The Effect of the Conformation Process on the Physicochemical Properties of Carboxymethylcellulose–Starch Hydrogels
Abstract
1. Introduction
2. Results and Discussion
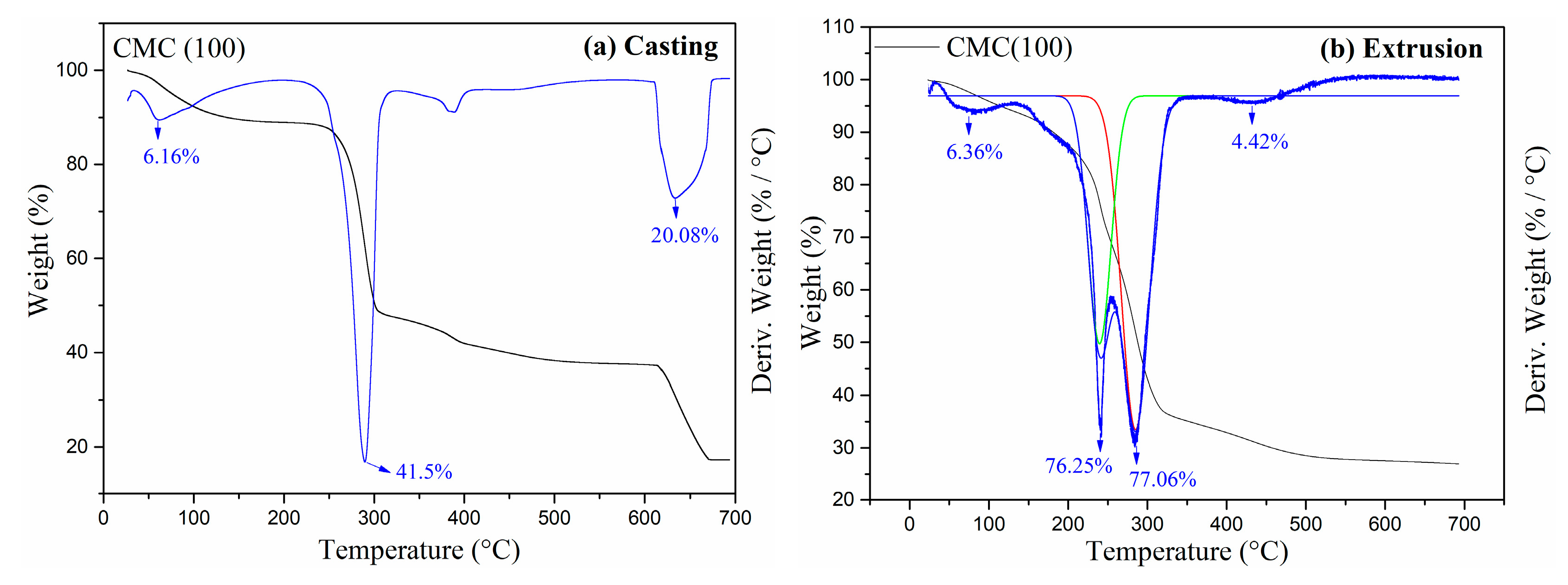
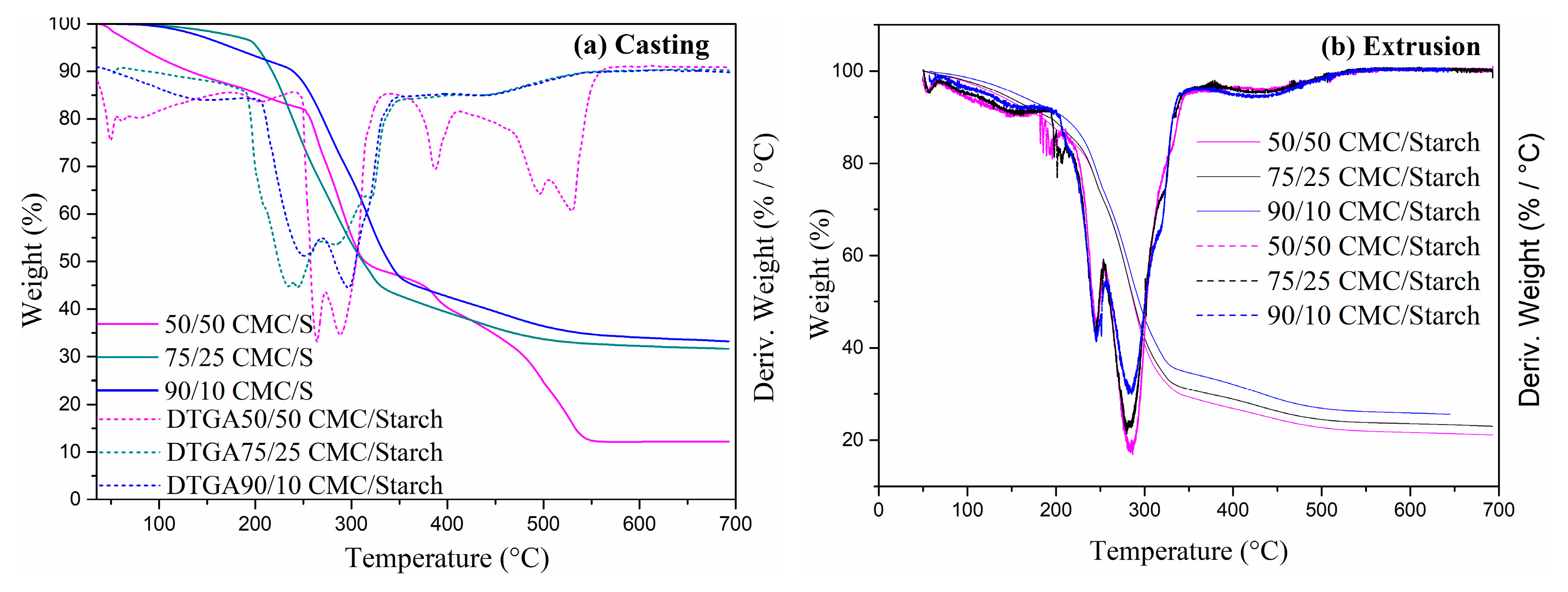
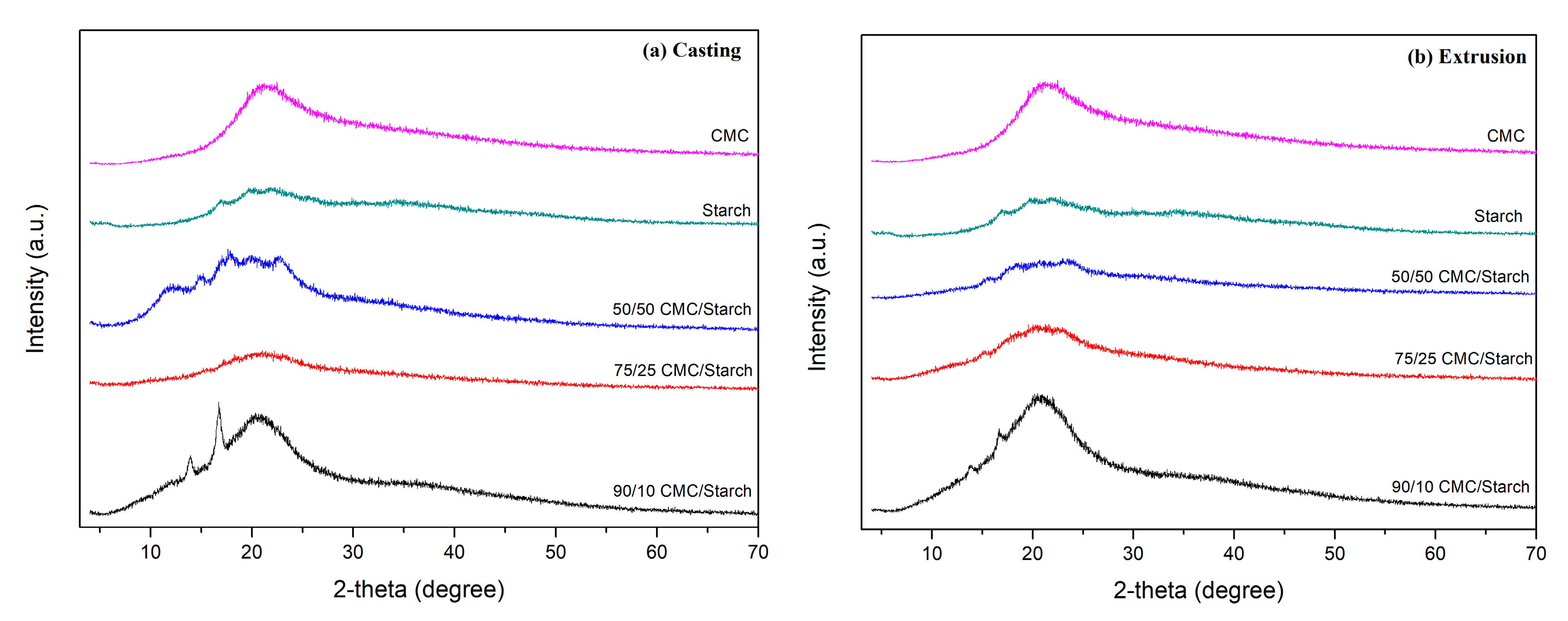
2.1. Characterization
2.2. Mechanical Properties
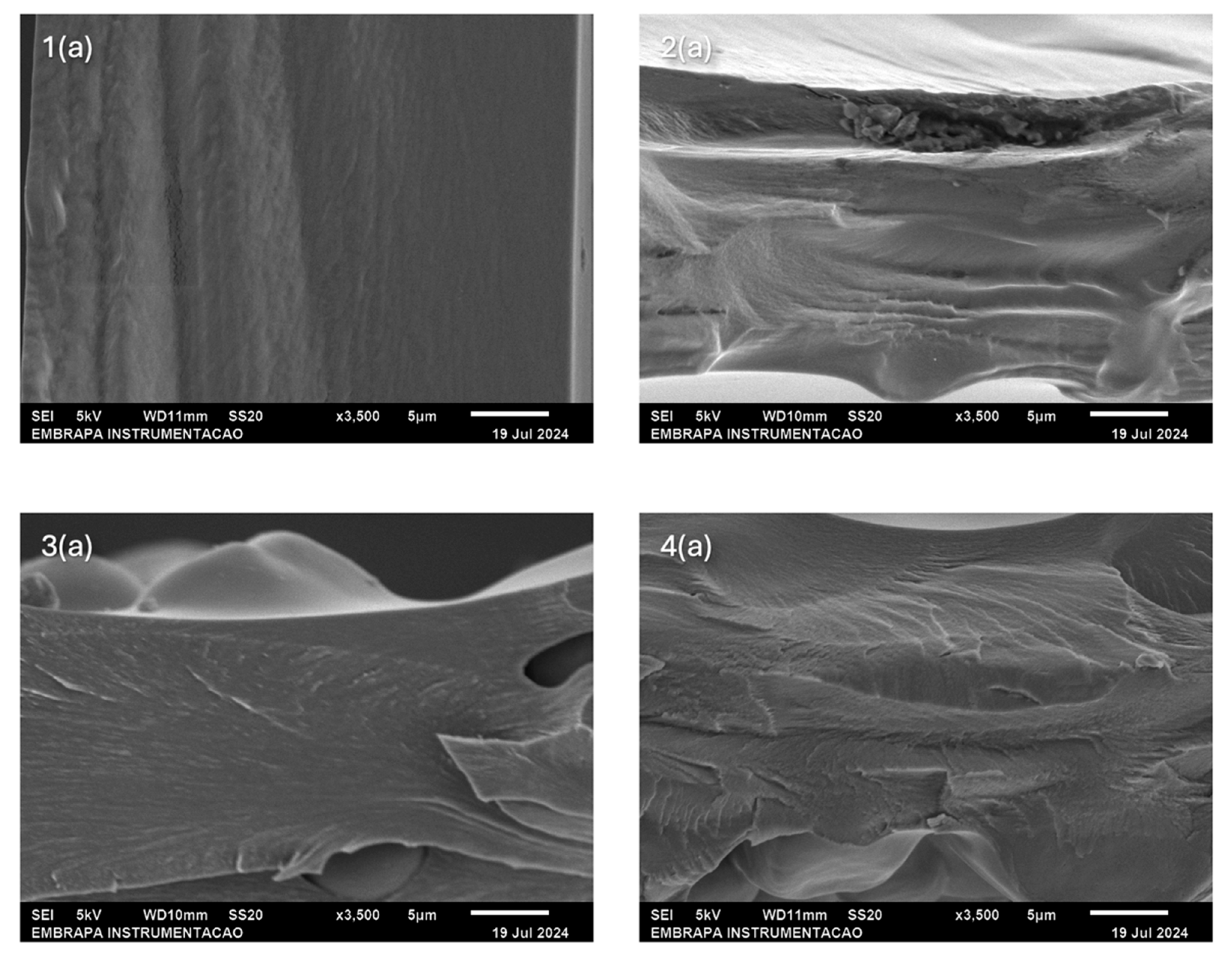
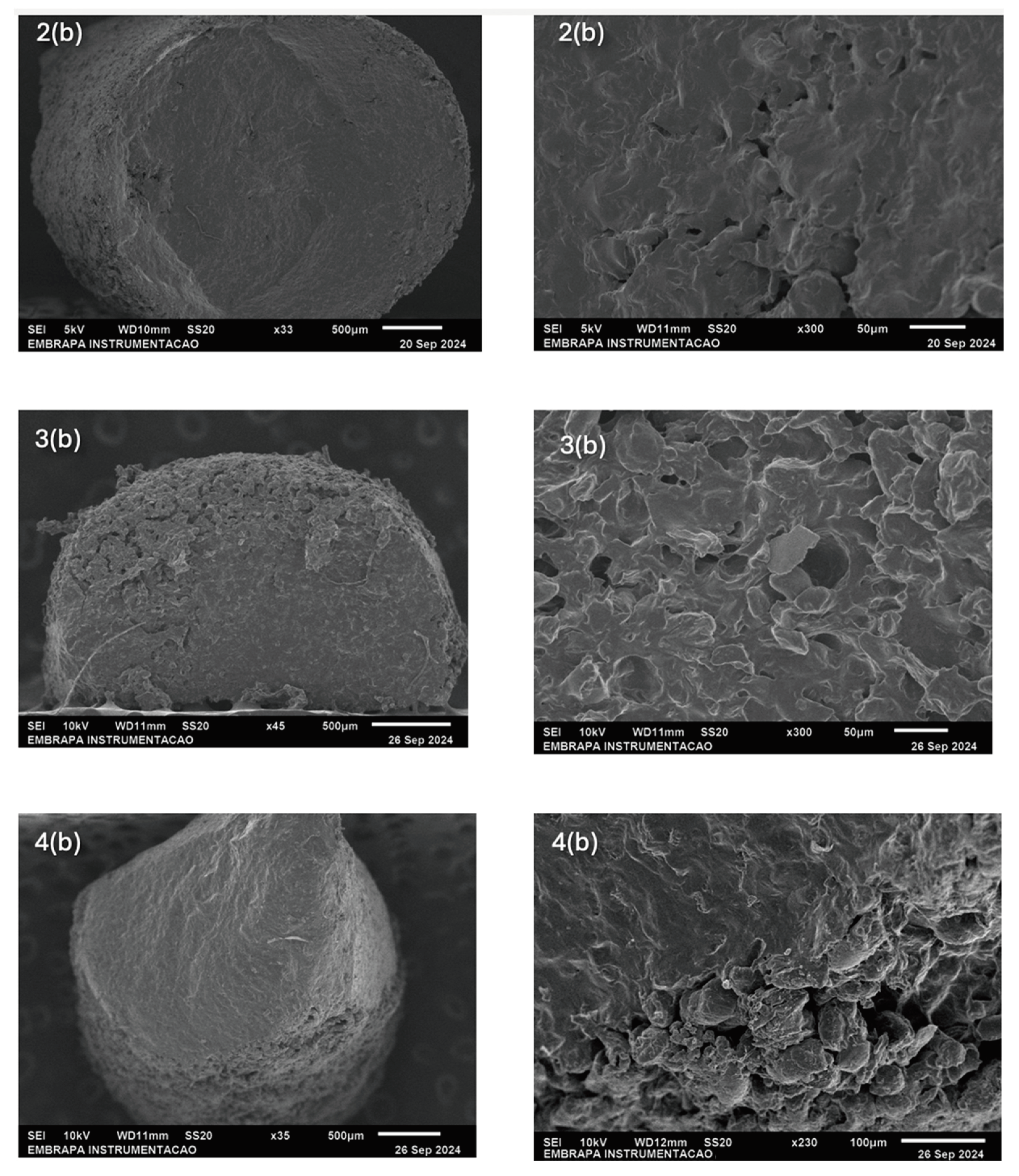
2.3. Morphology
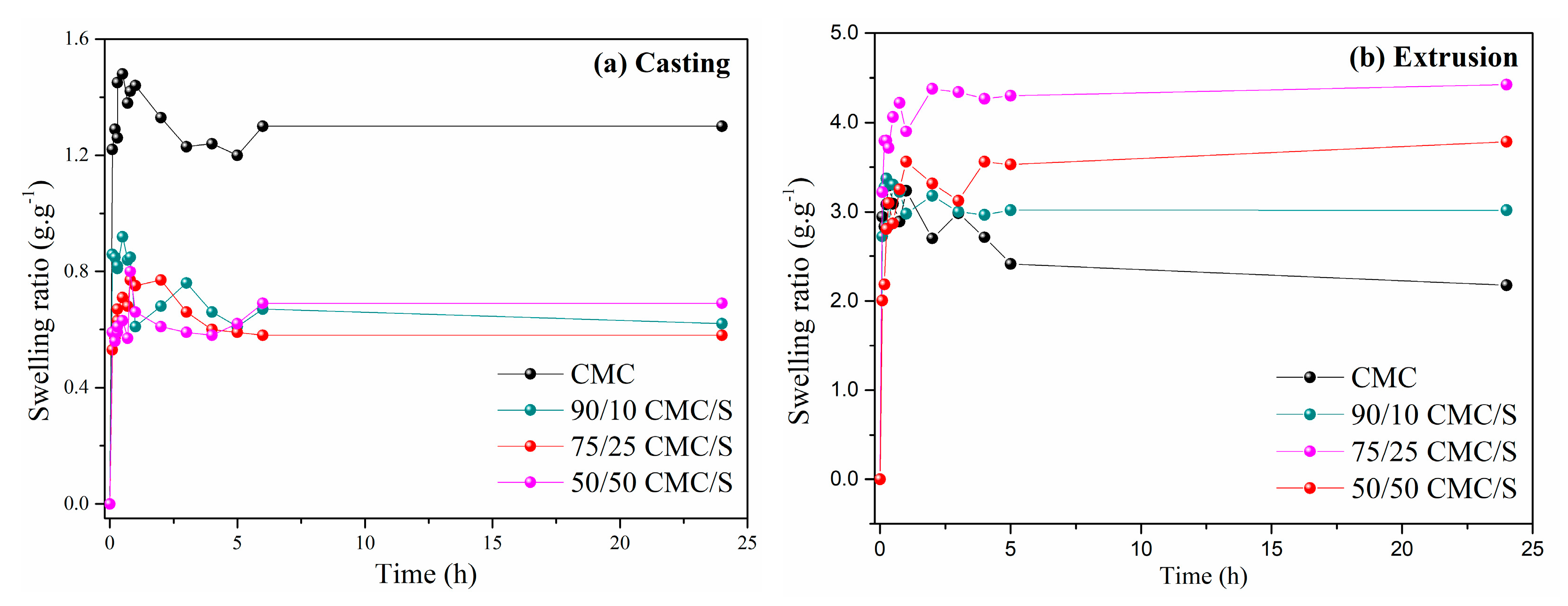
2.4. Degree of Swelling
2.5. Porosity Measurement
3. Conclusions
4. Materials and Methods
4.1. Reagents
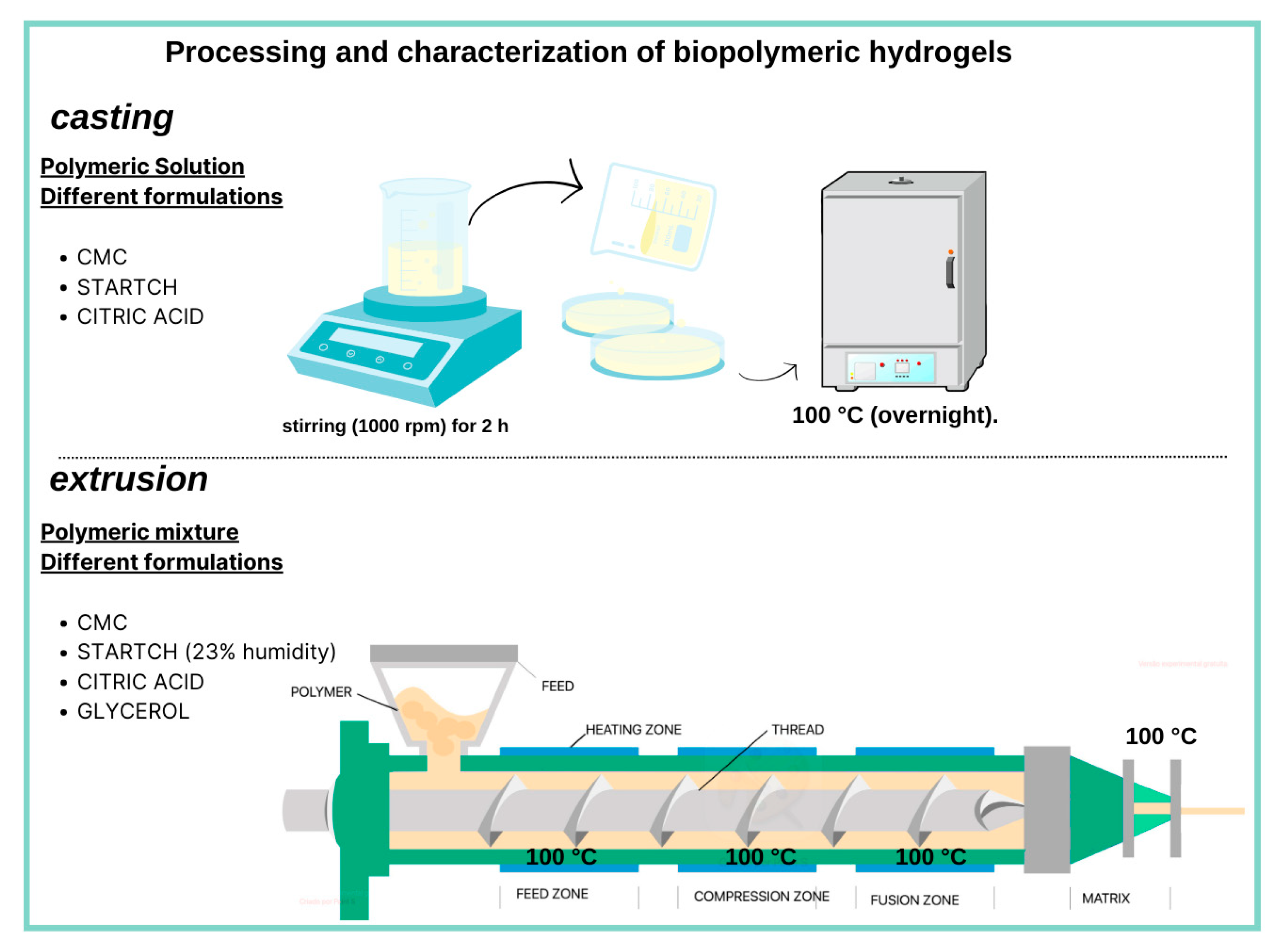
4.2. Preparation of Hydrogels with Carboxymethylcellulose (CMC) and Starch (S) by Casting
4.3. Preparation of Hydrogels with Carboxymethylcellulose (CMC) and Starch (S) by Extrusion
4.4. Fourier Transform Infrared Spectrometry (FTIR)
4.5. Thermal Properties
4.6. Reological Measurements
4.7. Scanning Electron Microscopy
4.8. X-Ray Diffraction
4.9. Degree of Swelling
4.10. Porosity Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, C.; Carmo, M. Why Nonconventional Materials Are Answers for Sustainable Agriculture. MRS Energy Sustain. 2019, 6, 7. Available online: https://www.cambridge.org/core/journals/mrs-energy-and-sustainability/article/abs/why-nonconventional-materials-are-answers-for-sustainable-agriculture/D56DD645DD2057C1F546BB6795E752D7 (accessed on 2 February 2025). [CrossRef]
