Transforming Wheat Straw into Superabsorbent Polymers for Sustainable Agricultural Management
Abstract
1. Introduction
2. Results and Discussion
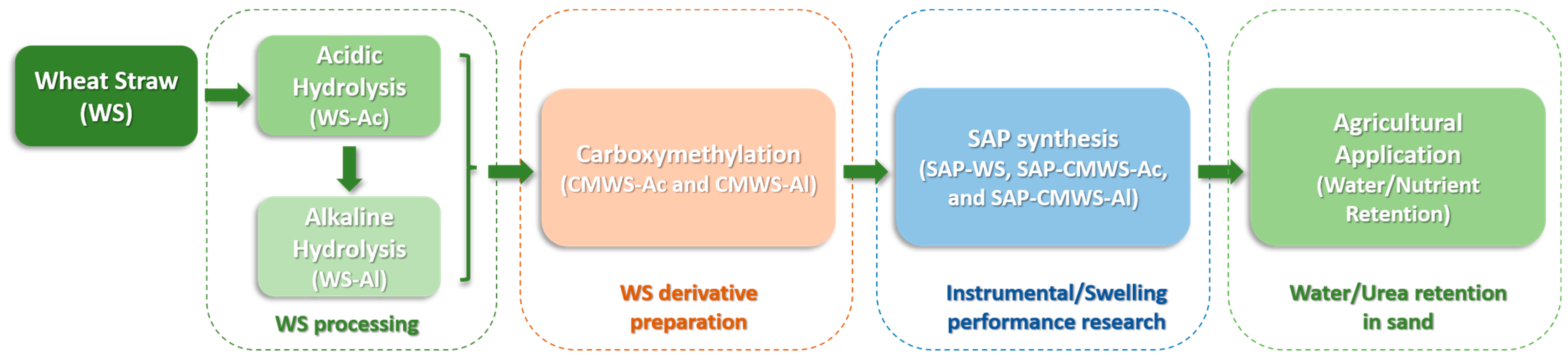
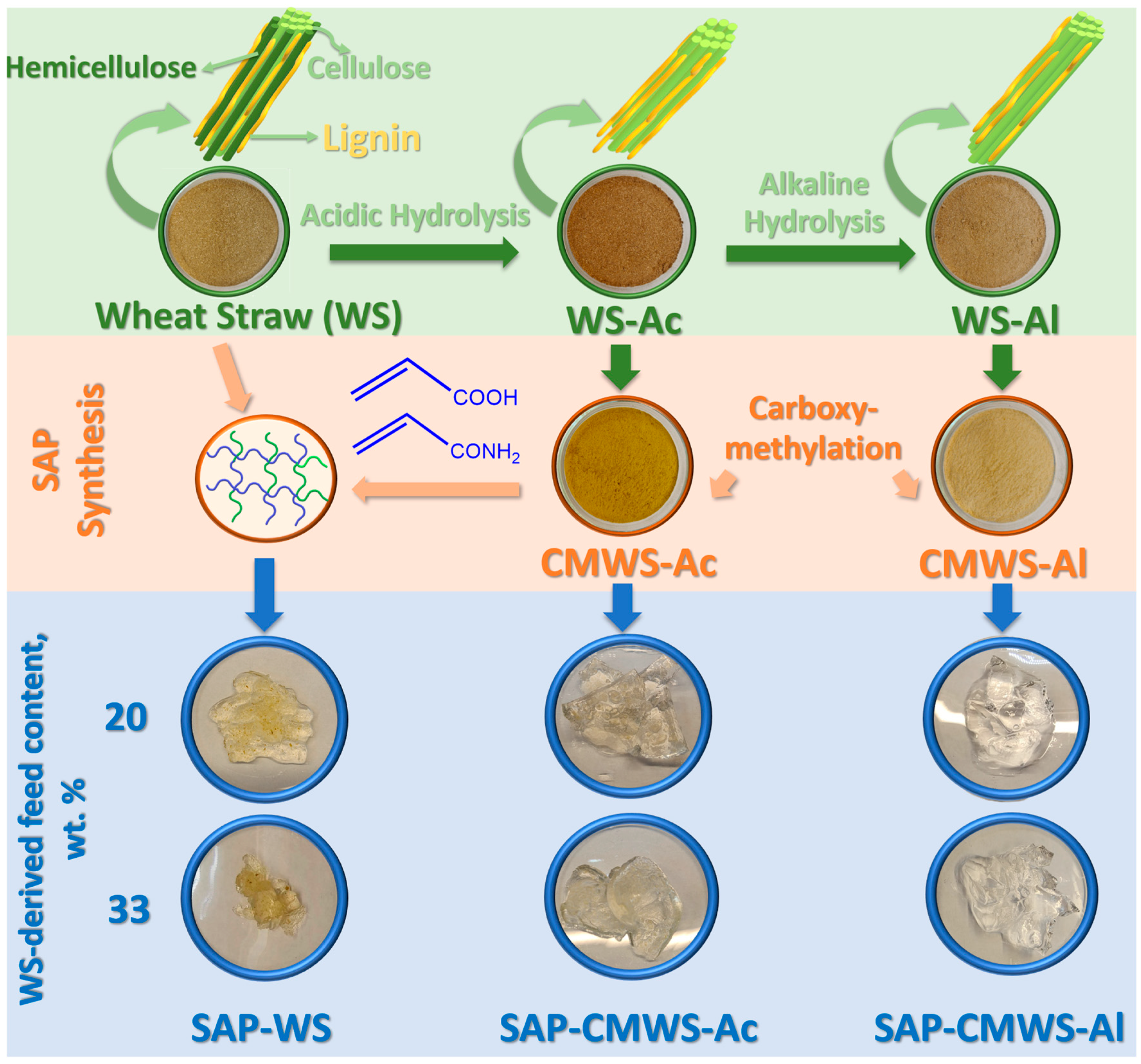
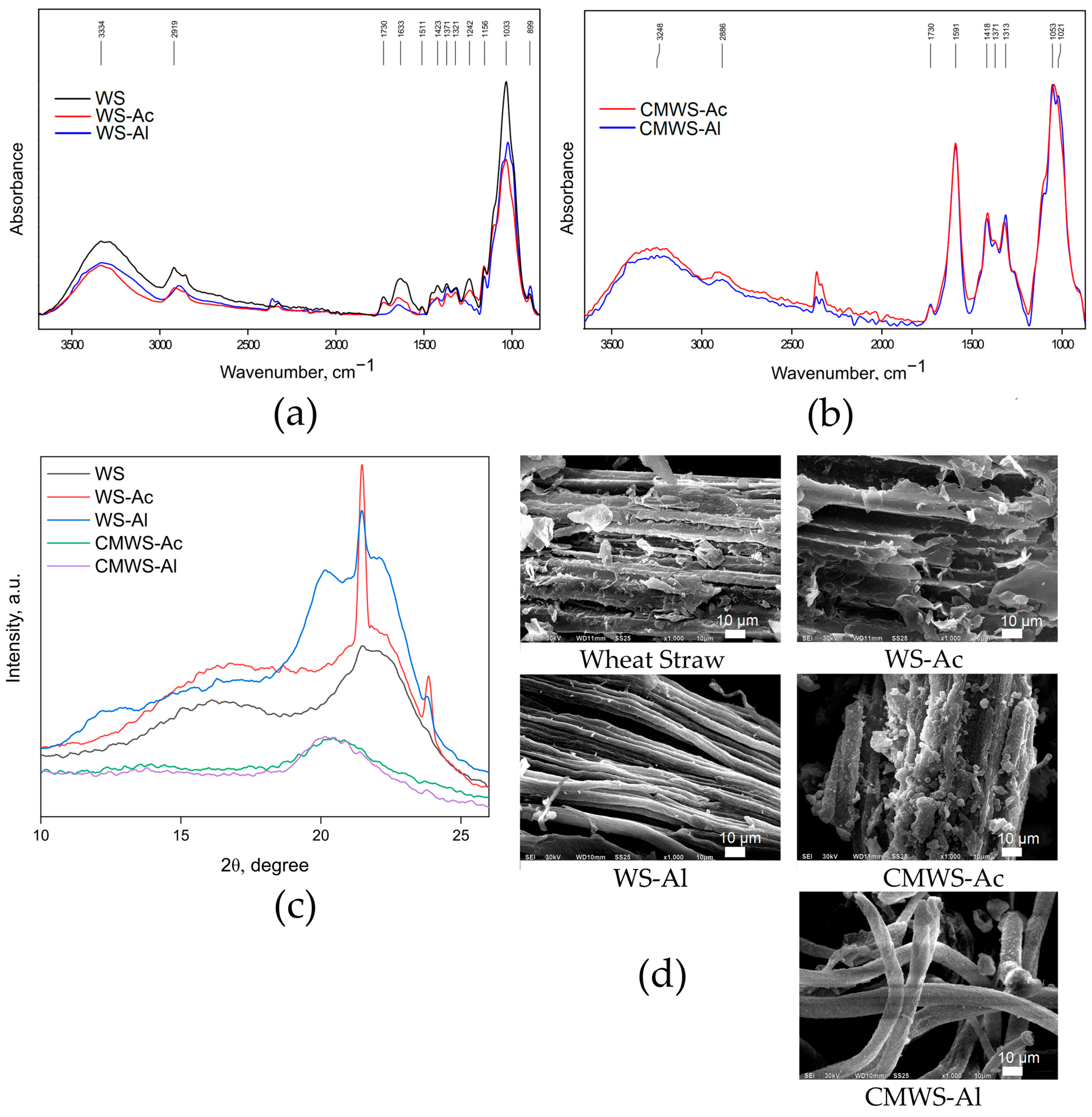
2.1. Wheat Straw Processing: Hydrolysis and Carboxymethylation
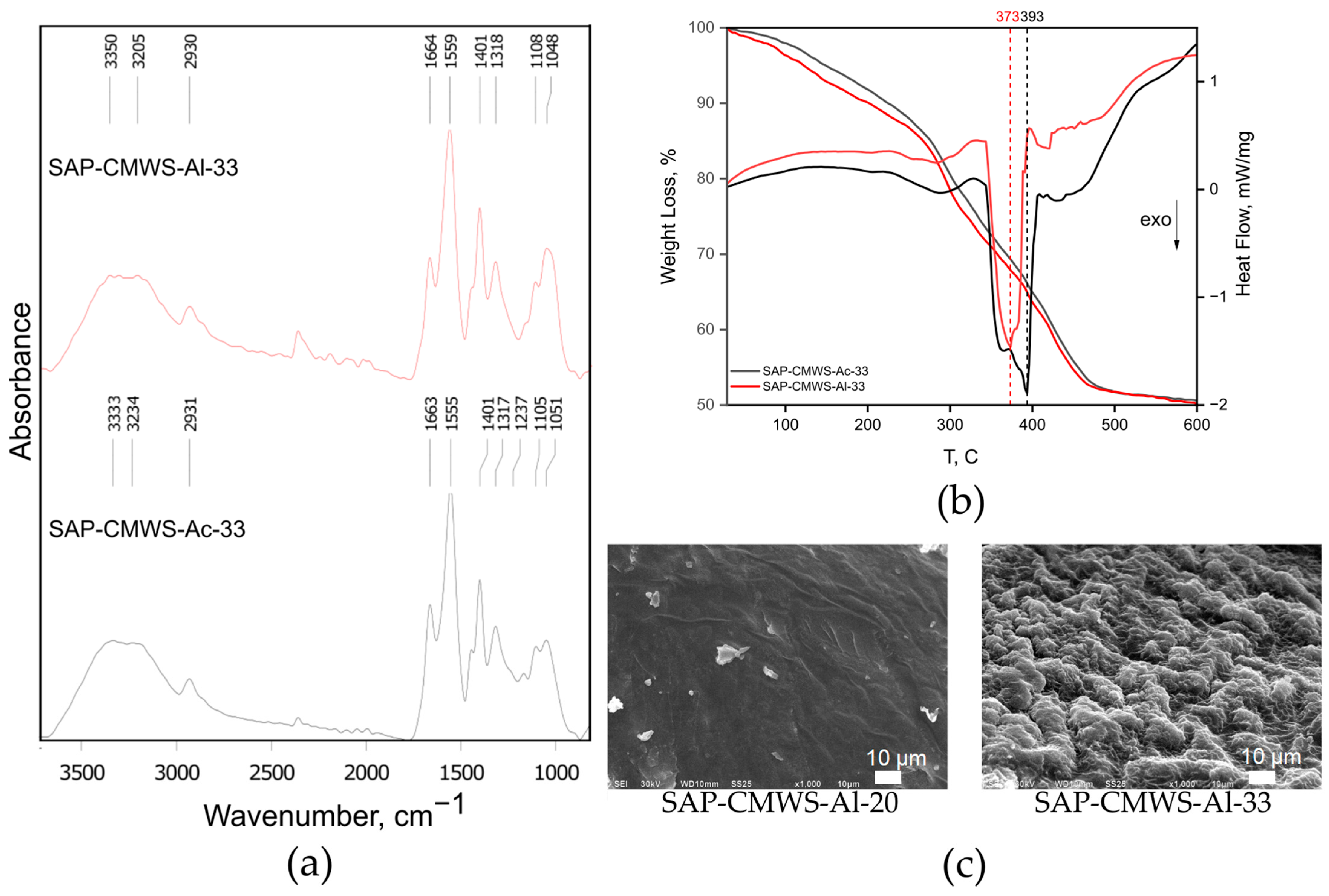
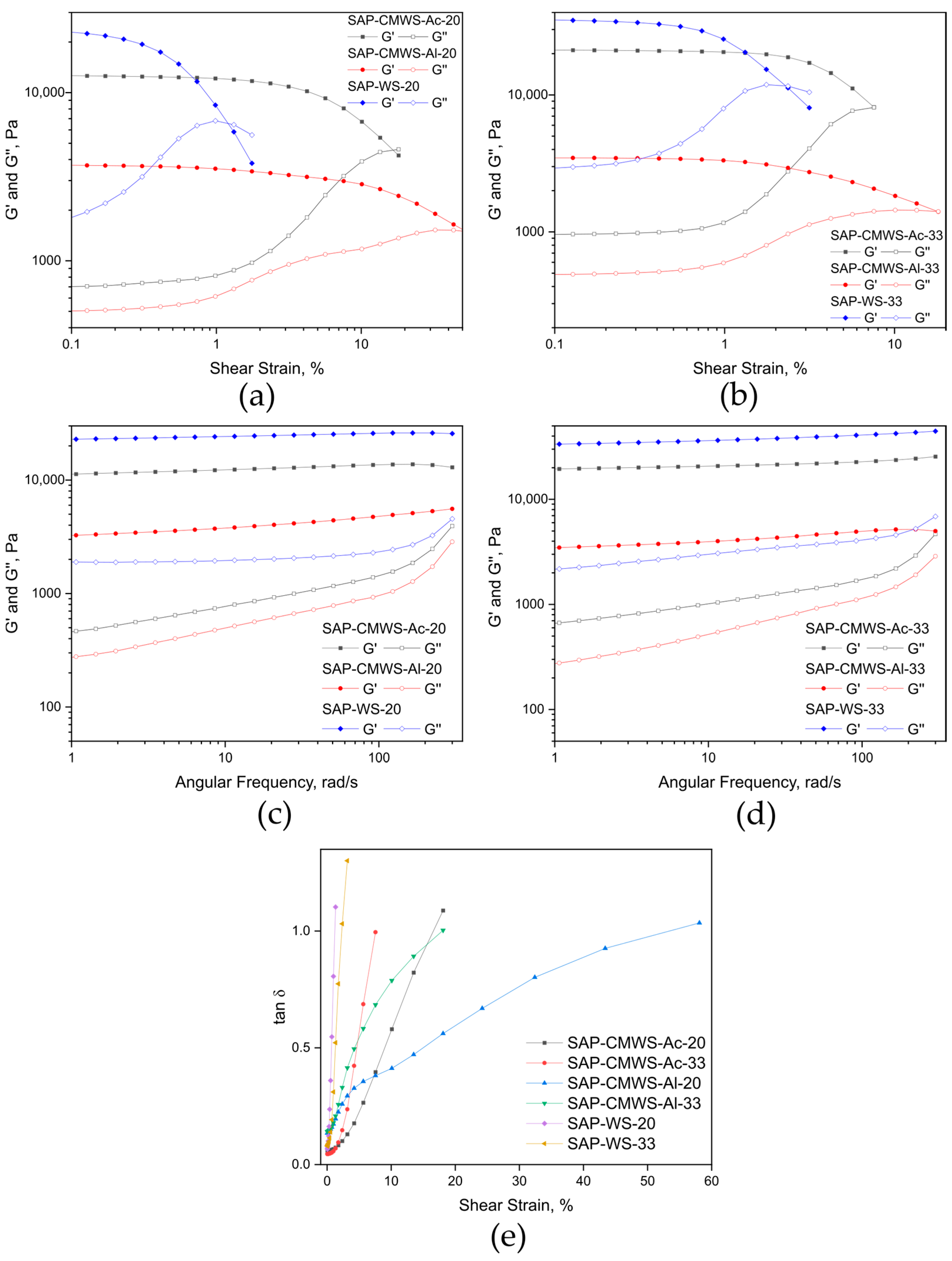
2.2. Superabsorbent Synthesis and Instrumental Characterization
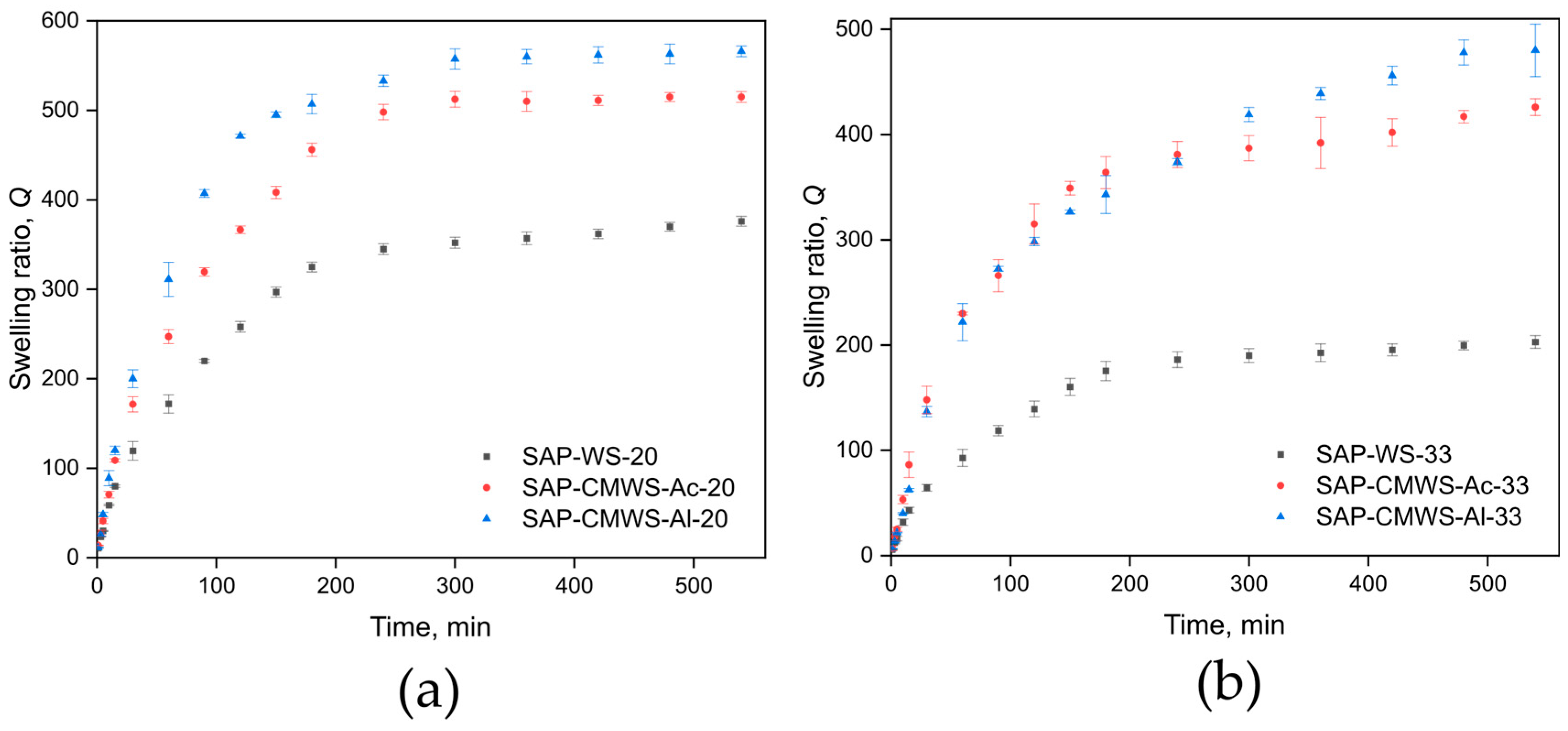
2.3. Superabsorbent Swelling Performance
2.4. Urea Retention in Soil
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Wheat Straw Processing
4.2.1. Wheat Straw Acidic Hydrolysis
4.2.2. Wheat Straw Alkaline Hydrolysis
4.2.3. Carboxymethylation
4.2.4. Lignin Content Assay
4.3. Superabsorbent Synthesis
4.4. Instrumental Characterization
4.4.1. Fourier-Transform Infrared Spectroscopy
4.4.2. Scanning Electron Microscopy
4.4.3. X-Ray Diffraction
4.4.4. Thermogravimetric Analysis and Differential Scanning Calorimetry
4.4.5. Rheological Studies
4.5. Investigating of the SAP Swelling Performance
4.6. Nitrogen Fertilizer Retention
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawrence, D.; Coe, M.; Walker, W.; Verchot, L.; Vandecar, K. The Unseen Effects of Deforestation: Biophysical Effects on Climate. Front. For. Glob. Change 2022, 5, 756115. [Google Scholar] [CrossRef]
- Jakhar, R.; Samek, L.; Styszko, K. A Comprehensive Study of the Impact of Waste Fires on the Environment and Health. Sustainability 2023, 15, 14241. [Google Scholar] [CrossRef]
- Petukhova, M.S. Innovative Development of the Russian Grain Sector. Rus. J. Econ. 2022, 8, 49–59. [Google Scholar] [CrossRef]
- Andreenko, T.I.; Kiseleva, S.V.; Rafikova, Y.Y. Agricultural Waste from Crop Production as an Energy Resource. IOP Conf. Ser. Earth Environ. Sci. 2022, 1116, 012054. [Google Scholar] [CrossRef]
- Marinchenko, T. Raw Material Resources for Extruding. KnE Life Sci. 2021, 6, 384–393. [Google Scholar] [CrossRef]
