Spray-Drying Microencapsulation of Grape Pomace Extracts with Alginate-Based Coatings and Bioaccessibility of Phenolic Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Encapsulation Efficiency
2.2. Physicochemical Properties of Microcapsules
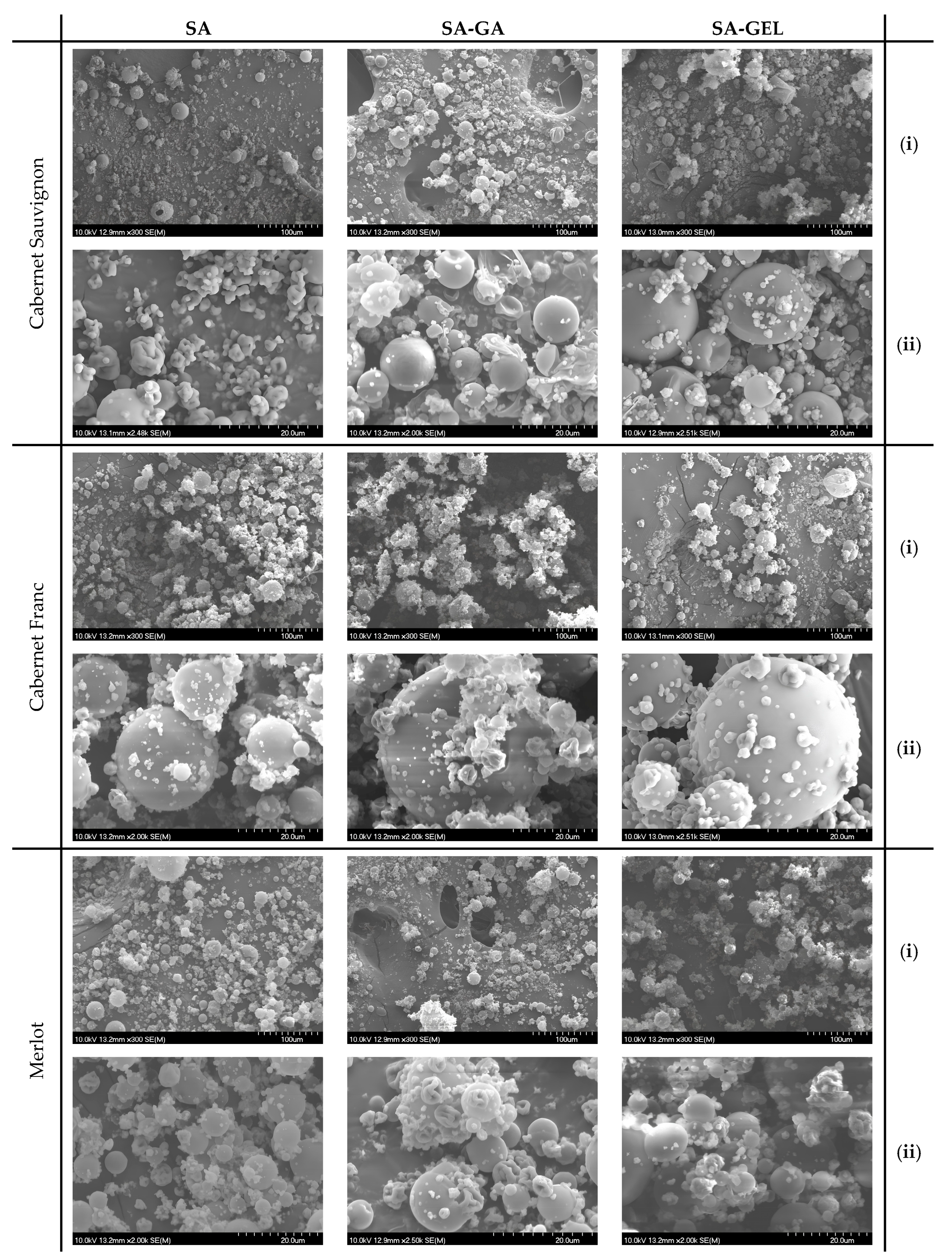
2.2.1. Scanning Electron Microscopy of Microcapsules
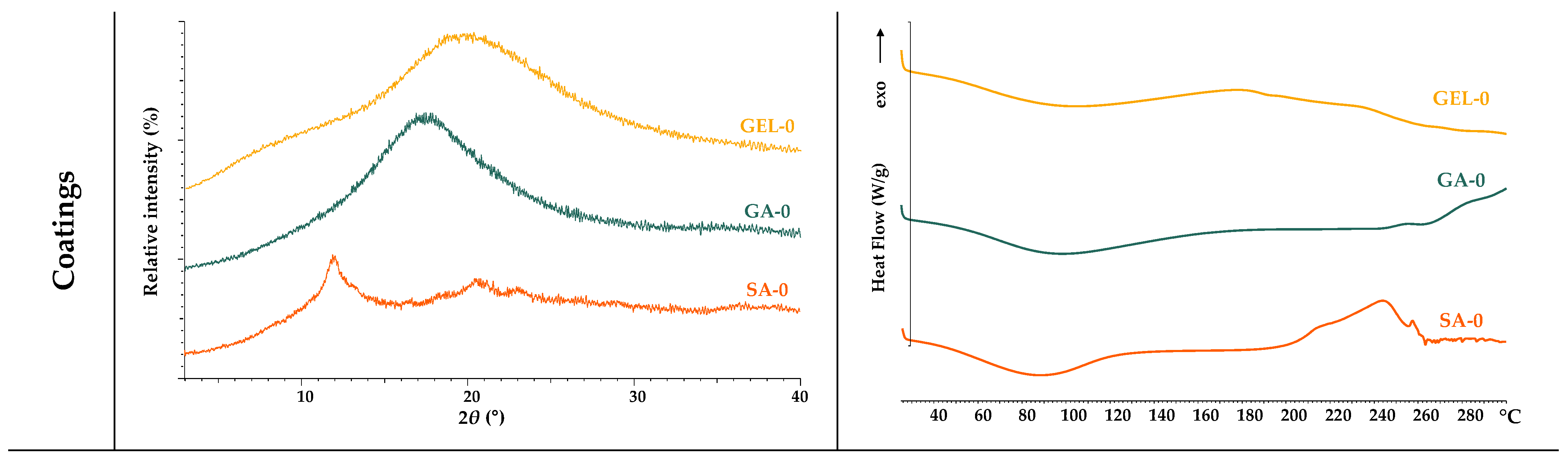
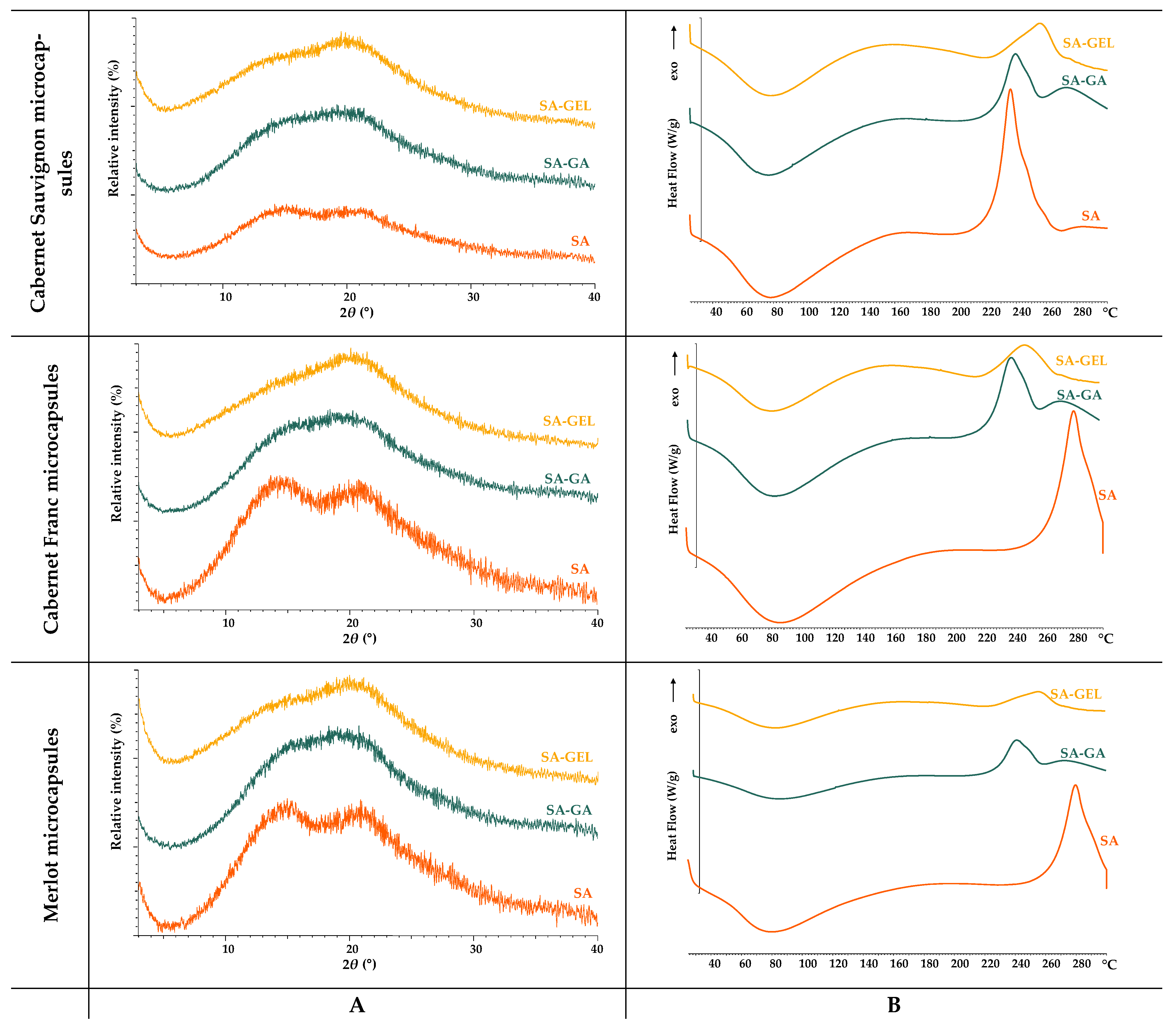
2.2.2. Thermal and Structural Analysis of Coatings and Microcapsules
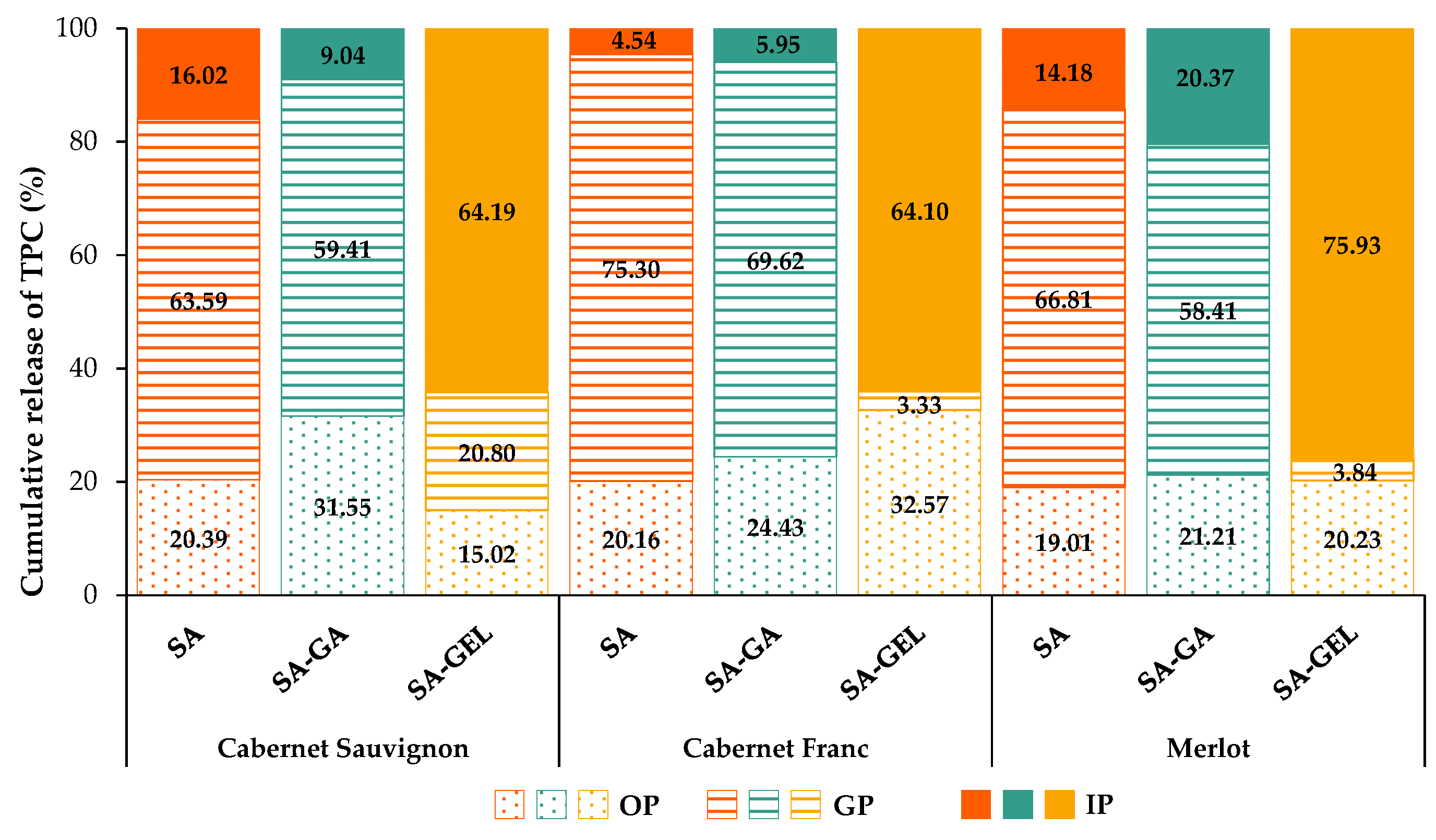
2.3. Enzyme-Free Release Study of Phenolic Compounds
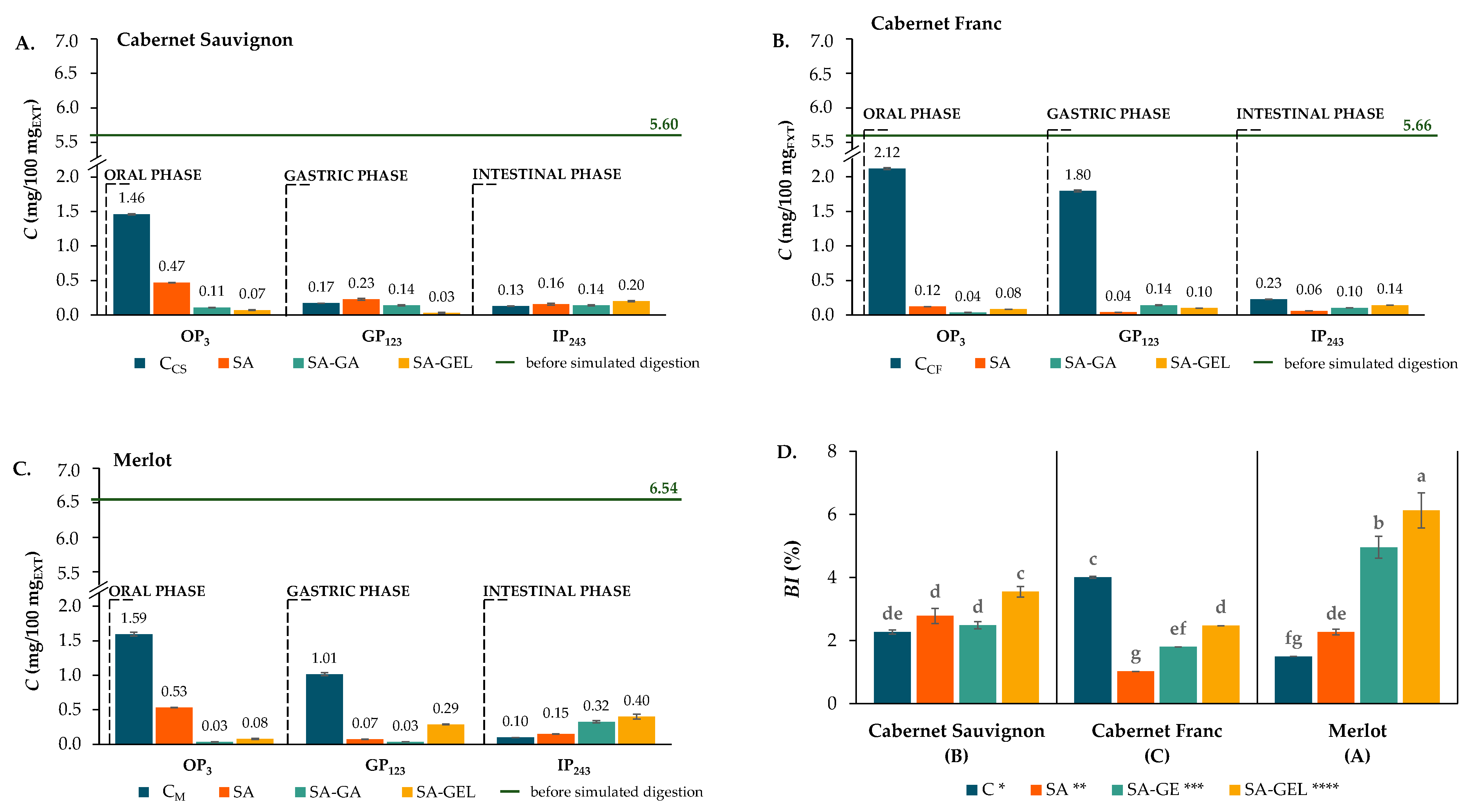
2.4. Bioaccessibility of Phenolic Compounds
2.4.1. Total Phenolics, Total Flavonoids and Total Proanthocyanidins
2.4.2. Bioaccessibility of Individual Phenolics
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Extraction of Phenolic Compounds from Grape Pomace
4.3. Spray-Drying Microencapsulation
4.4. Characterization of Microcapsules
4.4.1. Encapsulation Efficiency Determination
4.4.2. Scanning Electron Microscopy
4.4.3. Particle Size Analysis
4.4.4. X-Ray Powder Diffraction
4.4.5. Differential Scanning Calorimetry
4.5. Determination of Phenolic Compounds
4.5.1. Total Phenolic Content
4.5.2. Total Flavonoid Content
4.5.3. Total Extractable Proanthocyanidin Content
4.5.4. Individual Phenolic Compound Determination
4.6. Phenolic Compound Release and Bioaccessibility Studies
4.6.1. Enzyme-Free Release Study
4.6.2. In Vitro Simulated Digestion and Determination of Bioaccessibility Index
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; Castro-López, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Martínez-Ávila, G.C.G. Grape Pomace—Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. ISBN 978-0-12-814774-0. [Google Scholar]
- Mišković Špoljarić, K.; Šelo, G.; Pešut, E.; Martinović, J.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Antioxidant and Antiproliferative Potentials of Phenolic-Rich Extracts from Biotransformed Grape Pomace in Colorectal Cancer. BMC Complement. Med. Ther. 2023, 23, 29. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape Pomace as a Source of Phenolic Compounds and Diverse Bioactive Properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Hassan, S.T.S.; Tenore, G.C.; Novellino, E. Effect of Grape Pomace Polyphenols With or Without Pectin on TMAO Serum Levels Assessed by LC/MS-Based Assay: A Preliminary Clinical Study on Overweight/Obese Subjects. Front. Pharmacol. 