Strategies for Regulating the Release Kinetics of Bioactive Compounds from Biopolymeric Hydrogels
Abstract
1. Introduction
2. Absorption of Bioactive Compounds
3. Factors Influencing Stability and Bioavailability of Natural Bioactive Absorption of Bioactive Compounds
4. Entrapment of Bioactive Compounds
4.1. Polymers Used for Entrapment of Bioactive Compounds
4.2. Fabrication of Biopolymer Matrices
4.2.1. Single-Polymer Hydrogel
4.2.2. Multi-Polymer Hydrogel
Hybrid Crystalline–Hydrogel Composites
5. Key Factors Regulating the Release Rate of Bioactive Compounds
5.1. Conditions of Release Media
5.1.1. Boundary Conditions
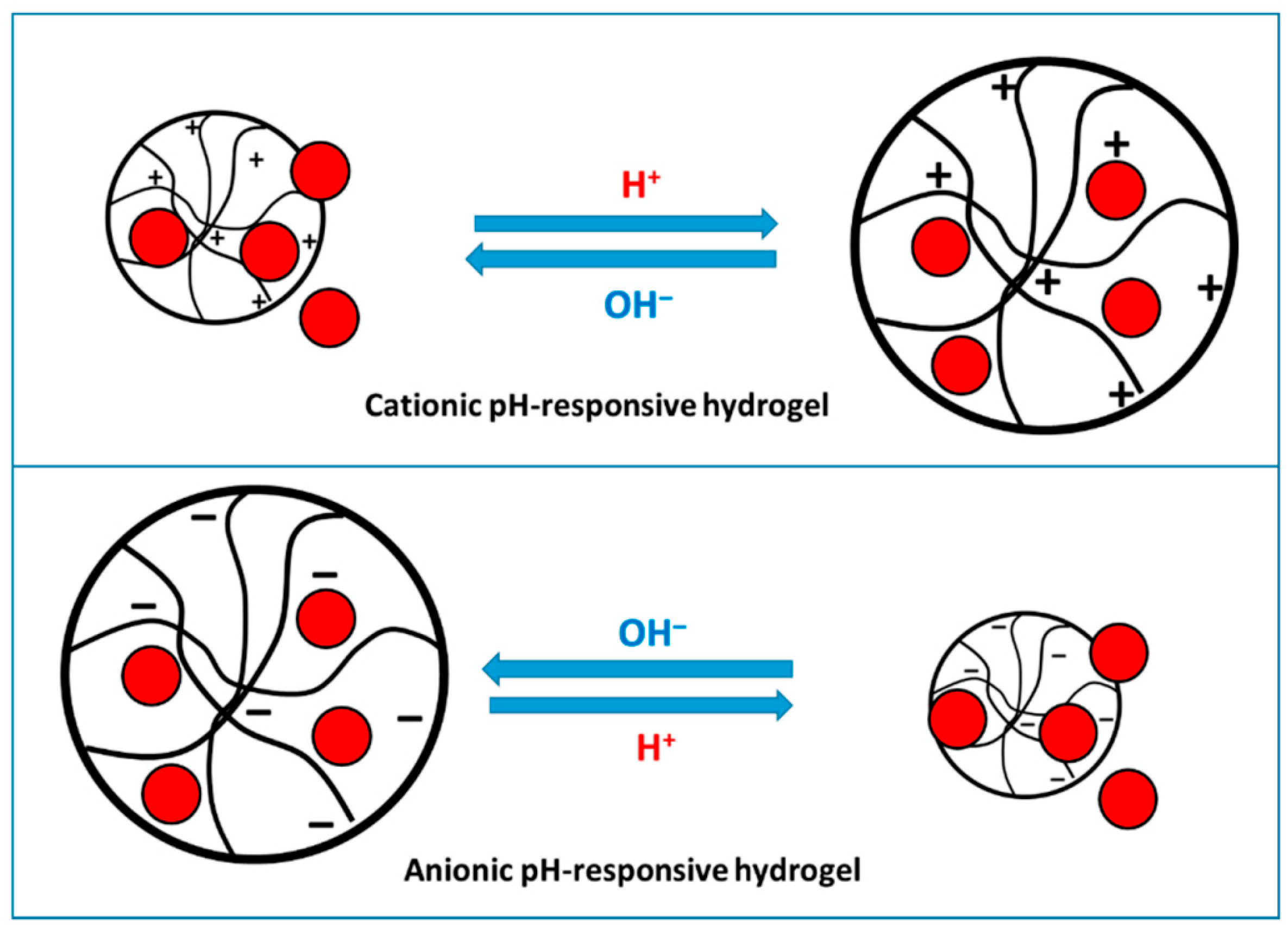
5.1.2. pH
5.1.3. Ionic Profile
5.1.4. Nature of Solvent
5.1.5. Temperature
5.2. Hydrogel Properties
5.2.1. Polymer Types
5.2.2. Polymer Crosslinking
Chemical Crosslinking
Dual Crosslinking
Physical Crosslinking
5.2.3. Effect of Plasticisers
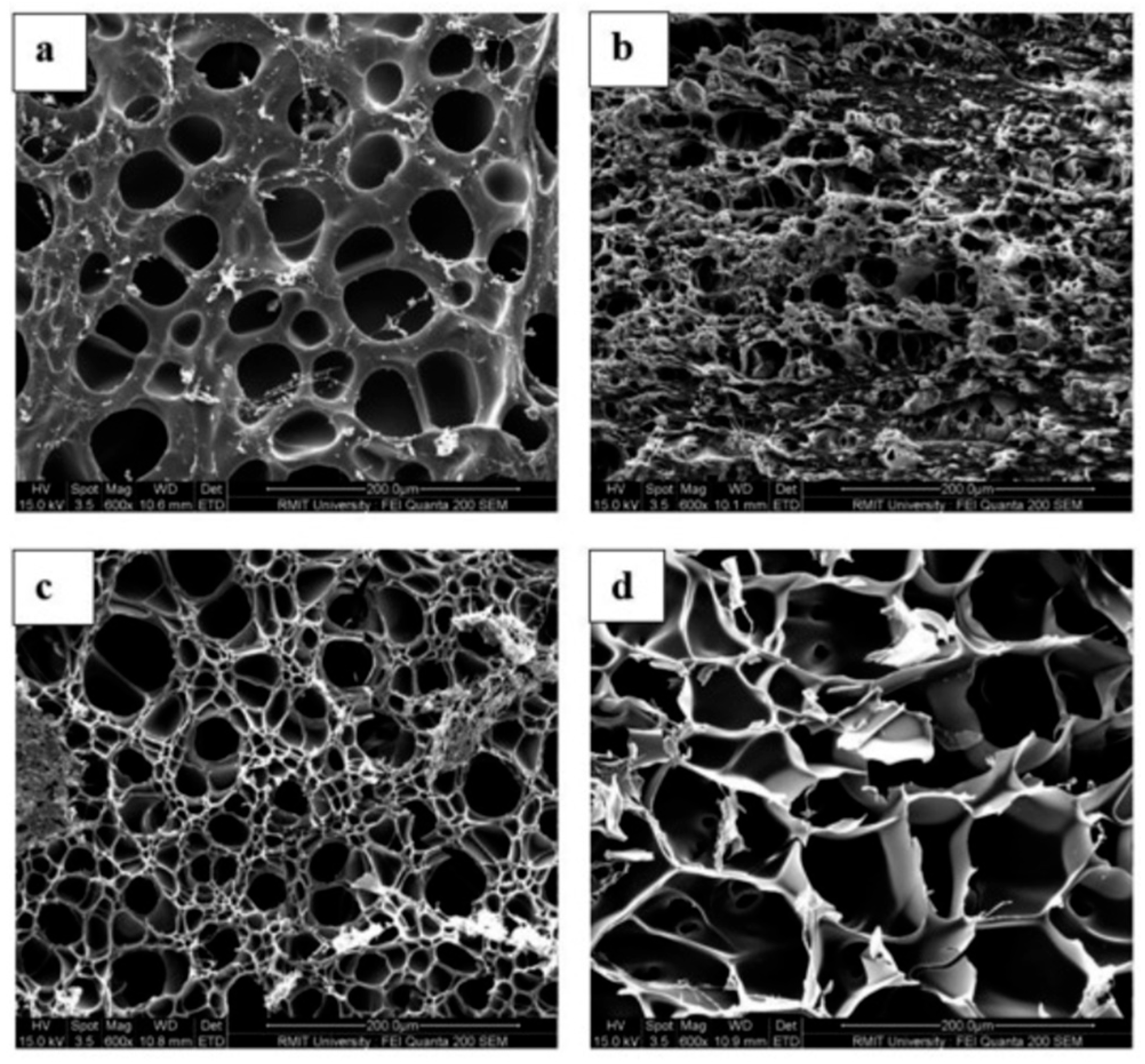
5.2.4. Swelling and Microstructural Properties
5.3. Characteristics of Bioactive Compounds
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.; Gonçalves, A.; Jesus, F.; Simões, M.; Silva, L. Bioactive Compounds: Sources, Properties and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2017. [Google Scholar]
- Joye, I.J.; Nelis, V.A.; McClements, D.J. Gliadin-based nanoparticles: Fabrication and stability of food-grade colloidal delivery systems. Food Hydrocoll. 2015, 44, 86–93. [Google Scholar] [CrossRef]
- Vozza, G.; Khalid, M.; Byrne, H.J.; Ryan, S.M.; Frias, J.M. Nutraceutical formulation, characterisation, and in-vitro evaluation of methylselenocysteine and selenocystine using food derived chitosan: Zein nanoparticles. Food Res. Int. 2019, 120, 295–304. [Google Scholar] [CrossRef]
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Norde, W.; Li, Y. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef]
- Costa, A.M.; Nunes, J.; Lima, B.; Pedrosa, C.; Calado, V.; Torres, A.; Pierucci, A. Effective stabilization of CLA by microencapsulation in pea protein. Food Chem. 2015, 168, 157–166. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in encapsulation technologies for delivery of food bioactive compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Opriș, O.; Mormile, C.; Lung, I.; Stegarescu, A.; Soran, M.-L.; Soran, A. An overview of biopolymers for drug delivery applications. Appl. Sci. 2024, 14, 1383. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Encapsulation and controlled release of bioactive compounds in lactoferrin-glycomacropeptide nanohydrogels: Curcumin and caffeine as model compounds. J. Food Eng. 2016, 180, 110–119. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Llavata-Cabrero, B.; Martínez-Sanz, M.; Fabra, M.J.; López-Rubio, A. Self-assembled gelatin-ι-carrageenan encapsulation structures for intestinal-targeted release applications. J. Colloid Interface Sci. 2018, 517, 113–123. [Google Scholar] [CrossRef]
- Fazal, T.; Murtaza, B.N.; Shah, M.; Iqbal, S.; Rehman, M.-u.; Jaber, F.; Dera, A.A.; Awwad, N.S.; Ibrahium, H.A. Recent developments in natural biopolymer based drug delivery systems. RSC Adv. 2023, 13, 23087–23121. [Google Scholar] [CrossRef]
- Dumontel, B.; Conejo-Rodríguez, V.; Vallet-Regí, M.; Manzano, M. Natural biopolymers as smart coating materials of mesoporous silica nanoparticles for drug delivery. Pharmaceutics 2023, 15, 447. [Google Scholar] [CrossRef]
- Che, E.; Gao, Y.; Wan, L.; Zhang, Y.; Han, N.; Bai, J.; Li, J.; Sha, Z.; Wang, S. Paclitaxel/gelatin coated magnetic mesoporous silica nanoparticles: Preparation and antitumor efficacy in vivo. Microporous Mesoporous Mater. 2015, 204, 226–234. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Kuai, L.; Liu, F.; Chiou, B.-S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled release of antioxidants from active food packaging: A review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Far, B.F.; Naimi-Jamal, M.R.; Jahanbakhshi, M.; Rostamani, H.; Karimi, M.; Keihankhadiv, S. Synthesis and characterization of chitosan/collagen/polycaprolactone hydrogel films with enhanced biocompatibility and hydrophilicity for artificial tendon applications. Int. J. Biol. Macromol. 2023, 253, 127448. [Google Scholar]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Alammar, A.; Park, S.-H.; Ibrahim, I.; Arun, D.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Song, Y.; He, X.; Xu, T.; Zhang, X. Hydrogel-functionalized bandages with Janus wettability for efficient unidirectional drug delivery and wound care. ACS Nano 2024, 18, 3468–3479. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, C.; Wang, X.; Wang, Y.; Sun, K.; Li, L.; Fan, Y.; Hu, L. A hydrogel-based biosensor for stable detection of glucose. Biosens. Bioelectron. 2023, 221, 114908. [Google Scholar] [CrossRef]
- Dragan, E.S.; Perju, M.M.; Dinu, M.V. Preparation and characterization of IPN composite hydrogels based on polyacrylamide and chitosan and their interaction with ionic dyes. Carbohydr. Polym. 2012, 88, 270–281. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P. Drug delivery strategies and biomedical significance of hydrogels: Translational considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Sabaghi, M.; Tavasoli, S.; Hoseyni, S.Z.; Mozafari, M.; Degraeve, P.; Katouzian, I. A critical review on approaches to regulate the release rate of bioactive compounds from biopolymeric matrices. Food Chem. 2022, 382, 132411. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D.; Regenstein, J.M.; Xia, W.; Dong, J. A comprehensive review on natural bioactive films with controlled release characteristics and their applications in foods and pharmaceuticals. Trends Food Sci. Technol. 2021, 112, 690–707. [Google Scholar] [CrossRef]
- Faisant, N.; Akiki, J.; Siepmann, F.; Benoit, J.; Siepmann, J. Effects of the type of release medium on drug release from PLGA-based microparticles: Experiment and theory. Int. J. Pharm. 2006, 314, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Effect of crosslinker on drug delivery properties of curcumin loaded starch coated iron oxide nanoparticles. Int. J. Biol. Macromol. 2016, 93, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking strategies for 3D bioprinting of polymeric hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Debeaufort, F. Bioactive edible films for food applications: Influence of the bioactive compounds on film structure and properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 1137–1153. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 70–77. [Google Scholar] [CrossRef]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A update: Forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Guéant, J.-L.; Guéant-Rodriguez, R.-M.; Alpers, D.H. Vitamin B12 absorption and malabsorption. Vitam. Horm. 2022, 119, 241–274. [Google Scholar]
- Temova Rakuša, Ž.; Roškar, R.; Hickey, N.; Geremia, S. Vitamin B12 in foods, food supplements, and medicines—A review of its role and properties with a focus on its stability. Molecules 2022, 28, 240. [Google Scholar] [CrossRef]
- Bianchi, J.; Wilson, F.A.; Rose, R.C. Dehydroascorbic acid and ascorbic acid transport systems in the guinea pig ileum. Am. J. Physiol.-Gastrointest. Liver Physiol. 1986, 250, G461–G468. [Google Scholar] [CrossRef]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Recent advances in water-soluble vitamins delivery systems prepared by mechanical processes (electrospinning and spray-drying techniques) for food and nutraceuticals applications—A review. Foods 2022, 11, 1271. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.; Furlanetto, T.W. Intestinal absorption of vitamin D: A systematic review. Nutr. Rev. 2018, 76, 60–76. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Pišlar, M.; Kristl, A.; Roškar, R. Comprehensive stability study of vitamin D3 in aqueous solutions and liquid commercial products. Pharmaceutics 2021, 13, 617. [Google Scholar] [CrossRef]
- Reboul, E. Vitamin E bioavailability: Mechanisms of intestinal absorption in the spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, S.; Ji, Y.; Chen, H.; Zhang, H.; Chen, W.; Gu, Z.; Chen, Y.Q. Dietary intake of n-3 PUFAs modifies the absorption, distribution and bioavailability of fatty acids in the mouse gastrointestinal tract. Lipids Health Dis. 2017, 16, 10. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019, 10, 6999–7021. [Google Scholar] [CrossRef]
- Reddy, V.S.; Shiva, S.; Manikantan, S.; Ramakrishna, S. Pharmacology of caffeine and its effects on the human body. Eur. J. Med. Chem. Rep. 2024, 10, 100138. [Google Scholar] [CrossRef]
- Piscopo, R.; Coppola, F.; Almeida, Â.; De Marchi, L.; Russo, T.; Esteves, V.I.; Soares, A.M.; Pretti, C.; Chiellini, F.; Polese, G. Effects of temperature on caffeine and carbon nanotubes co-exposure in Ruditapes philippinarum. Chemosphere 2021, 271, 129775. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tang, K.; Wu, S.; Liu, L.; Qiang, C.; Lin, X.; Liu, B. Astragalus polysaccharides lowers plasma cholesterol through mechanisms distinct from statins. PLoS ONE 2011, 6, e27437. [Google Scholar] [CrossRef] [PubMed]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.; Gomez-Cabrera, M.; Vina, J. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Chen, Y.; Teng, W.; Wang, J.; Wang, Y.; Zhang, Y.; Cao, J. The intestinal delivery systems of ferulic acid: Absorption, metabolism, influencing factors, and potential applications. Food Front. 2024, 5, 1126–1144. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Digestion and absorption of ferulic acid sugar esters in rat gastrointestinal tract. J. Agric. Food Chem. 2003, 51, 5534–5539. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Matsui, T. Current knowledge of intestinal absorption of bioactive peptides. Food Funct. 2017, 8, 4306–4314. [Google Scholar] [CrossRef]
- Gammeltoft, S. Receptor-mediated endocytosis and degradation of polypeptide hormones, growth factors, and neuropeptides. In Degradation of Bioactive Substances; CRC Press: Boca Raton, FL, USA, 2024; pp. 81–111. [Google Scholar]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Aslam, S.; Ahmad, M.; Riaz, M. Stability of carotenoids. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 251–315. [Google Scholar]