- Jena, S.; Singh, R. Agricultural Crop Waste Materials—A Potential Reservoir of Molecules. Environ. Res. 2022, 206, 112284. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, Q.; Xin, Y.; Liu, Y. Comprehensive Review in Moisture Retention Mechanism of Polysaccharides from Algae, Plants, Bacteria and Fungus. Arab. J. Chem. 2022, 15, 104163. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, J.; Zhao, S. Design, Synthesis and Characterization of a Starch-Based Superabsorbent Polymer and Its Impact on Autogenous Shrinkage of Cement Paste. Constr. Build. Mater. 2024, 415, 134986. [Google Scholar] [CrossRef]
- Matmin, J.; Ibrahim, S.I.; Mohd Hatta, M.H.; Ricky Marzuki, R.; Jumbri, K.; Nik Malek, N.A.N. Starch-Derived Superabsorbent Polymer in Remediation of Solid Waste Sludge Based on Water–Polymer Interaction. Polymers 2023, 15, 1471. [Google Scholar] [CrossRef]
- Supare, K.; Mahanwar, P.A. Starch-Derived Superabsorbent Polymers in Agriculture Applications: An Overview. Polym. Bull. 2022, 79, 5795–5824. [Google Scholar] [CrossRef]
- Tan, Q.; Chen, H.; Chang, H.; Woo, M.W.; Liu, W. Current Advances in Design of Starch-Based Superabsorbent Polymers and Their Removal of Typical Pollutants from Tannery Wastewater. Starch-Stärke 2023, 75, 2300004. [Google Scholar] [CrossRef]
- Omidian, H.; Akhzarmehr, A.; Chowdhury, S.D. Advancements in Cellulose-Based Superabsorbent Hydrogels: Sustainable Solutions across Industries. Gels 2024, 10, 174. [Google Scholar] [CrossRef]
- Zhang, Z.; Abidi, N.; Lucia, L.; Chabi, S.; Denny, C.T.; Parajuli, P.; Rumi, S.S. Cellulose/Nanocellulose Superabsorbent Hydrogels as a Sustainable Platform for Materials Applications: A Mini-Review and Perspective. Carbohyd. Polym. 2023, 299, 120140. [Google Scholar] [CrossRef]
- Tanpichai, S.; Phoothong, F.; Boonmahitthisud, A. Superabsorbent Cellulose-Based Hydrogels Cross-Liked with Borax. Sci. Rep. 2022, 12, 8920. [Google Scholar] [CrossRef]
