Toward Sustainable Green and Intelligent Profile Control Gels: An ETI–CFI-Based Structure–Environment Evaluation Framework
Abstract
1. Introduction
2. Results and Discussion
2.1. Development and Bottlenecks of Mainstream Gel Systems
- (i)
- (ii)
- High ecological risk: Systems based on Cr(III), Cr(Ⅵ), or phenol–formaldehyde organic crosslinkers exhibit inherent toxicity and are increasingly restricted by regulations such as the EU REACH directive [61] and China’s Measures for the Environmental Management of New Chemical Substances [62,63,64].
- (iii)
2.1.1. Transition-Metal Gels: Structural Optimization and Ecological Risks
- (i)
- Backbone modification for thermo–salt resistance. Incorporating hydrophobic monomers (e.g., styrene and alkyl acrylates) induces intra- and intermolecular associations, whereas grafting polar monomers (e.g., AMPS, NVP, and guanidinium) enhances chain rigidity and hydration stability [74,75,76,77,78,79]. For instance, Gumerov et al. [80] confirmed via DPD simulations that amphiphilic PVCL/TBCHA microgels with higher hydrophobic content exhibit stronger nanoscale interfacial bonding and improved thermo-responsive resilience. Similarly, Sarsenbekuly et al. [81] developed a hydrophobically modified polyacrylamide (RH-4) that maintained viscosity under 80,000 mg L−1 salinity, and Yang et al. [82] reported amphiphilic polymers maintaining > 90% volumetric stability and minimal shrinkage at 120 °C and salinity of 1.5 × 105 mg/L. These results verify that molecular structure engineering is key to achieving thermo-salt-resistant backbones.
- (ii)
- Interfacial crosslink regulation for mechanical reinforcement. Beyond backbone stabilization, fine-tuning of crosslink density and topology effectively improves mechanical integrity. Host–guest inclusion and branched architectures enhance network compactness and self-recovery. For example, β-cyclodextrin inclusion increased crosslink density and gel strength even at low dosage in [83]; grafting acrylamide onto CMC backbones yielded rigid frameworks with >30% branching degree in [84]; and Biswas et al. [78] achieved multilayer amphiphilic gels through sequential FRP–ATRP polymerization, where interlayer covalent coupling endowed high strength and elasticity. Collectively, these studies highlight that hierarchical polymer design, rather than single-component modification, dictates 3D network deformability under HT/HS stress.
- (iii)
- Nanocomposite reinforcement for structural durability. To further compensate for backbone fragility, rigid–flexible hybridization using nanoparticles has gained attention. Inorganic fillers such as SiO2, montmorillonite, and cellulose nanocrystals impart rigidity and thermal resistance, while flexible polymers (e.g., PEG and polyglutamic acid) provide elasticity and interfacial adhesion [85,86]. Recent studies have confirmed the universality of this strategy across multiple systems. Sarvesh et al. [87] incorporated Laponite® nanoclay into ABA-type PLA–PEO–PLA hydrogels, achieving nearly an order-of-magnitude increase in storage modulus. Das et al. [88] introduced graphene nanosheets into PAM matrices to enhance tensile strength, and Yang et al. [89] fabricated core–shell SiO2–PAM nanocomposites via in situ polymerization, markedly improving fracture strength. Hyperbranched silica nanoparticles (HBSPs) further reduced network density while preserving high deformability [90]. These results confirm that nano–macro-hierarchical coupling—rather than the mere addition of fillers—governs the enhancement of strength, elasticity, and thermal stability in metal-ion gels. Nevertheless, achieving uniform dispersion and interfacial compatibility at high nanoparticle loadings remains challenging, as agglomeration often deteriorates performance and limits large-scale application.
- (1)
- Low-toxicity ligand chelation. Organic acids bearing carboxyl/hydroxyl groups—such as acetic, propionic, and citric acids—serve as eco-friendly ligands that coordinate metal ions and enable delayed/controlled crosslinking [104]. As shown in Figure 3, their functional groups underpin chelation in gel networks. Notably, citric acid, due to its tri-carboxylic configuration, affords higher complex stability, promotes the reduction of Cr(Ⅵ) into the less toxic Cr(III), and suppresses the leaching/mobility of chromium species, thereby mitigating ecological risk—consistent with Lockhart’s ligand-exchange theory [105]. Representative coordination strengths and thermal stabilities are summarized in Table 3.
- (2)
- Multivalent metal substitution. Replacing toxic chromium with lower-toxicity multivalent cations—e.g., Al(III), Zr(IV), and Ti(IV)—enables controllable gelation and improved thermal endurance. Practical implementations combine delayed-release complexants (e.g., lactate/citrate) with nano-enhancement to sustain long-term integrity under high-temperature/high-salinity conditions. Representative formulations and operating windows are compiled in Table 4.
2.1.2. Evolution and Green Transition of Organic Gels
- (1)
- Deviation between “green” labels and actual environmental behavior. Current green evaluations of organic gels primarily emphasize “metal-free” or “low-toxicity” labeling while overlooking their real environmental behavior under subsurface conditions. Most degradation assessments are performed under ambient temperature and neutral pH, failing to capture the actual degradation pathways, migration of byproducts, and ecological risks that occur under high-temperature and high-salinity environments. For instance, tannic acid-based crosslinkers, though commonly regarded as eco-friendly, may generate phenolic intermediates during degradation, potentially leading to groundwater contamination and secondary environmental hazards [148,149].
- (2)
- Trade-off between performance and degradability. Flexible organic gels possess excellent hydrophilicity and biocompatibility but often suffer from insufficient mechanical strength, brittleness, and short service lifetimes under high-temperature and high-pressure conditions [150,151]. For instance, polysaccharide-based gels generally exhibit a loose three-dimensional network and limited toughness due to their high water content [152]. Enhancing thermal stability and plugging efficiency typically requires an increase in crosslinking density or the incorporation of rigid monomers. Although these strategies significantly improve mechanical robustness, they inevitably compromise biodegradability. Conversely, excessive network softening enhances degradability but reduces mechanical endurance, resulting in a clear mismatch between gel strength and degradability (Figure 5a) [153].
- (3)
- Lack of a “structure–property–environment” model. Current design approaches for organic gels remain largely empirical, lacking predictive frameworks capable of describing the coupling effects among molecular structure, performance, and environmental fate. For example, polyethyleneimine (PEI)-based systems can mitigate heavy-metal contamination; however, their long-term environmental behavior and potential toxicological impacts under reservoir conditions remain poorly understood [154,155,156]. This theoretical gap reflects the absence of an integrated understanding of the “green structure–service performance–environmental adaptability” relationship in the existing literature on organic gels.
- (4)
- Need for a coupled “structure–performance–degradation” model. To achieve both structural reliability and environmental compatibility, it is essential to develop a coupled “structure–performance–degradation behavior” model under realistic reservoir boundary conditions. Such a model would provide molecular-level guidance for rational gel design, enabling dynamic optimization between mechanical strength and degradability, and ultimately advancing the green and sustainable transformation of organic gel systems (Figure 5b).
- (1)
- Employing dynamic covalent bonds (e.g., Schiff bases and cleavable ester bonds) in synergy with physical association units (e.g., hydrophobic associations and host–guest recognition) to construct reversible networks, thus improving structural reversibility and service stability [71,165,166,167,168,169];
- (2)
- (3)
- Leveraging environmental stimuli such as temperature, pH, salinity, and shear disturbances to trigger network reconstruction or phase transitions, thereby promoting dynamic coupling between material performance and multi-field evolution (seepage–mechanical–chemical).
2.1.3. Profile Control Failure Driven by Reservoir Dynamic Evolution
- Seepage–structure disequilibrium: Pore structure reconstruction facilitates agent escape, reducing plugging efficiency;
- Seepage diversion enhances bypass flow, increases injection energy consumption, and necessitates excessive chemical dosage, thereby elevating unit oil-production carbon emissions, aggravating oily wastewater burdens, and increasing the risk of formation dissolution and reservoir integrity loss [239,240,241,242,243].
2.2. Green Grading Results
- Class I (Conventional): Cr(III)-HPAM, exhibiting strong plugging capacity but associated with high toxicity, poor degradability, and a significant carbon footprint.
- Class II (Low-Toxicity): Al-PAM and Zr-PAM, with reduced toxicity compared to Cr-based gels, yet still carbon-intensive.
- Class III (Eco-Friendly): Citric acid–chitosan gels, derived from renewable raw materials, biodegradable, and low in toxicity.
- Class IV (Intelligent Green): pH-responsive nanogels, integrating environmental adaptability, degradability, and functional responsiveness with a low-carbon synthesis pathway.
2.3. Case Studies
- Example A. Cr(III)-HPAM Gel (Conventional, Class I)
- A1. ETI Calculation (weights: toxicity 0.4, persistence 0.2, degradability 0.3, regulation 0.1)
- Chemical toxicity, S1 = 0.85: Cr compounds exhibit significant acute and chronic toxicity, while residual acrylamide monomers pose occupational hazards (data source: ECHA/EPA databases);
- Environmental persistence, S2 = 0.70: Cr is classified as environmentally persistent with bioaccumulation concerns;
- Biodegradability, S3 = 1.00: HPAM networks are non-biodegradable;
- Regulatory concern, S4 = 1.00: Cr salts are widely listed as substances of very high concern (SVHC) or subject to strict EPA regulation.
- A2. CFI Calculation (weights: raw materials 0.5, synthesis 0.2, use 0.2, disposal 0.1)
- Raw material carbon footprint, : The baseline HPAM footprint () was conservatively upscaled to by allocating upstream contributions from Cr-salt production and chelation co-reagents, as well as packaging and transportation. This upscaling was based on existing research and LCA data from reputable sources, including the Ecoinvent and ELCD databases, which provide comprehensive life cycle assessments of similar chemicals and their associated environmental impacts [244,245,246].
- Synthesis energy demand, S2 = 0.80: Polymerization and post-processing steps are energy-intensive.
- Operational energy demand, S3 = 0.75: High injection pressure and polymer dosage increase energy input.
- Disposal impact, S4 = 1.00: Disposal mainly relies on incineration or landfilling with limited valorization pathways.
- Classification: ETI = 1.45, CFI = 9.1 ⇒ Class Ⅰ (Conventional).
- Interpretation: This system combines effective plugging performance with substantial environmental burdens. The high ETI reflects acute/chronic toxicity, non-biodegradability, and stringent regulatory restrictions. The elevated CFI is driven by raw-material carbon intensity, energy-intensive synthesis, and limited end-of-life valorization. Overall, it constitutes a “performance-oriented but environmentally unsustainable” gel system.
- Example B. Al–PAM/Zr–PAM Gel (Low-Toxicity; Class II)
- B1. ETI Calculation
- Chemical toxicity, S1 = 0.30: Al/Zr salts are far less acutely toxic compared with Cr.
- Environmental persistence, S2 = 0.20: Low persistence and bioaccumulation concern.
- Biodegradability, S3 = 0.55: PAM backbone remains largely non-degradable.
- Regulatory concern, S4 = 0.15: Generally subject to standard regulatory oversight.
- B2. CFI Calculation
- Raw material carbon footprint, : Lower-range PAM values with moderate adjustment for Al/Zr salt preparation.
- Synthesis energy demand, S2 = 0.60: Partly requires high-temperature and aqueous processing.
- Operational energy demand, S3 = 0.60: Moderate injection pressure and dosage.
- Disposal impact, S4 = 0.50: Mainly landfilling with partial valorization options.
- Classification: ETI = 0.68, CFI = 4.3 ⇒ Class II (Low-Toxicity System).
- Interpretation: This system demonstrates reduced toxicological risks compared with Cr-based gels, as reflected in its lower ETI score. The substitution of Al/Zr crosslinkers decreases acute toxicity and ecological persistence, yet the use of a non-biodegradable PAM backbone remains a limiting factor. The moderate CFI arises from raw-material requirements, partially energy-intensive synthesis, and disposal routes dominated by landfilling. Overall, it represents a “toxicity-mitigated but still carbon-intensive” gel system, suitable as a transitional alternative but not fully aligned with long-term sustainability targets.
- Example C. Citric Acid-Chitosan Gel (Eco-Friendly, Class III)
- C1. ETI Calculation
- Chemical toxicity, S1 = 0.20: Citric acid and chitosan are considered non-toxic with low occupational exposure risks.
- Environmental persistence, S2 = 0.10: Not classified as persistent or bioaccumulative.
- Biodegradability, S3 = 0.10: Chitosan is biodegradable, and the crosslinked network retains degradability.
- Regulatory concern, S4 = 0.10: Neither component is listed under SVHC or EPA high-priority categories.
- C2. CFI Calculation
- Raw material carbon footprint, : Bio-based chitosan and citric acid; mid-range values from LCA databases and the literature.
- Synthesis energy demand, S2 = 0.20: Gel formation occurs in mild aqueous conditions at room temperature.
- Operational energy demand, S3 = 0.30: Relatively low injection pressure and polymer concentration.
- Disposal impact, S4 = 0.10: Biodegradable with potential for resource recovery.
- Classification: ETI = 0.42, CFI = 2.1 ⇒ Class III (Eco-Friendly).
- Interpretation: This system exemplifies the benefits of bio-based feedstocks and degradable crosslinkers. The low ETI reflects the non-toxic nature and biodegradability of citric acid and chitosan, as well as their exclusion from major regulatory concern lists. The moderate CFI results from renewable raw materials, mild aqueous synthesis under ambient conditions, relatively low operational energy demands, and environmentally benign disposal pathways. Overall, it constitutes a “biodegradable and carbon-mitigated” gel system, highlighting its strong potential for sustainable oilfield applications.
