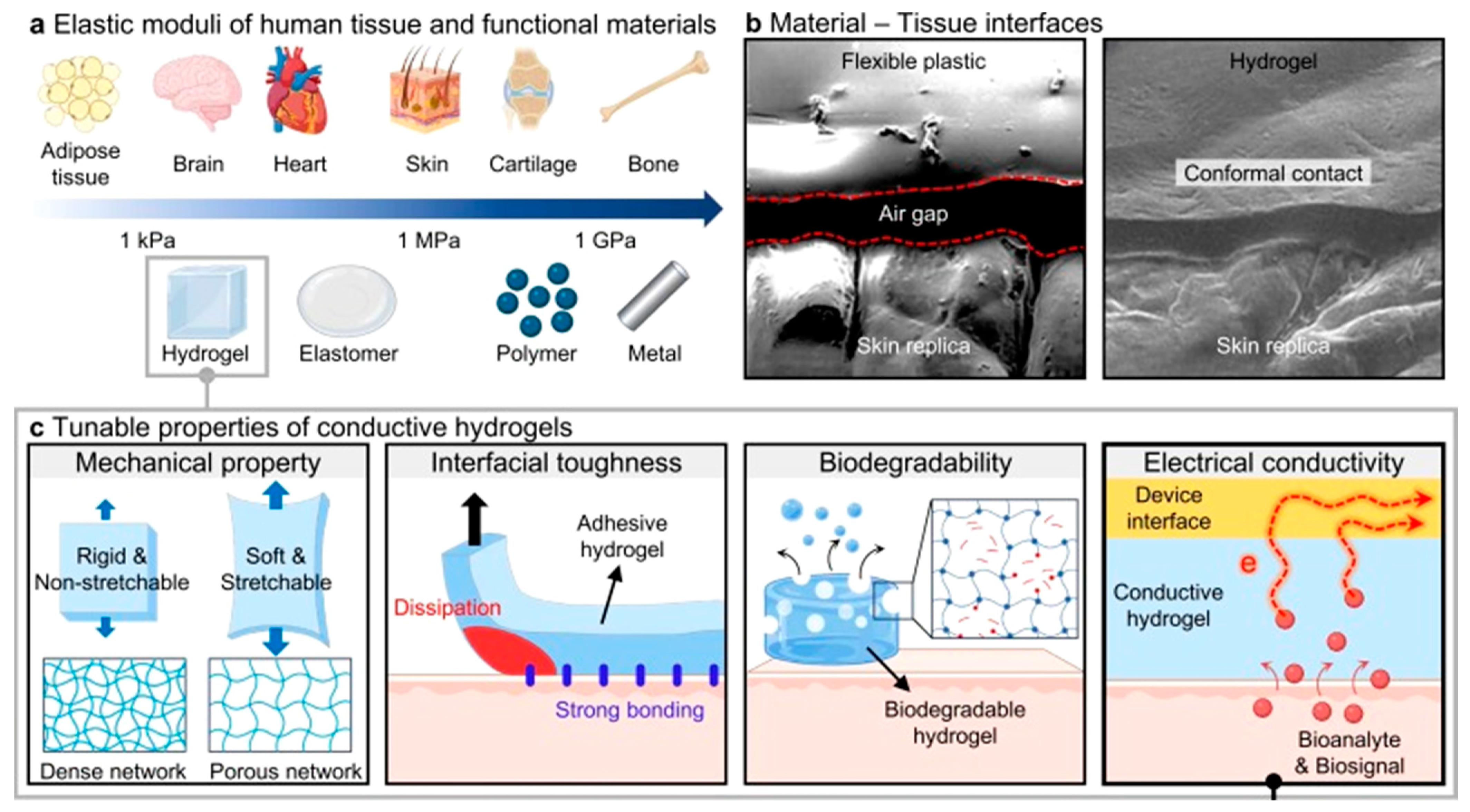
4. Functional Properties of Eco-Friendly CHG for WE
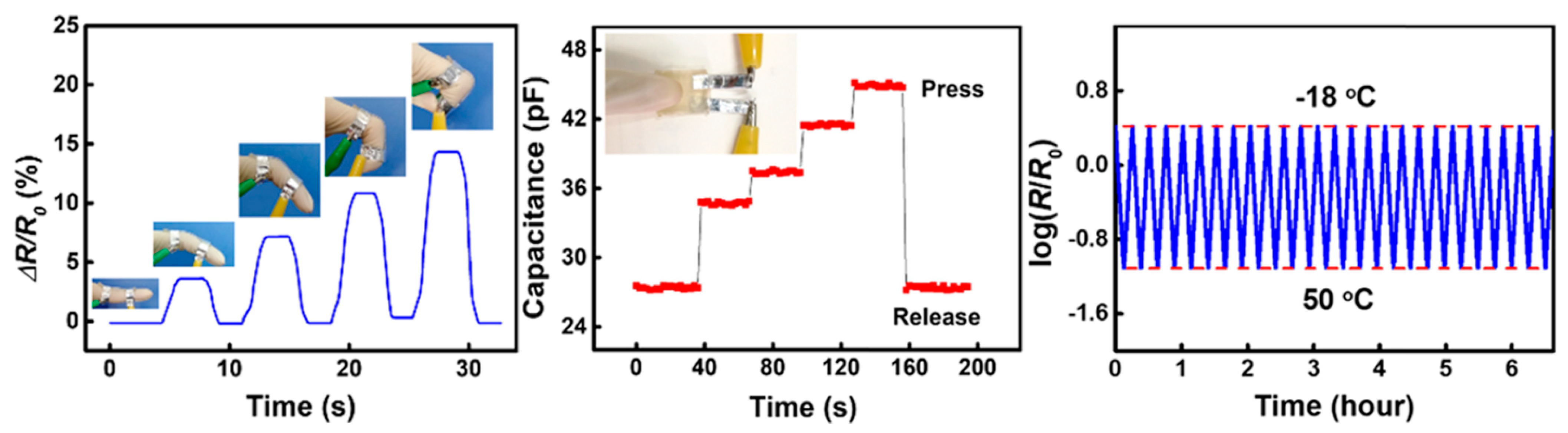
4.1. Electrical Conductivity
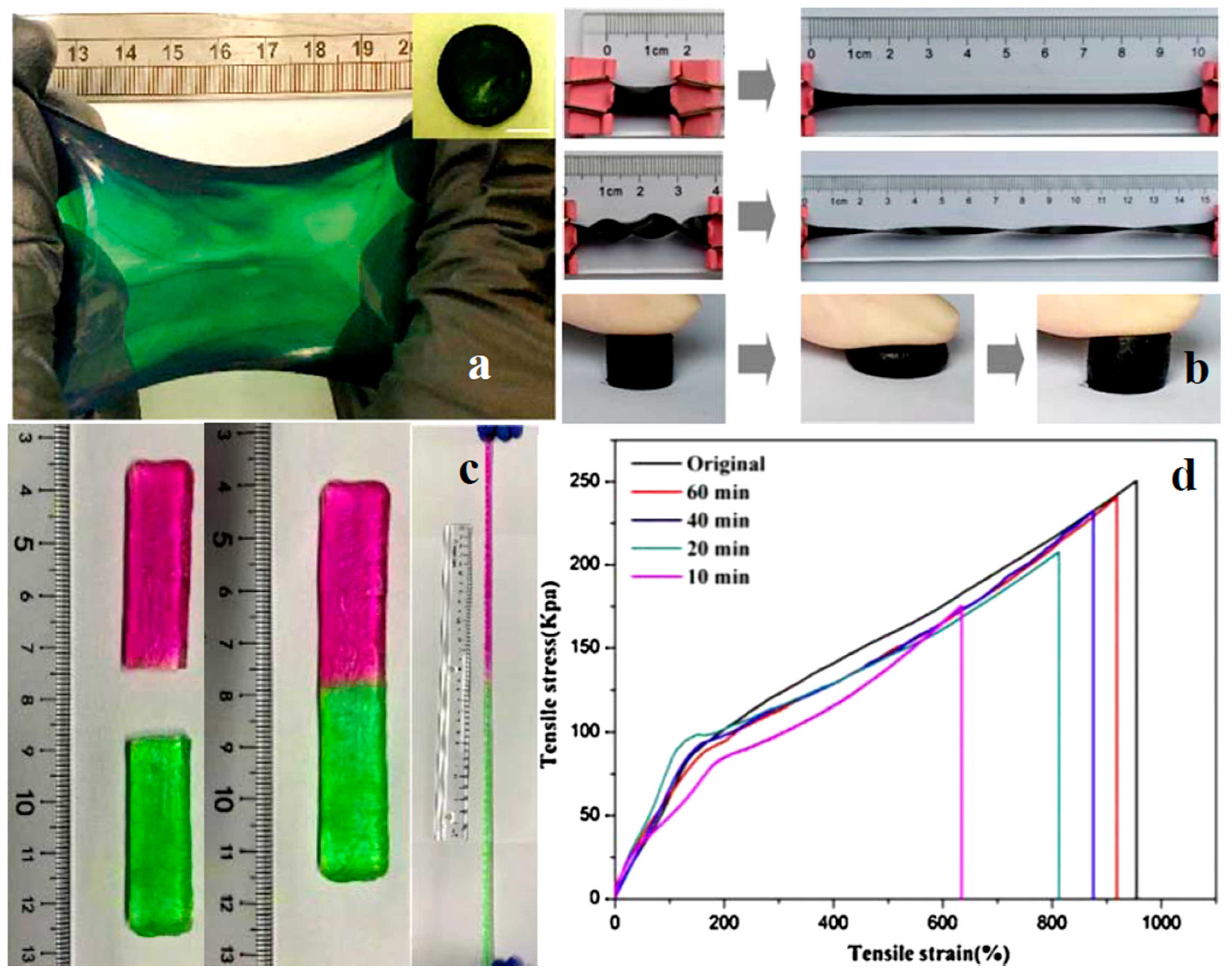
4.2. Mechanical Properties and Stretchability
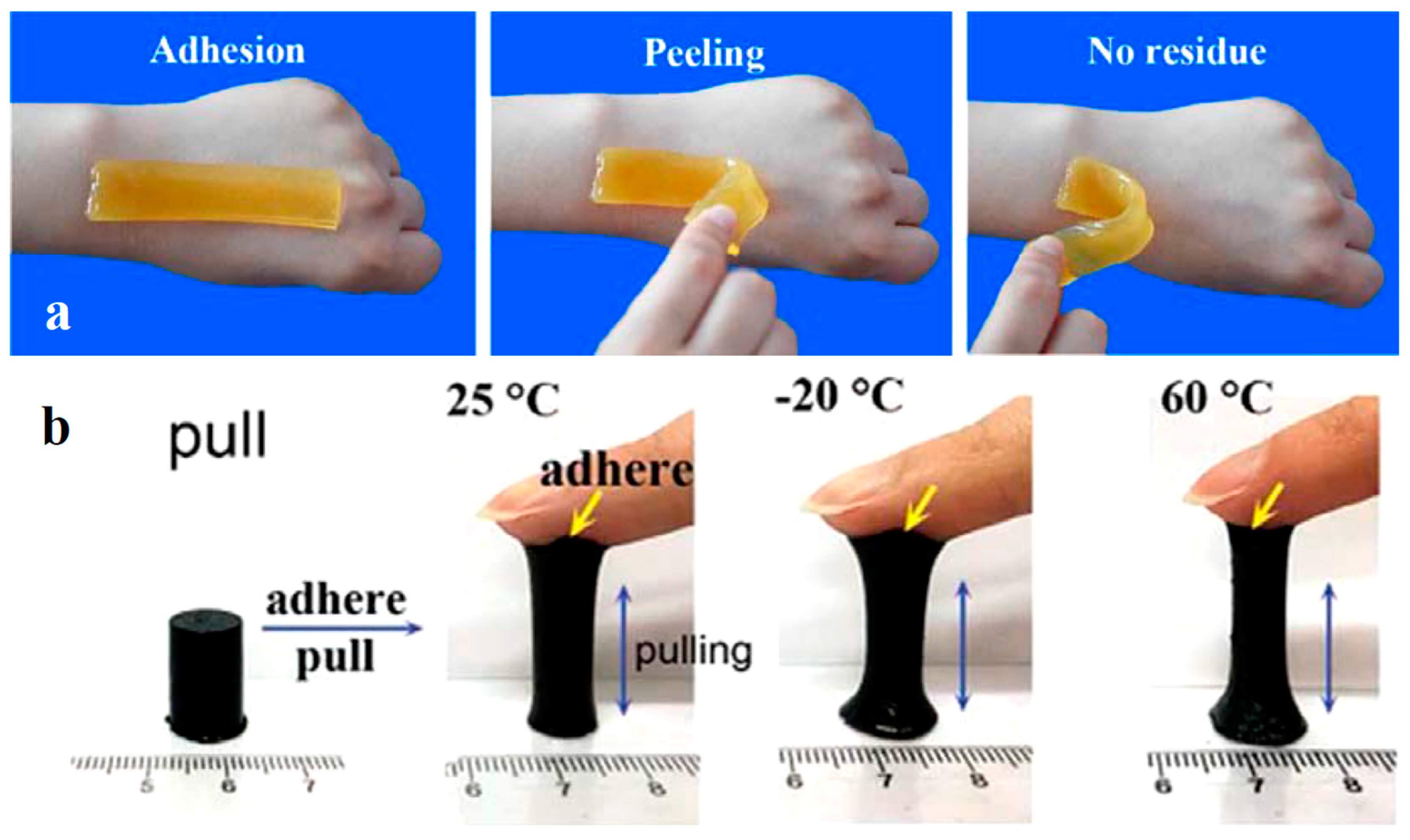
4.3. Skin-Friendliness
4.4. Swelling Behavior and Stimuli Responsive Properties
5. Applications in WE for Healthcare
5.1. ECG Sensors
5.2. EEG Sensors
5.3. Electromyogram (EMG) Sensors
5.4. Glucose Monitoring
5.5. Sweat Analysis
5.6. Soft Pressure and Motion Sensors
5.7. Temperature and Thermal Therapy Monitoring
5.8. Smart Bandages
5.9. Electronic Skin
5.10. UV and Pollution Sensors
5.11. Breath Sensors and Toxin Detection
6. Challenges and Limitations
6.1. Trade-Offs Between Performance and Ecofriendliness
6.2. Scalability of Green Synthesis Methods
- Many green synthesis methods depend on natural polymers (e.g., cellulose, chitosan, alginate) or bio-derived conductive materials (e.g., carbon nanodots, polydopamine). Ensuring a stable supply chain for biodegradable and renewable raw materials with consistent properties is critical for scalability. Variability in natural sources, extraction efficiency, and purity can lead to inconsistencies in hydrogel properties, affecting performance and reproducibility.