- Wang, Y.; Torres, J.A.; Shviro, M.; Carmo, M.; He, T.; Ribeiro, C. Photocatalytic Materials Applications for Sustainable Agriculture. Prog. Mater. Sci. 2022, 130, 100965. [Google Scholar] [CrossRef]
- Kearney, J. Food Consumption Trends and Drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent Polymers Used for Agricultural Water Retention. Polym. Test. 2021, 94, 107021. [Google Scholar] [CrossRef]
- Tariq, Z.; Iqbal, D.N.; Rizwan, M.; Ahmad, M.; Faheem, M.; Ahmed, M. Significance of Biopolymer-Based Hydrogels and Their Applications in Agriculture: A Review in Perspective of Synthesis and Their Degree of Swelling for Water Holding. RSC Adv. 2023, 13, 24731–24754. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, S.; Jain, A.; Khan, S. 3-Dimensional Cross Linked Hydrophilic Polymeric Network “Hydrogels”: An Agriculture Boom. Agric. Water Manag. 2021, 253, 106939. [Google Scholar] [CrossRef]
- Felippe, D.; Navroski, M.C.; de Oliveira Pereira, M.; de Paula Baptista, K.R.S. Hydrogel in the Seedling Growth of Eucalyptus Dunnii Maiden under Different Irrigation Management. Rev. Ambiente Água 2021, 16, e2582. [Google Scholar] [CrossRef]