- Example D. pH-responsive nanogels (Intelligent green; Class IV)
- D1. ETI Calculation
- Chemical toxicity, S1 = 0.10: Derived from natural or bio-based monomers, inherently low toxicity.
- Environmental persistence, S2 = 0.10: Low persistence with reversible hydration/dehydration.
- Biodegradability, S3 = 0.20: Introduction of hydrolysable/cleavable linkages enables partial degradability.
- Regulatory concern, S4 = 0.05: No inclusion of SVHC or high-priority substances.
- D2. CFI Calculation
- Raw material carbon footprint, : Bio-based feedstocks or low-carbon synthetic precursors.
- Synthesis energy demand, S2 = 0.15: Typically prepared under mild aqueous conditions.
- Operational energy demand, S3 = 0.25: Low concentration and injection pressure, with potential for self-adaptive swelling/plugging.
- Disposal impact, S4 = 0.10: Biodegradable with potential for recycling or valorization.
- Classification: ETI = 0.25, CFI = 1.2 ⇒ Class IV (Intelligent Green System)
- Interpretation: This system integrates environmental friendliness with functional responsiveness. The very low ETI reflects the use of benign, bio-derived precursors and the absence of major toxicological or regulatory concerns. The low CFI is due to natural raw materials, mild synthesis conditions, minimal injection energy requirements, and fully degradable or recyclable end-of-life pathways. In addition, the pH-responsive network provides controllable plugging and adaptive behavior under reservoir conditions. Overall, it represents a “functionally adaptive and environmentally sustainable” gel system, aligning closely with long-term low-carbon and green development goals.
2.4. Framework Validation
2.5. Engineering and Academic Implications
- Quantitative benchmark: Establishes a standardized and transparent metric for evaluating environmental performance across gel systems.
- Design guideline: Provides a systematic reference for reconciling plugging efficiency and structural stability with degradability and environmental compatibility.
- Screening tool: Serves as a practical method for identifying suitable gel systems under dual-carbon policy constraints and environmental regulations.
- Theoretical foundation: Links material innovation with low-carbon development strategies, offering a scientific basis for sustainable substitution and performance optimization in profile control applications.
3. Conclusions
4. Materials and Methods
4.1. Green Grading Framework
- Toxicological and Regulatory Screening (ETI): The intrinsic environmental hazards of gel components are assessed through acute and chronic toxicity (e.g., LD50, LC50, NOEC), ecological persistence and bioaccumulation, biodegradability, and regulatory concern (e.g., inclusion in REACH SVHC or EPA priority lists). These indicators are aggregated to yield the ETI, which reflects the overall toxicological risk.
- Carbon Footprint Accounting (CFI): Using life cycle assessment (LCA), greenhouse gas emissions associated with raw material acquisition, synthesis, application, and end-of-life treatment are quantified. The results are expressed as standardized emissions per unit mass of gel (kg CO2e kg−1), thereby capturing both material-specific burdens and process-related energy inputs.
- Comprehensive Grading and Classification: ETI and CFI values are jointly mapped onto a dual-axis evaluation framework. This enables systematic comparison of different gel systems and classification into distinct green performance levels, ranging from traditional heavy-metal gels to organic crosslinked systems, bio-based degradable gels, and intelligent green gels.
- Threshold Definition and Classification Criteria
4.2. Environmental Friendliness Evaluation
4.2.1. Calculation Logic of Environmental Toxicity Index (ETI)
- Degradability: Chemical and biological degradability, based on standardized OECD 301B tests (e.g., chitosan is degradable, while HPAM is non-degradable);
4.2.2. Carbon Footprint Intensity (CFI) Calculation Logic
- Raw material footprint (kg CO2e kg−1): Data are obtained from established life cycle assessment (LCA) databases—such as Ecoinvent (https://ecoinvent.org), GaBi LCA (https://sphera.com/gabi/, accessed on 11 October 2025), and ELCD (https://eplca.jrc.ec.europa.eu/, accessed on 11 October 2025)—or derived from reports from the literature. For reference, typical values include HPAM ≈ 5–7 kg CO2e kg−1 and chitosan ≈ 2 kg CO2e kg−1 [264].
- Synthesis energy consumption: Determined by whether high-temperature/high-pressure reactions are required (e.g., HPAM synthesis involves energy-intensive conditions, while chitosan–citric acid gels can be formed under ambient conditions).
- Operational energy consumption: Accounts for injection pressure and concentration requirements during field application (polymer-based systems typically require higher injection pressure).
- End-of-life treatment: Considers degradability and potential for resource recovery (e.g., chitosan gels are biodegradable, whereas Cr-based gels present significant disposal challenges).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ETI | Environmental Toxicity Index |
| CFI | Carbon Footprint Intensity |
| HPAM | Hydrolyzed Polyacrylamide |
| LCA | Life Cycle Assessment |
| REACH | Registration, Evaluation, Authorisation and Restriction of Chemicals |
| SVHC | Substances of Very High Concern |
| OECD | Organisation for Economic Co-operation and Development |
References
- Liang, B.; Chen, C.; Jia, C.; Wang, C.; Wang, X.; Zha, Y.; Wang, R.; Meng, Z.; Wang, H. Carbon capture, utilization and storage (CCUS) in oil and gas reservoirs in China: Status, opportunities and challenges. Fuel 2024, 375, 132353. [Google Scholar] [CrossRef]
- Zhao, S.; Song, Q.; Liu, L.; Li, J.; Zhao, D. Uncovering the lifecycle carbon emissions and its reduction pathways: A case study of petroleum refining enterprise. Energy Convers. Manag. 2024, 301, 118048. [Google Scholar] [CrossRef]
- Shang, L.; Lyu, Z.; Sun, N.; Shen, G.; Shen, Q.; Guo, R.; Wei, W. Pathways for supply security and carbon-neutral transition in the oil products industry: A comprehensive technology portfolio evaluation. J. Clean. Prod. 2025, 499, 145241. [Google Scholar] [CrossRef]
- Zou, C.; Lin, M.; Ma, F.; Liu, H.; Yang, Z.; Zhang, G.; Yang, Y.; Guan, C.; Liang, Y.; Wang, Y.; et al. Development, challenges and strategies of natural gas industry under carbon neutral target in China. Pet. Explor. Dev. 2024, 51, 476–497. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.; Andrew, R.; Bakker, D.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Luijkx, I.; Peters, G.; et al. Global Carbon Budget 2023. Earth Syst. Sci. Data 2023, 15, 5301–5369. [Google Scholar] [CrossRef]
- Cointe, B.; Guillemot, H. A history of the 1.5 °C target. WIREs Clim. Change 2023, 14, e824. [Google Scholar] [CrossRef]
- Kuyper, J.; Schroeder, H.; Linnér, B.-O. The Evolution of the UNFCCC. Annu. Rev. Environ. Resour. 2018, 43, 343–368. [Google Scholar] [CrossRef]
- Schipper, E.L.F. Conceptual History of Adaptation in the UNFCCC Process. Rev. Eur. Community Int. Environ. Law 2006, 15, 82–92. [Google Scholar] [CrossRef]
- IEA, Global Energy Review 2025; IEA: Paris, France, 2025; Available online: https://www.iea.org/reports/global-energy-review-2025 (accessed on 11 October 2025).
- Finkbeiner, M.; Inaba, A.; Tan, R.; Christiansen, K.; Klüppel, H.-J. The New International Standards for Life Cycle Assessment: ISO 14040 and ISO 14044. Int. J. Life Cycle Assess. 2006, 11, 80–85. [Google Scholar] [CrossRef]
- Wu, P.; Xia, B.; Wang, X. The contribution of ISO 14067 to the evolution of global greenhouse gas standards—A review. Renew. Sustain. Energy Rev. 2015, 47, 142–150. [Google Scholar] [CrossRef]
- Rebitzer, G.; Ekvall, T.; Frischknecht, R.; Hunkeler, D.; Norris, G.; Rydberg, T.; Schmidt, W.P.; Suh, S.; Weidema, B.P.; Pennington, D.W. Life cycle assessment: Part 1: Framework, goal and scope definition, inventory analysis, and applications. Environ. Int. 2004, 30, 701–720. [Google Scholar] [CrossRef]
- Schrijvers, D.; Loubet, P.; Sonnemann, G. Archetypes of Goal and Scope Definitions for Consistent Allocation in LCA. Sustainability 2020, 12, 5587. [Google Scholar] [CrossRef]
- Van Hoof, G.; Vieira, M.; Gausman, M.; Weisbrod, A. Indicator selection in life cycle assessment to enable decision making: Issues and solutions. Int. J. Life Cycle Assess. 2013, 18, 1568–1580. [Google Scholar] [CrossRef]
- Goedkoop, M.; Heijungs, R.; Huijbregts, M.; De Schryver, A.; Struijs, J.; Van Zelm, R. ReCiPe 2008. A life cycle impact assessment method which comprises harmonised category indicators at the midpoint and the endpoint level. Impact World 2009, 1, 1–126. [Google Scholar]
- Owsianiak, M.; Hauschild, M.Z.; Posthuma, L.; Saouter, E.; Vijver, M.G.; Backhaus, T.; Douziech, M.; Schlekat, T.; Fantke, P. Ecotoxicity characterization of chemicals: Global recommendations and implementation in USEtox. Chemosphere 2023, 310, 136807. [Google Scholar] [CrossRef]
- Rosenbaum, R.K.; Bachmann, T.M.; Gold, L.S.; Huijbregts, M.A.J.; Jolliet, O.; Juraske, R.; Koehler, A.; Larsen, H.F.; MacLeod, M.; Margni, M.; et al. USEtox—The UNEP-SETAC toxicity model: Recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int. J. Life Cycle Assess. 2008, 13, 532–546. [Google Scholar] [CrossRef]
- Henderson, A.D.; Hauschild, M.Z.; van de Meent, D.; Huijbregts, M.A.J.; Larsen, H.F.; Margni, M.; McKone, T.E.; Payet, J.; Rosenbaum, R.K.; Jolliet, O. USEtox fate and ecotoxicity factors for comparative assessment of toxic emissions in life cycle analysis: Sensitivity to key chemical properties. Int. J. Life Cycle Assess. 2011, 16, 701–709. [Google Scholar] [CrossRef]
- Rosenbaum, R.K.; Huijbregts, M.A.J.; Henderson, A.D.; Margni, M.; McKone, T.E.; van de Meent, D.; Hauschild, M.Z.; Shaked, S.; Li, D.S.; Gold, L.S.; et al. USEtox human exposure and toxicity factors for comparative assessment of toxic emissions in life cycle analysis: Sensitivity to key chemical properties. Int. J. Life Cycle Assess. 2011, 16, 710–727. [Google Scholar] [CrossRef]
- Belyanovskaya, A.; Laratte, B.; Perry, N.; Baranovskaya, N. A regional approach for the calculation of characteristic toxicity factors using the USEtox model. Sci. Total Environ. 2019, 655, 676–683. [Google Scholar] [CrossRef]
- Bare, J.C. Traci. J. Ind. Ecol. 2002, 6, 49–78. [Google Scholar] [CrossRef]
- Bare, J. TRACI 2.0: The tool for the reduction and assessment of chemical and other environmental impacts 2.0. Clean Technol. Environ. Policy 2011, 13, 687–696. [Google Scholar] [CrossRef]
- Sydansk, R.D.; Southwell, G. More than 12 years’ experience with a successful conformance-control polymer-gel technology. SPE Prod. Fac. 2000, 15, 270–278. [Google Scholar] [CrossRef]
- Frampton, H.; Morgan, J.C.; Cheung, S.K.; Munson, L.; Chang, K.T.; Williams, D. Development of a novel waterflood conformance control system. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, Oklahoma, 17–21 April 2004. [Google Scholar]
- Sagbana, P.I.; Abushaikha, A.S. A comprehensive review of the chemical-based conformance control methods in oil reservoirs. J. Pet. Explor. Prod. Technol. 2021, 11, 2233–2257. [Google Scholar] [CrossRef]
- Imqam, A.; Bai, B.; Delshad, M. Micro-particle gel transport performance through unconsolidated sandstone and its blocking to water flow during conformance control treatments. Fuel 2018, 231, 479–488. [Google Scholar] [CrossRef]
- Sheng, J.J.; Leonhardt, B.; Azri, N. Status of Polymer-Flooding Technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Ismail, S.; Sultan, A.S. Crosslinked Polyacrylamide Composite Hydrogels Impregnated with Fly Ash: Synthesis, Characterization and Their Application as Fractures Sealant for High Water Producing Zones in Oil and Gas Wells. J. Polym. Environ. 2018, 26, 3294–3306. [Google Scholar] [CrossRef]
- Ghosh, B.; Ali, S.A.; Belhaj, H. Controlling excess water production in fractured carbonate reservoirs: Chemical zonal protection design. J. Pet. Explor. Prod. Technol. 2020, 10, 1921–1931. [Google Scholar] [CrossRef]
- Song, R.; Jiang, G.; Wang, K. Gelation mechanism and rheological properties of polyacrylamide crosslinking with polyethyleneimine and its plugging performance in air-foam displacement. J. Appl. Polym. Sci. 2018, 135, 45778. [Google Scholar] [CrossRef]
- Wang, K.; Luo, M.; Li, M.; Gu, X.; Li, X.; Fan, Q.; Pu, C.; Wang, L. Gelation and Plugging Performance of Low-Concentration Partially Hydrolyzed Polyacrylamide/Polyethyleneimine System at Moderate Temperature and in Fractured Low-Permeability Reservoir. Polymers 2024, 16, 1585. [Google Scholar] [CrossRef]
- Niu, C.; Fan, S.; Chen, X.; He, Z.; Dai, L.; Wen, Z.; Li, M. Preparation and Performance Evaluation of a Supramolecular Polymer Gel-Based Temporary Plugging Agent for Heavy Oil Reservoir. Gels 2024, 10, 536. [Google Scholar] [CrossRef]
- Sang, Q.; Li, Y.; Yu, L.; Li, Z.; Dong, M. Enhanced oil recovery by branched-preformed particle gel injection in parallel-sandpack models. Fuel 2014, 136, 295–306. [Google Scholar] [CrossRef]
- Tongwa, P.; Baojun, B. A more superior preformed particle gel with potential application for conformance control in mature oilfields. J. Pet. Explor. Prod. Technol. 2015, 5, 201–210. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. npj Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Meng, M.; Niu, D.; Shang, W. CO2 emissions and economic development: China’s 12th five-year plan. Energy Policy 2012, 42, 468–475. [Google Scholar] [CrossRef]
- SY/T 6788-2020; Technical Evaluation Methods for Environmental Protection of Water-Soluble Oilfield Chemicals. National Energy Administration: Beijing, China, 2020.