- Rocha, H.R.; Coelho, M.C.; Gomes, A.M.; Pintado, M.E. Carotenoids diet: Digestion, gut microbiota modulation, and inflammatory diseases. Nutrients 2023, 15, 2265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, R.; Tian, Y.; Xu, S.; Meng, X. Curcumin nanopreparations: Recent advance in preparation and application. Biomed. Mater. 2024, 19, 052009. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; García-Viguera, C. In vitro gastrointestinal digestion study of broccoli inflorescence phenolic compounds, glucosinolates, and vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Abdulkarim, M.; Agulló, N.; Cattoz, B.; Griffiths, P.; Bernkop-Schnürch, A.; Borros, S.G.; Gumbleton, M. Nanoparticle diffusion within intestinal mucus: Three-dimensional response analysis dissecting the impact of particle surface charge, size and heterogeneity across polyelectrolyte, pegylated and viral particles. Eur. J. Pharm. Biopharm. 2015, 97, 230–238. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Shan, W.; Huang, Y. Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 2015, 10, 275–282. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.-P.; Aura, A.-M.; Hollman, P.C.; Gruppen, H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl) acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone as the major metabolites. J. Agric. Food Chem. 2009, 57, 1084–1092. [Google Scholar] [CrossRef]
- Hai, Y.; Zhang, Y.; Liang, Y.; Ma, X.; Qi, X.; Xiao, J.; Xue, W.; Luo, Y.; Yue, T. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives: Absorption, metabolism and function of quercetin. Food Front. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Momma, T.Y.; Heiss, C.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free. Radic. Biol. Med. 2011, 50, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Renard, C.M. A Perspective on Carotenoids: Z/E-Isomerization, Extraction by Deep Eutectic Solvents and Applications. In Dietary Carotenoids-Sources, Properties, and Role in Human Health; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Yuan, D.; Guo, Y.; Pu, F.; Yang, C.; Xiao, X.; Du, H.; He, J.; Lu, S. Opportunities and challenges in enhancing the bioavailability and bioactivity of dietary flavonoids: A novel delivery system perspective. Food Chem. 2024, 430, 137115. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, U.; Jaiswal, S.G.; Dave, J.; Wei, S.; Hailu, G.G. Recent trends in the encapsulation of functional lipids: Comprehensive review. Sustain. Food Technol. 2024, 2, 1610–1630. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, J.; Chandrapala, J.; Majzoobi, M.; Brennan, C.; Zeng, X.A.; Sun, B. Recent progress of food-derived bioactive peptides: Extraction, purification, function, and encapsulation. Food Front. 2024, 5, 1240–1264. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, L.; Chai, J.; Bao, C.; Jiang, P.; Li, Y. Encapsulation and targeted release. In Food Hydrocolloids: Functionalities and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 369–407. [Google Scholar]
- Niaounakis, M. Biopolymers: Applications and Trends; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of bioactive compounds for food and agricultural applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Dergunov, S.A.; Mun, G.A. γ-irradiated chitosan-polyvinyl pyrrolidone hydrogels as pH-sensitive protein delivery system. Radiat. Phys. Chem. 2009, 78, 65–68. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Teimouri, S.; Kasapis, S. Morphology of genipin-crosslinked BSA networks yields a measurable effect on the controlled release of vitamin B6. Food Chem. 2020, 314, 126204. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, S.; Morrish, C.; Panyoyai, N.; Small, D.M.; Kasapis, S. Diffusion and relaxation contributions in the release of vitamin B6 from a moving boundary of genipin crosslinked gelatin matrices. Food Hydrocoll. 2019, 87, 839–846. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Young, S.A.; Kasapis, S. Structural relaxation and glass transition in high-solid gelatin systems crosslinked with genipin. Int. J. Biol. Macromol. 2019, 141, 867–875. [Google Scholar] [CrossRef]
- Dimida, S.; Barca, A.; Cancelli, N.; De Benedictis, V.; Raucci, M.G.; Demitri, C. Effects of genipin concentration on cross-linked chitosan scaffolds for bone tissue engineering: Structural characterization and evidence of biocompatibility features. Int. J. Polym. Sci. 2017, 2017, 8410750. [Google Scholar] [CrossRef]
- Morrish, C.; Teimouri, S.; Kasapis, S. Structural manipulation of the gelatin/genipin network to inform the molecular transport of caffeine. Food Hydrocoll. 2023, 140, 108616. [Google Scholar] [CrossRef]
- Qu, X.; Wirsén, A.; Albertsson, A.C. Structural change and swelling mechanism of pH-sensitive hydrogels based on chitosan and D, L-lactic acid. J. Appl. Polym. Sci. 1999, 74, 3186–3192. [Google Scholar] [CrossRef]
- Willfahrt, A.; Steiner, E.; Hötzel, J.; Crispin, X. Printable acid-modified corn starch as non-toxic, disposable hydrogel-polymer electrolyte in supercapacitors. Appl. Phys. A 2019, 125, 474. [Google Scholar] [CrossRef]
- Ku, J.; Seonwoo, H.; Park, S.; Jang, K.-J.; Lee, J.; Lee, M.; Lim, J.W.; Kim, J.; Chung, J.H. Cell-laden thermosensitive chitosan hydrogel bioinks for 3D bioprinting applications. Appl. Sci. 2020, 10, 2455. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Dehabadi, L.; Wilson, L.D. Renewable starch carriers with switchable adsorption properties. ACS Sustain. Chem. Eng. 2018, 6, 4603–4613. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Lima, D.C.; Junior, M.D.M.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E. Hydrogels based on ozonated cassava starch: Effect of ozone processing and gelatinization conditions on enhancing 3D-printing applications. Int. J. Biol. Macromol. 2019, 138, 1087–1097. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Tunable collagen I hydrogels for engineered physiological tissue micro-environments. PLoS ONE 2015, 10, e0122500. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Moshayedi, S.; Sarpoolaky, H.; Khavandi, A. Fabrication, swelling behavior, and water absorption kinetics of genipin-crosslinked gelatin–chitosan hydrogels. Polym. Eng. Sci. 2021, 61, 3094–3103. [Google Scholar] [CrossRef]
- Sethi, S.; Saruchi; Kaith, B.S.; Kaur, M.; Sharma, N.; Kumar, V. Cross-linked xanthan gum–starch hydrogels as promising materials for controlled drug delivery. Cellulose 2020, 27, 4565–4589. [Google Scholar] [CrossRef]
- Bayat, M.; Nasri, S. Injectable microgel–hydrogel composites “plum pudding gels”: New system for prolonged drug delivery. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 343–372. [Google Scholar]
- Zhang, Y.; Wu, F.; Li, M.; Wang, E. pH switching on-off semi-IPN hydrogel based on cross-linked poly (acrylamide-co-acrylic acid) and linear polyallyamine. Polymer 2005, 46, 7695–7700. [Google Scholar] [CrossRef]
- Liou, G.-S.; Lin, P.-H.; Yen, H.-J.; Yu, Y.-Y.; Tsai, T.-W.; Chen, W.-C. Highly flexible and optical transparent 6F-PI/TiO2 optical hybrid films with tunable refractive index and excellent thermal stability. J. Mater. Chem. 2010, 20, 531–536. [Google Scholar] [CrossRef]
- Swain, S.; Bal, T. Carrageenan-guar gum microwave irradiated micro-porous interpenetrating polymer network: A system for drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 256–265. [Google Scholar] [CrossRef]
- Roy, D.; Bal, T.; Swain, S. Fabrication and evaluation of pH-sensitive biocompatible microwave irradiated moringa barkgum-carrageenan (MOG-CRG-IPN) interpenetrating isotropic polymeric network for controlled delivery of pharmaceuticals. Sustain. Chem. Pharm. 2020, 18, 100325. [Google Scholar] [CrossRef]
- Pechenov, S.; Shenoy, B.; Yang, M.X.; Basu, S.K.; Margolin, A.L. Injectable controlled release formulations incorporating protein crystals. J. Control. Release 2004, 96, 149–158. [Google Scholar] [CrossRef]
- Bianco, S.; Hasan, M.; Ahmad, A.; Richards, S.-J.; Dietrich, B.; Wallace, M.; Tang, Q.; Smith, A.J.; Gibson, M.I.; Adams, D.J. Mechanical release of homogenous proteins from supramolecular gels. Nature 2024, 631, 544–548. [Google Scholar] [CrossRef]
- Contreras-Montoya, R.; Arredondo-Amador, M.; Escolano-Casado, G.; Manas-Torres, M.C.; González, M.; Conejero-Muriel, M.; Bhatia, V.; Díaz-Mochón, J.J.; Martínez-Augustin, O.; de Medina, F.S. Insulin crystals grown in short-peptide supramolecular hydrogels show enhanced thermal stability and slower release profile. ACS Appl. Mater. Interfaces 2021, 13, 11672–11682. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Li, X.; Zhang, Z.; Wei, Z.; Xing, Z.; Deng, S.; Duan, F. Okra polysaccharides/gelatin complex coacervate as pH-responsive and intestine-targeting delivery protects isoquercitin bioactivity. Int. J. Biol. Macromol. 2020, 159, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Jindal, R.; Jindal, D. RSM-CCD optimized microwave-assisted synthesis of chitosan and gelatin-based pH sensitive, inclusion complexes incorporated hydrogels and their use as controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 48, 161–173. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zhang, W.; Wang, X.; Zhao, Z.; Wang, Z.; Zhang, L. Multifunctional self-healing and pH-responsive hydrogel dressing based on cationic guar gum and hyaluronic acid for on-demand drug release. Int. J. Biol. Macromol. 2025, 301, 140326. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Network formation of Moringa oleifera gum by radiation induced crosslinking: Evaluation of drug delivery, network parameters and biomedical properties. Int. J. Biol. Macromol. 2018, 108, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Singh, S.K.; Mishra, S.; Pandey, J.P.; Sen, G. Gum ghatti based hydrogel: Microwave synthesis, characterization, 5-Fluorouracil encapsulation and ‘in vitro’drug release evaluation. Carbohydr. Polym. 2019, 222, 114979. [Google Scholar] [CrossRef]
- Shi, M.; Bai, J.; Zhao, L.; Yu, X.; Liang, J.; Liu, Y.; Nord, W.; Li, Y. Co-loading and intestine-specific delivery of multiple antioxidants in pH-responsive microspheres based on TEMPO-oxidized polysaccharides. Carbohydr. Polym. 2017, 157, 858–865. [Google Scholar] [CrossRef]
- Nisar, S.; Pandit, A.H.; Nadeem, M.; Pandit, A.H.; Rizvi, M.M.A.; Rattan, S. γ-Radiation induced L-glutamic acid grafted highly porous, pH-responsive chitosan hydrogel beads: A smart and biocompatible vehicle for controlled anti-cancer drug delivery. Int. J. Biol. Macromol. 2021, 182, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Gao, M.; Ren, Y.; Lou, R.; Xie, H.; Yu, W.; Liu, X.; Ma, X. An improved pH-responsive carrier based on EDTA-Ca-alginate for oral delivery of Lactobacillus rhamnosus ATCC 53103. Carbohydr. Polym. 2017, 155, 329–335. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Z.; Wang, X.; Yin, X.; Fu, M.; Qin, T.; Ji, X.; Yang, G.; Sun, S. A physical crosslinked pH-sensitive hydrogel based on hemicellulose/graphene oxide for controlled oral drug delivery. Int. J. Biol. Macromol. 2025, 289, 138875. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, B.; Jiang, Y.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. pH-responsive UV crosslinkable chitosan hydrogel via “thiol-ene” click chemistry for active modulating opposite drug release behaviors. Carbohydr. Polym. 2021, 251, 117101. [Google Scholar] [CrossRef]
- Chen, N.; Nicolai, T.; Chassenieux, C.; Wang, Y. pH and ionic strength responsive core-shell protein microgels fabricated via simple coacervation of soy globulins. Food Hydrocoll. 2020, 105, 105853. [Google Scholar] [CrossRef]