- Montesano, F.F.; Parente, A.; Santamaria, P.; Sannino, A.; Serio, F. Biodegradable Superabsorbent Hydrogel IncreasesWater Retention Properties of Growing Media and Plant Growth. Agric. Agric. Sci. Procedia 2015, 4, 451–458. [Google Scholar] [CrossRef]
- El-Asmar, J.; Jaafar, H.; Bashour, I.; Farran, M.T.; Saoud, I.P. Hydrogel Banding Improves Plant Growth, Survival, and Water Use Efficiency in Two Calcareous Soils. Clean 2017, 45, 1700251. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, M.; Luan, Q.; Tang, H.; Huang, F.; Xiang, X.; Yang, C.; Bao, Y. Cellulose Anionic Hydrogels Based on Cellulose Nanofibers As Natural Stimulants for Seed Germination and Seedling Growth. J. Agric. Food Chem. 2017, 65, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- González Gómez, H.; Ramírez Godina, F.; Ortega Ortiz, H.; Benavides Mendoza, A.; Robledo Torres, V.; Cabrera De la Fuente, M. Use of Chitosan-PVA Hydrogels with Copper Nanoparticles to Improve the Growth of Grafted Watermelon. Molecules 2017, 22, 1031. [Google Scholar] [CrossRef] [PubMed]