- Lei, S.; Sun, J.; Lv, K.; Zhang, Q.; Yang, J. Types and Performances of Polymer Gels for Oil-Gas Drilling and Production: A Review. Gels 2022, 8, 386. [Google Scholar] [CrossRef]
- Burrafato, G.; Carminati, S.; Bonaccorsi, F.; Lockhart, T.P. Evidence for molecular Cr3+ cross-links in Cr3+/polyacrylamide gels. Macromolecules 1990, 23, 2402–2406. [Google Scholar] [CrossRef]
- te Nijenhuis, K. Crosslink nature in Cr(III)-polyacrylamide gels. Macromol. Symp. 2001, 171, 189–200. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, J.; Gu, Y.; Zhao, Q.; Yang, R.; Yang, Y. Polyacrylamide gel formed by Cr(III) and phenolic resin for water control in high-temperature reservoirs. J. Pet. Sci. Eng. 2020, 194, 107423. [Google Scholar] [CrossRef]
- Shaiful Bahari, A.M.; Othman, S.Z.; Mohamad Fadli, M.F.; Zulkifli, M.Z.A.; Biyamin, S.A.; Islam, M.A.; Aspanut, Z.; Amin, N.; Misran, H. Facile synthesis of Zr-based metal-organic gel (Zr-MOG) using “green” sol-gel approach. Surf. Interfaces 2021, 27, 101469. [Google Scholar] [CrossRef]
- Dietrich, D.; Licht, C.; Nuhnen, A.; Höfert, S.-P.; De Laporte, L.; Janiak, C. Metal–Organic Gels Based on a Bisamide Tetracarboxyl Ligand for Carbon Dioxide, Sulfur Dioxide, and Selective Dye Uptake. ACS Appl. Mater. Interfaces 2019, 11, 19654–19667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, H.; Sarsenbekuly, B.; Zhang, M.; Jiang, H.; Kang, W.; Aidarova, S. The advances of organic chromium based polymer gels and their application in improved oil recovery. Adv. Colloid Interface Sci. 2020, 282, 102214. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, S.; Shi, C.; Huo, M.; Lin, Y. Progress in Research and Application of Metal–Organic Gels: A Review. Nanomaterials 2023, 13, 1178. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Zhang, H.; Ikeda, S. Hydrocolloid gels of polysaccharides and proteins. Curr. Opin. Colloid Interface Sci. 2000, 5, 195–201. [Google Scholar] [CrossRef]
- Clark, A.H. Biopolymer gels. Curr. Opin. Colloid Interface Sci. 1996, 1, 712–717. [Google Scholar] [CrossRef]
- Chowhan, A.; Giri, T.K. Polysaccharide as renewable responsive biopolymer for in situ gel in the delivery of drug through ocular route. Int. J. Biol. Macromol. 2020, 150, 559–572. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Davletshin, A.; Li, Z.; You, J.; Tan, S. Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers 2020, 12, 1860. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Gbonhinbor, J.; Onyekonwu, M. Natural polymer flow behaviour in porous media for enhanced oil recovery applications: A review. J. Pet. Explor. Prod. Technol. 2018, 8, 1349–1362. [Google Scholar] [CrossRef]
- Irzhak, V.I.; Uflyand, I.E.; Dzhardimalieva, G.I. Self-Healing of Polymers and Polymer Composites. Polymers 2022, 14, 5404. [Google Scholar] [CrossRef]
- Kang, C.; Guo, J.; Kiyingi, W.; Li, J.; Xue, P. A New Self-Healing Green Polymer Gel with Dynamic Networks for Flow Control in Harsh Reservoirs. Adv. Funct. Mater. 2025, 35, 2423892. [Google Scholar] [CrossRef]
- Kim, J.R.; Netravali, A.N. Self-healing green polymers and composites. In Advanced Green Composites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 135–185. [Google Scholar]
- Kang, W.; Kang, X.; Lashari, Z.A.; Li, Z.; Zhou, B.; Yang, H.; Sarsenbekuly, B.; Aidarova, S. Progress of polymer gels for conformance control in oilfield. Adv. Colloid Interface Sci. 2021, 289, 102363. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Aldhaheri, M.; Wei, M.; Zhang, N.; Bai, B. Field design guidelines for gel strengths of profile-control gel treatments based on reservoir type. J. Pet. Sci. Eng. 2020, 194, 107482. [Google Scholar] [CrossRef]
- Vargas-Vasquez, S.M.; Romero-Zerón, L.B. A Review of the Partly Hydrolyzed Polyacrylamide Cr(III) Acetate Polymer Gels. Pet. Sci. Technol. 2008, 26, 481–498. [Google Scholar] [CrossRef]
- Vossoughi, S. Profile modification using in situ gelation technology—A review. J. Pet. Sci. Eng. 2000, 26, 199–209. [Google Scholar] [CrossRef]
- Pu, W.; Wen, C.; Liu, R.; Jin, F.; Wang, C.; Liao, Z. Evaluation of a novel profile control agent for enhancing an oil-recovery application. J. Appl. Polym. Sci. 2016, 133, 43756. [Google Scholar] [CrossRef]
- EC. Registration, Evaluation, Authorization and Restriction of Chemicals (REACH). regulation (EC) no. 1907/2006 of the European Parliament and of the Council. Off. J. Eur. Commun 2006, 396, 1–849. [Google Scholar]
- Reddy, B.R.; Eoff, L.; Dalrymple, E.D.; Black, K.; Brown, D.; Rietjens, M. A Natural Polymer-Based Cross-Linker System for Conformance Gel Systems. Spe J. 2003, 8, 99–106. [Google Scholar] [CrossRef]
- China, Ministry of Environmental Protection, Order No. 12 of the Ministry of Environmental Protection of the People’s Republic of China: Measures for the Environmental Management of New Chemical Substances. 2020. Available online: www.gov.cn (accessed on 29 April 2020).
- Wang, H.; Yan, Z.-G.; Li, H.; Yang, N.-Y.; Leung, K.M.Y.; Wang, Y.-Z.; Yu, R.-Z.; Zhang, L.; Wang, W.-H.; Jiao, C.-Y.; et al. Progress of environmental management and risk assessment of industrial chemicals in China. Environ. Pollut. 2012, 165, 174–181. [Google Scholar] [CrossRef]
- Correia, M.G.; Maschio, C.; Schiozer, D.J. Development of complex layered and fractured reservoir models for reservoir simulation. J. Braz. Soc. Mech. Sci. Eng. 2017, 39, 219–233. [Google Scholar] [CrossRef]
- Amirsardari, M.; Dabir, B.; Naderifar, A. Development of a flow based dynamic gridding approach for fluid flow modeling in heterogeneous reservoirs. J. Nat. Gas Sci. Eng. 2016, 31, 715–729. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H.W.; Li, H.T.; Liu, X.J.; Tan, Y.S.; Chen, S.N.; Cai, J.C. A brief review of dynamic capillarity effect and its characteristics in low permeability and tight reservoirs. J. Pet. Sci. Eng. 2020, 189, 106959. [Google Scholar] [CrossRef]
- Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Needham, R.B.; Threlkeld, C.B.; Gall, J.W. Control of Water Mobility Using Polymers and Multivalent Cations. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, 22–24 April 1974. [Google Scholar]
- Fang, J.; Zhang, X.; Li, L.; Zhang, J.; Shi, X.; Hu, G. Research Progress of High-Temperature Resistant Functional Gel Materials and Their Application in Oil and Gas Drilling. Gels 2023, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, R.O.; Oluyemi, G.F.; Officer, S.; Ugwu, J.O. Hydrophobically associating polymers for enhanced oil recovery—Part A: A review on the effects of some key reservoir conditions. J. Pet. Sci. Eng. 2019, 180, 681–698. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, Z.; Yang, G.; Lü, H.; Xia, X. Research on feasibility of amphiphilic polymer for chemical flooding in heterogeneous heavy oil reservoir. Pet. Geol. Recovery Effic. 2015, 22, 116–120. [Google Scholar]
- Candau, F.; Selb, J. Hydrophobically-modified polyacrylamides prepared by micellar polymerization. Adv. Colloid Interface Sci. 1999, 79, 149–172. [Google Scholar] [CrossRef]
- Ida, S.; Nishisako, D.; Fujiseki, A.; Kanaoka, S. Thermoresponsive properties of polymer hydrogels induced by copolymerization of hydrophilic and hydrophobic monomers: Comprehensive study of monomer sequence and water affinity. Soft Matter 2021, 17, 6063–6072. [Google Scholar] [CrossRef]
- Vázquez, B.; San Roman, J.; Peniche, C.; Cohen, M.E. Polymeric Hydrophilic Hydrogels with Flexible Hydrophobic Chains. Control of the Hydration and Interactions with Water Molecules. Macromolecules 1997, 30, 8440–8446. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, X.; Fu, M.; Zhao, H.; Li, G.; Zuo, J. Experimental study of calcium-enhancing terpolymer hydrogel for improved oil recovery in ultrodeep carbonate reservoir. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 251–259. [Google Scholar] [CrossRef]
- Su, X.; Hao, D.; Xu, X.; Guo, X.; Li, Z.; Jiang, L. Hydrophilic/Hydrophobic Heterogeneity Anti-Biofouling Hydrogels with Well-Regulated Rehydration. ACS Appl. Mater. Interfaces 2020, 12, 25316–25323. [Google Scholar] [CrossRef]
- Biswas, S.; Singh, A.; Beziau, A.; Kowalewski, T.; Matyjaszewski, K.; Balazs, A.C. Modeling the formation of layered, amphiphilic gels. Polymer 2017, 111, 214–221. [Google Scholar] [CrossRef]
- Patrickios, C.S.; Georgiou, T.K. Covalent amphiphilic polymer networks. Curr. Opin. Colloid Interface Sci. 2003, 8, 76–85. [Google Scholar] [CrossRef]
- Gumerov, R.A.; Gau, E.; Xu, W.; Melle, A.; Filippov, S.A.; Sorokina, A.S.; Wolter, N.A.; Pich, A.; Potemkin, I.I. Amphiphilic PVCL/TBCHA microgels: From synthesis to characterization in a highly selective solvent. J. Colloid Interface Sci. 2020, 564, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Sarsenbekuly, B.; Kang, W.; Fan, H.; Yang, H.; Dai, C.; Zhao, B.; Aidarova, S.B. Study of salt tolerance and temperature resistance of a hydrophobically modified polyacrylamide based novel functional polymer for EOR. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 91–97. [Google Scholar] [CrossRef]
- Yang, H.B.; Iqbal, M.W.; Lashari, Z.A.; Cao, C.X.; Tang, X.C.; Kang, W.L. Experimental research on amphiphilic polymer/organic chromium gel for high salinity reservoirs. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 582, 123900. [Google Scholar] [CrossRef]
- Zhang, H.W.; Yang, H.B.; Zhou, B.B.; Li, X.X.; Zhao, H.; Wang, F.; Kang, W.L.; Sarsenbekuly, B.; Aidarova, S.; Gabdullin, M. Effects of cyclodextrin polymer on the gelation of amphiphilic polymer in inclusion complex. J. Mol. Liq. 2020, 305, 112850. [Google Scholar] [CrossRef]
- Singh, R.; Kamla, K.; and Mahto, V. Study of the Gelation and Rheological Behavior of Carboxymethyl Cellulose-Polyacrylamide Graft Copolymer Hydrogel. J. Dispers. Sci. Technol. 2015, 36, 877–884. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Nanoparticle Reinforced Polymers. Polymers 2019, 11, 625. [Google Scholar] [CrossRef]
- Awasthi, S.; Gaur, J.K.; Bobji, M.S.; Srivastava, C. Nanoparticle-reinforced polyacrylamide hydrogel composites for clinical applications: A review. J. Mater. Sci. 2022, 57, 8041–8063. [Google Scholar] [CrossRef]
- Agrawal, S.K.; Sanabria-DeLong, N.; Tew, G.N.; Bhatia, S.R. Nanoparticle-Reinforced Associative Network Hydrogels. Langmuir 2008, 24, 13148–13154. [Google Scholar] [CrossRef]
- Das, S.; Irin, F.; Ma, L.; Bhattacharia, S.K.; Hedden, R.C.; Green, M.J. Rheology and Morphology of Pristine Graphene/Polyacrylamide Gels. ACS Appl. Mater. Interfaces 2013, 5, 8633–8640. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.-R.; Duan, J.-F.; Xu, F.; Sun, R.-C. Interaction of Silica Nanoparticle/Polymer Nanocomposite Cluster Network Structure: Revisiting the Reinforcement Mechanism. J. Phys. Chem. C 2013, 117, 8223–8230. [Google Scholar] [CrossRef]
- Meng, X.; Qiao, Y.; Do, C.; Bras, W.; He, C.; Ke, Y.; Russell, T.P.; Qiu, D. Hysteresis-Free Nanoparticle-Reinforced Hydrogels. Adv. Mater. 2022, 34, 2108243. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, H.; Li, X.; Li, Z.; Bauyrzhan, S.; Ning, C.; Shen, J.; Wang, H.; Jiang, H.; Kang, W. Strong thickening performance and mechanism of supramolecular system constructed by β-cyclodextrin polymer included adamantane polymer. J. Mol. Liq. 2023, 391, 123313. [Google Scholar] [CrossRef]
- Rasouli, S.; Hashemianzadeh, S.M. Thermal behavior of cyclodextrin/adamantane host/guest inclusion complex in an aqueous media. J. Mol. Liq. 2023, 390, 123096. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, H.; Gao, J.; Gu, X.; Yu, X.; Su, G.; Jiang, Z.; Zhu, Y. Synthesis and molecular dynamics simulation of amphoteric hydrophobically associating polymer. J. Mol. Liq. 2023, 388, 122751. [Google Scholar] [CrossRef]