- Das, D.; Prakash, P.; Rout, P.K.; Bhaladhare, S. Synthesis and Characterization of Superabsorbent Cellulose-Based Hydrogel for Agriculture Application. Starch-Stärke 2021, 73, 1900284. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, J.; Wang, M.; Wang, Z.; Wang, W.; Shen, Y.; Xue, Y.; Li, B.; Ma, Y.; Yao, Y.; et al. Superabsorbent Carboxymethyl Cellulose–Based Hydrogel Fabricated by Liquid-Metal-Induced Double Crosslinking Polymerisation. Carbohyd. Polym. 2024, 331, 121910. [Google Scholar] [CrossRef]
- Lavlinskaya, M.S.; Sorokin, A.V. Enhancement of Water Uptake in Composite Superabsorbents Based on Carboxymethyl Cellulose Through Porogen Incorporation and Lyophilization. Gels 2024, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of Carboxymethylcellulose/Starch Superabsorbent Hydrogels by Gamma-Irradiation. Chem. Cent. J. 2017, 11, 46. [Google Scholar] [CrossRef]
- Omer, A.M.; Tamer, T.M.; Hassan, M.E.; Khalifa, R.E.; Abd El-Monaem, E.M.; Eltaweil, A.S.; Mohy Eldin, M.S. Fabrication of Grafted Carboxymethyl Cellulose Superabsorbent Hydrogel for Water Retention and Sustained Release of Ethephon in Sandy Soil. Arab. J. Sci. Eng. 2023, 48, 561–572. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Roh, H.; Oh, S.; Kim, S.; Kim, M.; Kim, D.; Park, J. Preparation and Characterization of Superabsorbent Polymers Based on Starch Aldehydes and Carboxymethyl Cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef]
- Islam, F.; Wong, S.Y.; Li, X.; Arafat, M.T. Pectin and Mucin Modified Cellulose-Based Superabsorbent Hydrogel for Controlled Curcumin Release. Cellulose 2022, 29, 5207–5222. [Google Scholar] [CrossRef]
- Zhou, P.; Li, X.; Jiang, Z.; Zhou, J.; He, G.; Qu, L. An Approach of Pectin from Citrus Aurantium L. for Superabsorbent Resin with Superior Quality for Hygiene Products: Salt Resistance, Antibacterial, Nonirritant and Biodegradability. Int. J. Biol. Macromol. 2023, 227, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Sengoku, K.; Fujioka, R. Pectin-Based Surperabsorbent Hydrogels Crosslinked by Some Chemicals: Synthesis and Characterization. Polym. Bull. 2005, 55, 123–129. [Google Scholar] [CrossRef]