2019, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Parisi, O.I.; Puoci, F.; Restuccia, D.; Farina, G.; Iemma, F.; Picci, N. Polyphenols and Their Formulations. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 29–45. ISBN 978-0-12-398456-2. [Google Scholar]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Dumont, M.-J.; Orsat, V. Encapsulation of Phenolic Compounds Present in Plants Using Protein Matrices. Food Biosci. 2016, 15, 87–104. [Google Scholar] [CrossRef]
- Neilson, A.P.; Goodrich, K.M.; Ferruzzi, M.G. Bioavailability and Metabolism of Bioactive Compounds From Foods. In Nutrition in the Prevention and Treatment of Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 301–319. ISBN 978-0-12-802928-2. [Google Scholar]
- Lorenzo, J.M.; Estévez, M.; Barba, F.J.; Thirumdas, R.; Franco, D.; Munekata, P.E.S. Polyphenols: Bioaccessibility and Bioavailability of Bioactive Components. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–332. ISBN 978-0-12-814174-8. [Google Scholar]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and Delivery of Bioactive Compounds Using Spray and Freeze-Drying Techniques: A Review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Bernal-Millán, M.D.J.; Gutiérrez-Grijalva, E.P.; Contreras-Angulo, L.; Muy-Rangel, M.D.; López-Martínez, L.X.; Heredia, J.B. Spray-Dried Microencapsulation of Oregano (Lippia Graveolens) Polyphenols with Maltodextrin Enhances Their Stability during In Vitro Digestion. J. Chem. 2022, 2022, 8740141. [Google Scholar] [CrossRef]
- Perković, G.; Planinić, M.; Šelo, G.; Martinović, J.; Nedić, R.; Puš, M.; Bucić-Kojić, A. Optimisation of the Encapsulation of Grape Pomace Extract by Spray Drying Using Goat Whey Protein as a Coating Material. Coatings 2024, 14, 1101. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef]
- Gim, S.; Zhu, Y.; Seeberger, P.H.; Delbianco, M. Carbohydrate-based Nanomaterials for Biomedical Applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1558. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Peighambardoust, S.H.; Sarabandi, K.; Jafari, S.M. Spray Drying Encapsulation of Bioactive Compounds within Protein-Based Carriers; Different Options and Applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef]
- Madkhali, O.A. Drug Delivery of Gelatin Nanoparticles as a Biodegradable Polymer for the Treatment of Infectious Diseases: Perspectives and Challenges. Polymers 2023, 15, 4327. [Google Scholar] [CrossRef] [PubMed]