- Singh, R.; Mahto, V.; Vuthaluru, H. Development of a novel fly ash-polyacrylamide nanocomposite gel system for improved recovery of oil from heterogeneous reservoir. J. Pet. Sci. Eng. 2018, 165, 325–331. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Synthesis and Solution Properties of Zwitterionic Polymers. Chem. Rev. 2002, 102, 4177–4190. [Google Scholar] [CrossRef]
- Ntente, C.; Iatridi, Z.; Theodoropoulou, M.; Bokias, G.; Tsakiroglou, C. Anionic amphiphilic copolymers as potential agents for enhanced oil recovery. React. Funct. Polym. 2023, 184, 105521. [Google Scholar] [CrossRef]
- Yang, J.-B.; Sun, J.-S.; Bai, Y.-R.; Lv, K.-H.; Li, J.; Li, M.-C.; Zhu, Y.-C. Preparation and characterization of supramolecular gel suitable for fractured formations. Pet. Sci. 2023, 20, 2324–2342. [Google Scholar] [CrossRef]
- Yang, H.; Lv, Z.; Zhang, M.; Jiang, J.; Xu, B.; Shen, J.; Jiang, H.; Kang, W. A novel active amphiphilic polymer for enhancing heavy oil recovery: Synthesis, characterization and mechanism. J. Mol. Liq. 2023, 391, 123210. [Google Scholar] [CrossRef]
- Pandit, Y.K.; Kumar, A.; Mahto, V.; Gopalakrishnan Nair, U.; Matey, S.; Dhandi, M. Experimental Investigation of a Novel Alumina Nanomaterial Reinforced Particle Gel System for Water Shut-off Jobs in Heterogeneous Reservoirs: Fabrication, Characterization, and Performance Assessment. Ind. Eng. Chem. Res. 2024, 63, 15665–15682. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Xing, J.; Li, J.; Yang, F.; Fu, Y.; Huang, J.; Bai, Y.; Bai, B. Cyclic enrichment of chromium based on valence state transformation in metal-free photocatalytic reductive imprinted composite hydrogel. Sci. Total Environ. 2022, 839, 156367. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A Critical Assessment of Chromium in the Environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Barnhart, J. Chromium chemistry and implications for environmental fate and toxicity. J. Soil Contam. 1997, 6, 561–568. [Google Scholar] [CrossRef]
- Dastidar, P.; Ganguly, S.; Sarkar, K. Metallogels from Coordination Complexes, Organometallic, and Coordination Polymers. Chem. Asian J. 2016, 11, 2484–2498. [Google Scholar] [CrossRef]
- Lockhart, T.P. Chemical Properties of Chromium/Polyacrylamide Gels. SPE Adv. Technol. Ser. 1994, 2, 199–205. [Google Scholar] [CrossRef]
- Yi, G.; Sayer, M. An acetic acid/water based sol-gel PZT process I: Modification of Zr and Ti alkoxides with acetic acid. J. Sol-Gel Sci. Technol. 1996, 6, 65–74. [Google Scholar] [CrossRef]
- Yi, G.; Sayer, M. An acetic acid/water based sol-gel PZT process II: Formation of a water based solution. J. Sol-Gel Sci. Technol. 1996, 6, 75–82. [Google Scholar] [CrossRef]
- Kang, C.-H.; Guo, J.-X.; Fei, D.-T.; Kiyingi, W. Intelligent responsive self-assembled micro-nanocapsules: Used to delay gel gelation time. Pet. Sci. 2024, 21, 2433–2443. [Google Scholar] [CrossRef]
- Mumallah, N.A. Chromium (III) Propionate: A Crosslinking Agent for Water-Soluble Polymers in Hard Oilfield Brines. SPE Reserv. Eng. 1988, 3, 243–250. [Google Scholar] [CrossRef]
- Kaddouri, A.; Mazzocchia, C. Thermoanalytic study of some metal propionates synthesised by sol–gel route: A kinetic and thermodynamic study. J. Anal. Appl. Pyrolysis 2002, 65, 253–267. [Google Scholar] [CrossRef]
- Gel, B.A. Boric Acid Gel. 1993. Available online: https://www.sigmaaldrich.cn/deepweb/assets/sigmaaldrich/product/documents/312/932/al_techbull_al102.pdf (accessed on 14 November 2025).
- Shah, S.N.; Lord, D.L.; Rao, B.N. Borate-Crosslinked Fluid Rheology Under Various pH, Temperature, and Shear History Conditions. In Proceedings of the SPE Production Operations Symposium, Oklahoma City, OK, USA, 9–11 March 1997. [Google Scholar]
- Brannon, H.D.; Ault, M.G. New, Delayed Borate-Crosslinked Fluid Provides Improved Fracture Conductivity in High-Temperature Applications. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 6–9 October 1991. [Google Scholar]
- Harris, P.C. Chemistry and Rheology of Borate-Crosslinked Fluids at Temperatures to 300F. J. Pet. Technol. 1993, 45, 264–269. [Google Scholar] [CrossRef]
- de Jong, S.J.; van Eerdenbrugh, B.; van Nostrum, C.F.; Kettenes-van den Bosch, J.J.; Hennink, W.E. Physically crosslinked dextran hydrogels by stereocomplex formation of lactic acid oligomers: Degradation and protein release behavior. J. Control. Release 2001, 71, 261–275. [Google Scholar] [CrossRef]
- Niu, C.; Zhang, N.; Hu, C.; Zhang, C.; Zhang, H.; Xing, Y. Preparation of a novel citric acid-crosslinked Zn-MOF/chitosan composite and application in adsorption of chromium(VI) and methyl orange from aqueous solution. Carbohydr. Polym. 2021, 258, 117644. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, S.; Sodesawa, T.; Suzuki, M.; Ichikuni, N. Ni/SiO2 prepared by sol–gel process using citric acid. Microporous Mesoporous Mat. 2003, 66, 197–208. [Google Scholar] [CrossRef]
- Shibayama, M.; Shudo, Y.; Izumi, A. Phenolic Resins—Recent Progress of Structure and Properties Investigations. Macromol. Symp. 2019, 385, 1800156. [Google Scholar] [CrossRef]
- Ran, Y.; Zhang, G.; Jiang, P.; Pei, H. Study on Water-Soluble Phenolic Resin Gels for High-Temperature and High-Salinity Oil Reservoir. Gels 2023, 9, 489. [Google Scholar] [CrossRef]
- Nichifor, M. Role of Hydrophobic Associations in Self-Healing Hydrogels Based on Amphiphilic Polysaccharides. Polymers 2023, 15, 1065. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Guo, H.; Wang, J.; Zhang, Y. Preparation of hydrophobic association polyacrylamide in a new micellar copolymerization system and its hydrophobically associative property. Macromolecules 2008, 41, 2890–2897. [Google Scholar] [CrossRef]
- Jiang, H.; Duan, L.; Ren, X.; Gao, G. Hydrophobic association hydrogels with excellent mechanical and self-healing properties. Eur. Polym. J. 2019, 112, 660–669. [Google Scholar] [CrossRef]
- Kakuta, T.; Takashima, Y.; Harada, A. Highly elastic supramolecular hydrogels using host–guest inclusion complexes with cyclodextrins. Macromolecules 2013, 46, 4575–4579. [Google Scholar] [CrossRef]
- You, Q.; Zhang, P.; Bai, S.; Huang, W.; Jia, Z.; Zhou, C.; Li, D. Supramolecular linear polymer formed by host–guest interactions of β-cyclodextrin dimers and polyacrylamide end-capped with adamantane. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 130–135. [Google Scholar] [CrossRef]
- Tungala, K.; Kumar, K.; Sonker, E.; Krishnamoorthi, S. Micellization of amphiphilic host–guest inclusion complexes of polymers based on β–cyclodextrin trimer and adamantane. React. Funct. Polym. 2020, 157, 104771. [Google Scholar] [CrossRef]
- Chen, J.H.; Rong, M.Z.; Ruan, W.H.; Zhang, M.Q. Interfacial enhancement of nano-SiO2/polypropylene composites. Compos. Sci. Technol. 2009, 69, 252–259. [Google Scholar] [CrossRef]
- Dellatolas, I.; Bantawa, M.; Damerau, B.; Guo, M.; Divoux, T.; Del Gado, E.; Bischofberger, I. Local Mechanism Governs Global Reinforcement of Nanofiller-Hydrogel Composites. ACS Nano 2023, 17, 20939–20948. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Samanta, S.K. Soft-Nanocomposites of Nanoparticles and Nanocarbons with Supramolecular and Polymer Gels and Their Applications. Chem. Rev. 2016, 116, 11967–12028. [Google Scholar] [CrossRef]
- Debertrand, L.; Zhao, J.; Creton, C.; Narita, T. Swelling and Mechanical Properties of Polyacrylamide-Derivative Dual-Crosslink Hydrogels Having Metal–Ligand Coordination Bonds as Transient Crosslinks. Gels 2021, 7, 72. [Google Scholar] [CrossRef]
- te Nijenhuis, K.; Mensert, A.; Zitha, P.L.J. Viscoelastic behaviour of partly hydrolysed polyacrylamide/chromium(III) gels. Rheol. Acta 2003, 42, 132–141. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Li, L.; Wu, R.; Liu, D.; Wu, J.; Wu, W. High-temperature-resistant polymer gel system with metal–organic mixed cross-linking agents. J. Appl. Polym. Sci. 2015, 132, 42261. [Google Scholar] [CrossRef]
- Cai, W.; Huang, R. Study on gelation of partially hydrolyzed polyacrylamide with titanium(IV). Eur. Polym. J. 2001, 37, 1553–1559. [Google Scholar] [CrossRef]
- Kedir, A.S.; Seland, J.G.; Skauge, A.; Skauge, T. Nanoparticles for Enhanced Oil Recovery: Influence of pH on Aluminum-Cross-linked Partially Hydrolyzed Polyacrylamide-Investigation by Rheology and NMR. Energy Fuels 2014, 28, 2343–2351. [Google Scholar] [CrossRef]
- Karatum, O.; Bhuiya, M.M.H.; Carroll, M.K.; Anderson, A.M.; Plata, D.L. Life Cycle Assessment of Aerogel Manufacture on Small and Large Scales: Weighing the Use of Advanced Materials in Oil Spill Remediation. J. Ind. Ecol. 2018, 22, 1365–1377. [Google Scholar] [CrossRef]
- Garrido, R.; Silvestre, J.D.; Flores-Colen, I. Economic and Energy Life Cycle Assessment of aerogel-based thermal renders. J. Clean. Prod. 2017, 151, 537–545. [Google Scholar] [CrossRef]
- Pinto, I.; Silvestre, J.D.; de Brito, J.; Júlio, M.F. Environmental impact of the subcritical production of silica aerogels. J. Clean. Prod. 2020, 252, 119696. [Google Scholar] [CrossRef]
- Turhan Kara, I.; Kiyak, B.; Colak Gunes, N.; Yucel, S. Life cycle assessment of aerogels: A critical review. J. Sol-Gel Sci. Technol. 2024, 111, 618–649. [Google Scholar] [CrossRef]
- Enríquez-Martínez, V.; Niembro-García, I.J.; Marmolejo-Saucedo, J.A. A Life Cycle Assessment (LCA) of Antibacterial Gel Production. In Proceedings of the Computer Science and Engineering in Health Services, Virtual Event, 29 July 2021; Springer: Cham, Switzerland, 2021; pp. 12–27. [Google Scholar]
- Silva, D.A.L. Life Cycle Assessment (LCA)—Definition of Goals and Scope. In Life Cycle Engineering and Management of Products: Theory and Practice; de Oliveira, J.A., Lopes Silva, D.A., Puglieri, F.N., Saavedra, Y.M.B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 45–69. [Google Scholar]
- Leroy-Parmentier, N.; Valdivia, S.; Sonnemann, G. Defining the goal and scope for life cycle sustainability assessment. In Handbook on Life Cycle Sustainability Assessment; Edward Elgar Publishing: Cheltenham, UK, 2024; pp. 136–143. [Google Scholar]
- Toffoletto, L.; Bulle, C.; Godin, J.; Reid, C.; Deschênes, L. LUCAS—A New LCIA Method Used for a Canadian-Specific Context. Int. J. Life Cycle Assess. 2007, 12, 93–102. [Google Scholar] [CrossRef]
- Reddy, B.R.; Eoff, L.; Crespo, F.; Lewis, C. Recent Advances in Organically Crosslinked Conformance Polymer Systems. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013. [Google Scholar]
- Hutchins, R.D.; Dovan, H.T.; Sandiford, B.B. Field Applications of High Temperature Organic Gels for Water Control. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 21–24 April 1996. [Google Scholar]
- Salgaonkar, L.; Das, P. Laboratory Evaluation of Organically Crosslinked Polymer for Water Shutoff in High-Temperature Well Applications. In Proceedings of the SPE Kuwait International Petroleum Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2012. [Google Scholar]
- Shehbaz, S.M.; Bera, A. Effects of nanoparticles, polymer and accelerator concentrations, and salinity on gelation behavior of polymer gel systems for water shut-off jobs in oil reservoirs. Pet. Res. 2023, 8, 234–243. [Google Scholar] [CrossRef]
- Duceac, I.A.; Coseri, S. Chitosan Schiff-Base Hydrogels—A Critical Perspective Review. Gels 2022, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ding, M.; Hu, Y.; Wang, Y.; Dong, J. Optimization of the Methods to Develop Stable Polymer Gels for Water Management in Medium- and Ultra-High-Salinity Reservoirs. Gels 2023, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Boczkaj, G. Paving the way for green cross-linker substances for the fabrication of polymer membranes—A review. Curr. Opin. Chem. Eng. 2025, 47, 101097. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Zhu, Q.; Yang, M.; Li, J.; Zhang, J.; Yang, B.; Zhao, X. Identification, Formation, and Predicted Toxicity of Halogenated DBPs Derived from Tannic Acid and Its Biodegradation Products. Environ. Sci. Technol. 2019, 53, 13019–13030. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef]