- Tahmasebi, S.; Farmanbordar, H.; Mohammadi, R. Synthesis of magnetic bio-nanocomposite hydrogel beads based on sodium alginate and β-cyclodextrin: Potential pH-responsive oral delivery anticancer systems for colorectal cancer. Int. J. Biol. Macromol. 2025, 305, 140748. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, Q. Developing organogel-based Pickering emulsions with improved freeze-thaw stability and hesperidin bioaccessibility. Food Hydrocoll. 2019, 93, 68–77. [Google Scholar] [CrossRef]
- de Farias, N.S.; Silva, B.; de Oliveira Costa, A.C.; Müller, C.M.O. Alginate based antioxidant films with yerba mate (Ilex paraguariensis St. Hil.): Characterization and kinetics of phenolic compounds release. Food Packag. Shelf Life 2021, 28, 100548. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohydr. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef]
- Qiao, Z.; Liu, H.-Y.; Zha, J.-C.; Mao, X.-X.; Yin, J. Completely degradable backbone-type hydrogen peroxide responsive curcumin copolymer: Synthesis and synergistic anticancer investigation. Polym. Chem. 2019, 10, 4305–4313. [Google Scholar] [CrossRef]
- Bernardos, A.; Božik, M.; Montero, A.; Pérez-Esteve, É.; García-Casado, E.; Lhotka, M.; Fraňková, A.; Marcos, M.D.; Barat, J.M.; Martínez-Máñez, R. Secreted enzyme-responsive system for controlled antifungal agent release. Nanomaterials 2021, 11, 1280. [Google Scholar] [CrossRef]
- Coulter, S.M.; Pentlavalli, S.; An, Y.; Vora, L.K.; Cross, E.R.; Moore, J.V.; Sun, H.; Schweins, R.; McCarthy, H.O.; Laverty, G. In situ forming, enzyme-responsive peptoid-peptide hydrogels: An advanced long-acting injectable drug delivery system. J. Am. Chem. Soc. 2024, 146, 21401–21416. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.A.; Nayak, R.R. Supramolecular phenoxy-alkyl maleate-based hydrogels and their enzyme/pH-responsive curcumin release. New J. Chem. 2019, 43, 5559–5567. [Google Scholar] [CrossRef]
- de Abreu Figueiredo, J.; Teixeira, M.A.; Campelo, P.H.; Lago, A.M.T.; de Souza, T.P.; Yoshida, M.I.; de Oliveira, C.R.; Pereira, A.P.A.; Pastore, G.M.; Sanches, E.A. Encapsulation of camu-camu extracts using prebiotic biopolymers: Controlled release of bioactive compounds and effect on their physicochemical and thermal properties. Food Res. Int. 2020, 137, 109563. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Hu, X.; Zhu, J.; Wang, Z.; Wang, X.; Wang, M. Release properties of tannic acid from hydrogen bond driven antioxidative cellulose nanofibrous films. Int. J. Biol. Macromol. 2016, 91, 68–74. [Google Scholar] [CrossRef]
- Emam, H.E.; Shaheen, T.I. Design of a dual pH and temperature responsive hydrogel based on esterified cellulose nanocrystals for potential drug release. Carbohydr. Polym. 2022, 278, 118925. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, M.; Chen, H.; Xu, Z.; Li, J.; Kong, Y.; Zuo, X. Synthesis of stimuli-responsive copolymeric hydrogels for temperature, reduction and pH-controlled drug delivery. J. Ind. Eng. Chem. 2025, 143, 252–261. [Google Scholar] [CrossRef]
- Chou, K.F.; Chiu, H.S.; Lin, J.H.; Huang, W.Y.; Chen, P.Y.; Xiao, W.L.; Chen, T.K.; Wang, L.W. The effect of microwave treatment on the drug release property of gelatin microspheres. In Proceedings of the 15th International Conference on Biomedical Engineering: ICBME 2013, Singapore, 4–7 December 2013; pp. 726–729. [Google Scholar]
- Chauhan, R.; Kinney, K.; Akalkotkar, A.; Nunn, B.M.; Keynton, R.S.; Soucy, P.A.; O’Toole, M.G. Radiation-induced curcumin release from curcumin–chitosan polymer films. RSC Adv. 2020, 10, 16110–16117. [Google Scholar] [CrossRef]
- Gulfam, M.; Jo, S.-H.; Vu, T.T.; Ali, I.; Rizwan, A.; Joo, S.-B.; Park, S.-H.; Lim, K.T. NIR-degradable and biocompatible hydrogels derived from hyaluronic acid and coumarin for drug delivery and bio-imaging. Carbohydr. Polym. 2023, 303, 120457. [Google Scholar] [CrossRef]
- Han, Y.; Shchukin, D.; Fernandes, P.; Mutihac, R.-C.; Möhwald, H. Mechanism and kinetics of controlled drug release by temperature stimuli responsive protein nanocontainers. Soft Matter 2010, 6, 4942–4947. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Modeling the permeability of multiaxial electrospun poly (ε-caprolactone)-gelatin hybrid fibers for controlled doxycycline release. Mater. Sci. Eng. C 2017, 76, 161–170. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Young, S.A.; Kasapis, S. Swelling behaviour and glass transition in genipin-crosslinked chitosan systems. Int. J. Biol. Macromol. 2020, 164, 3075–3083. [Google Scholar] [CrossRef]
- Erdagi, S.I.; Ngwabebhoh, F.A.; Yildiz, U. Genipin crosslinked gelatin-diosgenin-nanocellulose hydrogels for potential wound dressing and healing applications. Int. J. Biol. Macromol. 2020, 149, 651–663. [Google Scholar] [CrossRef]
- Teimouri, S.; Kasapis, S.; Dokouhaki, M. Diffusional characteristics of food protein-based materials as nutraceutical delivery systems: A review. Trends Food Sci. Technol. 2022, 122, 201–210. [Google Scholar] [CrossRef]
- Kelly, S.M.; Upadhyay, A.K.; Mitra, A.; Narasimhan, B. Analyzing drug release kinetics from water-soluble polymers. Ind. Eng. Chem. Res. 2019, 58, 7428–7437. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Gowd, V.; Suo, H.; Wang, M.; Wang, Q.; Chen, F.; Cheng, K.W. Advances in smart delivery of food bioactive compounds using stimuli-responsive carriers: Responsive mechanism, contemporary challenges, and prospects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5449–5488. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Shin, H.S.; Park, S.N. A novel pH-responsive hydrogel based on carboxymethyl cellulose/2-hydroxyethyl acrylate for transdermal delivery of naringenin. Carbohydr. Polym. 2018, 200, 341–352. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Fen, L.S.; Hui, N.H.; Mustapha, N.A. Genipin-cross-linked kappa-carrageenan/carboxymethyl cellulose beads and effects on beta-carotene release. Carbohydr. Polym. 2011, 83, 1207–1212. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.-H.; Yin, S.-W.; Yang, X.-Q.; Qi, J.-R. Genipin-crosslinked gelatin films as controlled releasing carriers of lysozyme. Food Res. Int. 2013, 51, 321–324. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.; Zhang, J.; Zhu, Y.; Wang, J. Self-assembled pH-responsive supramolecular hydrogel for hydrophobic drug delivery. RSC Adv. 2018, 8, 31581–31587. [Google Scholar] [CrossRef]
- Xu, W.; Huang, L.; Jin, W.; Ge, P.; Shah, B.R.; Zhu, D.; Jing, J. Encapsulation and release behavior of curcumin based on nanoemulsions-filled alginate hydrogel beads. Int. J. Biol. Macromol. 2019, 134, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, S.; Morrish, C.; Kasapis, S. Release profile of vitamin B6 from a pH-responsive BSA network crosslinked with genipin. LWT 2020, 128, 109458. [Google Scholar] [CrossRef]
- Sabaghi, M.; Tavasoli, S.; Taheri, A.; Jamali, S.N.; Faridi Esfanjani, A. Controlling release patterns of the bioactive compound by structural and environmental conditions: A review. J. Food Meas. Charact. 2023, 17, 2261–2284. [Google Scholar] [CrossRef]
- Seeli, D.S.; Dhivya, S.; Selvamurugan, N.; Prabaharan, M. Guar gum succinate-sodium alginate beads as a pH-sensitive carrier for colon-specific drug delivery. Int. J. Biol. Macromol. 2016, 91, 45–50. [Google Scholar] [CrossRef]
- Mondal, S.; Li, C.; Wang, K. Bovine serum albumin adsorption on gluteraldehyde cross-linked chitosan hydrogels. J. Chem. Eng. Data 2015, 60, 2356–2362. [Google Scholar] [CrossRef]
- Kono, H.; Otaka, F.; Ozaki, M. Preparation and characterization of guar gum hydrogels as carrier materials for controlled protein drug delivery. Carbohydr. Polym. 2014, 111, 830–840. [Google Scholar] [CrossRef]
- Yang, Y.; Decker, E.A.; Xiao, H.; McClements, D.J. Enhancing vitamin E bioaccessibility: Factors impacting solubilization and hydrolysis of α-tocopherol acetate encapsulated in emulsion-based delivery systems. Food Funct. 2015, 6, 83–96. [Google Scholar] [CrossRef]
- Akar, E.; Altınışık, A.; Seki, Y. Preparation of pH-and ionic-strength responsive biodegradable fumaric acid crosslinked carboxymethyl cellulose. Carbohydr. Polym. 2012, 90, 1634–1641. [Google Scholar] [CrossRef]
- Ponnusami, V. Polyelectrolyte complex-based ionically gelled biopolymeric systems for sustained drug release. In Ionically Gelled Biopolysaccharide Based Systems in Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2021; pp. 105–120. [Google Scholar]
- Assifaoui, A.; Chambin, O.; Cayot, P. Drug release from calcium and zinc pectinate beads: Impact of dissolution medium composition. Carbohydr. Polym. 2011, 85, 388–393. [Google Scholar] [CrossRef]
- Sepe, F.; Valentino, A.; Marcolongo, L.; Petillo, O.; Conte, R.; Margarucci, S.; Peluso, G.; Calarco, A. Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds. Int. J. Mol. Sci. 2025, 26, 764. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C 2016, 63, 231–239. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone) nanofibres. Application in starch multilayer films. Food Hydrocoll. 2018, 79, 158–169. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Kashiri, M.; Shakeri, A. Evaluation of release mechanism of catechin from chitosan-polyvinyl alcohol film by exposure to gamma irradiation. Carbohydr. Polym. 2020, 230, 115589. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Medina-Jaramillo, C.; Piñeros-Hernandez, D.; Goyanes, S. Cassava starch films containing rosemary nanoparticles produced by solvent displacement method. Food Hydrocoll. 2017, 71, 26–34. [Google Scholar] [CrossRef]
- Rezaeinia, H.; Ghorani, B.; Emadzadeh, B.; Tucker, N. Electrohydrodynamic atomization of Balangu (Lallemantia royleana) seed gum for the fast-release of Mentha longifolia L. essential oil: Characterization of nano-capsules and modeling the kinetics of release. Food Hydrocoll. 2019, 93, 374–385. [Google Scholar] [CrossRef]
- Panyoyai, N.; Kasapis, S. A free-volume interpretation of the decoupling parameter in bioactive-compound diffusion from a glassy polymer. Food Hydrocoll. 2016, 54, 338–341. [Google Scholar] [CrossRef]
- Bakshi, A.S.; Singh, R.P. Kinetics of water diffusion and starch gelatinization during rice parboiling. J. Food Sci. 1980, 45, 1387–1392. [Google Scholar] [CrossRef]
- Nicolin, D.J.; Neto, R.M.; Paraíso, P.R.; Jorge, R.M.M.; Jorge, L.M.M. Analytical solution and experimental validation of a model for hydration of soybeans with variable mass transfer coefficient. J. Food Eng. 2015, 149, 17–23. [Google Scholar] [CrossRef]
- Paramita, V.D.; Bannikova, A.; Kasapis, S. Release mechanism of essential fatty acids in polysaccharide matrices undergoing glass transition. In Gums and Stabilisers for the Food Industry 18: Hydrocolloid Functionality for Affordable and Sustainable Global Food Solutions; Royal Society of Chemistry: London, UK, 2016; Volume 18, p. 157. [Google Scholar]