- Adapting and scaling low-energy, cost-effective fabrication techniques that maintain performance while remaining eco-friendly is an ongoing challenge. Some bio-based synthesis methods still rely on harsh conditions, high temperatures, or significant water consumption, which may offset their eco-friendly advantages. Developing low-energy, water-efficient, and waste-minimizing hydrogel production processes is crucial for large-scale implementation. Sustainable synthesis approaches, such as enzyme-assisted polymerization, solvent-free processing, or supramolecular self-assembly, are difficult to scale due to their complexity and cost (e.g., longer reaction times, precise pH conditions, temperature control). Bio-based crosslinking agents (e.g., genipin, citric acid) and natural dopants may be more expensive than synthetic alternatives, limiting commercial viability.
- Adapting green hydrogel processing to existing manufacturing infrastructure (e.g., 3D printing, roll-to-roll processing) requires further optimization, due to limited industrial compatibility of eco-friendly hydrogels. Traditional hydrogel fabrication methods, such as chemical crosslinking with toxic reagents (e.g., glutaraldehyde), are well-established for mass production. Green alternatives, such as photo-crosslinking, ionic crosslinking, or biodegradable linkers, often result in weaker mechanical properties or slower gelation rates.
6.3. Durability and Long-Term Stability
7. Future Directions and Opportunities
7.1. Integration of Multi-Functional Materials
7.2. Advances in Recycling and Reusability and Potential for Circular Economies in WE
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHG | Conductive Hydrogels |
| WE | Wearable Electronics |
| CNTs | Carbon Nanotubes |
| ECG | Electrocardiogram |
| EEG | Electroencefalogram |
| PVA | Polyvinyl Alcohol |
| PPy | Polypyrrole |
| PANI | Polyaniline |
| PEDOT:PSS | Poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate) |
| EMG | Electromyogram |
| TENGs | Triboelectric Nanogenerators |
| EOG/IOP | Electrooculogram/Intraocular Pressure |
References
- Meena, J.S.; Choi, S.B.; Jung, S.-B.; Kim, J.-W. Electronic textiles: New age of wearable technology for healthcare and fitness solutions. Mater. Today Bio 2023, 19, 100565. [Google Scholar] [CrossRef]
- Vo, D.-K.; Trinh, K.T.L. Advances in Wearable Biosensors for Healthcare: Current Trends, Applications, and Future Perspectives. Biosensors 2024, 14, 560. [Google Scholar] [CrossRef]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.-K.; Jung, J. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef]
- Hua, J.; Su, M.; Sun, X.; Li, J.; Sun, Y.; Qiu, H.; Shi, Y.; Pan, L. Hydrogel-Based Bioelectronics and Their Applications in Health Monitoring. Biosensors 2023, 13, 696. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, H.; Gao, L.; Hao, R.; Shi, Y.; Yang, J.; Hao, Y.; Chen, J. Wearable hydrogel-based health monitoring systems: A new paradigm for health monitoring? Chem. Eng. J. 2024, 495, 153382. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, Y.; Zhang, Y.; Zhu, P.; Mao, Y. Recent Advances of Stretchable Nanomaterial-Based Hydrogels for Wearable Sensors and Electrophysiological Signals Monitoring. Nanomaterials 2024, 14, 1398. [Google Scholar] [CrossRef]
- Lin, A.; Xu, H.; Chen, Z.; Wearable, A.R. System for Real-Time Pedestrian Conflict Alerts Using Live Roadside Data. Electronics 2025, 14, 99. [Google Scholar] [CrossRef]
- Si, J.; Duan, R.; Zhang, M.; Liu, X. Recent Progress Regarding Materials and Structures of Triboelectric Nanogenerators for AR and VR. Nanomaterials 2022, 12, 1385. [Google Scholar] [CrossRef]
- Hu, J.; Meng, H.; Li, G.; Ibekwe, S. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21, 053001. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, H.S.; Jeong, H.; Kim, D.-H. Recent advances in conductive hydrogels for soft biointegrated electronics: Materials, properties, and device applications. Wearable Electron. 2024, 1, 255–280. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, T.; Plotka, B.; Janik, S.; Canini, L.; Mäntele, W. Evaluation of a Novel Noninvasive Blood Glucose Monitor Based on Mid-Infrared Quantum Cascade Laser Technology and Photothermal Detection. J. Diabetes Sci. Technol. 2021, 15, 6–10. [Google Scholar] [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Ankhili, A.; Tao, X.; Cochrane, C.; Coulon, D.; Koncar, V. Washable and Reliable Textile Electrodes Embedded into Underwear Fabric for Electrocardiography (ECG) Monitoring. Materials 2018, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Tseng, R.C.; Chen, C.-C.; Hsu, S.-M.; Chuang, H.-S. Contact-Lens Biosensors. Sensors 2018, 18, 2651. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Solino, C.; Lorenzo, M.D. Enzymatic Fuel Cells: Towards Self-Powered Implantable and Wearable Diagnostics. Biosensors 2018, 8, 11. [Google Scholar] [CrossRef]
- Rodrigues, D.; Barbosa, A.I.; Rebelo, R.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Skin-Integrated Wearable Systems and Implantable Biosensors: A Comprehensive Review. Biosensors 2020, 10, 79. [Google Scholar] [CrossRef]
- Zhou, B.; Sundholm, M.; Cheng, J.; Cruz, H.; Lukowicz, P. Measuring muscle activities during gym exercises with textile pressure mapping sensors. Pervasive Mob. Comput. 2017, 38, 331–345. [Google Scholar] [CrossRef]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef]
- Rodgers, M.M.; Alon, G.; Pai, V.M.; Conroy, R.S. Wearable technologies for active living and rehabilitation: Current research challenges and future opportunities. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319839607. [Google Scholar] [CrossRef]
- Kang, H.S.; Exworthy, M. Wearing the Future-Wearables to Empower Users to Take Greater Responsibility for Their Health and Care: Scoping Review. MIR mHealth uHealth 2022, 10, e35684. [Google Scholar] [CrossRef]
- Zhan, T.; Yin, K.; Xiong, J.; He, Z.; Wu, S.-T. Augmented Reality and Virtual Reality Displays: Perspectives and Challenges. iScience 2020, 23, 101397. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, H.T.; Yoo, B. Virtual Reality Sickness: A Review of Causes and Measurements. Int. J. Hum. Comput. Interact. 2020, 36, 1658–1682. [Google Scholar] [CrossRef]
- Dopsaj, M.; Tan, W.; Perovic, V.; Stajic, Z.; Milosavljevic, N.; Paessler, S.; Makishima, T. Novel neurodigital interface reduces motion sickness in virtual reality. Neurosci. Lett. 2024, 825, 137692. [Google Scholar] [CrossRef] [PubMed]
- Canali, S.; Schiaffonati, V.; Aliverti, A. Challenges and recommendations for wearable devices in digital health: Data quality, interoperability, health equity, fairness. PLOS Digit. Health. 2022, 1, e0000104. [Google Scholar] [CrossRef]
- Shih, J.J.; Krusienski, D.J.; Wolpaw, J.R. Brain-computer interfaces in medicine. Mayo Clin. Proc. 2012, 87, 268–279. [Google Scholar] [CrossRef]