- Kazemzadeh, B.; Hosseinzadeh, H.; Babazadeh, M. Synthesis of a Novel Pectin-Based Superabsorbent Hydrogel with Salt and pH-Responsiveness Properties. Biomed. Pharmacol. J. 2013, 6, 41–48. [Google Scholar] [CrossRef]
- Mota, H.P.; Fajardo, A.R. Development of Superabsorbent Hydrogel Based on Gum Arabic for Enhanced Removal of Anxiolytic Drug from Water. J. Environ. Manag. 2021, 288, 112455. [Google Scholar] [CrossRef]
- Bachra, Y.; Grouli, A.; Damiri, F.; Talbi, M.; Berrada, M. A Novel Superabsorbent Polymer from Crosslinked Carboxymethyl Tragacanth Gum with Glutaraldehyde: Synthesis, Characterization, and Swelling Properties. Int. J. Biomater. 2021, 2021, 5008833. [Google Scholar] [CrossRef] [PubMed]
- De Lima, I.S.; Sousa, H.R.; Silva, A.S.; De Oliveira, L.H.; Muniz, E.C.; Osajima, J.A.; Silva-Filho, E.C. Superabsorbent Hydrogel from Cassava Gum (Manihot Esculenta) to Release Water and Macronutrients. Ind. Crops Prod. 2024, 219, 119045. [Google Scholar] [CrossRef]
- Sorze, A.; Valentini, F.; Dorigato, A.; Pegoretti, A. Development of a Xanthan Gum Based Superabsorbent and Water Retaining Composites for Agricultural and Forestry Applications. Molecules 2023, 28, 1952. [Google Scholar] [CrossRef]
- Pal, I.; Chopra, L.; Ganesan, S.; Sharma, G.C.; Bhowmik, A.; Santhosh, A.J. Guar Gum: Superabsorbent Hydrogels for Dye Remediation. Polym. Adv. Techs 2025, 36, e70080. [Google Scholar] [CrossRef]
- Sharma, P.; Dagar, A.; Sapna; Vyas, A.; Sand, A. Superabsorbent Composites (SACs) Based on Xanthan Gum-g-Poly (Itaconic Acid)/Kaolinite. Polym. Bull. 2021, 78, 6441–6454. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Agricultural Waste-Derived Superabsorbent Hydrogels: Preparation, Performance, and Socioeconomic Impacts. J. Clean. Prod. 2020, 251, 119669. [Google Scholar] [CrossRef]
- Dodangeh, F.; Nabipour, H.; Rohani, S.; Xu, C. Applications, Challenges and Prospects of Superabsorbent Polymers Based on Cellulose Derived from Lignocellulosic Biomass. Bioresour. Technol. 2024, 408, 131204. [Google Scholar] [CrossRef] [PubMed]