- Kieserling, H.; De Bruijn, W.J.C.; Keppler, J.; Yang, J.; Sagu, S.T.; Güterbock, D.; Rawel, H.; Schwarz, K.; Vincken, J.; Schieber, A.; et al. Protein–Phenolic Interactions and Reactions: Discrepancies, Challenges, and Opportunities. Comp. Rev. Food Sci. Food Safe. 2024, 23, e70015. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. In Vitro Bioaccessibility Assessment of Phenolic Compounds from Encapsulated Grape Pomace Extract by Ionic Gelation. Molecules 2023, 28, 5285. [Google Scholar] [CrossRef]
- Martinović, J.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. Microencapsulation of Grape Pomace Extracts with Alginate-Based Coatings by Freeze-Drying: Release Kinetics and In Vitro Bioaccessibility Assessment of Phenolic Compounds. Gels 2024, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, Y.; Chen, M.; Ren, Z.; Chen, L.; Fu, C.; Ma, Z.F.; Li, Z. Advancement of Protein- and Polysaccharide-Based Biopolymers for Anthocyanin Encapsulation. Front. Nutr. 2022, 9, 938829. [Google Scholar] [CrossRef]
- Cortés-Morales, E.A.; Mendez-Montealvo, G.; Velazquez, G. Interactions of the Molecular Assembly of Polysaccharide-Protein Systems as Encapsulation Materials. A Review. Adv. Colloid Interface Sci. 2021, 295, 102398. [Google Scholar] [CrossRef]
- Motlagh, S.; Ravines, P.; Karamallah, K.A.; Ma, Q. The Analysis of Acacia Gums Using Electrophoresis. Food Hydrocoll. 2006, 20, 848–854. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. Optimization of the Arabic Gum Based Edible Coating Formulations with Sodium Caseinate and Tulsi Extract for Guava. LWT—Food Sci. Technol. 2017, 80, 271–279. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe Oleraceae Mart.) Powder Produced by Spray Drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Botrel, D.A.; De Barros Fernandes, R.V.; Borges, S.V.; Yoshida, M.I. Influence of Wall Matrix Systems on the Properties of Spray-Dried Microparticles Containing Fish Oil. Int. Food Res. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Cao, J.; Zhao, S.; Wang, W. Microencapsulation of Ginger Volatile Oil Based on Gelatin/Sodium Alginate Polyelectrolyte Complex. Chem. Pharm. Bull. 2016, 64, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhong, Q. The Improved Thermal Stability of Anthocyanins at pH 5.0 by Gum Arabic. LWT—Food Sci. Technol. 2015, 64, 706–712. [Google Scholar] [CrossRef]