- Shi, W.; Weitz, D.A. Polymer phase separation in a microcapsule shell. Macromolecules 2017, 50, 7681–7686. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Paramita, V.D.; Piccolo, J.D.L.; Kasapis, S. Effect of co-solute concentration on the diffusion of linoleic acid from whey protein matrices. Food Hydrocoll. 2017, 70, 277–285. [Google Scholar] [CrossRef]
- Bouman, J.; Belton, P.; Venema, P.; Van Der Linden, E.; De Vries, R.; Qi, S. Controlled release from zein matrices: Interplay of drug hydrophobicity and pH. Pharm. Res. 2016, 33, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Thakur, D.; Ghoshal, G.; Katare, O.; Shivhare, U. Microencapsulation by complex coacervation using whey protein isolates and gum acacia: An approach to preserve the functionality and controlled release of β-carotene. Food Bioprocess Technol. 2015, 8, 1635–1644. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Leena, M.M.; Moses, J.; Anandharamakrishnan, C. Micro-and nano-encapsulation of β-carotene in zein protein: Size-dependent release and absorption behavior. Food Funct. 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Yan, J.-K.; Qiu, W.-Y.; Wang, Y.-Y.; Wu, J.-Y. Biocompatible polyelectrolyte complex nanoparticles from lactoferrin and pectin as potential vehicles for antioxidative curcumin. J. Agric. Food Chem. 2017, 65, 5720–5730. [Google Scholar] [CrossRef]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Teng, M.-J.; Wei, Y.-S.; Hu, T.-G.; Zhang, Y.; Feng, K.; Zong, M.-H.; Wu, H. Citric acid cross-linked zein microcapsule as an efficient intestine-specific oral delivery system for lipophilic bioactive compound. J. Food Eng. 2020, 281, 109993. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, Z.-L.; Li, S.; Xu, C.-Z.; Hou, Y.-J.; Liao, L.-X.; Xu, Y.-L.; Zhang, J.-T.; Wei, B.-M.; Wen, W. Recent advances on collagen biomaterial: From extraction, cross-linking to tissue regeneration. Polym. Rev. 2024, 64, 1031–1059. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. Am. J. Polym. Sci 2014, 4, 25–31. [Google Scholar]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wu, Z.; Deng, D.; Li, J.; Qi, X.; Song, M.; Liu, Y.; Wu, Q.; Xie, X.; Chen, Z. Improved cytocompatibility and reduced calcification of glutaraldehyde-crosslinked bovine pericardium by modification with glutathione. Front. Bioeng. Biotechnol. 2022, 10, 844010. [Google Scholar] [CrossRef]
- Ahmed, R.; ul ain Hira, N.; Wang, M.; Iqbal, S.; Yi, J.; Hemar, Y. Genipin, a natural blue colorant precursor: Source, extraction, properties, and applications. Food Chem. 2024, 434, 137498. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Zhang, Y.; Nardin, C. Core-shell gelatin-chitosan nanoparticles with lysozyme responsiveness formed via pH-drive and transglutaminase cross-linking. Int. J. Biol. Macromol. 2025, 292, 138802. [Google Scholar] [CrossRef]
- Wen, X.; Jin, F.; Regenstein, J.M.; Wang, F. Transglutaminase induced gels using bitter apricot kernel protein: Chemical, textural and release properties. Food Biosci. 2018, 26, 15–22. [Google Scholar] [CrossRef]
- Mzoughi, J.; Tran, Q.H.; Schrodj, G.; Vandamme, T.; Luchnikov, V. Rolled-up gastroretentive oral dosages for controlled release of riboflavin and propranolol. J. Drug Deliv. Sci. Technol. 2024, 95, 105563. [Google Scholar] [CrossRef]
- Yin, W.; Su, R.; Qi, W.; He, Z. A casein-polysaccharide hybrid hydrogel cross-linked by transglutaminase for drug delivery. J. Mater. Sci. 2012, 47, 2045–2055. [Google Scholar] [CrossRef]
- Lin, Y.; Roos, Y.H.; Miao, S. Transglutaminase crosslinked fish gelatin emulsion gels: Structure, rheology behaviors, and delivery functionality. Food Hydrocoll. 2025, 162, 111001. [Google Scholar] [CrossRef]
- De Souza, P.M.; Fernández, A.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Modified sodium caseinate films as releasing carriers of lysozyme. Food Hydrocoll. 2010, 24, 300–306. [Google Scholar] [CrossRef]
- Fang, M.; Ma, F.; Yu, L.; Wang, D.; Wen, C.; Zhang, L.; Li, P. Fabrication of resveratrol-loaded soy peptide nanogels with transglutaminase and in vitro gastrointestinal delivery and cholesterol-lowering effect. Food Hydrocoll. 2024, 157, 110437. [Google Scholar] [CrossRef]
- Wu, X.; Wang, K.; Liu, Y.; Liu, A.; Ye, R. Microstructure of transglutaminase-induced gelatin-natamycin fungistatic composite films. Int. J. Food Prop. 2017, 20, 3191–3203. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Wang, P.; Zhang, M.; Liu, S.; Wang, R.; Li, Y.; Ren, F.; Fang, B. Improvement in the Sustained-Release Performance of Electrospun Zein Nanofibers via Crosslinking Using Glutaraldehyde Vapors. Foods 2024, 13, 1583. [Google Scholar] [CrossRef]
- Jayanudin; Fahrurrozi, M.; Wirawan, S.K.; Rochmadi. Preparation of chitosan microcapsules containing red ginger oleoresin using emulsion crosslinking method. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018809917. [Google Scholar] [CrossRef]
- Ofokansi, K.; Kenechukwu, F.; Isah, A.; Okigbo, E. Formulation and evaluation of glutaraldehyde-crosslinked chitosan microparticles for the delivery of ibuprofen. Trop. J. Pharm. Res. 2013, 12, 19–25. [Google Scholar] [CrossRef]
- Liao, J.; Lin, Y.; Xu, M.; Luo, Z.; Jiang, G.; Chen, F.; Li, H.; Yang, L. Preparation of hydrophobic hard gelatin capsules for slow-release fertilizers. Polym. Test. 2024, 134, 108427. [Google Scholar] [CrossRef]
- Hiwale, P.; Lampis, S.; Conti, G.; Caddeo, C.; Murgia, S.; Fadda, A.M.; Monduzzi, M. In vitro release of lysozyme from gelatin microspheres: Effect of cross-linking agents and thermoreversible gel as suspending medium. Biomacromolecules 2011, 12, 3186–3193. [Google Scholar] [CrossRef]
- Fatima, W.; Batool, S.R.; Mushtaq, F.; Aslam, M.; Raza, Z.A.; Nazeer, M.A. Controlled release of doxorubicin from gelatin-based nanoparticles: Theoretical and experimental approach. Mater. Adv. 2024, 5, 2347–2358. [Google Scholar] [CrossRef]
- Wang, Y.X.; Qin, Y.P.; Kong, Z.J.; Wang, Y.J.; Ma, L. Glutaraldehyde cross-linked silk fibroin films for controlled release. Adv. Mater. Res. 2014, 887, 541–546. [Google Scholar] [CrossRef]
- Pichayakorn, W.; Boonme, P. Evaluation of cross-linked chitosan microparticles containing metronidazole for periodontitis treatment. Mater. Sci. Eng. C 2013, 33, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, E.; Cavusoglu, K. Glutaraldehyde cross-linked agarose carriers: Design, characterization and insulin release behaviour. Turk. J. Biochem 2008, 33, 148–153. [Google Scholar]
- Tseng, T.-H.; Chang, J.-H.; Chang, L.-C.; Wang, M.-L.; Yang, S.-J.; Chang, C.-H. Indocyanine green-mediated photothermal release of lidocaine from genipin-crosslinked gelatin hydrogel in nerve block. Int. J. Biol. Macromol. 2025, 297, 139518. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Yuan, G. Chitosan/squid ring teeth protein hydrogels for the controlled release of curcumin. Int. J. Biol. Macromol. 2025, 291, 139163. [Google Scholar] [CrossRef]
- Li, F.; Jin, H.; Xiao, J.; Yin, X.; Liu, X.; Li, D.; Huang, Q. The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food Res. Int. 2018, 111, 351–360. [Google Scholar] [CrossRef]
- Li, M.; Wang, K.; Wang, Y.; Han, Q.; Ni, Y.; Wen, X. Effects of genipin concentration on cross-linked β-casein micelles as nanocarrier of naringenin: Colloidal properties, structural characterization and controlled release. Food Hydrocoll. 2020, 108, 105989. [Google Scholar] [CrossRef]
- Thakare, S.; Gorle, A. Design of Jackfruit Gum-Based Genipin Crosslinked Nanoparticles for Sustained Release of Curcumin: Optimization and in Vitro Characterization. Cellul. Chem. Technol. 2024, 58, 67–79. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Paramita, V.D.; Teimouri, S.; Young, S.; Kasapis, S. Controlled release of ascorbic acid from genipin-crosslinked gelatin matrices under moving boundary conditions. Food Hydrocoll. 2019, 89, 171–179. [Google Scholar] [CrossRef]
- Uddin, M.S.; Khand, S.; Dong, C. Effect of crosslinking agents on chitosan hydrogel carriers for drug loading and release for targeted drug delivery. Gels 2024, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Fraj, J.; Petrović, L.; Đekić, L.; Budinčić, J.M.; Bučko, S.; Katona, J. Encapsulation and release of vitamin C in double W/O/W emulsions followed by complex coacervation in gelatin-sodium caseinate system. J. Food Eng. 2021, 292, 110353. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. β-Glycerol phosphate/genipin chitosan hydrogels: A comparative study of their properties and diclofenac delivery. Carbohydr. Polym. 2020, 248, 116811. [Google Scholar] [CrossRef]
- Zhu, N.; Bi, D.; Huang, J.; Yao, L.; Wu, Y.; Jiang, Z.; Hu, Z.; Zhu, B.; Li, S.; Xu, X. Genipin crosslinked sodium caseinate-chitosan oligosaccharide nanoparticles for optimizing β-carotene stability and bioavailability. Int. J. Biol. Macromol. 2025, 297, 139626. [Google Scholar] [CrossRef]
- Ubaid, M.; Murtaza, G. Fabrication and characterization of genipin cross-linked chitosan/gelatin hydrogel for pH-sensitive, oral delivery of metformin with an application of response surface methodology. Int. J. Biol. Macromol. 2018, 114, 1174–1185. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, C.; Sun, X.; Zhang, S.; Yuan, Y.; Wang, D.; Xu, Y. Fabrication and characterization of cold-gelation whey protein-chitosan complex hydrogels for the controlled release of curcumin. Food Hydrocoll. 2020, 103, 105619. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, R.; Yu, S.; Li, J.; Wang, Y.; Song, Y.; Yang, X.; Pan, W.; Li, S. Nanostructured lipid carrier-based pH and temperature dual-responsive hydrogel composed of carboxymethyl chitosan and poloxamer for drug delivery. Int. J. Biol. Macromol. 2018, 114, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Philip, J.; Rodrigues, F.C.; Thakur, G. Transport of curcumin from cross-linked chitosan matrices: A comparative study. IOP Conf. Ser. Mater. Sci. Eng. 2019, 561, 012029. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Shi, Y.; Cai, T.; Miao, Q.; Gao, Z.; Cui, Z. Lightweight pH-responsive chitosan hydrogel iron fertilizer: Efficient performance, controlled-release, and tomato application. J. Environ. Chem. Eng. 2024, 12, 113428. [Google Scholar] [CrossRef]
- Chen, B.Z.; Ashfaq, M.; Zhu, D.D.; Zhang, X.P.; Guo, X.D. Controlled Delivery of Insulin Using Rapidly Separating Microneedles Fabricated from Genipin-Crosslinked Gelatin. Macromol. Rapid Commun. 2018, 39, 1800075. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Akkaramongkolporn, P.; Jaewjira, S.; Opanasopit, P. Design of alpha mangostin-loaded chitosan/alginate controlled-release nanoparticles using genipin as crosslinker. J. Drug Deliv. Sci. Technol. 2018, 46, 312–321. [Google Scholar] [CrossRef]
- Koc, F.E.; Altıncekic, T.G. Investigation of gelatin/chitosan as potential biodegradable polymer films on swelling behavior and methylene blue release kinetics. Polym. Bull. 2021, 78, 3383–3398. [Google Scholar] [CrossRef]