- El Idrissi, A.; Channab, B.; Essamlali, Y.; Zahouily, M. Superabsorbent Hydrogels Based on Natural Polysaccharides: Classification, Synthesis, Physicochemical Properties, and Agronomic Efficacy under Abiotic Stress Conditions: A Review. Int. J.Biol. Macromol. 2024, 258, 128909. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and Characterization of a Novel Super-Absorbent Based on Wheat Straw. Biores. Technol. 2011, 102, 2853–2858. [Google Scholar] [CrossRef]
- Liang, R.; Yuan, H.; Xi, G.; Zhou, Q. Synthesis of Wheat Straw-g-Poly(Acrylic Acid) Superabsorbent Composites and Release of Urea from It. Carbohyd. Polym. 2009, 77, 181–187. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Rashid, T.; Kait, C.F.; Murugesan, T. A “Fourier Transformed Infrared” Compound Study of Lignin Recovered from a Formic Acid Process. Procedia Eng. 2016, 148, 1312–1319. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Goncharova, S.S.; Lavlinskaya, M.S.; Holyavka, M.G.; Faizullin, D.A.; Kondratyev, M.S.; Kannykin, S.V.; Zuev, Y.F.; Artyukhov, V.G. Carboxymethyl Cellulose-Based Polymers as Promising Matrices for Ficin Immobilization. Polymers 2023, 15, 649. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on Cellulose Nanocrystals Produced from Cellulose Sources with Various Polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Sorokin, A.; Sukhanov, P.; Popov, V.; Kannykin, S.; Lavlinskaya, M. A New Approach to Increasing the Equilibrium Swelling Ratio of the Composite Superabsorbents Based on Carboxymethyl Cellulose Sodium Salt. Cellulose 2022, 29, 159–173. [Google Scholar] [CrossRef]
- Sorokin, A.; Lavlinskaya, M. Synthesis of the Superabsobents Enriched in Chitosan Derivatives with Excellent Water Absorption Properties. Polym. Bull. 2022, 79, 407–427. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Mu, B.; Liu, Y.; Wang, A. From the Waste Semicoke to Superabsorbent Composite: Synthesis, Characterization and Performance Evaluation. J. Polym. Environ. 