- Barra, P.A.; Márquez, K.; Gil-Castell, O.; Mujica, J.; Ribes-Greus, A.; Faccini, M. Spray-Drying Performance and Thermal Stability of L-Ascorbic Acid Microencapsulated with Sodium Alginate and Gum Arabic. Molecules 2019, 24, 2872. [Google Scholar] [CrossRef]
- Dai, C.; Chen, Y.; Liu, M. Thermal Properties Measurements of Renatured Gelatin Using Conventional and Temperature Modulated Differential Scanning Calorimetry. J. Appl. Polym. Sci. 2006, 99, 1795–1801. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Mohan, A.M. Novel pH Switchable Gelatin Based Hydrogel for the Controlled Delivery of the Anti Cancer Drug 5-Fluorouracil. RSC Adv. 2014, 4, 12109. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for Preservation of Functionality and Targeted Delivery of Bioactive Food Components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Gu, L. Absorption and Metabolism of Proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Niwano, Y.; Kohzaki, H.; Shirato, M.; Shishido, S.; Nakamura, K. Metabolic Fate of Orally Ingested Proanthocyanidins through the Digestive Tract. Antioxidants 2022, 12, 17. [Google Scholar] [CrossRef]
- Giuliani, C.; Marzorati, M.; Innocenti, M.; Vilchez-Vargas, R.; Vital, M.; Pieper, D.H.; Van De Wiele, T.; Mulinacci, N. Dietary Supplement Based on Stilbenes: A Focus on Gut Microbial Metabolism by the in Vitro Simulator M-SHIME®. Food Funct. 2016, 7, 4564–4575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Ensunsa, J.L.; Hammerstone, J.F.; Schmitz, H.H.; Keen, C.L. Stability of the Flavan-3-Ols Epicatechin and Catechin and Related Dimeric Procyanidins Derived from Cocoa. J. Agric. Food Chem. 2002, 50, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.G.; Cazarin, C.B.B.; Marostica Junior, M.R. Anthocyanins: New Techniques and Challenges in Microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food Processing Strategies to Enhance Phenolic Compounds Bioaccessibility and Bioavailability in Plant-Based Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Sugier, D.; Świeca, M.; Gawlik-Dziki, U. The Effect of in Vitro Digestion, Food Matrix, and Hydrothermal Treatment on the Potential Bioaccessibility of Selected Phenolic Compounds. Food Chem. 2021, 344, 128581. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.-S.; Vallons, K.J.R.; Arendt, E.K. Influence of Gallic Acid and Tannic Acid on the Mechanical and Barrier Properties of Wheat Gluten Films. J. Agric. Food Chem. 2012, 60, 6157–6163. [Google Scholar] [CrossRef]