- Ghaleshahi, A.Z.; Rajabzadeh, G. The influence of sodium alginate and genipin on physico-chemical properties and stability of WPI coated liposomes. Food Res. Int. 2020, 130, 108966. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Jin, L.; Niu, C.; Liang, H.; Chen, C.; Ban, Z. Development of controlled-release soy β-conglycinin nanoparticles containing curcumin using genipin as crosslinker. Food Biosci. 2023, 54, 102826. [Google Scholar] [CrossRef]
- Tuan Mohamood, N.F.A.-Z.; Abdul Halim, A.H.; Zainuddin, N. Carboxymethyl cellulose hydrogel from biomass waste of oil palm empty fruit bunch using calcium chloride as crosslinking agent. Polymers 2021, 13, 4056. [Google Scholar] [CrossRef]
- O-chongpian, P.; Na Takuathung, M.; Chittasupho, C.; Ruksiriwanich, W.; Chaiwarit, T.; Baipaywad, P.; Jantrawut, P. Composite nanocellulose fibers-based hydrogels loading clindamycin HCl with Ca2+ and citric acid as crosslinking agents for pharmaceutical applications. Polymers 2021, 13, 4423. [Google Scholar] [CrossRef]
- Neto, L.A.A.; Silva, L.P. Influence of biopolymer composition and crosslinking agent concentration on the micro-and nanomechanical properties of hydrogel-based filaments. J. Mech. Behav. Biomed. Mater. 2024, 150, 106316. [Google Scholar]
- Alves-Silva, G.F.; Romani, V.P.; Martins, V.G. Different crosslinking as a strategy to improve films produced from external mesocarp of pequi (Caryocar brasiliense). Food Chem. 2024, 432, 137202. [Google Scholar] [CrossRef] [PubMed]
- Medina-Jaramillo, C.; Quintero-Pimiento, C.; Díaz-Díaz, D.; Goyanes, S.; López-Córdoba, A. Improvement of andean blueberries postharvest preservation using carvacrol/alginate-edible coatings. Polymers 2020, 12, 2352. [Google Scholar] [CrossRef] [PubMed]
- Kimi, M.; Chong, C.J. Facile Preparation of Chitosan-Alginate Crosslinked with Calcium Chloride Hydrogel as Sustained Release Fertilizers. Res. Sq. 2024. preprint. [Google Scholar] [CrossRef]
- Saad, S.; Alali, H. Developing and Evaluating a Novel Drug Delivery System, Calcium-Alginate Beads loaded with Valsartan. Iraqi J. Pharm. Sci. 2024, 33, 146–153. [Google Scholar] [CrossRef]
- Sumitha, N.S.; Prakash, P.; Nair, B.N.; Sailaja, G.S. Degradation-dependent controlled delivery of doxorubicin by glyoxal cross-linked magnetic and porous chitosan microspheres. ACS Omega 2021, 6, 21472–21484. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Szczepańska, B.; Mazur, O.; Michalska-Sionkowska, M.; Łukowicz, K.; Osyczka, A.M. The preparation and characterization of chitosan-based hydrogels cross-linked by glyoxal. Materials 2021, 14, 2449. [Google Scholar] [CrossRef]
- Krishna, D.V.; Sankar, M.R.; Reddy, T.N. Effect of glyoxal concentration and nanoparticles reinforcement on the functional properties of composite hydrogel for biomedical applications. Macromol. Res. 2025, 33, 569–592. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Durpekova, S.; Filatova, K.; Cisar, J.; Ronzova, A.; Kutalkova, E.; Sedlarik, V. A novel hydrogel based on renewable materials for agricultural application. Int. J. Polym. Sci. 2020, 2020, 8363418. [Google Scholar] [CrossRef]
- Abraham, S.; Rajamanickam, D.; Srinivasan, B. Research article preparation, characterization and cross-linking of chitosan by microwave assisted synthesis. Sci. Int 2018, 6, 18–30. [Google Scholar] [CrossRef]
- Motiei, M.; Sedlařík, V.; Lucia, L.A.; Fei, H.; Münster, L. Stabilization of chitosan-based polyelectrolyte nanoparticle cargo delivery biomaterials by a multiple ionic cross-linking strategy. Carbohydr. Polym. 2020, 231, 115709. [Google Scholar] [CrossRef]
- Shi, X.; Xu, S.; Xu, J.; He, J. Preparation and properties of a multi-crosslinked chitosan/sodium alginate composite hydrogel. Mater. Lett. 2024, 354, 135414. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Yang, W.; Gong, Y.; Zhang, X.; Li, J.; Wu, D. Dual cross-linking with tannic acid and transglutaminase improves microcapsule stability and encapsulates lemon essential oil for food preservation. Food Chem. 2025, 465, 142173. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Altintas, Z. Integrated ultrasonic-transglutaminase modification of lesser mealworm protein isolate: A pioneering cobalamin delivery vehicle in gluten-free breads. Food Chem. 2024, 448, 139069. [Google Scholar] [CrossRef] [PubMed]
- Arjeh, E.; Rostami, H.; Pirsa, S.; Fathi, M. Synthesis and characterization of novel Spirulina protein isolate (SPI)-based hydrogels through dual-crosslinking with genipin/Zn2+. Food Res. Int. 2022, 162, 112107. [Google Scholar] [CrossRef]
- Song, F.; Zhang, L.-M. Gelation modification of soy protein isolate by a naturally occurring cross-linking agent and its potential biomedical application. Ind. Eng. Chem. Res. 2009, 48, 7077–7083. [Google Scholar] [CrossRef]
- Li, Z.; Lu, F.; Liu, Y. A review of the mechanism, properties, and applications of hydrogels prepared by enzymatic cross-linking. J. Agric. Food Chem. 2023, 71, 10238–10249. [Google Scholar] [CrossRef]
- Mirabelli, V.; Majidi Salehi, S.; Angiolillo, L.; Belviso, B.D.; Conte, A.; Del Nobile, M.A.; Di Profio, G.; Caliandro, R. Enzyme crystals and hydrogel composite membranes as new active food packaging material. Glob. Chall. 2018, 2, 1700089. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.; Liu, K.; Liu, X.; Zhang, X.; Wang, H.; Lu, F. High-level expression of the Streptomyces mobaraense CICC 11018 transglutaminase in Corynebacterium glutamicum ATCC 13032. Appl. Biochem. Microbiol. 2014, 50, 456–462. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, W.; Li, Y.; Gao, G.; Zhang, K.; Zhou, J.; Wu, Z. Cross-linking and film-forming properties of transglutaminase-modified collagen fibers tailored by denaturation temperature. Food Chem. 2019, 271, 527–535. [Google Scholar] [CrossRef]
- Feng, S.; Yang, W.; Zhang, L.; Wang, X.; Wang, S.; Tao, S. Casein-hydroxyapatite composite microspheres for strontium-containing wastewater treatment. ACS EST Water 2021, 1, 900–909. [Google Scholar] [CrossRef]
- Lee, S.; Rosenberg, M. Preparation and properties of glutaraldehyde cross-linked whey protein-based microcapsules containing theophylline. J. Control. Release 1999, 61, 123–136. [Google Scholar] [CrossRef]
- Gan, C.-Y.; Cheng, L.-H.; Phuah, E.-T.; Chin, P.-N.; AlKarkhi, A.F.; Easa, A.M. Combined cross-linking treatments of bovine serum albumin gel beadlets for controlled-delivery of caffeine. Food Hydrocoll. 2009, 23, 1398–1405. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Altintas, Z. Ultrasound-assisted alcoholic extraction of lesser mealworm larvae oil: Process optimization, physicochemical characteristics, and energy consumption. Antioxidants 2022, 11, 1943. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Chambin, O.; Karbowiak, T.; Debeaufort, F. Release behavior of quercetin from chitosan-fish gelatin edible films influenced by electron beam irradiation. Food Control 2016, 66, 315–319. [Google Scholar] [CrossRef]
- Abu Ghalia, M.; Dahman, Y. Radiation crosslinking polymerization of poly (vinyl alcohol) and poly (ethylene glycol) with controlled drug release. J. Polym. Res. 2015, 22, 218. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Assifaoui, A.; Karbowiak, T.; Debeaufort, F.; Chambin, O. Controlled release of tyrosol and ferulic acid encapsulated in chitosan–gelatin films after electron beam irradiation. Radiat. Phys. Chem. 2016, 118, 81–86. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Park, J.-S.; Kim, Y.-A.; Yang, S.-J.; Jeong, S.-I.; Lee, J.-Y.; Lim, Y.-M. Gamma ray-induced polymerization and cross-linking for optimization of PPy/PVP hydrogel as biomaterial. Polymers 2020, 12, 111. [Google Scholar] [CrossRef]
- Huang, T.; Fang, Z.; Zhao, H.; Xu, D.; Yang, W.; Yu, W.; Zhang, J. Physical properties and release kinetics of electron beam irradiated fish gelatin films with antioxidants of bamboo leaves. Food Biosci. 2020, 36, 100597. [Google Scholar] [CrossRef]
- Lin, J.; Yong, K.Y.A.; Zhou, Y.; Wang, Y.; Zhou, W. Improved in vitro bioaccessibility of quercetin by nanocomplexation with high-intensity ultrasound treated soy protein isolate. Food Chem. 2023, 406, 135004. [Google Scholar] [CrossRef]
- De Oliveira, F.C.; Olvera, D.; Sawkins, M.J.; Cryan, S.-A.; Kimmins, S.D.; Da Silva, T.E.; Kelly, D.J.; Duffy, G.P.; Kearney, C.; Heise, A. Direct UV-Triggered thiol–ene cross-linking of electrospun polyester fibers from unsaturated poly (macrolactone) s and their drug loading by solvent swelling. Biomacromolecules 2017, 18, 4292–4298. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-H.; Tsai, W.-B. In situ UV-crosslinking gelatin electrospun fibers for tissue engineering applications. Biofabrication 2013, 5, 035008. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Dong, P.; Gravesande, J.; Cheng, B.; Xing, J. UV-mediated solid-state cross-linking of electrospinning nanofibers of modified collagen. Int. J. Biol. Macromol. 2018, 120, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Microwave-assisted synthesis of nutgall tannic acid–based salecan polysaccharide hydrogel for tunable release of β-lactoglobulin. Int. J. Biol. Macromol. 2020, 161, 1431–1439. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Li, S.; Zeng, R.; Zhang, F.; Wang, Z.; Guan, S. Corrosion resistance and drug release profile of gentamicin-loaded polyelectrolyte multilayers on magnesium alloys: Effects of heat treatment. J. Colloid Interface Sci. 2019, 547, 309–317. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Zaman, S.; Perveen, A.; Jahan, R.A.; Islam, M.F.; Arafat, M.T. Controlled release of curcumin from electrospun fiber mats with antibacterial activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]
- Sullivan, S.T.; Tang, C.; Kennedy, A.; Talwar, S.; Khan, S.A. Electrospinning and heat treatment of whey protein nanofibers. Food Hydrocoll. 2014, 35, 36–50. [Google Scholar] [CrossRef]
- Rimdusit, S.; Somsaeng, K.; Kewsuwan, P.; Jubsilp, C.; Tiptipakorn, S. Comparison of gamma radiation crosslinking and chemical crosslinking on properties of methylcellulose hydrogel. Eng. J. 2012, 16, 15–28. [Google Scholar] [CrossRef]
- Basu, T.; Bhutani, U.; Majumdar, S. Cross-linker-free sodium alginate and gelatin hydrogels: A multiscale biomaterial design framework. J. Mater. Chem. B 2022, 10, 3614–3623. [Google Scholar] [CrossRef] [PubMed]
- Basu, T.; Goswami, D.; Majumdar, S. Fabrication of crosslinker free hydrogels with diverse properties: An interplay of multiscale physical forces within polymer matrix. iScience 2024, 27, 111227. [Google Scholar] [CrossRef]
- Kayati, F.N.; Purnomo, C.W.; Kusumastuti, Y. Physical properties comparison of hydrogel from cassava starch using two different non toxic crosslinkers. Next Sustain. 2024, 4, 100043. [Google Scholar] [CrossRef]