- Abdulkader, S.N.; Atia, A.; Mostafa, M.-S.M. Brain computer interfacing: Applications and challenges. Egypt. Inform. J. 2015, 16, 213–230. [Google Scholar] [CrossRef]
- Kawala-Sterniuk, A.; Browarska, N.; Al-Bakri, A.; Pelc, M.; Zygarlicki, J.; Sidikova, M.; Martinek, R.; Gorzelanczyk, E.J. Summary of over Fifty Years with Brain-Computer Interfaces—A Review. Brain Sci. 2021, 11, 43. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Liu, B.; Liang, L.; Wang, Y.; Gao, S.; Gao, X. Signal acquisition of brain–computer interfaces: A medical-engineering crossover perspective review. Fundam. Res. 2025, 5, 3–16. [Google Scholar] [CrossRef]
- An, Y.; Wong, J.; Ling, S.H. Development of real-time brain-computer interface control system for robot. Appl. Soft Comput. 2024, 159, 111648. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.-Z.; Yuan, M. Requirements, challenges, and novel ideas for wearables on power supply and energy harvesting. Nano Energy 2023, 115, 108715. [Google Scholar] [CrossRef]
- Zheng, C.; Yue, Y.; Gan, L.; Xu, X.; Mei, C.; Han, J. Highly Stretchable and Self-Healing Strain Sensors Based on Nanocellulose-Supported Graphene Dispersed in Electro-Conductive Hydrogels. Nanomaterials 2019, 9, 937. [Google Scholar] [CrossRef]
- Costa, J.C.; Spina, F.; Lugoda, P.; Garcia-Garcia, L.; Roggen, D.; Münzenrieder, N. Flexible Sensors—From Materials to Applications. Technologies 2019, 7, 35. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Mariatti, M. Printed graphene and hybrid conductive inks for flexible, stretchable, and wearable electronics: Progress, opportunities, and challenges. J. Sci. Adv. Mater. Devices 2022, 7, 100435. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y. Flexible, Stretchable Sensors for Wearable Health Monitoring: Sensing Mechanisms, Materials, Fabrication Strategies and Features. Sensors 2018, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.G.; Silva, A.P.; Nunes-Pereira, J. Current On-Skin Flexible Sensors, Materials, Manufacturing Approaches, and Study Trends for Health Monitoring: A Review. ACS Sens. 2024, 9, 1104–1133. [Google Scholar] [CrossRef]
- Ali, A.; Shaukat, H.; Bibi, S.; Altabey, W.A.; Noori, M.; Kouritem, S.A. Recent progress in energy harvesting systems for wearable technology. Energy Strategy Rev. 2023, 49, 101124. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Chen, X. Supercapacitors for renewable energy applications: A review. Micro Nano Eng. 2023, 21, 100229. [Google Scholar] [CrossRef]
- Devi, D.H.; Duraisamy, K.; Armghan, A.; Alsharari, M.; Aliqab, K.; Sorathiya, V.; Das, S.; Rashid, N. 5G Technology in Healthcare and Wearable Devices: A Review. Sensors 2023, 23, 2519. [Google Scholar] [CrossRef] [PubMed]
- LaBoone, P.A.; Marques, O. Overview of the future impact of wearables and artificial intelligence in healthcare workflows and technology. Int. J. Inf. Manag. Data Insights 2024, 4, 100294. [Google Scholar] [CrossRef]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G.; et al. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef]
- Fernández-Caballero, A.; Martínez-Rodrigo, A.; Pastor, J.M.; Castillo, J.C.; Lozano-Monasor, E.; López, M.T.; Zangróniz, R.; Latorre, J.M.; Alicia Fernández-Sotos, A. Smart environment architecture for emotion detection and regulation. J. Biomed. Inform. 2016, 64, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Cilliers, L. Wearable devices in healthcare: Privacy and information security issues. Health Inf. Manag. 2020, 49, 150–156. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Srivastava, G.; Dhar, S.; Singh, R. A Decentralized Privacy-Preserving Healthcare Blockchain for IoT. Sensors 2019, 19, 326. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.J.; Fernandes, C.I.; Rammal, H.G.; Veiga, P.M. Wearable technology and consumer interaction: A systematic review and research agenda. Comput. Hum. Behav. 2021, 118, 106710. [Google Scholar] [CrossRef]
- Imani, K.B.C.; Dodda, J.M.; Yoon, J.; Torres, F.G.; Imran, A.B.; Deen, G.R.; Al-Ansari, R. Seamless Integration of Conducting Hydrogels in Daily Life: From Preparation to Wearable Application. Adv. Sci. 2024, 11, 2306784. [Google Scholar] [CrossRef]
- Thirumalai, D.; Santhamoorthy, M.; Kim, S.-C.; Lim, H.-R. Conductive Polymer-Based Hydrogels for Wearable Electrochemical Biosensors. Gels 2024, 10, 459. [Google Scholar] [CrossRef]
- He, Q.; Cheng, Y.; Deng, Y.; Wen, F.; Lai, Y.; Li, H. Conductive Hydrogel for Flexible Bioelectronic Device: Current Progress and Future Perspective. Adv. Funct. Mater. 2024, 34, 2308974. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ohm, Y.; Liao, J.; Luo, Y.; Cheng, H.Y.; Won, P.; Roberts, P.; Carneiro, M.R.; Islam, M.F.; Ahn, J.H.; et al. A self-healing electrically conductive organogel composite. Nat. Electron. 2023, 6, 206–215. [Google Scholar]
- An, Y.H.; Lee, J.; Son, D.U.; Kang, D.H.; Park, M.J.; Cho, K.W.; Kim, S.; Kim, S.H.; Ko, J.; Jang, M.H.; et al. Facilitated transdermal drug delivery using nanocarriers-embedded electroconductive hydrogel coupled with reverse electrodialysis-driven iontophoresis. ACS Nano 2020, 14, 4523–4535. [Google Scholar] [CrossRef]
- Wang, L.; Xu, T.; Zhang, X. Multifunctional conductive hydrogel-based flexible wearable sensors. TrAC Trends Anal. Chem. 2021, 134, 116130. [Google Scholar] [CrossRef]
- Lim, C.; Hong, Y.J.; Jung, J.; Shin, Y.; Sunwoo, S.H.; Baik, S.; Park, O.K.; Choi, S.H.; Hyeon, T.; Kim, J.H.; et al. Tissue-like skin-device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels. Sci. Adv. 2021, 7, eabd3716. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Dervin, S.; Ganguly, P.; Dahiya, R. Biodegradable Materials for Sustainable Health Monitoring Devices. ACS Appl. Bio Mater. 2021, 4, 163–194. [Google Scholar] [CrossRef]
- Devi, V.K.A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Taylor, D.L.; in H$et Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Y.; Poisson, J.; He, Y.; Zhang, H.; Zhang, Y.; Bao, Y.; Chen, S.; Chen, Y.M.; Zhang, K. Recent Advances in Biopolymer-Based Hydrogel Electrolytes for Flexible Supercapacitors. ACS Energy Lett. 2024, 9, 1803–1825. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Chen, J.; Lee, P.S. Sustainable wearable energy storage devices self-charged by human-body bioenergy. Susmat 2021, 1, 285–302. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Li, Y.; Chao, M.; Li, M.; Wan, P.; Zhang, L. Healable, Degradable, and Conductive MXene Nanocomposite Hydrogel for Multifunctional Epidermal Sensors. ACS Nano 2021, 15, 7765–7773. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, X.; Wang, S.; Yuan, L.; Ge, L.; Li, D.; Mu, C. Facile Fabrication of Biocompatible Gelatin-Based Self-Healing Hydrogels. ACS Appl. Polym. Mater. 2019, 1, 1350–1358. [Google Scholar] [CrossRef]
- Lv, H.; Liang, Q.; He, Y.; Liang, H.; Xiang, S.; Yuan, W.; Li, S.; Hong, J.; Wu, J.; Zhao, L.; et al. A transparent, moisturizing, antibacterial and mechanically flexible hydrogel for emergency conservation of unearthed bone relics. Eur. Polym. J. 2025, 222, 113590. [Google Scholar] [CrossRef]