- Asghari-Varzaneh, E.; Sharifian-Mobarakeh, S.; Shekarchizadeh, H. Enhancing Hamburger Shelf Life and Quality Using Gallic Acid Encapsulated in Gelatin/Tragacanth Gum Complex Coacervate. Heliyon 2024, 10, e24917. [Google Scholar] [CrossRef]
- De Souza, A.G.; Cesco, C.T.; De Lima, G.F.; Artifon, S.E.S.; Rosa, D.D.S.; Paulino, A.T. Arabic Gum-Based Composite Hydrogels Reinforced with Eucalyptus and Pinus Residues for Controlled Phosphorus Release. Int. J. Biol. Macromol. 2019, 140, 33–42. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of Natural Polymer Gum Arabic: A Review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Martinović, J.; Perković, G.; Bucić-Kojić, A. Bioconversion of Grape Pomace with Rhizopus Oryzae under Solid-State Conditions: Changes in the Chemical Composition and Profile of Phenolic Compounds. Microorganisms 2023, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Encapsulation of Phenolic-Rich Extract from Banana (Musa Cavendish) Peel. J. Food Sci. Technol. 2020, 57, 2089–2098. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of Grape Polyphenols Using Maltodextrin and Gum Arabic as Two Alternative Coating Materials: Development and Characterization. J. Biotech. 2016, 239, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons Inc.: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total Phenolics and Total Flavonoids in Bulgarian Fruits and Vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Šelo, G.; Zelić, B.; Planinić, M.; Tišma, M. Recovery of Phenolic Acid and Enzyme Production from Corn Silage Biologically Treated by Trametes Versicolor. Appl. Biochem. Biotechnol. 2017, 181, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Bucić-Kojić, A.; Fernandes, F.; Silva, T.; Planinić, M.; Šelo, G.; Šibalić, D.; Pereira, D.M.; Andrade, P.B. Enhancement of the Anti-Inflammatory Properties of Grape Pomace Treated by Trametes Versicolor. Food. Funct. 2020, 11, 680–688. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Ozkan, G.; Isik, H.; Horoz, O.; Van Camp, J.; Capanoglu, E. Black Carrot Pomace as a Source of Polyphenols for Enhancing the Nutritional Value of Cake: An in Vitro Digestion Study with a Standardized Static Model. LWT—Food Sci. Technol. 2017, 77, 475–481. [Google Scholar] [CrossRef]



| Grape Variety | Coatings | Mean Diameter (µm) | D10 (µm) | D50 (µm) | D90 (µm) | Range (µm) |
|---|---|---|---|---|---|---|
| Cabernet Sauvignon | SA | 3.96 ± 2.20 | 2.08 | 3.03 | 6.95 | 1.97–11.98 |
| SA-GA | 2.61 ± 1.73 | 1.25 | 1.95 | 5.36 | 1.19–10.69 | |