- Sulastri, E.; Zubair, M.S.; Lesmana, R.; Mohammed, A.F.A.; Wathoni, N. Development and characterization of ulvan polysaccharides-based hydrogel films for potential wound dressing applications. Drug Des. Dev. Ther. 2021, 15, 4213–4226. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.S.; Bai, J.; Chang, Y. Antibacterial κ-carrageenan/konjac glucomannan-based edible hydrogel film containing Salmonella phage PBSE191 and its application in chicken meat. LWT 2023, 180, 114707. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P.; Yamaguchi, M. The effect of glycerol/water and sorbitol/water on the plasticization of hydroxyethylacryl chitosan/sodium alginate films. In Proceedings of the MATEC Web of Conferences, Singapore, 26–28 October 2015; p. 02006. [Google Scholar]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. The effect of glycerin content in sodium alginate/poly (vinyl alcohol)-based hydrogels for wound dressing application. Int. J. Mol. Sci. 2021, 22, 12022. [Google Scholar] [CrossRef]
- Xie, J.; Jin, X.; Cheng, H.; Chen, W.; Yu, W.; Wang, L. Molecular origin of the plasticizing effect difference of glycerol with other polyols on plasticizing polyvinyl alcohol (PVA) as elucidated by solid-state NMR. Ind. Crops Prod. 2024, 220, 119246. [Google Scholar] [CrossRef]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J. Polym. Environ. 2021, 29, 156–174. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef]
- Kadzińska, J.; Bryś, J.; Ostrowska-Ligęza, E.; Estéve, M.; Janowicz, M. Influence of vegetable oils addition on the selected physical properties of apple–sodium alginate edible films. Polym. Bull. 2020, 77, 883–900. [Google Scholar] [CrossRef]
- Sharmin, N.; Sone, I.; Walsh, J.L.; Sivertsvik, M.; Fernández, E.N. Effect of citric acid and plasma activated water on the functional properties of sodium alginate for potential food packaging applications. Food Packag. Shelf Life 2021, 29, 100733. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, S.; Liu, Y.; Chen, S.; Li, L. Modelling and assessment of plasticizer migration and structure changes in hydrophobic starch-based films. Int. J. Biol. Macromol. 2022, 195, 41–48. [Google Scholar] [CrossRef]
- Paramita, V.D.; Kasapis, S. Molecular dynamics of the diffusion of natural bioactive compounds from high-solid biopolymer matrices for the design of functional foods. Food Hydrocoll. 2019, 88, 301–319. [Google Scholar] [CrossRef]
- Wadkin-Snaith, D.; Mulheran, P.A.; Johnston, K. The impact of plasticisers on crystal nucleation, growth and melting in linear polymers. Polymer 2024, 304, 127095. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Mohamad, A.B. Study of the plasticising effect on polymer and its development in fuel cell application. Renew. Sustain. Energy Rev. 2017, 79, 794–805. [Google Scholar] [CrossRef]
- Jost, V.; Langowski, H.-C. Effect of different plasticisers on the mechanical and barrier properties of extruded cast PHBV films. Eur. Polym. J. 2015, 68, 302–312. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and water vapour transport properties in yeast biomass based films: A study of plasticizer content and thickness effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Yoshida, C.M.; Bastos, C.E.N.; Franco, T.T. Modeling of potassium sorbate diffusion through chitosan films. LWT-Food Sci. Technol. 2010, 43, 584–589. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tan, L.W.; Lee, K.V.; Beh, K.P.; Goh, C.F. Effects of polyol and surfactant plasticisers on lyophilised rice starch wafers for buccal drug delivery. Int. J. Biol. Macromol. 2024, 261, 129935. [Google Scholar] [CrossRef] [PubMed]
- Oustadi, F.; Imani, R.; Haghbin Nazarpak, M.; Sharifi, A.M. Genipin-crosslinked gelatin hydrogel incorporated with PLLA-nanocylinders as a bone scaffold: Synthesis, characterization, and mechanical properties evaluation. Polym. Adv. Technol. 2020, 31, 1783–1792. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2017, 20, S2822–S2832. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Han, Q. Modulation of spatial and topological inhomogeneities of linear polymer hydrogel. Mater. Today Commun. 2022, 31, 103700. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Whent, M.; Yu, L.L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surf. B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Chen, L.; Tong, Q.; McClements, D.J. Designing hydrogel particles for controlled or targeted release of lipophilic bioactive agents in the gastrointestinal tract. Eur. Polym. J. 2015, 72, 698–716. [Google Scholar] [CrossRef]
- Prusty, K.; Biswal, A.; Biswal, S.B.; Swain, S.K. Synthesis of soy protein/polyacrylamide nanocomposite hydrogels for delivery of ciprofloxacin drug. Mater. Chem. Phys. 2019, 234, 378–389. [Google Scholar] [CrossRef]
- Tong, X.; Lee, S.; Bararpour, L.; Yang, F. Long-Term controlled protein release from poly (ethylene glycol) hydrogels by modulating mesh size and degradation. Macromol. Biosci. 2015, 15, 1679–1686. [Google Scholar] [CrossRef]
- Schwartz, M.P.; Fairbanks, B.D.; Rogers, R.E.; Rangarajan, R.; Zaman, M.H.; Anseth, K.S. A synthetic strategy for mimicking the extracellular matrix provides new insight about tumor cell migration. Integr. Biol. 2010, 2, 32–40. [Google Scholar] [CrossRef]
- Nagy-Smith, K.; Yamada, Y.; Schneider, J.P. Protein release from highly charged peptide hydrogel networks. J. Mater. Chem. B 2016, 4, 1999–2007. [Google Scholar] [CrossRef]
- Yu, Y.; Chau, Y. Formulation of in situ chemically cross-linked hydrogel depots for protein release: From the blob model perspective. Biomacromolecules 2015, 16, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Adams, D.J.; Iggo, J.A. Analysis of the mesh size in a supramolecular hydrogel by PFG-NMR spectroscopy. Soft Matter 2013, 9, 5483–5491. [Google Scholar] [CrossRef]
- Waters, D.J.; Engberg, K.; Parke-Houben, R.; Hartmann, L.; Ta, C.N.; Toney, M.F.; Frank, C.W. Morphology of photopolymerized end-linked poly (ethylene glycol) hydrogels by small-angle X-ray scattering. Macromolecules 2010, 43, 6861–6870. [Google Scholar] [CrossRef]
- Saffer, E.M.; Lackey, M.A.; Griffin, D.M.; Kishore, S.; Tew, G.N.; Bhatia, S.R. SANS study of highly resilient poly (ethylene glycol) hydrogels. Soft Matter 2014, 10, 1905–1916. [Google Scholar] [CrossRef]
- Munoz-Pinto, D.J.; Samavedi, S.; Grigoryan, B.; Hahn, M.S. Impact of secondary reactive species on the apparent decoupling of poly (ethylene glycol) diacrylate hydrogel average mesh size and modulus. Polymer 2015, 77, 227–238. [Google Scholar] [CrossRef][Green Version]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef]
- Thakur, G.; Mitra, A.; Rousseau, D.; Basak, A.; Sarkar, S.; Pal, K. Crosslinking of gelatin-based drug carriers by genipin induces changes in drug kinetic profiles in vitro. J. Mater. Sci. Mater. Med. 2011, 22, 115–123. [Google Scholar] [CrossRef]
- Pal, K.; Paulson, A.T.; Rousseau, D. Biopolymers in controlled-release delivery systems. In Modern Biopolymer Science; Elsevier: Amsterdam, The Netherlands, 2009; pp. 519–557. [Google Scholar]
- Nie, X.; Gong, Y.; Wang, N.; Meng, X. Preparation and characterization of edible myofibrillar protein-based film incorporated with grape seed procyanidins and green tea polyphenol. LWT-Food Sci. Technol. 2015, 64, 1042–1046. [Google Scholar] [CrossRef]
- Friesen, K.; Chang, C.; Nickerson, M. Incorporation of phenolic compounds, rutin and epicatechin, into soy protein isolate films: Mechanical, barrier and cross-linking properties. Food Chem. 2015, 172, 18–23. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wang, B.-J.; Weng, Y.-M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT-Food Sci. Technol. 2015, 63, 115–121. [Google Scholar] [CrossRef]
- Yoon, S.-D. Cross-linked potato starch-based blend films using ascorbic acid as a plasticizer. J. Agric. Food Chem. 2014, 62, 1755–1764. [Google Scholar] [CrossRef]
- Ulbin-Figlewicz, N.; Zimoch-Korzycka, A.; Jarmoluk, A. Antibacterial activity and physical properties of edible chitosan films exposed to low-pressure plasma. Food Bioprocess Technol. 2014, 7, 3646–3654. [Google Scholar] [CrossRef]
- Song, F.; Zhang, L.-M.; Yang, C.; Yan, L. Genipin-crosslinked casein hydrogels for controlled drug delivery. Int. J. Pharm. 2009, 373, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Schweizer, K.S. Theory of activated penetrant diffusion in viscous fluids and colloidal suspensions. J. Chem. Phys. 2015, 143, 144906. [Google Scholar] [CrossRef]
- Rahman, M.; Teimouri, S.; Morrish, C.; Kasapis, S. A theoretical approach is presented that follows the effect of solute size on their diffusion through genipin-crosslinked gelatin networks. Food Hydrocoll. 2025, 170, 111567. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Leahy-Dios, A.; Firoozabadi, A. Molecular and Thermal Diffusion Coefficients of Alkane-Alkane and Alkane-Aromatic Binary Mixtures: Effect of Shape and Size of Molecules. J. Phys. Chem. B 2007, 111, 191–198. [Google Scholar] [CrossRef]

| Bioactive Compounds | Environmental Influence Factors | Absorption Mechanisms in Small Intestine | References |
|---|---|---|---|
| vitamin A | Light, Acid, Heat, Humidity | Retinol absorption may occur through active or passive mechanisms, and fatty meals can increase its uptake. | [36,37] |
| vitamin B12 | Light, Heat, pH | Involve three transport protein: intrinsic factor (IF), haptocorrin (HC) and transcobalamin (TC) and their respective receptors. | [38,39] |
| vitamin C | Heat, Light, Humidity | Na+-dependent transporter transportation | [40,41] |
| vitamin D | Light, pH, hydrophobicity | Passive diffusion and/or receptor-mediated transport | [42,43] |
| vitamin E | Heat, Light, Humidity | Passive diffusion, Receptor-mediated transport | [44] |
| β-Carotene | Light, Heat | By passive diffusion and/or transporter-mediated processes | [36,37] |
| w-3 fatty acid | Heat, Light | Carrier-mediated transport | [45] |
| Quercetin | Heat, Alkali | Passive diffusion | [46,47] |
| Caffeine | Temperature | 80% of caffeine is absorbed through the gastrointestinal trac, the remaining 20% is absorbed by the stomach. | [48,49] |
| Astragals polysaccharides | Stickiness, Low solubility | Carrier-mediated transport, Passive diffusion | [50] |
| Fungal polysaccharides | Heat, Light, Humidity | Clathrin-mediated endocytosis | [51] |
| Resveratrol | Heat, Light | Passive diffusion and/or transporter-mediated processes | [52] |
| Ferulic acid | Low solubility, pH, pKa, | Passive transcellular diffusion, monocarboxylic acid transporter (MCT) and an H+-dependent carrier | [53,54] |
| Polymers | Effective Factors | Bioactive Compounds | Main Achievements | Reference |
|---|---|---|---|---|
| Okra-gelatin | pH | Isoquercetin | Embedding inhibited the decomposition of isoquercetin caused by heat, acid, and enzymes. | [102] |
| Chitosan gelatin hydrogel | pH and Temperature | Antihypertensive drug ATL | Matrices fabricated by RSM design was found to be good device to release the ATL in a controlled pattern. | [103] |
| Polysaccharide hydrogel dressing | pH | Zn2+ and ferulic acid | Hydrogel has shown effectiveness in enhancing wound healing by influencing the immune microenvironment and facilitating the process of vascularization. | [104] |