- Babaluei, M.; Mottaghitalab, F.; Seifalian, A.; Farokhi, M. Injectable Multifunctional Hydrogel Based on Carboxymethylcellulose/Polyacrylamide/Polydopamine Containing Vitamin C and Curcumin Promoted Full-Thickness Burn Regeneration. Int. J. Biol. Macromol. 2023, 236, 124005. [Google Scholar] [CrossRef] [PubMed]
- Gregorova, A.; Saha, N.; Kitano, T.; Saha, P. Hydrothermal Effect and Mechanical Stress Properties of Carboxymethylcellulose Based Hydrogel Food Packaging. Carbohydr. Polym. 2015, 117, 559–568. [Google Scholar] [CrossRef]
- Pereira, J.F.; Marim, B.M.; Simões, B.M.; Yamashita, F.; Mali, S. Hydrogels Based on Gelatin, Xanthan Gum, and Cellulose Obtained by Reactive Extrusion and Thermopressing Processes. Prep. Biochem. Biotechnol. 2023, 53, 942–953. [Google Scholar] [CrossRef]
- Simões, B.M.; Cagnin, C.; Yamashita, F.; Olivato, J.B.; Garcia, P.S.; de Oliveira, S.M.; Eiras Grossmann, M.V. Citric Acid as Crosslinking Agent in Starch/Xanthan Gum Hydrogels Produced by Extrusion and Thermopressing. LWT 2020, 125, 108950. [Google Scholar] [CrossRef]
- Madhumitha, G.; Fowsiya, J.; Mohana Roopan, S.; Kumar Thakur, V. Recent Advances in Starch-Clay Nanocomposites. Int. J. Polym. Anal. Charact. 2018, 23, 331–345. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.M.; Reid, D.S.; McHugh, T.H.; Berrios, J.D.J.; Krochta, J.M. Thermal Transitions and Extrusion of Glycerol-Plasticized Whey Protein Mixtures. J. Food Sci. 2008, 73, E169–E175. [Google Scholar] [CrossRef]