| SA-GEL | 2.62 ± 1.91 | 1.24 | 1.82 | 5.35 | 1.18–12.02 | |
| Cabernet Franc | SA | 4.29 ± 2.26 | 2.10 | 3.46 | 7.37 | 1.97–11.90 |
| SA-GA | 2.59 ± 1.71 | 1.24 | 1.82 | 5.51 | 1.18–10.08 | |
| SA-GEL | 2.58 ± 1.81 | 1.24 | 1.87 | 5.21 | 1.18–12.17 | |
| Merlot | SA | 2.82 ± 2.12 | 1.24 | 1.95 | 5.81 | 1.19–12.23 |
| SA-GA | 2.53 ± 1.69 | 1.24 | 1.86 | 4.94 | 1.18–11.56 | |
| SA-GEL | 2.38 ± 1.72 | 1.18 | 1.67 | 4.57 | 1.18–12.31 |
| Mathematical Models | Release Rate Constants and Statistical Criteria of Model Approximation Success | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cabernet Sauvignon | Cabernet Franc | Merlot | ||||||||
| SA | SA-GA | SA-GEL | SA | SA-GA | SA-GEL | SA | SA-GA | SA-GEL | ||
| First order model | R2adj | 0.855 | 0.779 | 0.784 | 0.937 | 0.916 | 0.759 | 0.929 | 0.899 | 0.765 |
| AIC | 123.918 | 130.018 | 128.235 | 113.001 | 117.112 | 131.785 | 114.313 | 119.575 | 133.050 | |
| MSC | 1.421 | 0.813 | 1.225 | 2.272 | 1.921 | 1.115 | 2.140 | 1.846 | 1.198 | |
| k1 | 0.031 | 0.041 | 0.008 | 0.042 | 0.042 | 0.010 | 0.036 | 0.029 | 0.009 | |
| Higuchi model | R2adj | 0.707 | 0.418 | 0.852 | 0.788 | 0.693 | 0.833 | 0.806 | 0.887 | 0.801 |
| AIC | 135.664 | 145.806 | 122.179 | 132.628 | 138.007 | 125.930 | 130.329 | 121.545 | 130.400 | |
| MSC | 0.687 | −0.174 | 1.603 | 1.046 | 0.615 | 1.481 | 1.139 | 1.723 | 1.363 | |
| kH | 8.034 | 8.588 | 5.537 | 8.354 | 8.543 | 5.849 | 8.081 | 7.776 | 5.438 | |
| Hixson–Crowell model | R2adj | 0.006 | 0.362 | 0.721 | 0.744 | 0.639 | 0.727 | 0.772 | 0.816 | 0.721 |
| AIC | 0.679 | 147.195 | 132.287 | 135.667 | 140.634 | 133.734 | 132.901 | 129.430 | 135.799 | |
| MSC | 137.111 | −0.261 | 0.971 | 0.856 | 0.451 | 0.993 | 0.979 | 1.230 | 1.026 | |
| kHC | 0.596 | 0.007 | 0.003 | 0.007 | 0.007 | 0.003 | 0.007 | 0.006 | 0.003 | |
| Korsmeyer– Peppas model | R2adj | 0.913 | 0.886 | 0.741 | 0.872 | 0.873 | 0.688 | 0.939 | 0.974 | 0.652 |
| AIC | 117.081 | 119.646 | 132.021 | 122.718 | 122.287 | 136.782 | 112.575 | 98.676 | 140.272 | |
| MSC | 1.848 | 1.461 | 0.988 | 1.665 | 1.598 | 0.803 | 2.249 | 3.152 | 0.746 | |
| kKP | 20.189 | 35.888 | 10.596 | 18.357 | 26.022 | 12.519 | 21.047 | 17.533 | 7.605 | |
| n | 0.319 | 0.204 | 0.341 | 0.356 | 0.272 | 0.313 | 0.316 | 0.335 | 0.391 | |
| Phenolics | Sample | BI (%) * | ||||||
|---|---|---|---|---|---|---|---|---|
| CS | CF | M | ||||||
| Phenolic acids | Gallic acid | C | 22.4 ± 0.8 | c | 108.8 ± 4.9 | b | 47.4 ± 0.3 | c |