| Moringa oleifera gum polysaccharides-poly (acrylic acid) hydrogel | pH | Ciprofloxacin | Non-Fickian diffusion mechanism. The hydrogels were observed to be pH responsive, non-haemolytic, mucoadhesive non-thrombogenic, and antioxidant in nature. | [105] |
| Gum ghatti hydrogel | pH | 5-Fluorouracil | The release rate promoted as pH value rose. | [106] |
| Chitosan-oxidized Konjac glucomanan | pH | β-carotene | The structure may break down under neutral intestinal conditions (pH 7.0), resulting in the release of encapsulated antioxidants. | [107] |
| The glutamic acid grafted chitosan hydrogel beads | pH | Doxorubicin | Hydrogel beads showed pH-responsive, swelling and controlled release pattern. | [108] |
| Ethylenediaminetetraacetic acid-calcium-alginate | pH | Lactobacillus rhamnosus | The probiotic was successfully protected from the acidic environment of the stomach, allowing for a gradual release in the intestine. | [109] |
| Hemicellulose/graphene oxide (HC/GO) composite hydrogel (HGCH) | pH | B12 | HGCH had high adaptability to soluble drugs and pH sensitivity triggered release. | [110] |
| Chitosan hydrogel | pH | DOX and bovine serum albumin | pH-responsive shrinkage enhanced the release of DOX 81% but prolonged BSA release. | [111] |
| Soy protein | Ionic strength | Lysozyme | When the ionic strength was raised to 0.5 M, a significant amount of lysozyme was released. | [112] |
| SA bio-nanocomposite magnetic hydrogel bead | pH | DOX | The hydrogel bead exhibited remarkable cytotoxicity to HT-29 cells. | [113] |
| Ovotransferrin–lysozyme | Ionic strength | Fatty acids | The enhancement of ionic strength through the electrostatic screening effect resulted in a reduction in complex stability and an increase in the release of free fatty acids. | [114] |
| Alginate | Solvent | Yerba mate extract | During release experiments, the maximum release of PC was observed in a 10% ethanolic solution. | [115] |
| Starch-chitosan films | Solvent | Polyphenols | The highest number of released polyphenols was observed in acidic medium. | [116] |
| Poly(curcumin-co-oxalate) copolymer | Redox | Curcumin | The copolymer’s dissociation in the presence of H2O2 resulted in a rapid release of the core. | [117] |
| Silica nanoparticles (MSN)–starch/maltodextrin/ maltose | Enzyme | Eugenol | Exogenous enzymes degrade the attached saccharides, leading to the release of eugenol and effective suppression of fungal growth. | [118] |
| Peptide-Peptide Hydrogels | Enzyme | Zidovudine | The release of the drug occurred through the hydrolysis resulting in the release of the drug in its unaltered state and subsequently diminishing the initial drug burst. | [119] |
| Phenoxy-alkyl maleate | Enzyme | Curcumin | The hydrogel structure underwent hydrolysis in an acidic environment or through the action of lysozyme, resulting in the liberation of the bioactive compound. | [120] |
| Maltodextrin (MD), inulin (IN), and oligofructose (OL) | Physical factors Temperature | Camu-camu (Myrciaria dubia) extract | Raising the temperature from 25 °C to 35 °C affected the release behavior of the compounds. | [121] |
| Poly (ethylene glycol) | Temperature | Tannic acid | The release rate of TA was elevated by higher bilayer numbers, increased pH, and elevated temperature, whereas it declined with rising ionic strength. | [122] |
| Succinylated cellulose monocrystal (Su-CNC) hydrogel | Temperature | Famotidine | The matrices exhibited a significant reaction to temperature variations, as evidenced by a reduction in their swelling behaviour and hydrophilicity at temperatures of 35 °C and above. | [123] |
| Copolymeric hydrogels (CH) | Temperature-Reduction-pH | 5-fluorouracil | Temperature-, reduction-, and pH-responsive delivery of 5-fluorouracil from the CH system has been achieved. | [124] |
| Gelatine microsphere | Temperature | - | The diameter of microsphere increases with the increasing temperature. | [125] |
| Chitosan-poly dimethyl siloxane | Irradiation; Gamma | Curcumin | In a fibrous chitosan–curcumin polymer, exposure to 1 Gy gamma irradiation (137Cs) resulted in the release of 5 ± 1% of the conjugated curcumin, whereas 6 Gy irradiation released 98 ± 1%. | [126] |
| Hyaluronic acid-coumarin hydrogel | Near-infrared (NIR) | DOX | This injectable and biocompatible hydrogel may serve as an effective carrier for drug delivery responsive to near-infrared light, as well as for applications in bio-imaging. | [127] |
| Crosslinkers | Biopolymers | Bioactive Ingredients | Main Achievements | Reference |
|---|---|---|---|---|
| Transglutaminase enzyme | Gelatin and chitosan Core–shell nanoparticles | Curcumin | Core–shell nanoparticles were found effective delivery vehicle for curcumin. | [175] |
| Apricot kernel protein gels | Riboflavin | Riboflavin was protected from digestive degradation | [176] | |
| Gelatin film | Riboflavin and propranolol | Controlled release has been effectively demonstrated | [177] | |
| Casein/KGM hydrogels | Docetaxel | The hydrogel containing 1% KGM showed good drug release characteristics. | [178] | |
| Gelatin-based emulsion gels | β-carotene | Promising strategy for gelatin-emulsion gels with better stability, controlled release, and enhanced bioactive delivery system. | [179] | |
| Sodium caseinate film | Lysozyme | Lysozyme release was almost retarded | [180] | |
| Soy peptide nanogels | Resveratrol | This work provides a theoretical framework and technical aspects for the fabrication of peptide-based delivery systems. | [181] | |
| Gelatin film | Natamycin | Natamycin release was delayed and thus prolonged inhibition of Aspergillus ochraceus, Aspergillus niger, and Penicillium funlculosu. | [182] | |
| Glutaraldehyde (GTA) | zein nanofibers | Eugenol (EU) | Controlled release of EU from zein encapsulation was enhanced through GTA vapor crosslinking. | [183] |
| Chitosan microcapsules | Extract of red ginger oleoresin | Glutaraldehyde strengthened the bonds and lessened diameter of microspheres. | [184] | |
| Chitosan microcapsules | Ibuprofen | Drug release exhibited a biphasic pattern. | [185] | |
| Gelatin capsules | urea | Hydrophobically modification of gelatin shells for improved controlled release of urea. | [186] | |
| Chitosan microcapsules | Lysozyme | Release of lysozyme was successfully controlled. | [187] | |
| Gelatin-nanoparticles | Doxorubicin | Presented the fabrication of Ge-NPs for slow drug release and the application of ANN model | [188] | |
| Silk fibroin films | Rhodamine | Release rate increased linearly with the β-sheet content in the crosslinked film. | [189] | |
| Chitosan microparticles (Film and hydrogel types) | Metronidazole | The release profiles from hydrogel and film had better pattern compared to those containing drug powders, and hydrogel had preferrable outcome. | [190] | |
| Agarose films | Insulin | Prolonged insulin release was recorded. | [191] | |
| Genipin | Gelatin hydrogel | Lidocaine | The ICG@Lido/gGel expressed potential as photothermal-triggered release cargo for PNB (Peripheral nerve block (PNB)). | [192] |
| Chitosan/squid ring teeth protein hydrogels (CH/SRTs) | Curcumin | CH/SRTs delayed release of curcumin in a simulated gastrointestinal fluid. | [193] | |
| chitosan nanoparticles | catechin and quercetin | Both catechin and quercetin showed sustainable release profiles. | [194] | |
| β-casein micelles | Naringenin | Release of naringenin was delayed upon genipin crosslinking. | [195] | |
| Jackfruit gum-based nanoparticles | Curcumin | Offered high entrapment efficiency, controlled releases of curcumin, good mucoadhesive behaviour and enhanced anticancer activity. | [196] | |
| Gelatin hydrogel | Ascorbic acid | Infusion coefficient of water was the rate determining factor in this delivery vehicle. | [197] | |
| Chitosan Hydrogel | Thymoquinone, gefitinib, and erlotinib | Genipin-crosslinking showed stable loading and releasing with all three drugs. | [198] | |
| Gelatin-sodium caseinate microcapsules | Vitamin C and E | Fickian diffusion after crosslinking with genipin | [199] | |
| Chitosan hydrogel | Diclofenac | Hydrogels provided strong association with negatively charged diclofenac, limiting the release. | [200] | |
| Sodium caseinate-chitosan oligosaccharide nanoparticles | β-carotene | The β-carotene loaded had an enhanced anti-inflammatory activity. | [201] | |
| Chitosan-gelatin hydrogel | Metformin | Immediate release, with good safety profile, achieved by optimal genipin concentration | [202] | |
| Whey protein isolate chitosan hydrogel | Curcumin | Hydrogel had great advantages in continuous release of curcumin. | [203] | |
| Nanostructured lipid carrier-based hydrogel | Quercetin | Sustainable drug release was observed | [204] | |
| Chitosan hydrogels | Curcumin | Genipin cross-linked gels exhibited better release profile compared to glutaraldehyde crosslinked gels. | [205] | |
| Chitosan hydrogel | Iron fertilizer | GE-CSG@Fe could lower soil pH to preserve the activity of ferrous ion. | [206] | |
| Bovine serum albumin gel | Vitamin B6 | Network mesh size, regulated by crosslinking, influences diffusion kinetics. | [140] | |
| Gelatin microneedles | Insulin | A practical device designed for noninvasive, painless, and controlled insulin delivery. | [207] | |
| Chitosan/alginate nanoparticles | α–mangostin | Nanoparticles have been suggested as viable options for the oral administration of drugs targeting the colon. | [208] | |
| Gelatin/chitosan films | Methylene blue | At pH 8.0, release flux decreased markedly compared to pH 1.5, indicating a highly controllable release. | [209] | |
| Whey protein isolate-coated liposomes | Flaxseed oil | Coating and crosslinking improved the chemical stability of flaxseed oil and modified system properties. | [210] | |
| Soy β-conglycinin nanoparticles | Curcumin | This protein-based vehicle could successfully transport insoluble bioactive in the gastric conditions. | [211] | |
| Calcium chloride | Carboxymethyl cellulose (CMC) hydrogel | - | CMC hydrogel was successfully produced with CaCl2 as a crosslinker. | [212] |
| Nanocellulose fibres (CNFs)-low methoxy pectin (LMP)-sodium alginate (SA) hydrogel | Clindamycin hydrochloride (CM) | Hydrogels composed of CNFs/LMP/SA at 1:1:1 and 2:0.5:0.5 mass ratios were successfully developed as biocomposite CNFs-based systems. | [213] | |
| Sodium alginate and gelatin filament | - | Micro- and nanomechanical properties of matrices can be tuned by altering biopolymer and crosslinker concentrations. | [214] | |
| The pequi (Caryocar brasiliense) mesocarp film | - | Calcium chloride promoted a reduction in solubility and an enhanced elongation. | [215] | |
| Alginate coating | Applied on fruit | Decreased respiratory rate & weight loss | [216] | |
| Chitosan and alginate hydrogel | Fertilizer (urea) | The hydrogel regulated urea release and encapsulation efficiency without altering its swelling in water. | [217] | |