- Guo, H.; Ge, J.; Li, L.; Liu, M.; Wang, W. Development, evaluation and stability mechanism of high-strength gels in high-temperature and high-salinity reservoirs. J. Mol. Liq. 2024, 399, 124452. [Google Scholar] [CrossRef]
- Liu, Y.; Song, S.; Liu, S.; Zhu, X.; Wang, P. Application of Nanomaterial in Hydrogels Related to Wound Healing. J. Nanomater. 2022, 2022, 4656037. [Google Scholar] [CrossRef]
- Frazar, E.M.; Shah, R.A.; Dziubla, T.D.; Hilt, J.Z. Multifunctional temperature—Responsive polymers as advanced biomaterials and beyond. J. Appl. Polym. Sci. 2020, 137, 48770. [Google Scholar] [CrossRef]
- Soradech, S.; Williams, A.C.; Khutoryanskiy, V.V. Physically Cross-Linked Cryogels of Linear Polyethyleneimine: Influence of Cooling Temperature and Solvent Composition. Macromolecules 2022, 55, 9537–9546. [Google Scholar] [CrossRef]
- Lim, H.-S.; Chae, S.; Yan, L.; Li, G.; Feng, R.; Shin, Y.; Nie, Z.; Sivakumar, B.M.; Zhang, X.; Liang, Y.; et al. Crosslinked Polyethyleneimine Gel Polymer Interface to Improve Cycling Stability of RFBs. Energy Mater. Adv. 2022, 2022, 9863679. [Google Scholar] [CrossRef]
- Jia, H.; Chen, H. Using DSC technique to investigate the non-isothermal gelation kinetics of the multi-crosslinked Chromium acetate (Cr3+)-Polyethyleneimine (PEI)-Polymer gel sealant. J. Pet. Sci. Eng. 2018, 165, 105–113. [Google Scholar] [CrossRef]
- Duquette, D.; Dumont, M.-J. Comparative studies of chemical crosslinking reactions and applications of bio-based hydrogels. Polym. Bull. 2019, 76, 2683–2710. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, R.; Jena, T.; Pradhan, S.; Mohanty, S. Recent advancement of bio-based super absorbent polymer and its biodegradable and recycling behavior: A vision and future. Polym.-Plast. Technol. Mater. 2023, 62, 1290–1317. [Google Scholar] [CrossRef]
- Motornov, M.; Roiter, Y.; Tokarev, I.; Minko, S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci. 2010, 35, 174–211. [Google Scholar] [CrossRef]
- Takashima, Y.; Yonekura, K.; Koyanagi, K.; Iwaso, K.; Nakahata, M.; Yamaguchi, H.; Harada, A. Multifunctional Stimuli-Responsive Supramolecular Materials with Stretching, Coloring, and Self-Healing Properties Functionalized via Host–Guest Interactions. Macromolecules 2017, 50, 4144–4150. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Gao, C.; Qin, Z.; Mi, H.-Y.; Dong, B.; Jing, X.; Liu, C.; Shen, C. Impact-resistant, high-toughness, self-healable elastomers with physical-chemical dual-crosslinking networks for efficient energy absorption. Appl. Mater. Today 2024, 41, 102522. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Xu, D.; Dong, Q.-W.; Song, X.-J.; Chen, Y.-B.; Cui, Y.-L. Biomedical potentials of alginate via physical, chemical, and biological modifications. Int. J. Biol. Macromol. 2024, 277, 134409. [Google Scholar] [CrossRef]
- An, X.; Ma, C.; Gong, L.; Liu, C.; Li, N.; Liu, Z.; Li, X. Ionic-physical–chemical triple cross-linked all-biomass-based aerogel for thermal insulation applications. J. Colloid Interface Sci. 2024, 668, 678–690. [Google Scholar] [CrossRef]
- Becker, G.; Wurm, F.R. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem. Soc. Rev. 2018, 47, 7739–7782. [Google Scholar] [CrossRef]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Chen, J.-H.; Yuan, W.-Q.; Li, Y.-D.; Weng, Y.-X.; Zeng, J.-B. Malleable and Sustainable Poly(ester amide) Networks Synthesized via Melt Condensation Polymerization. ACS Sustain. Chem. Eng. 2019, 7, 15147–15153. [Google Scholar] [CrossRef]
- Qu, T.; West, K.N.; Rupar, P.A. Rapid synthesis of functional poly (ester amide) s through thiol–ene chemistry. RSC Adv. 2023, 13, 22928–22935. [Google Scholar] [CrossRef]
- Wei, Z.; He, J.; Liang, T.; Oh, H.; Athas, J.; Tong, Z.; Wang, C.; Nie, Z. Autonomous self-healing of poly (acrylic acid) hydrogels induced by the migration of ferric ions. Polym. Chem. 2013, 4, 4601–4605. [Google Scholar] [CrossRef]
- Liu, G.; Zou, F.; He, W.; Li, J.; Xie, Y.; Ma, M.; Zheng, Y. The controlled degradation of bacterial cellulose in simulated physiological environment by immobilization and release of cellulase. Carbohydr. Polym. 2023, 314, 120906. [Google Scholar] [CrossRef]
- Ginjupalli, K.; Shavi, G.V.; Averineni, R.K.; Bhat, M.; Udupa, N.; Nagaraja Upadhya, P. Poly(α-hydroxy acid) based polymers: A review on material and degradation aspects. Polym. Degrad. Stab. 2017, 144, 520–535. [Google Scholar] [CrossRef]
- Yao, F.; Bai, Y.; Chen, W.; An, X.; Yao, K.; Sun, P.; Lin, H. Synthesis and characterization of functional l-lactic acid/citric acid oligomer. Eur. Polym. J. 2004, 40, 1895–1901. [Google Scholar] [CrossRef]
- Yao, F.; Chen, W.; Liu, C.; De Yao, K. A novel amphoteric, pH-sensitive, biodegradable poly [chitosan-g-(l-lactic-co-citric) acid] hydrogel. J. Appl. Polym. Sci. 2003, 89, 3850–3854. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Reversible adsorption by a pH- and temperature-sensitive acrylic hydrogel. J. Control. Release 2002, 80, 247–257. [Google Scholar] [CrossRef]
- Osada, Y.; Gong, J. Stimuli-responsive polymer gels and their application to chemomechanical systems. Prog. Polym. Sci. 1993, 18, 187–226. [Google Scholar] [CrossRef]
- Goponenko, A.V.; Dzenis, Y.A. Role of mechanical factors in applications of stimuli-responsive polymer gels—Status and prospects. Polymer 2016, 101, 415–449. [Google Scholar] [CrossRef]
- Nakajima, T.; Sato, H.; Zhao, Y.; Kawahara, S.; Kurokawa, T.; Sugahara, K.; Gong, J.P. A Universal Molecular Stent Method to Toughen any Hydrogels Based on Double Network Concept. Adv. Funct. Mater. 2012, 22, 4426–4432. [Google Scholar] [CrossRef]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Xin, H. Double-Network Tough Hydrogels: A Brief Review on Achievements and Challenges. Gels 2022, 8, 247. [Google Scholar] [CrossRef]
- Zhang, M.; Choi, W.; Kim, M.; Choi, J.; Zang, X.; Ren, Y.; Chen, H.; Tsukruk, V.; Peng, J.; Liu, Y. Recent advances in environmentally friendly dual-crosslinking polymer networks. Angew. Chem. 2024, 136, e202318035. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, L.W.E.; Zhou, J. Recent developments of polysaccharide-based double-network hydrogels. J. Polym. Sci. 2023, 61, 7–43. [Google Scholar] [CrossRef]
- Yang, H.; van Ruymbeke, E.; Fustin, C.-A. Influence of Network Topology on the Viscoelastic Properties of Double Dynamics Hydrogels. Macromolecules 2022, 55, 5058–5070. [Google Scholar] [CrossRef]
- Rodin, M.; Li, J.; Kuckling, D. Dually cross-linked single networks: Structures and applications. Chem. Soc. Rev. 2021, 50, 8147–8177. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Q.; Chen, H.; Tang, Y.; Zheng, J. Spontaneous Macrophase Separation Strategy for Bridging Hydrogels from Bilayer to Double-Network Structure. Acc. Mater. Res. 2024, 5, 1415–1427. [Google Scholar] [CrossRef]
- Tominaga, T.; Tirumala, V.R.; Lee, S.; Lin, E.K.; Gong, J.P.; Wu, W.-l. Thermodynamic Interactions in Double-Network Hydrogels. J. Phys. Chem. B 2008, 112, 3903–3909. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Zhang, J.; Luo, H. Constructions and Properties of Physically Cross-Linked Hydrogels Based on Natural Polymers. Polym. Rev. 2023, 63, 574–612. [Google Scholar] [CrossRef]
- Šomvársky, J.; te Nijenhuis, K.; Ilavský, M. Polyfunctional Cross-Linking of Existing Polymer Chains. Macromolecules 2000, 33, 3659–3670. [Google Scholar] [CrossRef]
- Andrianov, K.A.; Emel’yanov, V.N. Some Aspects of the Theory of Gel Formation in Reactions of Polyfunctional Compounds. Russ. Chem. Rev. 1976, 45, 931. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Shi, Z.; Zhi, M.; Hong, Z. The investigation of an organic acid assisted sol–gel method for preparing monolithic zirconia aerogels. RSC Adv. 2018, 8, 8011–8020. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Kumar, G.; Bristow, J.F.; Smith, P.J.; Payne, G.F. Enzymatic gelation of the natural polymer chitosan. Polymer 2000, 41, 2157–2168. [Google Scholar] [CrossRef]
- Brotherton, E. Synthesis and Applications of New Hydrophilic Aldehyde-Functional Methacrylic Polymers. Doctoral Dissertation, University of Sheffield, Sheffield, UK, 2022. [Google Scholar]
- Dong, S.; He, L.; Li, L.; Wu, Y.; Wang, X. Investigation of Polyvinyl Alcohol–Phenolic Aldehyde–Polyacrylamide Gel for the Application in Saline Oil Reservoirs for Profile Modification. Energy Fuels 2023, 37, 13710–13720. [Google Scholar] [CrossRef]
- Brotherton, E.E.; Jesson, C.P.; Warren, N.J.; Smallridge, M.J.; Armes, S.P. New aldehyde-functional methacrylic water-soluble polymers. Angew. Chem. 2021, 133, 12139–12144. [Google Scholar] [CrossRef]
- Karlsson, S.; Backlund, S.; Eriksson, F.; Hedström, G. Enzymatic esterifications and transesterifications in AOT-based gels with different composition. Colloids Surf. B Biointerfaces 1998, 10, 137–147. [Google Scholar] [CrossRef]
- Matsuda, F.; Miyamoto, S.; Iizawa, T. Novel Synthesis of Gel Capsule by Esterification of Poly(acrylic acid) Gel. Polym. J. 1999, 31, 435–441. [Google Scholar] [CrossRef]
- Sun, S.; Sun, P.; Liu, D. The study of esterifying reaction between epoxy resins and carboxyl acrylic polymers in the presence of tertiary amine. Eur. Polym. J. 2005, 41, 913–922. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Liu, Z.; Yang, S.; Xu, N.; Zhao, W.; Luo, G.; Liu, S. Alleviation of the plastic deformation of gel ink under strong stress through an esterification of xanthan gum reinforcing its double helix structure. Chin. J. Chem. Eng. 2024, 67, 49–57. [Google Scholar] [CrossRef]
- Rivas, M.V.; Muñetón, M.J.A.; Bordoni, A.V.; Lombardo, M.V.; Spagnuolo, C.C.; Wolosiuk, A. Revisiting carboxylic group functionalization of silica sol–gel materials. J. Mater. Chem. B 2023, 11, 1628–1653. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.; Rietzler, B.; Pham, T.; Bechtold, T. Multivalent Ions as Reactive Crosslinkers for Biopolymers—A Review. Molecules 2020, 25, 1840. [Google Scholar] [CrossRef] [PubMed]
- Perić-Hassler, L.; Hünenberger, P.H. Interaction of alginate single-chain polyguluronate segments with mono-and divalent metal cations: A comparative molecular dynamics study. Mol. Simul. 2010, 36, 778–795. [Google Scholar] [CrossRef]
- Tavakoli, V. Carbonate Reservoir Heterogeneity: Overcoming the Challenges; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Duan, R.; Xu, Z.; Dong, Y.; Liu, W. Characterization and classification of pore structures in deeply buried carbonate rocks based on mono- and multifractal methods. J. Pet. Sci. Eng. 2021, 203, 108606. [Google Scholar] [CrossRef]
- Cicha-Szot, R.; Labus, K.; Leśniak, G. Pore-Scale Evolution of Carbonate and Sandstone Reservoirs Under CO2–Brine Interaction: Implications for Sustainable Carbon Storage. Sustainability 2025, 17, 9102. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Zhang, Z.; Yu, J.; Zhang, Y.; Li, X.; Zhao, H. Research on fluid mobility in tight-sandstone with a NMR fractal theory pore classification method. Front. Earth Sci. 2023, 10, 1035702. [Google Scholar] [CrossRef]
- Li, M.; Qu, Z.; Wang, M.; Ran, W. The Influence of Micro-Heterogeneity on Water Injection Development in Low-Permeability Sandstone Oil Reservoirs. Minerals 2023, 13, 1533. [Google Scholar] [CrossRef]