| SA | 42.8 ± 3.7 | c | 36.9 ± 0.4 | c | 26.9 ± 0.7 | c | ||
| SA-GA | 117.0 ± 4.4 | b | 18.2 ± 0.0 | c | 222.6 ± 7.3 | a | ||
| SA-GEL | 215.5 ± 4.4 | a | 95.7 ± 0.4 | a | 218.5 ± 27.7 | a | ||
| 3,4-Dihydroxybenzoic acid | C | 18.3 ± 0.6 | e | 70.6 ± 0.4 | cd | 12.3 ± 0.2 | e | |
| SA | 61.7 ± 2.9 | d | 67.1 ± 3.7 | cd | 66.9 ± 1.2 | cd | ||
| SA-GA | 77.0 ± 7.5 | c | 74.3 ± 2.8 | cd | 157.8 ± 2.7 | b | ||
| SA-GEL | 226.6 ± 3.8 | a | 149.0 ± 3.8 | b | 236.8 ± 5.7 | a | ||
| Syringic acid | C | 0.0 | d | 0.0 | d | 0.0 | d | |
| SA | 0.0 | d | 2.7 ± 0.0 | cd | 6.8 ± 0.2 | c | ||
| SA-GA | 0.0 | d | 1.6 ± 0.1 | cd | 94.0 ± 1.5 | a | ||
| SA-GEL | 0.0 | d | 0.0 | d | 28.0 ± 5.5 | b | ||
| Vanillic acid | C | 22.9 ± 1.1 | f | 29.6 ± 0.0 | f | 22.1 ± 0.7 | f | |
| SA | 53.0 ± 0.7 | de | 9.5 ± 0.8 | g | 49.3 ± 0.0 | e | ||
| SA-GA | 58.5 ± 2.0 | d | 22.6 ± 0.5 | f | 144.4 ± 4.1 | a | ||
| SA-GEL | 87.6 ± 2.8 | b | 24.0 ± 0.0 | f | 72.4 ± 4.6 | c | ||
| Ellagic acid | C | 1.7 ± 0.1 | d | 1.6 ± 0.0 | d | 15.5 ± 0.5 | cd | |
| SA | 9.0 ± 0.4 | d | 0.0 | d | 70.2 ± 2.3 | b | ||
| SA-GA | 15.0 ± 1.8 | cd | 7.9 ± 0.2 | d | 90.6 ± 7.7 | ab | ||
| SA-GEL | 38.1 ± 0.2 | c | 8.4 ± 0.4 | d | 107.9 ± 18.5 | a | ||
| o-Coumaric acid | C | 78.7 ± 12.4 | d | 48.1 ± 0.3 | d | 61.7 ± 0.6 | d | |
| SA | 191.0 ± 15.8 | c | 63.8 ± 3.0 | d | 57.8 ± 14.3 | d | ||
| SA-GA | 287.5 ± 37.2 | bc | 19.5 ± 1.7 | d | 325.7 ± 6.6 | b | ||
| SA-GEL | 337.2 ± 31.5 | b | 54.8 ± 1.8 | d | 464.2 ± 66.8 | a | ||
| p-Coumaric acid | C | 87.0 ± 3.3 | b | 272.8 ± 49.0 | a | 65.4 ± 2.5 | b | |
| SA | 0.0 | c | 0.0 | c | 0.0 | c | ||
| SA-GA | 0.0 | c | 0.0 | c | 0.0 | c | ||
| SA-GEL | 0.0 | c | 0.0 | c | 0.0 | c | ||
| Flavanols | Epicatechin | C | 49.8 ± 1.1 | c | 0.0 | d | 15.6 ± 0.2 | d |
| SA | 57.2 ± 1.2 | c | 3.6 ± 0.1 | d | 0.0 | d | ||
| SA-GA | 97.5 ± 13.0 | b | 4.9 ± 0.8 | d | 53.5 ± 0.7 | c | ||
| SA-GEL | 139.2 ± 1.8 | a | 8.8 ± 0.4 | d | 83.6 ± 4.7 | b | ||
| Epicatechin gallate | C | 116.8 ± 13.2 | cd | 98.8 ± 0.2 | cde | 88.7 ± 8.8 | cde | |
| SA | 113.6 ± 24.0 | cde | 5.8 ± 0.3 | e | 367.8 ± 45.1 | ab | ||
| SA-GA | 186.3 ± 28.5 | c | 36.7 ± 3.7 | de | 461.0 ± 25.8 | ab | ||
| SA-GEL | 356.4 ± 10.2 | b | 19.6 ± 1.6 | de | 476.4 ± 68.3 | a | ||
| Gallocatechin gallate | C | 251.4 ± 10.4 | h | 150.0 ± 4.7 | j | 234.0 ± 0.2 | hi | |
| SA | 692.4 ± 22.1 | e | 120.3 ± 0.5 | j | 580.7 ± 17.3 | f | ||
| SA-GA | 980.7 ± 17.9 | d | 197.6 ± 8.7 | i | 1342.7 ± 10.3 | c | ||