- Macron, J.; Gerratt, A.P.; Lacour, S.P. Thin Hydrogel–Elastomer Multilayer Encapsulation for Soft Electronics. Adv. Mater. Technol. 2019, 4, 1900331. [Google Scholar] [CrossRef]
- Yu, C.; Schimelman, J.; Wang, P.; Miller, K.L.; Ma, X.; You, S.; Guan, J.; Sun, B.; Zhu, W.; Chen, S. Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications. Chem. Rev. 2020, 120, 10695–10743. [Google Scholar] [CrossRef]
- Meng, L.; He, J.; Pan, C. Research Progress on Hydrogel–Elastomer Adhesion. Materials 2022, 15, 2548. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Choi, G.; Veerla, S.C.; Kim, S.; Kim, J.; Lee, H.J.; Kuzhiumparambil, U.; Ralph, P.J.; Yeo, J.; Jeong, H.E. Mussel-inspired resilient hydrogels with strong skin adhesion and high-sensitivity for wearable device. Nano Converg. 2024, 11, 12. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Zhou, J.; Zhao, C.; Li, J.; Wang, H. Conductive and Eco-friendly Biomaterials-based Hydrogels for Noninvasive Epidermal Sensors: A Review. ACS Biomater. Sci. Eng. 2024, 10, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Banitaba, S.N.; Khademolqorani, S.; Jadhav, V.V.; Chamanehpour, E.; Mishra, Y.K.; Mostafavi, E.; Kaushik, A. Recent progress of bio-based smart wearable sensors for healthcare applications. Mater. Today Electron. 2023, 5, 100055. [Google Scholar] [CrossRef]
- Cui, C.; Fu, Q.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Recent Progress in Natural Biopolymers Conductive Hydrogels for Flexible Wearable Sensors and Energy Devices: Materials, Structures, and Performance. ACS Appl. Bio Mater. 2021, 4, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: New biosynthesis methods, recent advances, and emerging applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef]
- Xie, F.; Gao, C.; Avérous, L. Alginate-based materials: Enhancing properties through multiphase formulation design and processing innovation. Mater. Sci. Eng. R Rep. 2024, 159, 100799. [Google Scholar] [CrossRef]
- Le, C.V.; Yoon, H. Advances in the Use of Conducting Polymers for Healthcare Monitoring. Int. J. Mol. Sci. 2024, 25, 1564. [Google Scholar] [CrossRef]
- Dulal, M.; Afroj, S.; Ahn, J.; Cho, Y.; Carr, C.; Kim, I.D.; Karim, N. Toward Sustainable Wearable Electronic Textiles. ACS Nano 2022, 16, 19755–19788. [Google Scholar] [CrossRef]
- Berradi, A.; Aziz, F.; Achaby, M.E.; Ouazzani, N.; Mandi, L. A Comprehensive Review of Polysaccharide-Based Hydrogels as Promising Biomaterials. Polymers 2023, 15, 2908. [Google Scholar] [CrossRef]
- Trache, D.; Thakur, V.K.; Boukherroub, R. Cellulose Nanocrystals/Graphene Hybrids—A Promising New Class of Materials for Advanced Applications. Nanomaterials 2020, 10, 1523. [Google Scholar] [CrossRef]
- Wang, H.; Biswas, S.K.; Zhu, S.; Lu, Y.; Yue, Y.; Han, J.; Xu, X.; Wu, Q.; Xiao, H. Self-Healable Electro-Conductive Hydrogels Based on Core-Shell Structured Nanocellulose/Carbon Nanotubes Hybrids for Use as Flexible Supercapacitors. Nanomaterials 2020, 10, 112. [Google Scholar] [CrossRef]
- Tong, R.; Chen, G.; Tian, J.; He, M. Highly Stretchable, Strain-Sensitive, and Ionic-Conductive Cellulose-Based Hydrogels for Wearable Sensors. Polymers 2019, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wang, Z.; Shen, Y.; Tang, L.; Zhang, P.; Wang, Y.; Chen, Y.; Huang, B.; Lu, B. Natural Skin-Inspired Versatile Cellulose Biomimetic Hydrogels. J. Mater. Chem. A 2019, 7, 26442–26455. [Google Scholar] [CrossRef]
- Xie, F. Natural polymer starch-based materials for flexible electronic sensor development: A review of recent progress. Carbohydr. Polym. 2024, 337, 122116. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M.; Popa, M.; Calderon Moreno, J.M. Chitosan Hydrogels for Water Purification Applications. Gels 2023, 9, 664. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, S.; Pramanik, N. Chitosan biopolymer and its composites: Processing, properties and applications—A comprehensive review. Hybrid. Adv. 2024, 6, 100265. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.; Qin, Z.; Che, P.; Yi, X.; Yu, Q.; Zhang, H.; Sun, X.; Yao, F.; Li, J. Fully physically crosslinked pectin-based hydrogel with high stretchability and toughness for biomedical application. Int. J. Biol. Macromol. 2020, 149, 707–716. [Google Scholar] [CrossRef]
- Roman-Benn, A.; Contador, C.A.; Li, M.-W.; Lam, H.-M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An overview of sources, extraction and applications in food products, biomedical, pharmaceutical and environmental issues. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef]
- He, F.; You, X.; Gong, H.; Yang, Y.; Bai, T.; Wang, W.; Guo, W.; Liu, X.; Ye, M. Stretchable, Biocompatible, and Multifunctional Silk Fibroin-Based Hydrogels toward Wearable Strain/Pressure Sensors and Triboelectric Nanogenerators. ACS Appl. Mater. Interfaces 2020, 12, 6442–6450. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 10, 1287–1301. [Google Scholar] [CrossRef]
- Shi, Z.; Gao, X.; Ullah, M.W.; Li, S.; Wang, Q.; Yang, G. Electroconductive natural polymer-based hydrogels. Biomaterials 2016, 111, 40–54. [Google Scholar] [CrossRef]
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with electrically conductive nanomaterials for biomedical applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.-A.; Maiti, T.K.; et al. Electroconductive multi-functional polypyrrole composites for biomedical applications. Appl. Mater. Today 2021, 24, 101117. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Mansour Lakouraj, M.; Mohseni, M. Biodegradable polypyrrole/dextrin conductive nanocomposite: Synthesis, characterization, antioxidant and antibacterial activity. Synth. Met. 2014, 187, 9–16. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.V.; Lee, S.; Lee, D.; Le, T.-H. Recent Developments and Implementations of Conductive Polymer-Based Flexible Devices in Sensing Applications. Polymers 2022, 14, 3730. [Google Scholar] [CrossRef]
- Goswami, S.; Nandy, S.; Fortunato, E.; Martins, R. Polyaniline and its composites engineering: A class of multifunctional smart energy materials. J. Solid. State Chem. 2023, 317A, 123679. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Song, X.; Du, W.; Zhao, X.; Zhang, D. Review on Carbon/Polyaniline Hybrids: Design and Synthesis for Supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Patel, M.; Koh, W.-G. Incorporation of Conductive Materials into Hydrogels for Tissue Engineering Applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Sun, X.; Yu, Q.; Zhang, H.; Wu, X.; Yao, M.; Liu, W.; Yao, F.; Li, J. Carbon Nanotubes/Hydrophobically Associated Hydrogels as Ultrastretchable, Highly Sensitive, Stable Strain, and Pressure Sensors. ACS Appl. Mater. Interfaces 2020, 12, 4944–4953. [Google Scholar] [CrossRef]