- Mohammadmoradi, P.; Bashtani, F.; Goudarzi, B.; Taheri, S.; Kantzas, A. Pore Network and Morphological Characterization of Pore-Level Structures. In Proceedings of the SPE Canada Heavy Oil Technical Conference, Calgary, AB, Canada, 15–16 February 2017. [Google Scholar]
- Wang, C.; Liu, X.; Wang, E.; Wang, M.; Liu, C. Dependence of connectivity dominance on fracture permeability and influence of topological centrality on the flow capacity of fractured porous media. J. Hydrol. 2023, 624, 129883. [Google Scholar] [CrossRef]
- Sarkar, P.; Singh, K.H.; Singh, T.N.; Ghosh, R. Pore Topology. In Laboratory Characterization of Shale: Measurement and Simulation; Sarkar, P., Singh, K.H., Singh, T.N., Ghosh, R., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 55–82. [Google Scholar]
- Stoop, N.; Waisbord, N.; Kantsler, V.; Heinonen, V.; Guasto, J.S.; Dunkel, J. Disorder-induced topological transition in porous media flow networks. J. Non-Newton. Fluid Mech. 2019, 268, 66–74. [Google Scholar] [CrossRef]
- Wang, M.; Cui, Z.; Xue, Y. Determination of Interfacial Tension of Nanomaterials and the Effect of Particle Size on Interfacial Tension. Langmuir 2021, 37, 14463–14471. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sang, Q.; Dong, M.; Yuan, Y. Effects of Interfacial Tension and Droplet Size on the Plugging Performance of Oil-in-Water Emulsions in Porous Media. Ind. Eng. Chem. Res. 2017, 56, 9237–9246. [Google Scholar] [CrossRef]
- von Nessen, K.; Karg, M.; Hellweg, T. Thermoresponsive poly-(N-isopropylmethacrylamide) microgels: Tailoring particle size by interfacial tension control. Polymer 2013, 54, 5499–5510. [Google Scholar] [CrossRef]
- Lepercq-Bost, É.; Giorgi, M.-L.; Isambert, A.; Arnaud, C. Use of the capillary number for the prediction of droplet size in membrane emulsification. J. Membr. Sci. 2008, 314, 76–89. [Google Scholar] [CrossRef]
- Style, R.W.; Hyland, C.; Boltyanskiy, R.; Wettlaufer, J.S.; Dufresne, E.R. Surface tension and contact with soft elastic solids. Nat. Commun. 2013, 4, 2728. [Google Scholar] [CrossRef]
- Mohammadmoradi, P.; Kantzas, A. Pore Scale Investigation of Wettability Effect on Waterflood Performance. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dubai, UAE, 26–28 September 2016. [Google Scholar]
- Lewis, M.G.; Sharma, M.M.; Dunlap, H.F. Wettability and Stress Effects on Saturation and Cementation Exponents. In Proceedings of the SPWLA 29th Annual Logging Symposium, San Antonio, TX, USA, 5–8 June 1988. [Google Scholar]
- Khurshid, I.; Al-Attar, H.; Alraeesi, A. Modeling cementation in porous media during waterflooding: Asphaltene deposition, formation dissolution and their cementation. J. Pet. Sci. Eng. 2018, 161, 359–367. [Google Scholar] [CrossRef]
- Barclay, S.A.; Worden, R.H. Effects of Reservoir Wettability on Quartz Cementation in Oil Fields. In Quartz Cementation in Sandstones; Wiley: New York, NY, USA, 2000; pp. 103–117. [Google Scholar]
- Changfu, X.; Hongxian, L.; Genbao, Q.; Jianhua, Q. Microcosmic mechanisms of water-oil displacement in conglomerate reservoirs in Karamay Oilfield, NW China. Pet. Explor. Dev. 2011, 38, 725–732. [Google Scholar] [CrossRef]
- Lin, Z.; Li, J.; Wang, M.; Zhang, P.; Lu, S.; Zhi, Q.; Wang, J.; Huang, H. Organic fluid migration in low permeability reservoirs restricted by pore structure parameters. J. Pet. Sci. Eng. 2022, 218, 111028. [Google Scholar] [CrossRef]
- Vafaie, A.; Kivi, I.R.; Moallemi, S.A.; Habibnia, B. Permeability prediction in tight gas reservoirs based on pore structure characteristics: A case study from South Western Iran. Unconv. Resour. 2021, 1, 9–17. [Google Scholar] [CrossRef]
- Mustafa, A.; Mahmoud, M.A.; Abdulraheem, A. A Review of Pore Structure Characterization of Unconventional Tight Reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 11–14 November 2019. [Google Scholar]
- Fan, C.; Cao, J.; Luo, J.; Li, S.; Wu, S.; Dai, L.; Hou, J.; Mao, Q. Heterogeneity and influencing factors of marine gravity flow tight sandstone under abnormally high pressure: A case study from the Miocene Huangliu Formation reservoirs in LD10 area, Yinggehai Basin, South China Sea. Pet. Explor. Dev. 2021, 48, 1048–1062. [Google Scholar] [CrossRef]
- Xiao, Z.; Ding, W.; Hao, S.; Taleghani, A.D.; Wang, X.; Zhou, X.; Sun, Y.; Liu, J.; Gu, Y. Quantitative analysis of tight sandstone reservoir heterogeneity based on rescaled range analysis and empirical mode decomposition: A case study of the Chang 7 reservoir in the Dingbian oilfield. J. Pet. Sci. Eng. 2019, 182, 106326. [Google Scholar] [CrossRef]
- Ju, Y.; Sun, Y.; Tan, J.; Bu, H.; Han, K.; Li, X.; Fang, L. The composition, pore structure characterization and deformation mechanism of coal-bearing shales from tectonically altered coalfields in eastern China. Fuel 2018, 234, 626–642. [Google Scholar] [CrossRef]
- Rachinsky, M.; Kerimov, V.Y. Fluid Dynamics of Oil and Gas Reservoirs; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mullins, O.C.; Zuo, J.Y.; Wang, K.; Hammond, P.S.; De Santo, I.; Dumont, H.; Mishra, V.K.; Chen, L.; Pomerantz, A.E.; Dong, C.; et al. The Dynamics of Reservoir Fluids and their Substantial Systematic Variations. Petrophysics—SPWLA J. 2014, 55, 96–112. [Google Scholar]
- Gussow, W.C. Migration of Reservoir Fluids. J. Pet. Technol. 1968, 20, 353–365. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Z.; Gao, L.; Li, C.; Li, H. Evolution of pore structure in gas shale related to structural deformation. Fuel 2017, 197, 310–319. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, Genesis, and Distribution of Nanometer-Scale Pores in Siliceous Mudstones of the Mississippian Barnett Shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Solano, N.; Bustin, R.M.; Bustin, A.M.M.; Chalmers, G.R.L.; He, L.; Melnichenko, Y.B.; Radliński, A.P.; Blach, T.P. Pore structure characterization of North American shale gas reservoirs using USANS/SANS, gas adsorption, and mercury intrusion. Fuel 2013, 103, 606–616. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Haghshenas, B.; Ghanizadeh, A.; Qanbari, F.; Williams-Kovacs, J.D.; Riazi, N.; Debuhr, C.; Deglint, H.J. Nanopores to megafractures: Current challenges and methods for shale gas reservoir and hydraulic fracture characterization. J. Nat. Gas Sci. Eng. 2016, 31, 612–657. [Google Scholar] [CrossRef]
- Cappa, F.; Guglielmi, Y.; Fénart, P.; Merrien-Soukatchoff, V.; Thoraval, A. Hydromechanical interactions in a fractured carbonate reservoir inferred from hydraulic and mechanical measurements. Int. J. Rock Mech. Min. Sci. 2005, 42, 287–306. [Google Scholar] [CrossRef]
- Wei, M.; Dai, F.; Ji, Y.; Wu, W. Effect of fluid pressure gradient on the factor of safety in rock stability analysis. Eng. Geol. 2021, 294, 106346. [Google Scholar] [CrossRef]
- Cornell, D.; Katz, D.L. Pressure Gradients in Natural Gas Reservoirs. J. Pet. Technol. 1953, 5, 61–70. [Google Scholar] [CrossRef]
- Montel, F.; Bickert, J.; Hy-Billiot, J.; Royer, M. Pressure and Compositional Gradients in Reservoirs. In Proceedings of the Nigeria Annual International Conference and Exhibition, Abuja, Nigeria, 4–6 August 2003. [Google Scholar]
- Sarma, H.K.; Bentsen, R.G. A study of the impact of instability on relative permeability and capillary pressure. J. Pet. Sci. Eng. 1989, 2, 311–330. [Google Scholar] [CrossRef]
- Abbasi, S.; Khamehchi, E. Investigation of permeability decline due to coupled precipitation/dissolution mechanism in carbonate rocks during low salinity co-water injection. Energy Rep. 2021, 7, 125–135. [Google Scholar] [CrossRef]
- Amour, F.; Bonto, M.; Hajiabadi, M.R.; Nick, H.M. Sensitivity Study of Chemical Effects on the Compaction Behavior of Reservoir Chalk (Dan Field, Danish North Sea). In Proceedings of the 55th U.S. Rock Mechanics/Geomechanics Symposium, Virtual, 18–25 June 2021. [Google Scholar]
- Zhou, L.; Wu, J.; Ji, J.-H.; Gao, J.; Liu, Y.-F.; Wang, B.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Characteristics of microbiota, core sulfate-reducing taxa and corrosion rates in production water from five petroleum reservoirs in China. Sci. Total Environ. 2023, 858, 159861. [Google Scholar] [CrossRef]
- Mangane, P.O.; Gouze, P.; Luquot, L. Permeability impairment of a limestone reservoir triggered by heterogeneous dissolution and particles migration during CO2-rich injection. Geophys. Res. Lett. 2013, 40, 4614–4619. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, Z. Permeability damage micro-mechanisms and stimulation of low-permeability sandstone reservoirs: A case study from Jiyang Depression, Bohai Bay Basin, China. Pet. Explor. Dev. 2020, 47, 374–382. [Google Scholar] [CrossRef]
- O’neill, T.J. Life Cycle Assessment and Environmental Impact of Polymeric Products; Rapra Technology Limited: Shrewsbury, UK, 2003. [Google Scholar]
- Ghosh, P.; Wilton, R.R.; Bowers, A.; O’Brien, T.; Cao, Y.; Wilson, C.; Metidji, M.O.; Dupuis, G.; Ravikiran, R. Re-Injection of Produced Polymer in EOR Projects to Improve Economics and Reduce Carbon Footprint. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 25–29 April 2022. [Google Scholar]
- Quintero, H.I.; Solorzano, P.L.; Barbosa, C.; Corredor, L.M.; Martinez, A.; Feriz, E.F.; HernÁNdez, R.; Vega, S.M.; Carrasca L, K.J.; Zapata, J.F.; et al. Carbon Footprint and Energy Intensity Assessment for Enhanced Oil Recovery (EOR) Based on Polymer Injection: A Colombian Case Study. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 22–25 April 2024. [Google Scholar]
- Sajid, M.; Płotka-Wasylka, J. Green analytical chemistry metrics: A review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef]
- Tufvesson, L.M.; Tufvesson, P.; Woodley, J.M.; Börjesson, P. Life cycle assessment in green chemistry: Overview of key parameters and methodological concerns. Int. J. Life Cycle Assess. 2013, 18, 431–444. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Judson, R.; Richard, A.; Dix, D.J.; Houck, K.; Martin, M.; Kavlock, R.; Dellarco, V.; Henry, T.; Holderman, T.; Sayre, P. The toxicity data landscape for environmental chemicals. Environ. Health Perspect. 2009, 117, 685–695. [Google Scholar] [CrossRef]
- Fantke, P.; Aurisano, N.; Provoost, J.; Karamertzanis, P.G.; Hauschild, M. Toward effective use of REACH data for science and policy. Environ. Int. 2020, 135, 105336. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Rattner, B.A.; Burton, G.A., Jr.; Cairns, J., Jr. Handbook of Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Faroon, O.; Mumtaz, M.; Wohlers, D. Toxicological Profile for Acrylamide; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.; Patlolla, A.; Sutton, D. Environmental toxicology. Mol. Clin. Environ. Toxicol. Exp. Suppl. 2012, 3, 133–164. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Lau, M.H.Y.; Leung, K.M.Y.; Wong, S.W.Y.; Wang, H.; Yan, Z.-G. Environmental policy, legislation and management of persistent organic pollutants (POPs) in China. Environ. Pollut. 2012, 165, 182–192. [Google Scholar] [CrossRef]
- Abelkop, A.D.; Graham, J.D.; Royer, T.V. Persistent, Bioaccumulative, and Toxic (PBT) Chemicals: Technical Aspects, Policies, and Practices; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Matthies, M.; Solomon, K.; Vighi, M.; Gilman, A.; Tarazona, J.V. The origin and evolution of assessment criteria for persistent, bioaccumulative and toxic (PBT) chemicals and persistent organic pollutants (POPs). Environ. Sci. Process. Impacts 2016, 18, 1114–1128. [Google Scholar] [CrossRef]
- Rücker, T. Safe Chemicals/REACH. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Springer: Berlin/Heidelberg, Germany, 2024; pp. 2689–2694. [Google Scholar]
- Prevention, P. TSCA Work Plan Chemical Risk Assessment. 2014. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/hhcb_wp_ra_final_08_27_14.pdf (accessed on 11 October 2025).