- Ochoa-Yepes, O.; Di Giogio, L.; Goyanes, S.; Mauri, A.; Famá, L. Influence of Process (Extrusion/Thermo-Compression, Casting) and Lentil Protein Content on Physicochemical Properties of Starch Films. Carbohydr. Polym. 2019, 208, 221–231. [Google Scholar] [CrossRef]
- Alam, M.S.; Pathania, S.; Sharma, A. Optimization of the Extrusion Process for Development of High Fibre Soybean-Rice Ready-to-Eat Snacks Using Carrot Pomace and Cauliflower Trimmings. LWT 2016, 74, 135–144. [Google Scholar] [CrossRef]
- Custódio, A.C.; Patrik, R.; Ribeiro, S.; Brunelle, T.; De Lima, S.; Silvano De Araújo, E.; Lopes Barros De Araújo, P. Purificação Simplificada Do Rejeito de Glicerina Bruta Da Produção de Biodiesel Da Biorrefinaria Berso-UFPE: Uma Prática Sustentável Simple Purification Process of Waste Glycerol from Berso-Ufpe Biorefinary Biodiesel Production: A Sustainable Practice. Rev. Bras. Geogr. Física 2022, 15, 2226–2237. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, B.; Li, C.; Kuang, W.; Zhang, J.; Xiong, Y.; Tan, S.; Cai, X.; Huang, L. Carboxymethyl Cellulose-Grafted Graphene Oxide for Efficient Antitumor Drug Delivery. Nanotechnol. Rev. 2018, 7, 291–301. [Google Scholar] [CrossRef]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose–Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-Based Bionanocomposite Films Incorporated with CMC and Nanoclay. Starch-Stärke 2020, 72, 1900121. [Google Scholar] [CrossRef]
- Parvaneh, S.; Pourmadadi, M.; Abdouss, M.; Pourmousavi, S.A.; Yazdian, F.; Rahdar, A.; Díez-Pascual, A.M. Carboxymethyl Cellulose/Starch/Reduced Graphene Oxide Composite as a PH-Sensitive Nanocarrier for Curcumin Drug Delivery. Int. J. Biol. Macromol. 2023, 241, 124566. [Google Scholar] [CrossRef] [PubMed]
- Simi, C.K.; Emilia Abraham, T. Hydrophobic Grafted and Cross-Linked Starch Nanoparticles for Drug Delivery. Bioprocess. Biosyst. Eng. 2007, 30, 173–180. [Google Scholar] [CrossRef]
- Cagnin, C.; Simões, B.M.; Yamashita, F.; Andrello, A.C.; de Carvalho, G.M.; Grossmann, M.V.E. Hydrogels of Starch/Carboxymethyl Cellulose Crosslinked with Sodium Trimetaphosphate via Reactive Extrusion. J. Appl. Polym. Sci. 2021, 138, 50194. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Rudhziah, S.; Ahmad, A.; Mohamed, N.S. Biopolymer Electrolyte Based on Derivatives of Cellulose from Kenaf Bast Fiber. Polymers 2014, 6, 2371–2385. [Google Scholar] [CrossRef]