| SA-GEL | 2028.7 ± 15.0 | a | 348.2 ± 0.9 | g | 1970.9 ± 1.7 | b | ||
| Procyanidin B1 | C | 0.0 | d | 0.0 | d | 0.0 | d | |
| SA | 71.2 ± 8.1 | c | 0.0 | d | 48.4 ± 4.5 | c | ||
| SA-GA | 130.4 ± 4.1 | b | 0.0 | d | 119.1 ± 7.1 | b | ||
| SA-GEL | 155.1 ± 6.2 | a | 0.0 | d | 135.4 ± 14.9 | ab | ||
| Procyanidin B2 | C | 0.0 | b | 0.00 | b | 0.0 | b | |
| SA | 0.0 | b | 11.1 ± 1.5 | b | 0.0 | b | ||
| SA-GA | 0.0 | b | 9.7 ± 0.1 | b | 283.8 ± 10.5 | a | ||
| SA-GEL | 0.0 | b | 0.0 | b | 0.0 | b | ||
| Flavonols | Rutin | C | 0.0 | b | 29.0 ± 2.8 | a | 0.0 | b |
| SA | 0.0 | b | 0.0 | b | 0.0 | b | ||
| SA-GA | 0.0 | b | 0.0 | b | 0.0 | b | ||
| SA-GEL | 0.0 | b | 0.0 | b | 0.0 | b | ||
| Anthocyanins | Oenin chloride | C | 34.4 ± 2.8 | bc | 32.7 ± 2.9 | bc | 62.6 ± 4.5 | a |
| SA | 9.1 ± 1.4 | e | 4.7 ± 0.1 | e | 14.9 ± 0.2 | de | ||
| SA-GA | 28.0 ± 3.6 | cd | 3.4 ± 0.3 | e | 40.1 ± 0.0 | bc | ||
| SA-GEL | 63.9 ± 10.5 | a | 10.2 ± 0.7 | e | 44.7 ± 4.6 | b | ||
| Peonidin-3-O-glucoside chloride | C | 24.7 ± 4.3 | b | 24.0 ± 2.9 | b | 42.4 ± 5.9 | a | |
| SA | 0.0 | c | 2.5 ± 0.3 | c | 0.0 | c | ||
| SA-GA | 0.0 | c | 0.0 | c | 0.0 | c | ||
| SA-GEL | 0.0 | c | 0.0 | c | 0.0 | c | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinović, J.; Ambrus, R.; Planinić, M.; Perković, G.; Šelo, G.; Klarić, A.-M.; Bucić-Kojić, A. Spray-Drying Microencapsulation of Grape Pomace Extracts with Alginate-Based Coatings and Bioaccessibility of Phenolic Compounds. Gels 2025, 11, 130. https://doi.org/10.3390/gels11020130
Martinović J, Ambrus R, Planinić M, Perković G, Šelo G, Klarić A-M, Bucić-Kojić A. Spray-Drying Microencapsulation of Grape Pomace Extracts with Alginate-Based Coatings and Bioaccessibility of Phenolic Compounds. Gels. 2025; 11(2):130. https://doi.org/10.3390/gels11020130
Chicago/Turabian StyleMartinović, Josipa, Rita Ambrus, Mirela Planinić, Gabriela Perković, Gordana Šelo, Ana-Marija Klarić, and Ana Bucić-Kojić. 2025. "Spray-Drying Microencapsulation of Grape Pomace Extracts with Alginate-Based Coatings and Bioaccessibility of Phenolic Compounds" Gels 11, no. 2: 130. https://doi.org/10.3390/gels11020130
APA StyleMartinović, J., Ambrus, R., Planinić, M., Perković, G., Šelo, G., Klarić, A.-M., & Bucić-Kojić, A. (2025). Spray-Drying Microencapsulation of Grape Pomace Extracts with Alginate-Based Coatings and Bioaccessibility of Phenolic Compounds. Gels, 11(2), 130. https://doi.org/10.3390/gels11020130