- Yameen, M.Z.; Naqvi, S.R.; Juchelková, D.; Khan, M.N.A. Harnessing the power of functionalized biochar: Progress, challenges, and future perspectives in energy, water treatment, and environmental sustainability. Biochar 2024, 6, 25. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Zhang, Q.; Zheng, S.; Zhang, Z.; Chen, S.; Wang, Z.; Zhang, D. Ionic conducting hydrogels as biomedical materials: Classification, design strategies, and skin tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2024, 1–24. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Xu, G.; Wang, Q.; Liu, S.; Li, J. A dynamic gel with reversible and tunable topological networks and performances. Matter 2020, 2, 390–403. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Zhou, W.; Han, X.; Yao, Y.; Wong, C.P. Realizing an All-Round Hydrogel Electrolyte toward Environmentally Adaptive Dendrite-Free Aqueous Zn-MnO2 Batteries. Adv. Mater. 2021, 33, e2007559. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Yoo, J.S.; Kim, Y.J.; Kim, S.H.; Choi, S.H. Study on genipin: A new alternative natural crosslinking agent for fixing heterograft tissue. Korean J. Thorac. Cardiovasc. Surg. 2011, 44, 197–207. [Google Scholar] [CrossRef]
- Khezerlou, A.; Sani, M.A.; Rhim, J.-W. Genipin crosslinked polysaccharide packaging films: An eco-friendly and innovative strategy to improve the performance of food packaging materials. Carbohydr. Polym. Technol. Appl. 2025, 9, 100732. [Google Scholar] [CrossRef]
- Ravishankar, K.; Dhamodharan, R. Advances in chitosan-based hydrogels: Evolution from covalently crosslinked systems to ionotropically crosslinked superabsorbents. React. Funct. Polym. 2020, 149, 104517. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Ezati, P.; Yang, D.-P.; Rhim, J.-W. Tannic acid: A green crosslinker for biopolymer-based food packaging films. Trends Food Sci. Technol. 2023, 136, 11–23. [Google Scholar] [CrossRef]
- Kim, E.; Kim, M.H.; Song, J.H.; Kang, C.; Park, W.H. Dual crosslinked alginate hydrogels by riboflavin as photoinitiator. Int. J. Biol. Macromol. 2020, 154, 989–998. [Google Scholar] [CrossRef]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.G.; Hwang, N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef]
- Dudeja, I.; Mankoo, R.K.; Singh, A.; Kaur, J. Citric acid: An ecofriendly cross-linker for the production of functional biopolymeric materials. Sustain. Chem. Pharm. 2023, 36, 101307. [Google Scholar] [CrossRef]
- Manarin, E.; Corsini, F.; Trano, S.; Fagiolari, L.; Amici, J.; Francia, C.; Bodoardo, S.; Turri, S.; Bella, F.; Griffini, G. Cardanol-Derived Epoxy Resins as Biobased Gel Polymer Electrolytes for Potassium-Ion Conduction. ACS Appl. Polym. Mater. 2022, 4, 3855–3865. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Avérous, L. Properties of glycerol-plasticized alginate films obtained by thermo-mechanical mixing. Food Hydrocoll. 2017, 63, 414–420. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A comprehensive review with the application of nanotechnology. J. Drug Deliv. Sci. Technol. 2023, 86, 104720. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: A comprehensive review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef]
- Trombino, S.; Sole, R.; Di Gioia, M.L.; Procopio, D.; Curcio, F.; Cassano, R. Green Chemistry Principles for Nano- and Micro-Sized Hydrogel Synthesis. Molecules 2023, 28, 2107. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M.; Popa, M.; Calderon Moreno, J. Aloe vera-Based Hydrogels for Wound Healing: Properties and Therapeutic Effects. Gels 2023, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Calderon Moreno, J.; Atkinson, I.; Pandele Cusu, J.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.-M.; Musuc, A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel functional natural biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424. [Google Scholar] [CrossRef]
- Sood, Y.; Singh, K.; Mudila, H.; Lokhande, P.E.; Singh, L.; Kumar, D.; Kumar, A.; Mubarak, N.M.; Dehghani, M.H. Insights into properties, synthesis and emerging applications of polypyrrole-based composites, and future prospective: A review. Heliyon 2024, 10, e33643. [Google Scholar] [CrossRef]
- Kausaite-minkstimiene, A.; Mazeiko, V.; Ramanaviciene, A.; Ramanavicius, A. Sensors and Actuators B: Chemical Evaluation of amperometric glucose biosensors based on glucose oxidase encapsulated within enzymatically synthesized polyaniline and polypyrrole. Sens. Actuators B Chem. 2011, 158, 278–285. [Google Scholar] [CrossRef]
- Kausaite, A.; Ramanaviciene, A.; Ramanavicius, A. Polyaniline synthesis catalysed by glucose oxidase. Polymer 2009, 50, 1846–1851. [Google Scholar] [CrossRef]
- Bouldin, R.; Ravichandran, S.; Kokil, A.; Garhwal, R.; Nagarajan, S.; Kumar, J.; Bruno, F.F.; Samuelson, L.A.; Nagarajan, R. Synthesis of polypyrrole with fewer structural defects using enzyme catalysis. Synth. Met. 2011, 161, 1611–1617. [Google Scholar] [CrossRef]
- ur Rahman, S.; Röse, P.; ul Haq Ali Shah, A.; Krewer, U.; Bilal, S. An Amazingly Simple, Fast and Green Synthesis Route to Polyaniline Nanofibers for Efficient Energy Storage. Polymers 2020, 12, 2212. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.A. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Guo, X.; Li, Y.; Li, L.; Pan, L. Recent Advances in Conductive Polymers-Based Electrochemical Sensors for Biomedical and Environmental Applications. Polymers 2024, 16, 1597. [Google Scholar] [CrossRef]
- Calderon Moreno, J.M.; Yoshimura, M. Hydrothermal processing of high-quality multiwall nanotubes from amorphous carbon. J. Am. Chem. Soc. 2001, 123, 741–742. [Google Scholar] [CrossRef]
- Motiei, M.; Hacohen, Y.R.; Calderon-Moreno, J.; Gedanken, A. Preparing carbon nanotubes and nested fullerenes from supercritical CO2 by a chemical reaction. J. Am. Chem. Soc. 2001, 123, 8624–8625. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, N.; Weng, X.; Li, C.; Chen, Z. Green reduction of graphene oxide using leaf extract and its application to remove dye. Eucalyptus 2018, 208, 417–424. [Google Scholar] [CrossRef]
- Chelu, M. Hydrogels with Essential Oils: Recent Advances in Designs and Applications. Gels 2024, 10, 636. [Google Scholar] [CrossRef]
- Chelu, M.; Calderon Moreno, J.M.; Musuc, A.M.; Popa, M. Natural Regenerative Hydrogels for Wound Healing. Gels 2024, 10, 547. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Kumar, H.; He, X.; Lu, Q.; Bai, H.; Kim, K.; Hu, J. The Effect of Crosslinking Degree of Hydrogels on Hydrogel Adhesion. Gels 2022, 8, 682. [Google Scholar] [CrossRef]