- McPartland, J.; Shaffer, R.M.; Fox, M.A.; Nachman, K.E.; Burke, T.A.; Denison, R.A. Charting a Path Forward: Assessing the Science of Chemical Risk Evaluations under the Toxic Substances Control Act in the Context of Recent National Academies Recommendations. Environ. Health Perspect. 2022, 130, 025003. [Google Scholar] [CrossRef]
- Elrod, A.A. The EPA and its regulations. In The Palgrave Handbook of Global Sustainability; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–19. [Google Scholar]
- Braun, O.; Coquery, C.; Kieffer, J.; Blondel, F.; Favero, C.; Besset, C.; Mesnager, J.; Voelker, F.; Delorme, C.; Matioszek, D. Spotlight on the Life Cycle of Acrylamide-Based Polymers Supporting Reductions in Environmental Footprint: Review and Recent Advances. Molecules 2022, 27, 42. [Google Scholar]

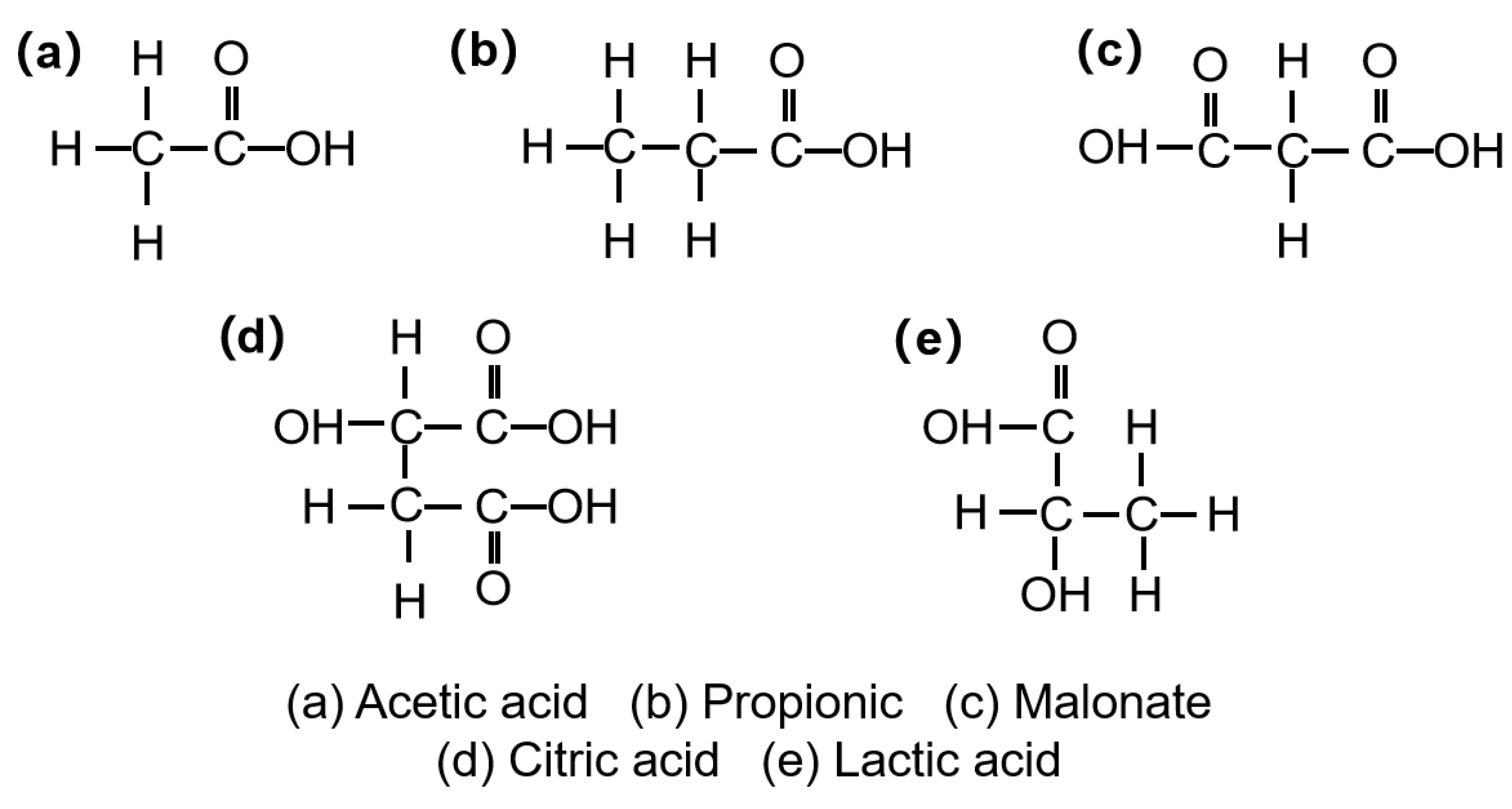
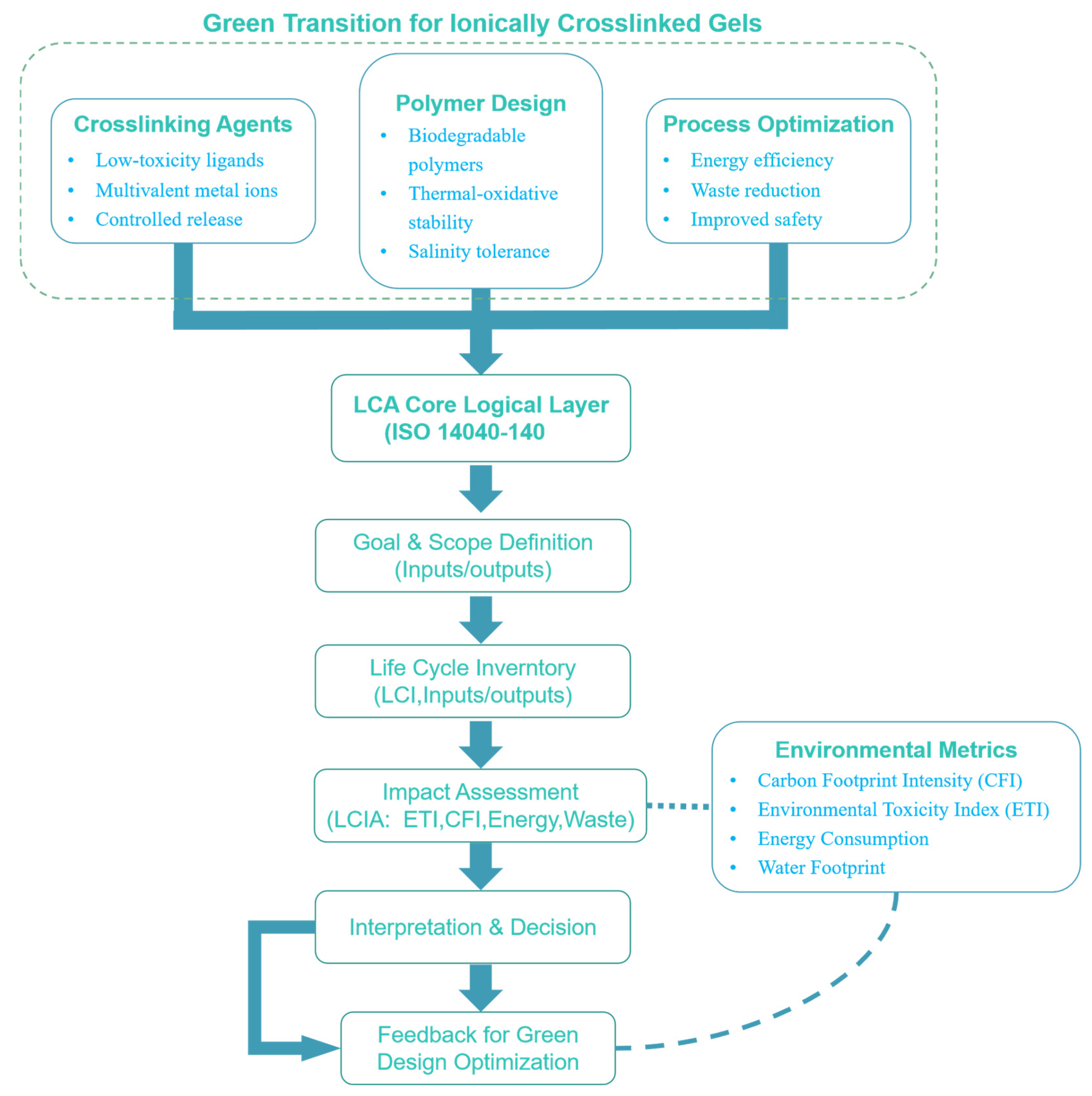


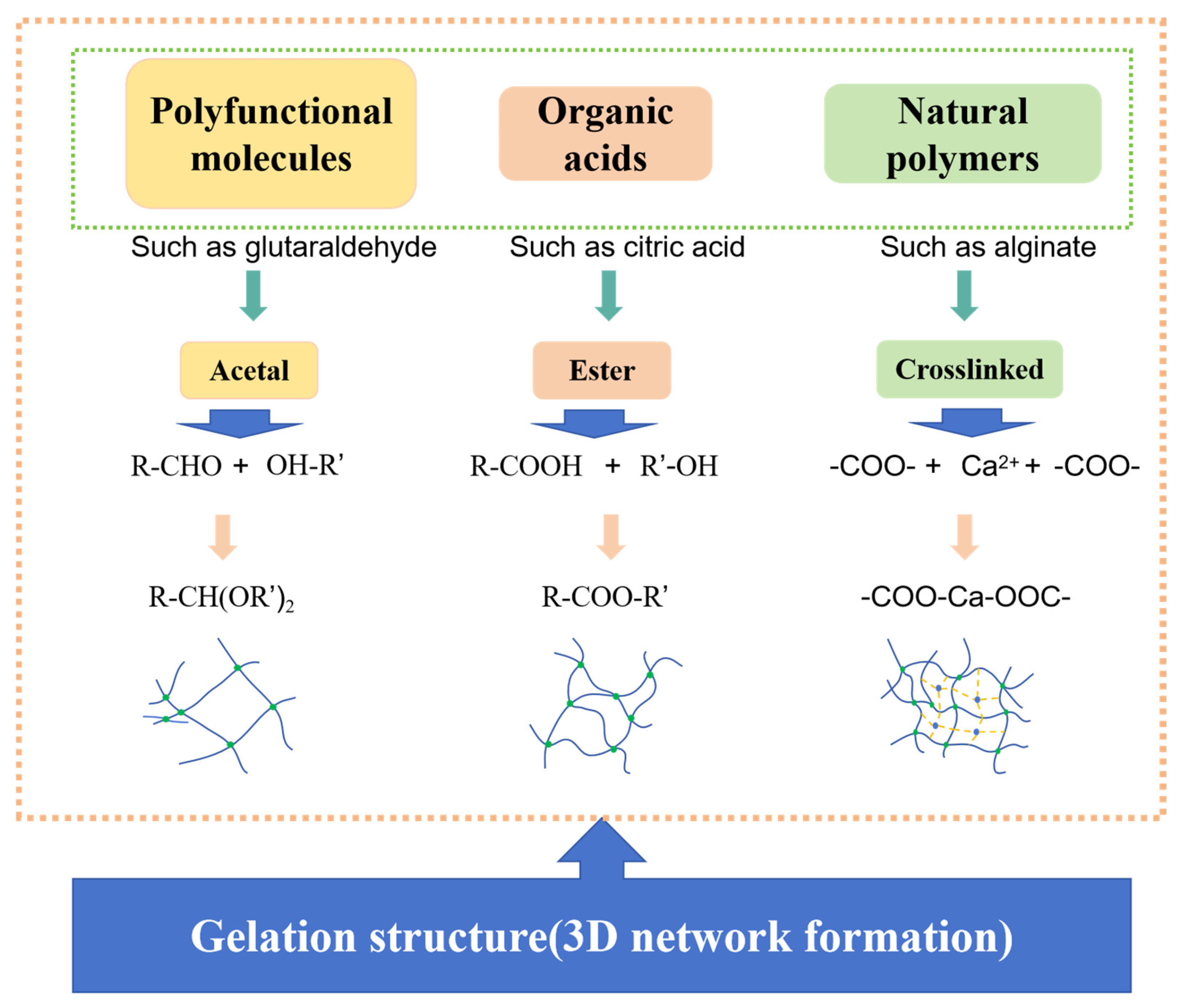

| Type | Definition and Characteristics | Representative Raw Materials/Crosslinkers | Environmental Performance and Challenges |
|---|---|---|---|
| Conventional (non-green) | Synthetic polymer networks crosslinked with Cr(III) or aldehydes; high stability but poor degradability [40,41,42]. | PAM with chromium/zirconium salts, glutaraldehyde. | High Cr(Ⅵ) toxicity, VOCs, and microplastic pollution; should be phased out. |
| Low-toxicity/low-pollution | Formulations using low-toxicity crosslinkers to reduce VOCs and improve polymer compatibility [43,44,45,46]. | Citric acid, aluminum salts, organic titanium. | Lower toxicity but limited durability and aging resistance. |
| Green eco-friendly | Renewable polymer matrices with biodegradable crosslinkers; naturally degradable after service [47,48,49,50,51]. | Alginate, chitosan, cellulose; citric or oxalic acids. | Excellent biodegradability but low salt tolerance and mechanical strength. |
| Intelligent green | Responsive gels with thermo-/pH-/shear-adaptive networks for targeted plugging [52,53,54]. | Responsive polysaccharides or peptide-based crosslinkers. | Good adaptability; complex synthesis and high cost. |
| Reinforcement Strategy | Key Mechanism/Technique | Representative Formulation | Applicable T/Environment | Application Scenario | Reference |
|---|---|---|---|---|---|
| Host–guest inclusion | β-Cyclodextrin–adamantane supramolecular interaction | PAAB: PAMN = 1:1 | 90 °C; salinity ≈ 32,900 mg L−1 | High-T, high-salinity reservoirs (EOR); drug delivery models | Zhou [91] |
| Thermo-responsive host–guest inclusion | Temperature-sensitive β-CD/Ad host–guest interaction; intermolecular binding energy analysis | β-CD/Ad complex | Stable at 330–340 K (57–67 °C) | Smart delivery systems; potential for thermo-responsive gels in EOR | Rasouli [92] |
| Zwitterionic hydrophobic monomer design | Sulfonic, phenyl, and long-alkyl monomer incorporation to enhance rigidity and salt tolerance | CQMP(AM/SSS/CQ) | Stable > 130 °C; decomposition ≈ 210 °C | High-T, high-salinity reservoirs (EOR) | Cheng [93] |
| Nano-fly ash composite | Nano-FA improves thermal stability and strength | AM/nano-FA/Cr(III) system | 90 °C; ΔP = 0.045 MPa; ηplug = 95.1% | Fractured reservoirs (water control) | Singh [94] |
| Zwitterionic copolymer reinforcement | Charge neutralization and ion-shielding compensation improve salinity tolerance and hydration stability | Poly(sulfobetaine-co-acrylamide) (P(SB-co-AM)); Poly(carboxybetaine-co-AMPS) | High salinity (>200,000 mg L−1 NaCl), 90–120 °C | High-salinity reservoirs; salt-resistant rheology modifiers | Lowe and McCormick [95] |
| Pickering emulsion flooding | Amphiphilic copolymer-stabilized anionic emulsion | AMPSA/AA/DMA | 94% displacement efficiency | Residual-oil recovery in porous media | Ntente [96] |
| Supramolecular dynamic network | Hydrophobic and H-bond crosslinking via methacrylate–ammonium system. | Methacrylate/DTAB | 135 °C; compressive strength ≈ 14.5 MPa | Cavernous/deep reservoirs | Yang [97] |
| Active amphiphilic polymer | C16 long-chain hydrophobic association. | AM/AA/C16DMAAC | >95% viscosity reduction at 60 °C | Heavy oil reservoirs (EOR) | Yang [98] |
| Al2O3 nanoparticle filling | Nanoparticles improve high-T/salinity tolerance and thermal stability | NaSS/DMA/Al2O3/Cr(III) | Stable 300 days at 150 °C | High-T aging reservoirs (plugging) | Pandit [99] |
| Organic Ligand | Structural Features | Chelation Ability | Release Control Ability | Thermal Stability | Representative References |
|---|---|---|---|---|---|
| Acetic acid | Monocarboxylic acid; limited coordination sites. | Medium | Medium | Medium | Guanghua Yi and Michael Sayer [106,107], Kang [108] |
| Propionic acid | Longer chain; slightly stronger chelation. | Medium | Medium | Medium | Mumallah [109], Kaddouri [110] |
| Boric acid | Trihydroxy structure; stable borate complexation. | Strong | Strong | Strong | Citeseer [111], Shah et al. [112], Brannon [113], Harris [114] |
| Lactic acid | α-Hydroxy and carboxyl groups; stabilizes Cr(III) complexes. | Strong | Relatively strong | Strong | Yang et al. [82], Jong et al. [115] |
| Citric acid | Tricarboxylic; multi-site coordination with hydroxyl groups. | Strong | Strong | Strong | Niu et al. [116], Takahashi [117] |
| Phenolic resin | Polyhydroxy aromatic; forms rigid chelated networks. | Strong | Relatively strong | Relatively strong | Shibayama [118], Ran et al. [119] |
| Enhancement Strategy | Key Technique/Mechanism | Representative Formulation | Applicable Conditions | Performance Advantages |