2021, 29, 4017–4026. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Díaz-Díaz, A.M.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. The Complexity of Lignin Thermal Degradation in the Isothermal Context. Processes 2021, 9, 1154. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, L.; Yan, Y.; Xu, C.; Liu, Z.; Yu, Z.; Li, J.; Hu, F. Rheological Characterization of Structural Stability for Black Soils from Northeast China. Agronomy 2025, 15, 1050. [Google Scholar] [CrossRef]
- Kawecka-Radomska, M.; Tomczyńska-Mleko, M.; Muszyński, S.; Wesołowska-Trojanowska, M.; Mleko, S. Viscoelastic Properties of Soil with Different Ammonium Nitrate Addition. Eurasian Soil Sci. 2017, 50, 1450–1454. [Google Scholar] [CrossRef]
- Peppas, N. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 2011; ISBN 9780198534112. [Google Scholar]
- Ma, S.-N.; Dong, X.-M.; Jeppesen, E.; Søndergaard, M.; Cao, J.-Y.; Li, Y.-Y.; Wang, H.-J.; Xu, J.-L. Responses of Coastal Sediment Phosphorus Release to Elevated Urea Loading. Mar. Pollut. Bull. 2022, 174, 113203. [Google Scholar] [CrossRef]
- Kalyuta, E.V.; Maltsev, M.I.; Markin, V.I.; Mashkina, E.I. Effect of Biopreparations Obtained from Carboxymethylated Plant Raw Material on the Wheat Growth, Crop Capacity, and Biochemical Parameters of Grain. Russ. J. Bioorg. Chem. 2022, 48, 1416–1421. [Google Scholar] [CrossRef]
- Stojanović, Ž.; Jeremić, K.; Jovanović, S.; Lechner, M.D. A Comparison of Some Methods for the Determination of the Degree of Substitution of Carboxymethyl Starch. Starch-Stӓrke 2005, 57, 79–83. [Google Scholar] [CrossRef]
- Bajpai, P. Pulp Bleaching. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 465–491. ISBN 9780128142400. [Google Scholar]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and Assessment of Methods for Cellulose Crystallinity Determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, M.; Ren, J.; Wang, J.; Fan, L.; Xu, Q. Preparation and Swelling Properties of Graphene Oxide/Poly(Acrylic Acid-Co-Acrylamide) Super-Absorbent Hydrogel Nanocomposites. Colloids Surfaces A Physicochem. Eng. Aspects 2012, 401, 97–106. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, X.; Zhen, J.; Lei, Z. Preparation of Superabsorbent Resin with Fast Water Absorption Rate Based on Hydroxymethyl Cellulose Sodium and Its Application. Carbohyd. Polym. 2019, 225, 115214. [Google Scholar] [CrossRef]
- Thakur, S.; Pandey, S.; Arotiba, O.A. Development of a Sodium Alginate-Based Organic/Inorganic Superabsorbent Composite Hydrogel for Adsorption of Methylene Blue. Carbohyd. Polym. 2016, 153, 34–46. [Google Scholar] [CrossRef]
- Lagergreen, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z. Chem. Ind. Kolloide 1907, 2, 15. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, Structure and Diffusional Behavior of Hydrogels in Controlled Release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Reyhanitabar, A. A Promising Porous Polymer-Nanoclay Hydrogel Nanocomposite as Water Reservoir Material: Synthesis and Kinetic Study. J. Porous Mater. 2018, 25, 665–675. [Google Scholar] [CrossRef]
- Watt, G.W.; Chrisp, J.D. Spectrophotometric Method for Determination of Urea. Anal. Chem. 1954, 26, 452–453. [Google Scholar] [CrossRef]
| SAPs | Equilibrium Swelling Ratio, Qe, g/g | |
|---|---|---|
| Distilled Water | 0.15 M NaCl | |
| SAP-WS-20 | 376 ± 5 | 38 ± 1 |
| SAP-WS-33 | 203 ± 6 | 34 ± 1 |
| SAP-CMWS-Ac-20 | 515 ± 7 | 44 ± 1 |
| SAP-CMWS-Ac-33 | 426 ± 8 | 40 ± 1 |
| SAP-CMWS-Al-20 | 566 ± 12 | 50 ± 2 |
| SAP-CMWS-Al-33 | 486 ± 17 | 46 ± 2 |
| Aquasorb® | 474 ± 29 | 55 ± 5 |
| Akvasin® | 459 ± 40 | 53 ± 4 |
| SAPs | Pseudo-First Order | Pseudo-Second Order | Ritger–Peppas Model | D·1010, cm2/min | |||
|---|---|---|---|---|---|---|---|
| R2 | k1, 1/min | R2 | k2, g/(mg min) | R2 | n | ||
| SAP-WS-20 | 0.98 | 0.008 | 0.99 | 0.002 | 0.99 | 0.68 | 0.221 ± 0.02 |
| SAP-WS-33 | 0.98 | 0.007 | 0.99 | 0.004 | 0.99 | 0.68 | 0.242 ± 0.01 |
| SAP-CMWS-Ac-20 | 0.95 | 0.010 | 0.99 | 0.002 | 0.99 | 0.73 | 0.241 ± 0.01 |