- Biswal, D.R.; Singh, R.P. Characterisation of Carboxymethyl Cellulose and Polyacrylamide Graft Copolymer. Carbohydr. Polym. 2004, 57, 379–387. [Google Scholar] [CrossRef]
- Kumar, B.; Deeba, F.; Priyadarshi, R.; Sauraj; Bano, S.; Kumar, A.; Negi, Y.S. Development of Novel Cross-Linked Carboxymethyl Cellulose/Poly(Potassium 1-Hydroxy Acrylate): Synthesis, Characterization and Properties. Polym. Bull. 2020, 77, 4555–4570. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Tohamy, H.-A.S.; Salama, A.; Kamel, S. Thermal Properties of Carboxymethyl Cellulose Acetate Butyrate. Cellulose Chem. Technol. 2019, 53, 667–675. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of Biodegradable Thermoplastic Pea Starch/Carboxymethyl Cellulose and Pea Starch/Microcrystalline Cellulose Composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Rohr, T.G. Ufrrj Instituto de Tecnologia Curso de Pós-Graduação em Engenharia Química Dissertação Estudo Reológico da Mistura Carboximetilcelulose/Amido e sua Utilização como Veículo de Inoculação Bacteriano. 2007. Available online: https://rima.ufrrj.br/jspui/handle/20.500.14407/13438 (accessed on 2 February 2025).