- Ali, F.; Khan, I.; Chen, J.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Emerging Fabrication Strategies of Hydrogels and Its Applications. Gels 2022, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Ručigaj, A.; Golobič, J.; Kopač, T. The role of multivalent cations in determining the cross-linking affinity of alginate hydrogels: A combined experimental and modeling study. Chem. Eng. J. Adv. 2024, 20, 100678. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- El Sayed, M.M. Production of Polymer Hydrogel Composites and Their Applications. J. Polym. Env. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Liu, J.; Su, C.; Chen, Y.; Tian, S.; Lu, C.; Huang, W.; Lv, Q. Current Understanding of the Applications of Photocrosslinked Hydrogels in Biomedical Engineering. Gels 2022, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Waresindo, W.X.; Luthfianti, H.R.; Priyanto, A.; Hapidin, D.A.; Edikresnha, D.; Aimon, A.H.; Suciati, T.; Khairurrijal, K. Freeze–thaw hydrogel fabrication method: Basic principles, synthesis parameters, properties, and biomedical applications. Mater. Res. Express 2023, 10, 024003. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Moreira Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Heck, T.; Faccio, G.; Richter, M.; Thöny-Meyer, L. Enzyme-catalyzed protein crosslinking. Appl. Microbiol. Biotechnol. 2013, 97, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Rachel, N.M.; Pelletier, J.N. Biotechnological Applications of Transglutaminases. Biomolecules 2013, 3, 870–888. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Jiang, Z.; Liu, Y.; Zhou, L.; Liu, Z.; Tang, L. Recent Advances of Conductive Hydrogels for Flexible Electronics. Electron. Mater. 2024, 5, 101–131. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, J.; Wang, T.; Peng, X.; Liu, J.; Wang, X.; Wang, J.; Zeng, H. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2020, 2, 843–865. [Google Scholar] [CrossRef]
- Khan, B.; Abdullah, S.; Khan, S. Current Progress in Conductive Hydrogels and Their Applications in Wearable Bioelectronics and Therapeutics. Micromachines 2023, 14, 1005. [Google Scholar] [CrossRef]
- Sui, X.; Guo, H.; Cai, C.; Li, Q.; Wen, C.; Zhang, X.; Wang, X.; Yang, J.; Zhang, L. Ionic conductive hydrogels with long-lasting antifreezing, water retention and self-regeneration abilities. Chem. Eng. J. 2021, 419, 129478. [Google Scholar]
- Wang, Y.; Zhang, L.; Lu, A. Transparent, Antifreezing, Ionic Conductive Cellulose Hydrogel with Stable Sensitivity at Subzero Temperature. ACS Appl. Mater. Interfaces 2019, 11, 41710–41716. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.G.; Lübben, J.F. Review on Hydrogel-Based Flexible Supercapacitors for Wearable Applications. Gels 2023, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, L.; Wang, S.; Zhang, L.; Chen, L.; Xu, X.; Song, Z.; Liu, H.; Chen, C. Strong, tough, ionic conductive, and freezing-tolerant all-natural hydrogel enabled by cellulose-bentonite coordination interactions. Nat. Commun. 2022, 13, 3408. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly stretchable, elastic, and ionic conductive hydrogel for artificial soft electronics. Adv. Funct. Mater. 2018, 29, 1806220. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, N.; Zhang, H.; Sun, Y.; Lin, Z.; Hou, L. Preparation and characterization of poly(vinyl alcohol)/sodium alginate hydrogel with high toughness and electric conductivity. Carbohydr. Polym. 2018, 186, 377–383. [Google Scholar] [CrossRef]
- Wu, A.; Luo, Y.; Cuthbert, T.J.; Shokurov, A.V.; Chu, P.K.; Feng, S.-P.; Menon, C. Hydrogels as Soft Ionic Conductors in Flexible and Wearable Triboelectric Nanogenerators. Adv. Sci. 2022, 9, 2106008. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Zhao, F.; Yu, G. Functional Hydrogels for Next-Generation Batteries and Supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar] [CrossRef]
- Ying, B.; Liu, X. Skin-like hydrogel devices for wearable sensing, soft robotics and beyond. iScience 2021, 24, 103174. [Google Scholar] [CrossRef]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef]
- Sun, X.; Qin, Z.; Ye, L.; Zhang, H.; Yu, Q.; Wu, X.; Li, J.; Yao, F. Carbon nanotubes reinforced hydrogel as flexible strain sensor with high stretchability and mechanically toughness. Chem. Eng. J. 2020, 382, 122832. [Google Scholar] [CrossRef]
- Pang, J.; Wang, L.; Xu, Y.; Wu, M.; Wang, M.; Liu, Y.; Yu, S.; Li, L. Skin-inspired cellulose conductive hydrogels with integrated self-healing, strain, and thermal sensitive performance. Carbohydr. Polym. 2020, 240, 116360. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Yin, S.; Guo, Y.; Zhao, F.; Guo, Q.; Zhang, X.; Zho, T.; Yu, G. Balancing the mechanical, electronic, and self-healing properties in conductive self-healing hydrogel for wearable sensor applications. Mater. Horiz. 2021, 8, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Li, H.; Yu, Z. A Review of Skin-Wearable Sensors for Non-Invasive Health Monitoring Applications. Sensors 2023, 23, 3673. [Google Scholar] [CrossRef]
- Fan, J.A.; Yeo, W.-H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S.-Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; et al. Fractal design concepts for stretchable electronics. Nat. Commun. 2014, 5, 3266. [Google Scholar] [CrossRef]
- Wan, Z.; Qu, R.; Sun, Y.; Gao, Y.; Gao, G.; Chen, K.; Liu, T. Physically crosslinked polyvinyl alcohol, phytic acid and glycerol hydrogels for wearable sensors with biocompatibility, antimicrobial stability and anti-freezing. Eur. Polym. J. 2024, 211, 112974. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Duan, L.; Gao, G. Ultra-stretchable wearable strain sensors based on skin-inspired adhesive, tough and conductive hydrogels. Chem. Eng. J. 2019, 365, 10–19. [Google Scholar] [CrossRef]
- Han, K.; Liu, K.; Wang, M.; Wang, K.; Fang, L.; Chen, H.; Zhou, J.; Lu, X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2018, 28, 1704195. [Google Scholar] [CrossRef]
- Mndlovu, H.; Kumar, P.; du Toit, L.C.; Choonara, Y.E. A review of biomaterial degradation assessment approaches employed in the biomedical field. npj Mater. Degrad. 2024, 8, 66. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Alabbosh, K.F.; Manan, S.; Khan, S.; Ahmad, F.; Ullah, M.W. Chitosan-based nanostructured biomaterials: Synthesis, properties, and biomedical applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 79–99. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Adv. Sci. 2023, 10, 2303326. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Posada-Quintero, H.F.; Chon, K.H. Innovations in Electrodermal Activity Data Collection and Signal Processing: A Systematic Review. Sensors 2020, 20, 479. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fang, Y.; Chen, J. Wearable Biosensors for Non-Invasive Sweat Diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef]
- Banerjee, H.; Suhail, M.; Ren, H. Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges. Biomimetics 2018, 3, 15. [Google Scholar] [CrossRef]
- Zhang, X.; Pint, C.L.; Lee, M.H.; Schubert, B.E.; Jamshidi, A.; Takei, K.; Ko, H.; Gillies, A.; Bardhan, R.; Urban, J.J.; et al. Optically-and thermally-responsive programmable materials based on carbon nanotube-hydrogel polymer composites. Nano Lett. 2011, 11, 3239–3244. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, P.; Lin, S.; Zhong, J.; Wang, Y. A Review of Conductive Hydrogel-Based Wearable Temperature Sensors. Adv. Health Mater. 2024, 13, e2401503. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Luo, Y.; Bai, B.; Cao, F. pH-Responsive Eco-Friendly Chitosan–Chlorella Hydrogel Beads for Water Retention and Controlled Release of Humic Acid. Water 2022, 14, 1190. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, L.; Chen, X.; Wu, W. Smart Wearable Systems for Health Monitoring. Sensors 2023, 23, 2479. [Google Scholar] [CrossRef] [PubMed]