| Alginate sodium beads | Valsartan | Beads followed the Korsmeyer–Peppas release mechanism, and finally showed extended-release beads | [218] | |
| Glyoxal | Magnetic chitosan microspheres (GMS) | Doxorubicin (DOX) | It conferred GMS a potential candidate for DOX delivery. | [219] |
| Chitosan hydrogel | Tannic acid | Tannic acid release from hydrogels was achieved, with its presence enhancing thermal stability. | [220] | |
| PVA/gelatin/guar gum hydrogels containing copper nanoparticles were developed as composites. | - | Hydrogels showed good blood compatibility, antibacterial, pH, and thermo-sensitive swelling behaviour. | [221] | |
| Citric acid (CA) | Carboxymethylcellulose (CMCNa) and hydroxyethylcellulose (HEC) hydrogel | - | The findings revealed CA’s successful application as a crosslinking agent for the first time. | [222] |
| Cellulose-based hydrogel | - | Citric acid increased the structural stability of the hydrogel. | [223] | |
| Nanocellulose fibres (CNFs)-low methoxy pectin (LMP)-sodium alginate (SA) hydrogel | Clindamycin hydrochloride (CM) | Hydrogels composed of CNFs/LMP/SA at 1:1:1 and 2:0.5:0.5 mass ratios were successfully developed as biocomposite CNFs-based systems. | [213] | |
| The pequi (Caryocar brasiliense) mesocarp film | - | Citric acid proved to be the optimal modifier for pequi mesocarp film properties. | [215] | |
| Vanillin | Chitosan hydrogel | Crosslinking was markedly increased under microwave irradiation. | [224] | |
| Multiple crosslinking agents | ||||
| Citric acid (CA) and Calcium chloride | Nanocellulose fibers (CNFs)-low methoxy pectin (LMP)-sodium alginate (SA) hydrogel | Clindamycin hydrochloride (CM) | Hydrogels composed of CNFs, LMP, and SA in 1:1:1 and 2:0.5:0.5 ratios were successfully developed as biocomposite systems. | [213] |
| The pequi (Caryocar brasiliense) mesocarp film | - | Citric acid was found to be the best agent to modify the properties of pequi mesocarp films. Calcium chloride promoted a reduction in solubility and an enhanced elongation. | [215] | |
| Tripolyphosphate and dextran sulfate | Chitosan-based core | Vitamin B3 | Controlled drug release in a pH-independent manner. | [225] |
| Epichlorohydrin, calcium chloride and tannic acid | Chitosan/sodium alginate /tannic acid (CST) composite hydrogels | - | CST-31 made of a mass ratio of chitosan sodium alginate at 3:1 found to have suitable mechanical behavior, high porosity, water holding capacity, good biocompatibility and antibacterial activity. | [226] |
| Transglutaminase (TGase) and tannic acid (TA) | Gelatin-chitosan microcapsules | Lemon essential oil (LEO) | The dual cross-linked exhibited excellent antibacterial and antioxidant characteristics. | [227] |
| Transglutaminase (TGase) and ultrasound (US) | Lesser mealworm protein bead gel (LMPG) | B12 | A high controlled release rate of B12 was achieved | [228] |
| Genipin/Zn2+. | Spirulina protein isolate (SPI) hydrogel | B6 | Release profile showed the best fit to the Peppas–Sahlin model | [229] |
| Genipin and disulfide | Chitosan Hydrogel | Thymoquinone, gefitinib, and erlotinib | Genipin-crosslinking showed stable loading and releasing with all three drugs. | [198] |
| Physical Crosslinkers | Biopolymers | Bioactive Ingredients | Main Achievements | Reference |
|---|---|---|---|---|
| Gamma ray | Chitosan and gelatin film | Quercetin | Irradiation prolonged the release and accelerated the retention of quercetin. | [239] |
| Polyvinyl alcohol -polyethylene glycol hydrogels | Sodium sulphate | The release was a non-Fickian diffusion type | [240] | |
| Chitosan and gelatin film | Ferulic acid and tyrosol | Ferulic acid along with irradiation induced higher retention and lower diffusion. | [241] | |
| Moringa oleifera gum polysaccharides-poly (acrylic acid) hydrogel | Ciprofloxacin | Release occurred via a non-Fickian mechanism, and the hydrogels were pH-responsive, mucoadhesive, non-haemolytic, non-thrombogenic, and antioxidant. | [105] | |
| The glutamic acid grafted chitosan hydrogel beads | Doxorubicin | Hydrogel beads showed pH-responsive, swelling and controlled release pattern. | [108] | |
| Chitosan-polyvinyl alcohol coated polylactic acid bilayer film | Catechin | Gamma irradiation at 60 kGy decreased catechin release in high-fat simulant. | [153] | |
| PPy/PVP hydrogel | - | 0.15PPy/PVP20 showed elevated compressive strength, reaching 51.96 ± 6.12 kPa. | [242] | |
| Fish gelatin film | Bamboo leave extract | Mechanical properties of matrices were enhanced with electron beam. Electron beam lessened the release rates. | [243] | |
| Ultrasound (US) | Lesser mealworm protein bead gel (LMPG) | B12 | A high controlled release rate of B12 was achieved | [228] |
| Soy protein isolate (SPI) nanoparticles | Quercetin | Quercetin’s bio accessibility greatly improved by ultrasound assisted encapsulation of SPI. | [244] | |
| Near-infrared (NIR) | Hyaluronic acid-coumarin hydrogel | DOX | This injectable and biocompatible hydrogel may serve as an effective carrier for drug delivery responsive to near-infrared light, as well as for applications in bio-imaging. | [127] |
| Ultraviolet ray | Chitosan hydrogel | DOX and bovine serum albumin | pH-responsive shrinkage enhanced the release of DOX 81% but prolonged BSA release. | [111] |
| Aliphatic poly-globalide nanofibers | Rhodamine B and indomethacin | Cross-linking retained fibre morphology during swelling. | [245] | |
| Gelatin electrospun fibers | - | L929 cells exhibited superior growth on the UV-crosslinked scaffolds in comparison to those crosslinked with glutaraldehyde. | [246] | |
| Collagen-based nanofibers | 5-Fluorouracil | UV-crosslinking prolonged the drug-release time from the fibers. | [247] | |
| Microwave | Gelatin microspheres | - | A brief microwave exposure increased the swelling ratio to approximately 25%. | [125] |
| Salecan-g-poly (N,N-dimethylaminoethyl acrylate) (PDMAEA) and nutgall tannic acid hydrogel | β-lactoglobulin (βlg) | βlg successfully accomplished both effective entrapment within the hydrogels and a release mechanism that is tunable and controlled by pH levels. | [248] | |
| Gum ghatti hydrogel | 5-Fluorouracil | The release rate increased as the mesh size and pH levels rose. | [106] | |
| Chitosan and gelatin hydrogel | Antihypertensive drug ATL. | Matrices fabricated by RSM design was found to be good device to release the ATL in a controlled pattern. | [103] | |
| Chitosan hydrogel | - | The microwave irradiation technique can serve as a swift, dependable, and cost-effective approach for the cross-linking of chitosan. | [224] | |
| Carrageenan-guar gum hydrogel | Metronidazole | Developed IPN could be targeted controlled drug delivery cargo, allowing for expected swelling and drug release. | [97] | |
| Heat Treatment | Polyelectrolyte multilayers on magnesium alloys | Gentamicin sulphate (GS) | This study demonstrated a better performance in antibacterial activities against S. aureus with a delayed release pattern of GS. | [249] |
| Nanofibers of polyvinyl alcohol (PVA) | Curcumin | Heat treatment limited the release to a maximum of 20%, while UV exposure restricted it to 9%, in comparison with the non-crosslinked samples. | [250] | |
| Whey protein electrospun nanofibers | Rhodamine B | Heat-treated mat had slightly slower release rate. | [251] |
| Plasticizers | Biopolymers | Results Achieved | Reference |
|---|---|---|---|
| Polyethylene glycol (PEG) and glycerol | Sodium alginate (SA) and gelatin hydrogel. | PEG creates a plasticizing layer at the interface between sodium alginate and gelatin, whereas glycerol modifies the overall structure of the polymer matrix. | [253] |
| Glycerol | Sodium alginate/gelatin hydrogel | Demonstrates that the creation of new materials might not always be essential, as significant variations can arise from the same polymeric matrix across various aspects. | [254] |
| Starch hydrogel | The degree of swelling was profoundly decreased in the presence of plasticizer. | [255] | |
| Ulvan hydrogel films | The swelling behaviour of the films were excellent and could be utilized as a wound dressing biopolymer. | [256] | |
| Sorbitol | K-carrageenan and konjac glucomannan-based hydrogel film | 40% (w/w) sorbitol-containing film demonstrated the most enhanced tensile strength. | [257] |
| Glycerol/sorbitol/water | Chitosan/sodium alginate films | Flexible films with suitable properties can be achieved through the utilization of either glycerol/water or sorbitol/water as combined plasticizers. | [258] |
| Glycerin | sodium alginate/poly(vinyl alcohol) (SA/PVA) hydrogel | The research indicates a notable reduction in the gel fraction, decreasing from 80.5 ± 2.1% to 45.0 ± 1.2% as the glycerin content is increased. | [259] |
| Ethylene glycol, glycerol, erythritol, xylitol, and sorbitol | Polyvinyl alcohol film (PVA) | Among the polyols tested, PVA plasticized with glycerol exhibited the greatest reduction in Young’s modulus, enhanced toughness, and the lowest melting temperature of 195 °C. | [260] |
| Essential oils (clove oil, oregano oil and tea tree oil) | Polyvinyl alcohol (PVA)-starch hydrogel | The hydrogel demonstrated excellent antibacterial, mechanical, and physical characteristics, making it suitable for use in wound dressing applications. | [261] |
| Glycerol (20–40%) and sorbitol (30–50% | Alginate | While glycerol demonstrates greater effectiveness as a plasticizer in terms of mass content, sorbitol exhibits superior plasticizing efficiency on a molecular level. | [262] |
| Glycerol, rapeseed oil, coconut oil, hazelnut oil, and sugars in the apple puree | Alginate and apple puree | The incorporation of vegetable oils and apple puree lowered the glass transition temperature (Tg), indicating their role as plasticizers. | [263] |
| Citric acid (CA) | Alginate | At high concentrations, CA exhibited a plasticizing effect. | [264] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.; Teimouri, S.; Roy, P.R.; Raposo, A.; Alturki, H.A.; Kasapis, S. Strategies for Regulating the Release Kinetics of Bioactive Compounds from Biopolymeric Hydrogels. Gels 2025, 11, 986. https://doi.org/10.3390/gels11120986
Rahman M, Teimouri S, Roy PR, Raposo A, Alturki HA, Kasapis S. Strategies for Regulating the Release Kinetics of Bioactive Compounds from Biopolymeric Hydrogels. Gels. 2025; 11(12):986. https://doi.org/10.3390/gels11120986
Chicago/Turabian StyleRahman, Mizanur, Shahla Teimouri, Poly Rani Roy, António Raposo, Hmidan A. Alturki, and Stefan Kasapis. 2025. "Strategies for Regulating the Release Kinetics of Bioactive Compounds from Biopolymeric Hydrogels" Gels 11, no. 12: 986. https://doi.org/10.3390/gels11120986
APA StyleRahman, M., Teimouri, S., Roy, P. R., Raposo, A., Alturki, H. A., & Kasapis, S. (2025). Strategies for Regulating the Release Kinetics of Bioactive Compounds from Biopolymeric Hydrogels. Gels, 11(12), 986. https://doi.org/10.3390/gels11120986