- Duquette, D.; Nzediegwu, C.; Portillo-Perez, G.; Dumont, M.J.; Prasher, S. Eco-Friendly Synthesis of Hydrogels from Starch, Citric Acid, and Itaconic Acid: Swelling Capacity and Metal Chelation Properties. Starch/Staerke 2020, 72, 1900008. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels: Present Challenges and Future Opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, P.; Zhang, J.; Wang, A. Study on Superabsorbent Composite XVI. Synthesis, Characterization and Swelling Behaviors of Poly(Sodium Acrylate)/Vermiculite Superabsorbent Composites. Eur. Polym. J. 2007, 43, 1691–1698. [Google Scholar] [CrossRef]
- Kirschner, C.M.; Anseth, K.S. Hydrogels in Healthcare: From Static to Dynamic Material Microenvironments. Acta Mater. 2013, 61, 931–944. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Luo, S.; Liu, W.; Chen, J.; Zeng, Z.; Liu, C. Properties of Starch after Extrusion: A Review. Starch-Stärke 2018, 70, 1700110. [Google Scholar] [CrossRef]
- Karathanos, V.T.; Saravacos, G.D. Porosity and Pore Size Distribution of Starch Materials. J. Food Eng. 1993, 18, 259–280. [Google Scholar] [CrossRef]
- De Albuquerque, G.; Neto, A.; Monteiro, M.; Dantas, I.; Da Silva, V.; Menegaz, C.; Cristiano, Z. Estudos de Degradação Térmica e no Solo de Filmes Formados por Goma Xantana e Carboximetilcelulose Reticulados com Ácido Cítrico. Rev. Iberoam. Polímeros y Mater. 2019, 20, 82–89. Available online: https://reviberpol.org/wp-content/uploads/2019/05/2019-20-2-82-89-andrade-y-col.pdf (accessed on 2 February 2025).
- Marim, B.M.; Mantovan, J.; Gil-Giraldo, G.A.; Pereira, J.F.; Simões, B.M.; Yamashita, F.; Mali, S. Reactive Extrusion-Assisted Process to Obtain Starch Hydrogels through Reaction with Organic Acids. Polysaccharides 2022, 3, 792–803. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsuo, K.; Fujioka, R. Novel Biodegradable Superabsorbent Hydrogels Derived from Cotton Cellulose and Succinic Anhydride: Synthesis and Characterization. J. Appl. Polym. Sci. 2006, 99, 3251–3256. [Google Scholar] [CrossRef]
- Vedovello, P.; Fernandes, C.; Tiritan, M.E.; Paranhos, C.M. Chiral Mixed Matrix Membranes with the (S,S)-Whelk-O®1 Selector Supported on Polyethersulfone/MCM-41. New J. Chem. 2024, 48, 9522–9533. [Google Scholar] [CrossRef]
- Chavda, H.; Patel, C. Effect of Crosslinker Concentration on Characteristics of Superporous Hydrogel. Int. J. Pharm. Investig. 2011, 1, 17. [Google Scholar] [CrossRef]



| Sample | CMC | Starch | Citric Acid |
|---|---|---|---|
| CMC | 100 | 0 | 20 |
| CMC/S | 90 | 10 | 20 |
| CMC/S | 75 | 25 | 20 |
| CMC/S | 50 | 50 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedovello, P.; Paiva, R.S.; Bortoletto-Santos, R.; Ribeiro, C.; Putti, F.F. The Effect of the Conformation Process on the Physicochemical Properties of Carboxymethylcellulose–Starch Hydrogels. Gels 2025, 11, 183. https://doi.org/10.3390/gels11030183
Vedovello P, Paiva RS, Bortoletto-Santos R, Ribeiro C, Putti FF. The Effect of the Conformation Process on the Physicochemical Properties of Carboxymethylcellulose–Starch Hydrogels. Gels. 2025; 11(3):183. https://doi.org/10.3390/gels11030183
Chicago/Turabian StyleVedovello, Priscila, Robert Silva Paiva, Ricardo Bortoletto-Santos, Caue Ribeiro, and Fernando Ferrari Putti. 2025. "The Effect of the Conformation Process on the Physicochemical Properties of Carboxymethylcellulose–Starch Hydrogels" Gels 11, no. 3: 183. https://doi.org/10.3390/gels11030183
APA StyleVedovello, P., Paiva, R. S., Bortoletto-Santos, R., Ribeiro, C., & Putti, F. F. (2025). The Effect of the Conformation Process on the Physicochemical Properties of Carboxymethylcellulose–Starch Hydrogels. Gels, 11(3), 183. https://doi.org/10.3390/gels11030183