|---|---|---|---|---|
| Hydrophobic association [120,121,122] | Styrene/acrylate monomers form hydrophobic domains | HPAM-St | 100–140 °C, high salinity | Improved elasticity and shear resistance. |
| Host–guest inclusion [123,124,125] | β-CD–adamantane supramolecular complexation | βCD-g-PAM | >90 °C, salinity 1.5 × 105 mg L−1 | Controllable density, low-dosage efficiency. |
| Nanofiller incorporation [126,127,128] | SiO2, montmorillonite, or fly ash reinforcement | HPAM/SiO2 | Up to 160 °C | Enhanced thermal strength and plugging. |
| Organic chelation [129,130,131] | Citric acid–Cr(III) coordination | HPAM-Cr(III)-citric acid | Delayed gelation (24–480 h) | Tunable gelation, reduced toxicity. |
| Metal substitution [132,133] | Ti(Ⅵ), Al(III), Fe(III) replacing Cr(III) | HPAM–Ti(IV) | Delayed gelation (24–480 h) | Tunable gelation; reduced toxicity |
| System Type | Cross Linking Mechanism | Technical Features | Limitations |
|---|---|---|---|
| Small-molecule aldehydes [192,193,194] | Schiff base condensation (–C=N–) | Adjustable gelation time (10–300 min) | Formaldehyde/glutaraldehyde residues (LC50 = 12 mg L−1) |
| Organic acid esterification [195,196,197,198,199] | Carboxylic ester bonding (–COO–) | pH responsiveness (swelling ratio > 300%) | Ester hydrolysis under high salinity (modulus loss > 60%) |
| Natural polymer ion-bridging [200,201] | Divalent cation coordination [Ca(II)/Mg(II)] | High biodegradability (>80%, OECD 301B) | Network dissociation at high temperature (>80 °C) |
| Model | Pore-Scale Heterogeneity | Microscopic Heterogeneity | Oil Recovery (Water-Free Stage, %) | Final Oil Recovery (%) | Relative Wettability Index | Water-Cut Variation Rate |
|---|---|---|---|---|---|---|
| X1 | High | Low | 6.3 | 25 | 0.17 | 3.5 |
| X2 | Low | High | 10.9 | 31.8 | 0.47 | 2.7 |
| X3 | Lowest | Lowest | 8.5 | 30.8 | 0.35 | 3.8 |
| X4 | Highest | Highest | 5.0 | 20.8 | 0.51 | 2.9 |
| X5 | Medium | Medium | 6.9 | 25 | 0.18 | 2.8 |
| Type | Representative System | ETI Score | CFI Score (kgCO2e/kg) | Classification Validation |
|---|---|---|---|---|
| Class Ⅰ Conventional | Cr(III)-PAM | 1.45 | 9.1 | Meets Class Ⅰ criteria |
| Class II Low-toxicity | Al-PAM/Zr-PAM | 0.68 | 4.3 | Meets Class II criteria |
| Class III Eco-friendly | Citric acid–chitosan | 0.42 | 2.1 | Meets Class III criteria |
| Class IV Intelligent green | PH-responsive nanogels | 0.25 | 1.2 | Meets Class IV criteria |
| Level | Definition | ETI Value | CFI (kgCO2e/kg) | Typical Features | Representative Systems |
|---|---|---|---|---|---|
| I. Conventional | High efficiency; high toxicity and carbon footprint. | >1.2 | >8 | Non-degradable; Cr(III) or aldehyde/phenolic crosslinkers. | Cr(III)-HPAM, glutaraldehyde–PAM gels |
| II. Low-toxicity | Lower toxicity; moderate carbon intensity. | 0.5–0.8 | 3–8 | Partial replacement of toxic ions or organics. | Al–PAM, Zr–HPAM, organic acid–PAM gels |
| III. Eco-friendly | Renewable and partially degradable networks. | 0.3–0.5 | 1.5–3 | Biopolymer matrix; low-toxicity organic crosslinkers. | Citric acid–chitosan, alginate, scleroglucan gels |
| IV. Intelligent green | Responsive, degradable, low-carbon systems. | <0.3 | <1.5 | Stimuli-responsive, self-healing, bio-based dual networks. | pH-responsive nanogels, double-network gels |
| Evaluation Dimension | Weight (Suggested) | Scoring Criteria (Example) | Score Range |
|---|---|---|---|
| Chemical Toxicity | 0.4 | LD50/LC50 < 50 mg/kg→ high score; higher LD50/LC50 → lower score | 0~1 |
| Ecological Persistence and Bioaccumulation | 0.2 | Persistent (P) + Bioaccumulative (B) → maximum score; no bioaccumulation → minimum score | 0~1 |
| Degradability | 0.3 | Completely degradable → minimum score; non-degradable → maximum score | 0~1 |
| Regulatory Concern | 0.1 | Listed in REACH SVHC or EPA priority list → maximum score | 0~1 |
| Evaluation Dimension | Weight (Suggested) | Scoring Criteria (Example) | Score Range |
|---|---|---|---|
| Raw material carbon footprint | 0.5 | Based on LCA database values, kg (CO2e kg−1) | 0–1 |
| Energy consumption during synthesis | 0.2 | High-temperature/high-pressure synthesis = high score; ambient-temperature synthesis = low score | 0–1 |
| Energy demand during application | 0.2 | High injection energy or difficulty = high score | 0–1 |
| Post-treatment carbon emissions | 0.1 | Incineration/landfilling = high score; biodegradable/recyclable = low score | 0–1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Xiao, H.; Chen, Z.; Wu, T.; Chen, H.; Wei, K. Toward Sustainable Green and Intelligent Profile Control Gels: An ETI–CFI-Based Structure–Environment Evaluation Framework. Gels 2025, 11, 952. https://doi.org/10.3390/gels11120952
Chen Q, Xiao H, Chen Z, Wu T, Chen H, Wei K. Toward Sustainable Green and Intelligent Profile Control Gels: An ETI–CFI-Based Structure–Environment Evaluation Framework. Gels. 2025; 11(12):952. https://doi.org/10.3390/gels11120952
Chicago/Turabian StyleChen, Qiang, Hanmin Xiao, Zhihua Chen, Tong Wu, Hao Chen, and Keqiang Wei. 2025. "Toward Sustainable Green and Intelligent Profile Control Gels: An ETI–CFI-Based Structure–Environment Evaluation Framework" Gels 11, no. 12: 952. https://doi.org/10.3390/gels11120952
APA StyleChen, Q., Xiao, H., Chen, Z., Wu, T., Chen, H., & Wei, K. (2025). Toward Sustainable Green and Intelligent Profile Control Gels: An ETI–CFI-Based Structure–Environment Evaluation Framework. Gels, 11(12), 952. https://doi.org/10.3390/gels11120952