- Güder, F.; Ainla, A.; Redston, J.; Mosadegh, B.; Glavan, A.; Martin, T.J.; Whitesides, G.M. Paper-Based Electrical Respiration Sensor. Angew. Chem. Int. Ed. 2016, 55, 5727–5732. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zimmerman, B.; Akhtar, A.; Yu, K.J.; Moore, M.; Wu, J.; Larsen, R.J.; Lee, J.W.; Li, J.; Liu, Y.; et al. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng. 2019, 3, 194–205. [Google Scholar] [CrossRef]
- Tao, L.Q.; Tian, H.; Liu, Y.; Ju, Z.Y.; Pang, Y.; Chen, Y.Q.; Wang, D.Y.; Tian, X.G.; Yan, J.C.; Deng, N.Q.; et al. An intelligent artificial throat with sound-sensing ability based on laser induced graphene. Nat. Commun. 2017, 8, 14579. [Google Scholar] [CrossRef] [PubMed]
- Araci, I.E.; Su, B.L.; Quake, S.R.; Mandel, Y. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat. Med. 2014, 20, 1074–1078. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef]
- Guo, L.; Li, Z.; Hu, W.; Liu, T.; Zheng, Y.; Yuan, M.; Dai, Y.; Ning, R.; Zhu, Y.; Tao, K.; et al. A flexible dual-structured MXene for ultra-sensitive and ultra-wide monitoring of anatomical and physiological movements. J. Mater. Chem. A 2021, 9, 26867–26874. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-Molded Flexible, Ultrasensitive, and Highly Stable Electronic Skin for Monitoring Human Physiological Signals. Adv. Mater. 2014, 26, 1336–1342. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, Z.; Wu, W.R.; Fu, Q.X.; Huang, K.; Nitin, N.; Ding, B.; Sun, G. Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci. Adv. 2018, 4, eaar5931. [Google Scholar] [CrossRef]
- Jung, D.; Lim, C.; Shim, H.J.; Kim, Y.; Park, C.; Jung, J.; Han, S.I.; Sunwoo, S.H.; Cho, K.W.; Cha, G.D.; et al. Highly conductive and elastic nanomembrane for skin electronics. Science 2021, 373, 1022–1026. [Google Scholar] [CrossRef]
- Nigusse, A.B.; Mengistie, D.A.; Malengier, B.; Tseghai, G.B.; Langenhove, L.V. Wearable Smart Textiles for Long-Term Electrocardiography Monitoring—A Review. Sensors 2021, 21, 4174. [Google Scholar] [CrossRef]
- Ankhili, A.; Tao, X.; Cochrane, C.; Koncar, V.; Coulon, D.; Tarlet, J.-M. Ambulatory Evaluation of ECG Signals Obtained Using Washable Textile-Based Electrodes Made with Chemically Modified PEDOT:PSS. Sensors 2019, 19, 416. [Google Scholar] [CrossRef]
- Castrillón, R.; Pérez, J.J.; Andrade-Caicedo, H. Electrical Performance of PEDOT: PSS-Based Textile Electrodes for Wearable ECGMonitoring: A Comparative Study. Bio Med. Eng. Online 2018, 17, 38. [Google Scholar] [CrossRef]
- Takamatsu, S.; Lonjaret, T.; Crisp, D.; Badier, J. Direct Patterning of Organic Conductors on Knitted Textiles for Long-Term Electrocardiography. Nat. Publ. Group. 2015, 5, 15003. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Chen, X.; Zhang, P.; Shan, L.; Duan, Q.; Xing, J.; Lan, Y.; Lu, B.; Liu, J. 3D Printed Implantable Hydrogel Bioelectronics for Electrophysiological Monitoring and Electrical Modulation. Adv. Funct. Mater. 2024, 34, 2314471. [Google Scholar] [CrossRef]
- Yu, J.; Tian, F.; Wang, W.; Wan, R.; Cao, J.; Chen, C.; Zhao, D.; Liu, J.; Zhong, J.; Wang, F.; et al. Design of Highly Conductive, Intrinsically Stretchable, and 3D Printable PEDOT:PSS Hydrogels via PSS-Chain Engineering for Bioelectronics. Chem. Mater. 2023, 35, 5936–5944. [Google Scholar] [CrossRef]
- Pedrosa, P.; Fiedler, P.; Schinaia, L.; Vasconcelos, B.; Martins, A.C.; Amaral, M.H.; Comani, S.; Haueisen, J.; Fonseca, C. Alginate-based hydrogels as an alternative to electrolytic gels for rapid EEG monitoring and easy cleaning procedures. Sens. Actuators B Chem. 2017, 247, 273–283. [Google Scholar] [CrossRef]
- Considine, C.; Besio, W. Conductive Hydrogel Tapes for Tripolar EEG: A Promising Solution to Paste-Related Challenges. Sensors 2024, 24, 4222. [Google Scholar] [CrossRef]
- Al-Ayyad, M.; Owida, H.A.; De Fazio, R.; Al-Naami, B.; Visconti, P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics 2023, 12, 1520. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; He, P.; Liu, K.; Ni, Y.; Chen, L.; Ouyang, X.; Huang, L.; Wang, H.; Xu, S. A bionic tactile plastic hydrogel-based electronic skin constructed by a nerve-like nanonetwork combining stretchable, compliant, and self-healing properties. Chem. Eng. J. 2020, 379, 122271. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Siyuan, L.; Zhang, S.; Li, Y.; Qu, Z.; Chen, Y.; Lu, B.; Wang, X.; Feng, X. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, G.; Jeon, C.; Han, H.H.; Kim, S.; Mok, J.W.; Joo, C.; Shin, S.; Sim, J.; Myung, D.; et al. Bimetallic Nanocatalysts immobilized in nanoporous hydrogels for long-term robust continuous glucose monitoring of smart contact lens. Adv. Mat. 2022, 34, 2110536. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef]
- Shinde, S.; Kim, K.H.; Park, S.Y.; Kim, J.H.; Kim, J.; Joe, D.J.; Lee, H.E. Wearable sweat-sensing patches for non-invasive and continuous health tracking. Sens. Actuators Rep. 2025, 9, 100265. [Google Scholar] [CrossRef]
- Lin, S.; Wang, B.; Zhao, Y.; Shih, R.; Cheng, X.; Yu, W.; Hojaiji, H.; Lin, H.; Hoffman, C.; Ly, D.; et al. Natural Perspiration Sampling and in Situ Electrochemical Analysis with Hydrogel Micropatches for User-Identifiable and Wireless Chemo/Biosensing. ACS Sens. 2020, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, N.; Tan, Y.; Fu, F.; Liu, G.; Fang, Y.; Zhang, X.X.; Liu, M.; Cheng, Y.; Yu, J. Sweat-resistant silk fibroin-based double network hydrogel adhesives, ACS Appl. Mater. Interfaces 2022, 14, 21945–21953. [Google Scholar] [CrossRef]
- Lin, P.H.; Sheu, S.C.; Chen, C.W.; Huang, S.C.; Li, B.R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]
- Assalve, G.; Lunetti, P.; Di Cagno, A.; De Luca, E.W.; Aldegheri, S.; Zara, V.; Ferramosca, A. Advanced Wearable Devices for Monitoring Sweat Biochemical Markers in Athletic Performance: A Comprehensive Review. Biosensors 2024, 14, 574. [Google Scholar] [CrossRef]
- Saha, T.; Songkakul, T.; Knisely, C.T.; Yokus, M.A.; Daniele, M.A.; Dickey, M.D.; Bozkurt, A.; Velev, O.D. Wireless Wearable Electrochemical Sensing Platform with Zero-Power Osmotic Sweat Extraction for Continuous Lactate Monitoring. ACS Sens. 2022, 7, 2037–2048. [Google Scholar] [CrossRef]
- Zhang, S.; Tu, T.; Li, T.; Cai, Y.; Wang, Z.; Zhou, Y.; Wang, D.; Fang, L.; Ye, X.; Liang, B. 3D MXene/PEDOT:PSS Composite Aerogel with a Controllable Patterning Property for Highly Sensitive Wearable Physical Monitoring and Robotic Tactile Sensing. ACS Appl. Mater. Interfaces 2022, 14, 23877–23887. [Google Scholar] [CrossRef]