| SAP-CMWS-Ac-33 | 0.97 | 0.006 | 0.99 | 0.002 | 0.99 | 0.88 | 0.324 ± 0.02 |
| SAP-CMWS-Al-20 | 0.98 | 0.012 | 0.99 | 0.002 | 0.99 | 0.83 | 0.324 ± 0.01 |
| SAP-CMWS-Al-33 | 0.88 | 0.007 | 0.99 | 0.002 | 0.99 | 0.87 | 0.338 ± 0.01 |
| SAP | C0, mM | C1, mM | V0, mL | V1, mL | Sorbed urea, % |
|---|---|---|---|---|---|
| Control (sand without SAP) | 92 ± 1 | 90 ± 0.8 | 25 | 22.0 ± 1.5 | 13.9 ± 1.5 |
| SAP-CMWS-Ac-20 | 86 ± 1.3 | 18.5 ± 1.0 | 30.8 ± 2.4 | ||
| SAP-CMWS-Al-20 | 80 ± 0.7 | 18.5 ± 1.1 | 35.7 ± 2.0 | ||
| SAP-CMWS-Ac-33 | 82 ± 1.1 | 17.0 ± 0.9 | 39.4 ± 2.3 | ||
| SAP-CMWS-Al-33 | 81 ± 0.6 | 12.5 ± 0.7 | 56.0 ± 2.5 |
| WS or WS-Derived Feed, g | WS or WS-Derived Feed in SAP, wt. % | AAm, g | AA *, g | MBAAm, g | MBAAm: Acrylate Comonomers Ratio (w/w) | PPS, g |
|---|---|---|---|---|---|---|
| 0.25 | 20% | 0.25 | 0.80 | 0.003 | 1:350 | 0.015 |
| 0.50 | 33% | 1:350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokin, A.V.; Kadyirov, A.I.; Saranov, I.A.; Tsimmer, E.M.; Kiselev, V.A.; Zhuravlev, I.A.; Lavlinskaya, M.S. Transforming Wheat Straw into Superabsorbent Polymers for Sustainable Agricultural Management. Gels 2025, 11, 953. https://doi.org/10.3390/gels11120953
Sorokin AV, Kadyirov AI, Saranov IA, Tsimmer EM, Kiselev VA, Zhuravlev IA, Lavlinskaya MS. Transforming Wheat Straw into Superabsorbent Polymers for Sustainable Agricultural Management. Gels. 2025; 11(12):953. https://doi.org/10.3390/gels11120953
Chicago/Turabian StyleSorokin, Andrey V., Aidar I. Kadyirov, Igor A. Saranov, Egor M. Tsimmer, Vladislav A. Kiselev, Ivan A. Zhuravlev, and Maria S. Lavlinskaya. 2025. "Transforming Wheat Straw into Superabsorbent Polymers for Sustainable Agricultural Management" Gels 11, no. 12: 953. https://doi.org/10.3390/gels11120953
APA StyleSorokin, A. V., Kadyirov, A. I., Saranov, I. A., Tsimmer, E. M., Kiselev, V. A., Zhuravlev, I. A., & Lavlinskaya, M. S. (2025). Transforming Wheat Straw into Superabsorbent Polymers for Sustainable Agricultural Management. Gels, 11(12), 953. https://doi.org/10.3390/gels11120953