- Huang, X.; Xue, Y.; Ren, S.; Wang, F. Sensor-Based Wearable Systems for Monitoring Human Motion and Posture: A Review. Sensors 2023, 23, 9047. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wu, S.; Qu, J.; Gong, L.; Tang, J. A Review of Conductive Hydrogel Used in Flexible Strain Sensor. Materials 2020, 13, 3947. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, J.; Li, M.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. MXene-coated air-permeable pressure-sensing fabric for smart wear. ACS Appl. Mater. Interfaces 2020, 12, 46446–46454. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, J.; Chen, G.; Hou, L.; Zhang, J.; Zhang, X.; Yang, X.; Guan, L.; Jiang, X.; Liu, H. Multifunctional Poly(vinyl alcohol) Nanocomposite Organohydrogel for Flexible Strain and Temperature Sensor. ACS Appl. Mater. Interfaces 2020, 12, 40815–40827. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, W.; Yeo, J.C.C.; Soo, X.Y.D.; Thitsartarn, W.; Liu, S.; Tan, B.H.; Suwardi, A.; Li, Z.; Zhu, Q.; et al. Responsive hydrogel dressings for intelligent wound management. BMEMat 2023, 1, e12021. [Google Scholar] [CrossRef]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B.; et al. Smart bandages for monitoring. Small 2018, 14, 1703509. [Google Scholar] [CrossRef]
- Du, S.; Suo, H.; Xie, G.; Lyu, Q.; Mo, M.; Xie, Z.; Zhou, N.; Zhang, L.; Tao, J.; Zhu, J. Self-powered and photothermal electronic skin patches for accelerating wound healing. Nano Energy 2022, 93, 106906. [Google Scholar] [CrossRef]
- Finny, A.S.; Jiang, C.; Andreescu, S. 3D Printed Hydrogel-Based Sensors for Quantifying UV Exposure. ACS Appl. Mater. Interfaces 2020, 12, 43911–43920. [Google Scholar] [CrossRef]
- Luo, Y.; Li, J.; Ding, Q.; Wang, H.; Liu, C.; Wu, J. Functionalized Hydrogel-Based Wearable Gas and Humidity Sensors. Nano-Micro Lett. 2023, 15, 136. [Google Scholar] [CrossRef]
- Cai, C.; Zhao, W.; Yang, J.; Zhang, L. Sensitive and flexible humidity sensor based on sodium hyaluronate/MWCNTs composite film. Cellulose 2021, 28, 6361–6371. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.; Sluyter, R.; Konstantinov, K.; Kim, J.; Kim, S.; Kim, Y.H. Wearable and implantable bioelectronics as eco-friendly and patient-friendly integrated nanoarchitectonics for next-generation smart healthcare technology. EcoMat. 2023, 5, e12356. [Google Scholar] [CrossRef]
- Chelu, M.; Stroescu, H.; Anastasescu, M.; Calderon-Moreno, J.M.; Preda, S.; Stoica, M.; Fogarassy, Z.; Petrik, P.; Gheorghe, M.; Parvulescu, C.; et al. High-quality PMMA/ZnO NWs piezoelectric coating on rigid and flexible metallic substrates. Appl. Surf. Sci. 2020, 529, 147135. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Y.; Deng, F.; Chen, Y.; Zhang, H. Eye-Movement-Controlled Wheelchair Based on Flexible Hydrogel Biosensor and WT-SVM. Biosensors 2021, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.-T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.; Zhou, Y.; Zhou, F.; Liu, W.; Wang, Z. Mussel-inspired hydrogels: From design principles to promising applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Gan, D.; Soubrier, M.; Chiang, H.; Yan, L.; Li, Y.; Li, J.; Yu, S.; Xia, Y.; et al. Bioadhesive and conductive hydrogel-integrated brain-machine interfaces for conformal and immune-evasive contact with brain tissue. Matter 2022, 5, 1204–1223. [Google Scholar] [CrossRef]
- Teo, M.Y.; Lim, K.; Aw, K.C.; Kee, S.; Stringer, J. Towards biodegradable conducting polymers by incorporating seaweed cellulose for decomposable wearable heaters. RSC Adv. 2023, 13, 26267–26274. [Google Scholar] [CrossRef]
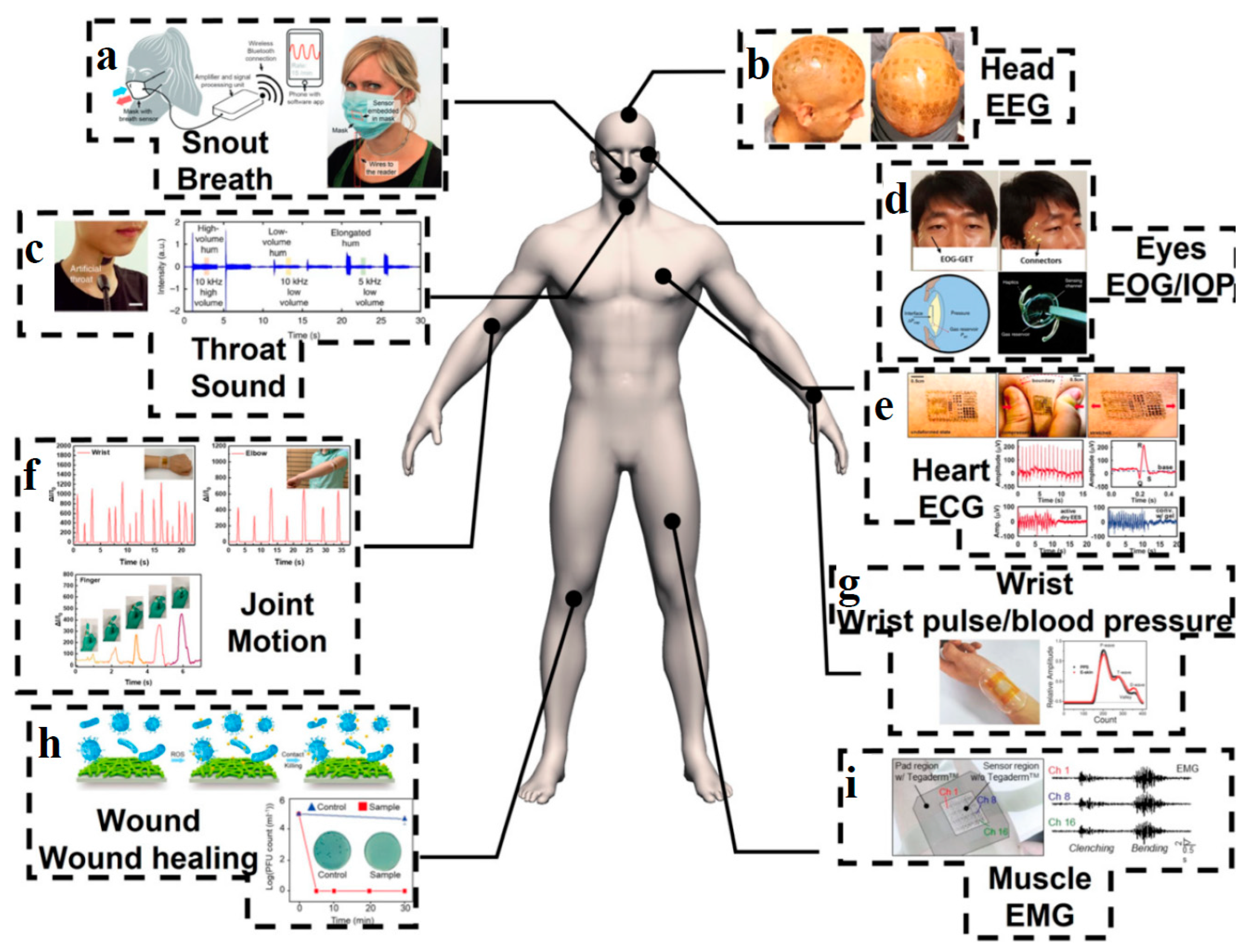

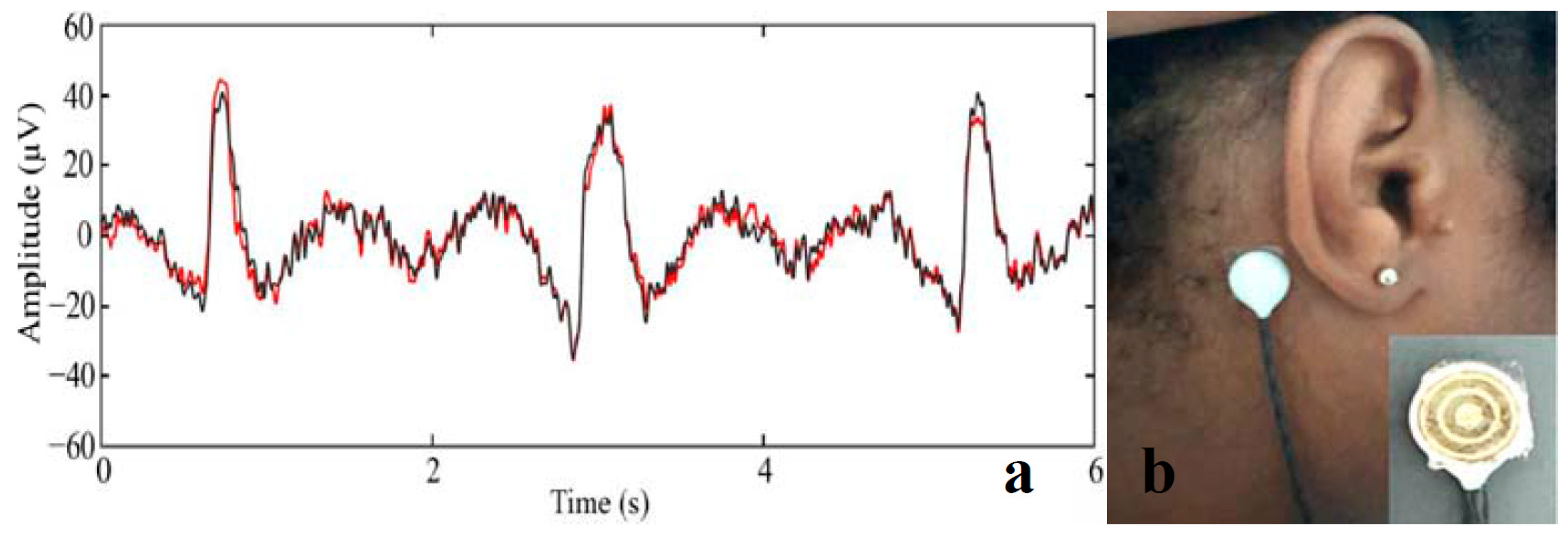
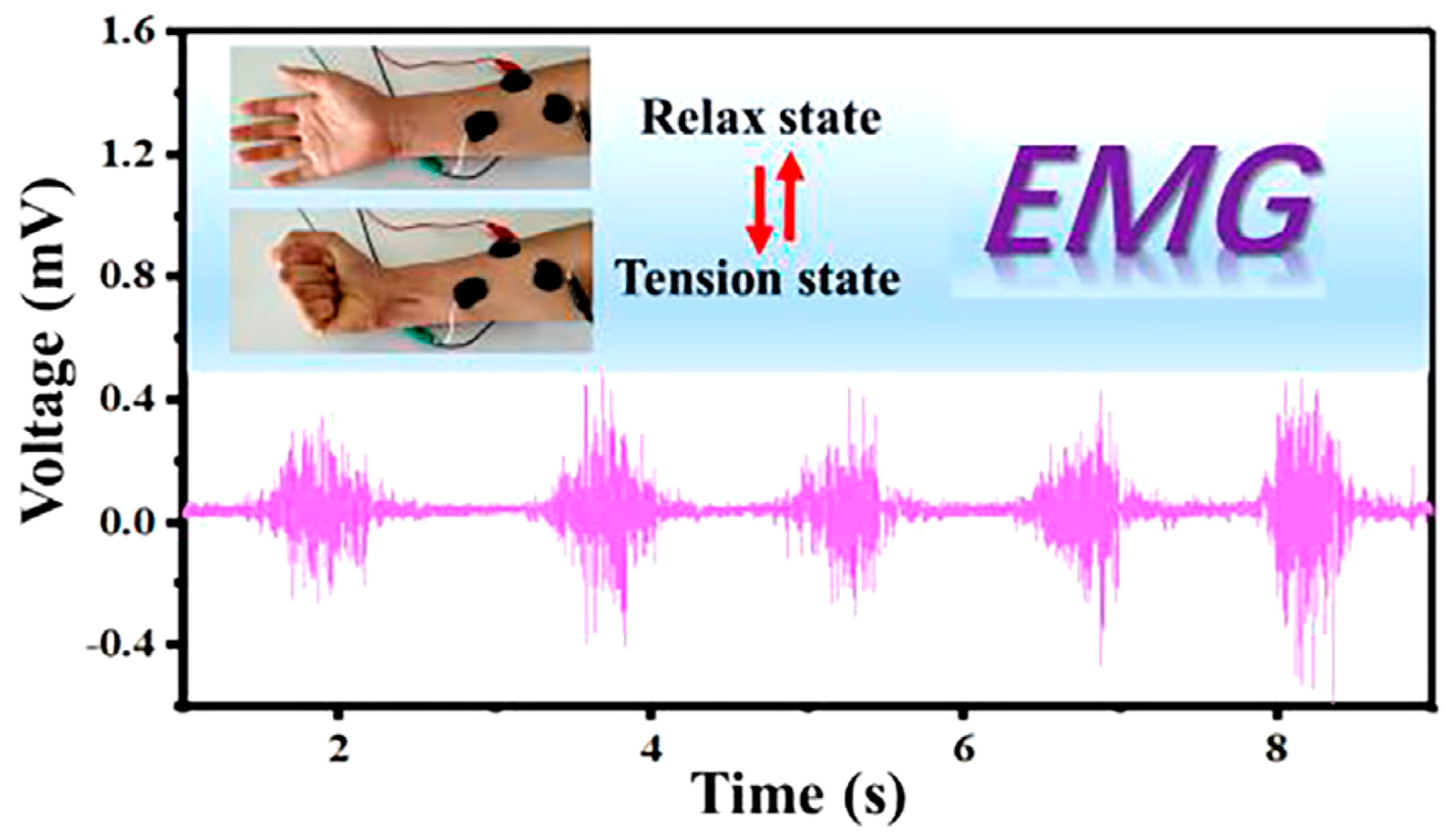

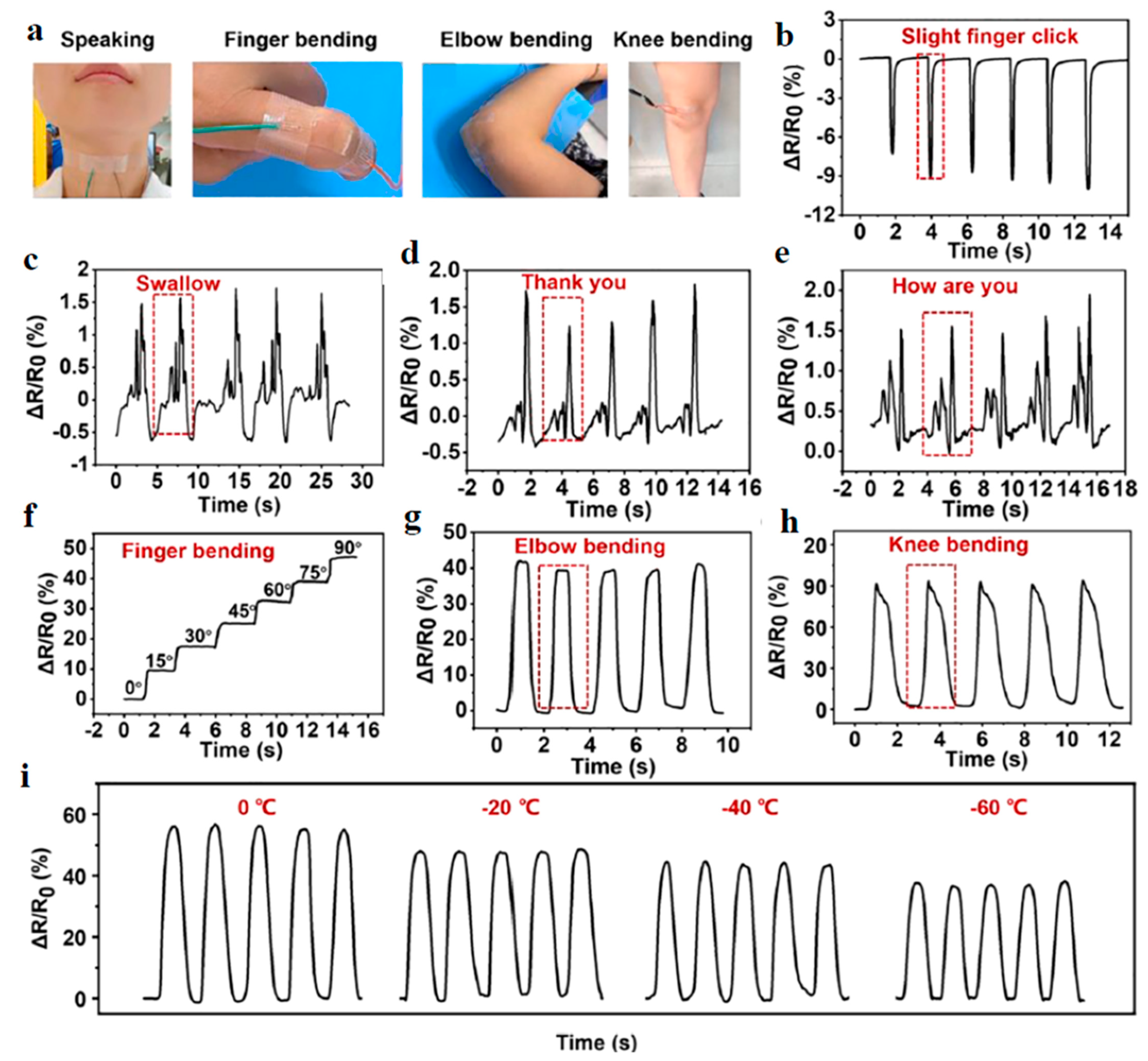

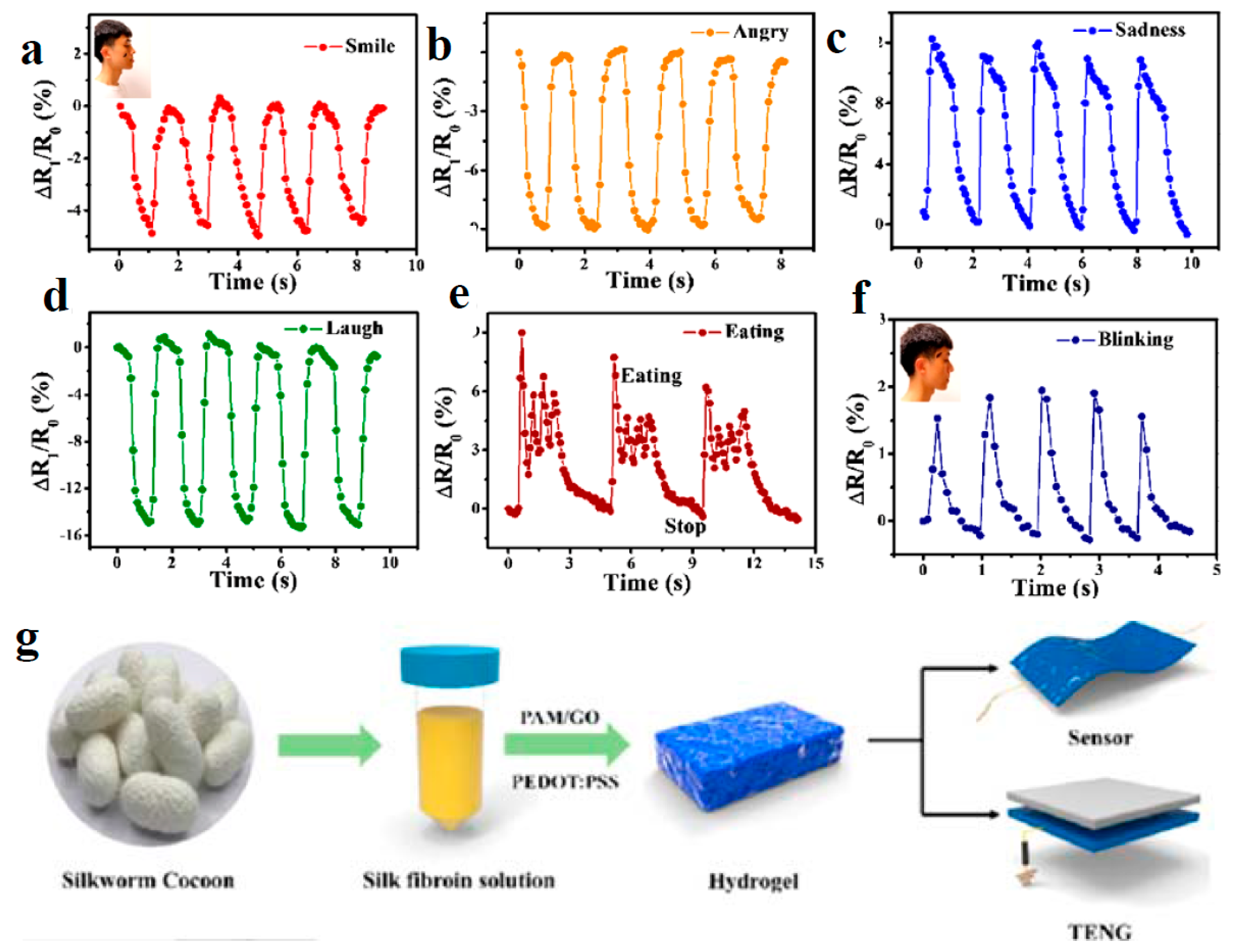
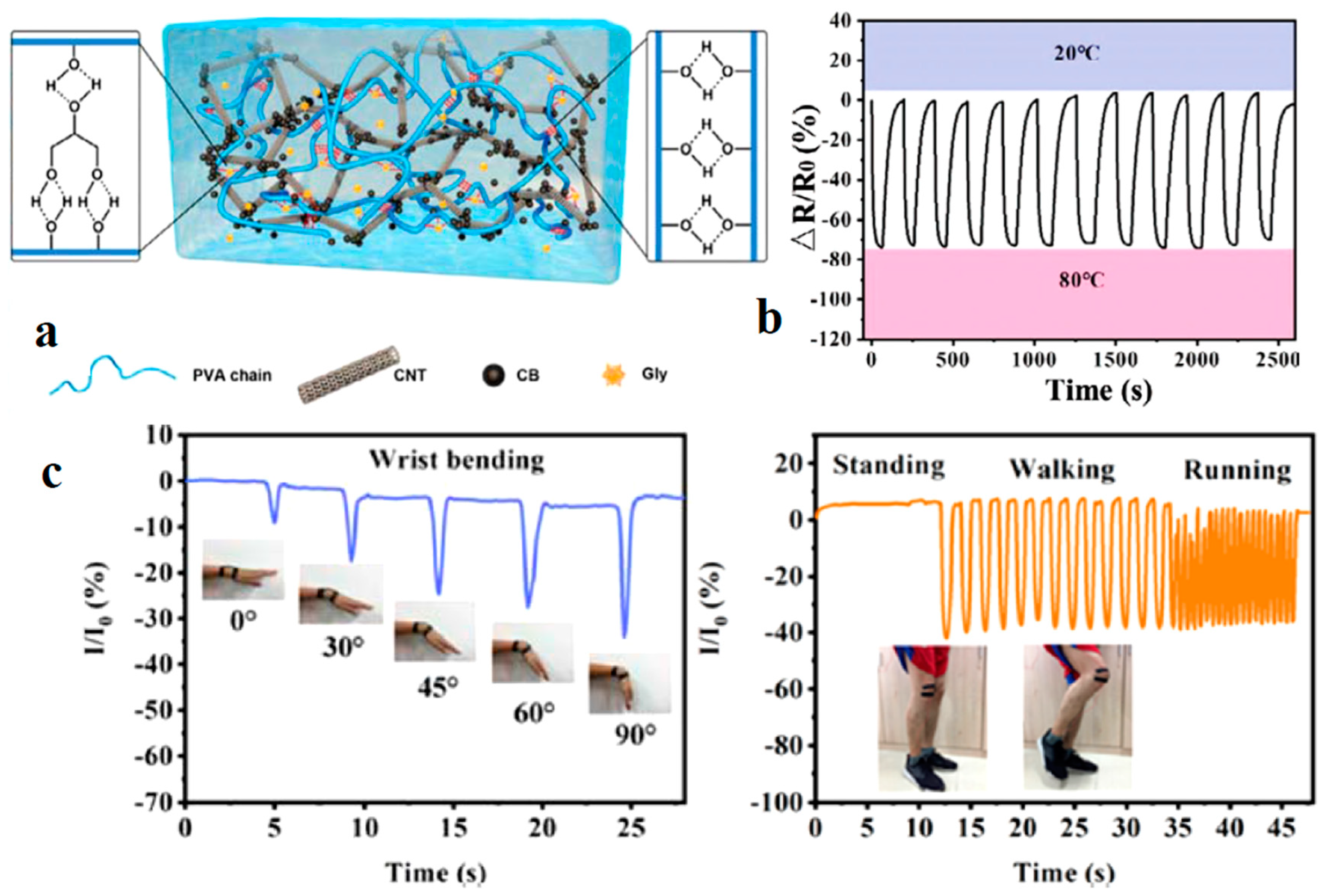
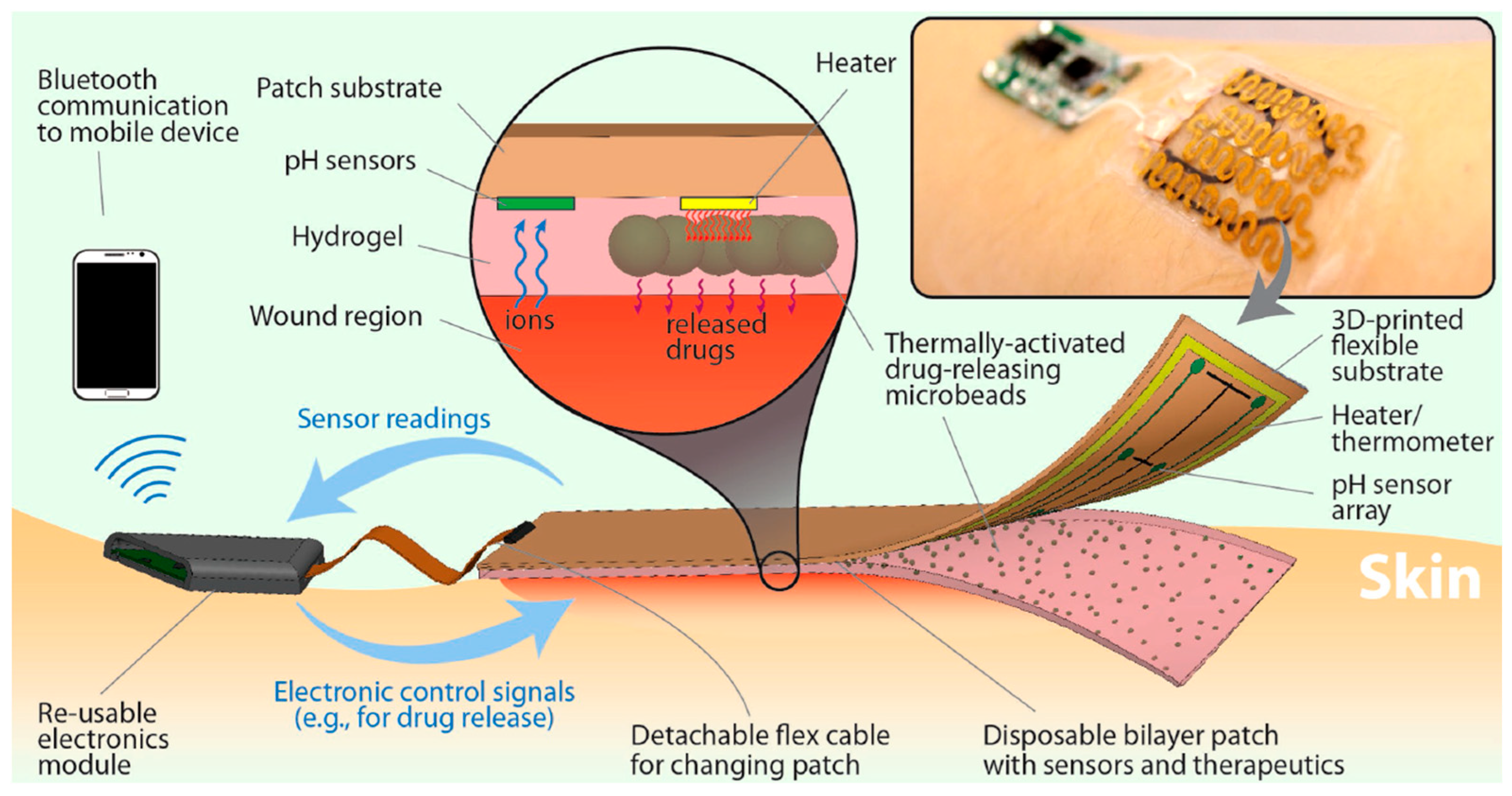
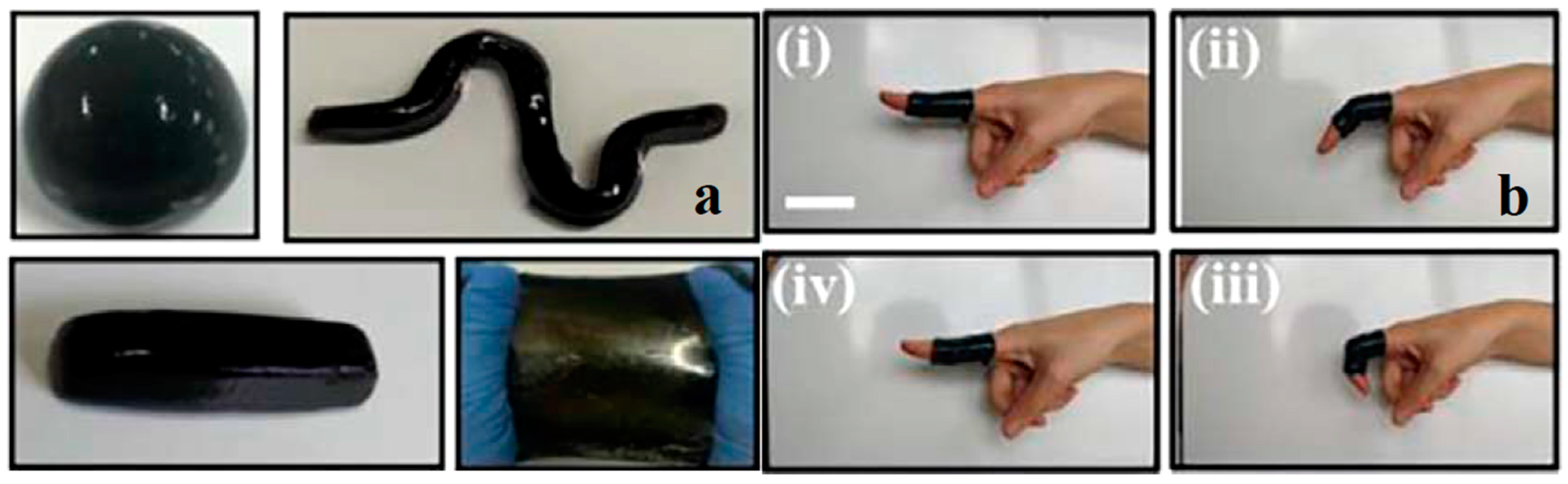

| Application | CHG | WE Description | Reference |
|---|---|---|---|
| ECG sensor | Polyamide, polyester, and cotton knitted fabrics coated with chemically modified PEDOT:PSS solution and silver-coated polyamide yarns used for signal transmission. | Low cost electrodes for cutaneous electrophysiology. | [16,202] |
| EEG sensor | Alginate-based hydrogels injected into the EEG electrode cavity. | Electrolytic gels for rapid EEG monitoring and easy cleaning procedures. | [205] |
| EMG and ECG sensor | Hydrogel-based electronic skin formed by proanthocyanins/reduced graphene oxide (PC/rGO) composite incorporated into glycerol-plasticized polyvinyl alcohol-borax (PVA-borax) hydrogel system. | Electronic skin, adhesive electrode. | [208] |
| Glucose monitoring | Gold platinum bimetallic nanocatalysts modified with hyaluronate immobilized in nanoporous hydrogels (HA-Au@Pt BiNCs). | Smart contact lenses for continuous tear glucose monitoring (CGM). | [215] |
| Sweat analysis | Silk fibroin-polyacrylamide (SF-PAAm) double network (DN) hydrogel adhesive. | Hydrogel patch-based sensor for real-time sweat detection on the body, biocompatible, with strong and durable adhesion to wet surfaces. | [215] |
| Soft pressure and motion sensor | Biomimetic hydrogel based on Ag/TA@CNC (cellulose nanocrystals (CNCs) decorated with tannic acid (TA) and Ag nanoparticles) nanohybrids and polyvinyl alcohol. | Soft artificial and intelligent material, similar to skin. | [82] |
| Temperature and tension sensor | Carbon nanotubes (CNT) and carbon black (CB) integrated into a poly(vinyl alcohol)/glycerol (PVA/Gly) nanocomposite organohydrogel. | Sensors with high sensitivity to stretch, strain and temperature. | [225] |
| UV and pollution sensor | 3D printed tattoo-type sensors, based on hydrogel ink containing alginate nanoparticles, gelatin, photoactive titanium dioxide and dyes (methyl orange, methylene blue and malachite green). | Measures sun exposure by decreasing the color of the printed material. | [229] |
| Breath sensors and toxin detection | Sodium hyaluronate (SH)/multi-walled carbon nanotubes (MWCNTs) composite film. | Flexible interdigital electrode for non-contact monitoring of respiration and detection of skin sweat evaporation. | [231] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón Moreno, J.M.; Chelu, M.; Popa, M. Eco-Friendly Conductive Hydrogels: Towards Green Wearable Electronics. Gels 2025, 11, 220. https://doi.org/10.3390/gels11040220
Calderón Moreno JM, Chelu M, Popa M. Eco-Friendly Conductive Hydrogels: Towards Green Wearable Electronics. Gels. 2025; 11(4):220. https://doi.org/10.3390/gels11040220
Chicago/Turabian StyleCalderón Moreno, José María, Mariana Chelu, and Monica Popa. 2025. "Eco-Friendly Conductive Hydrogels: Towards Green Wearable Electronics" Gels 11, no. 4: 220. https://doi.org/10.3390/gels11040220
APA StyleCalderón Moreno, J. M., Chelu, M., & Popa, M. (2025). Eco-Friendly Conductive Hydrogels: Towards Green Wearable Electronics. Gels, 11(4), 220. https://doi.org/10.3390/gels11040220









