Mechanically Reinforced Silica Aerogels via Thermally Induced Phase Separation of Poly(ethylene-co-vinyl Alcohol)
Abstract
1. Introduction
2. Results and Discussion
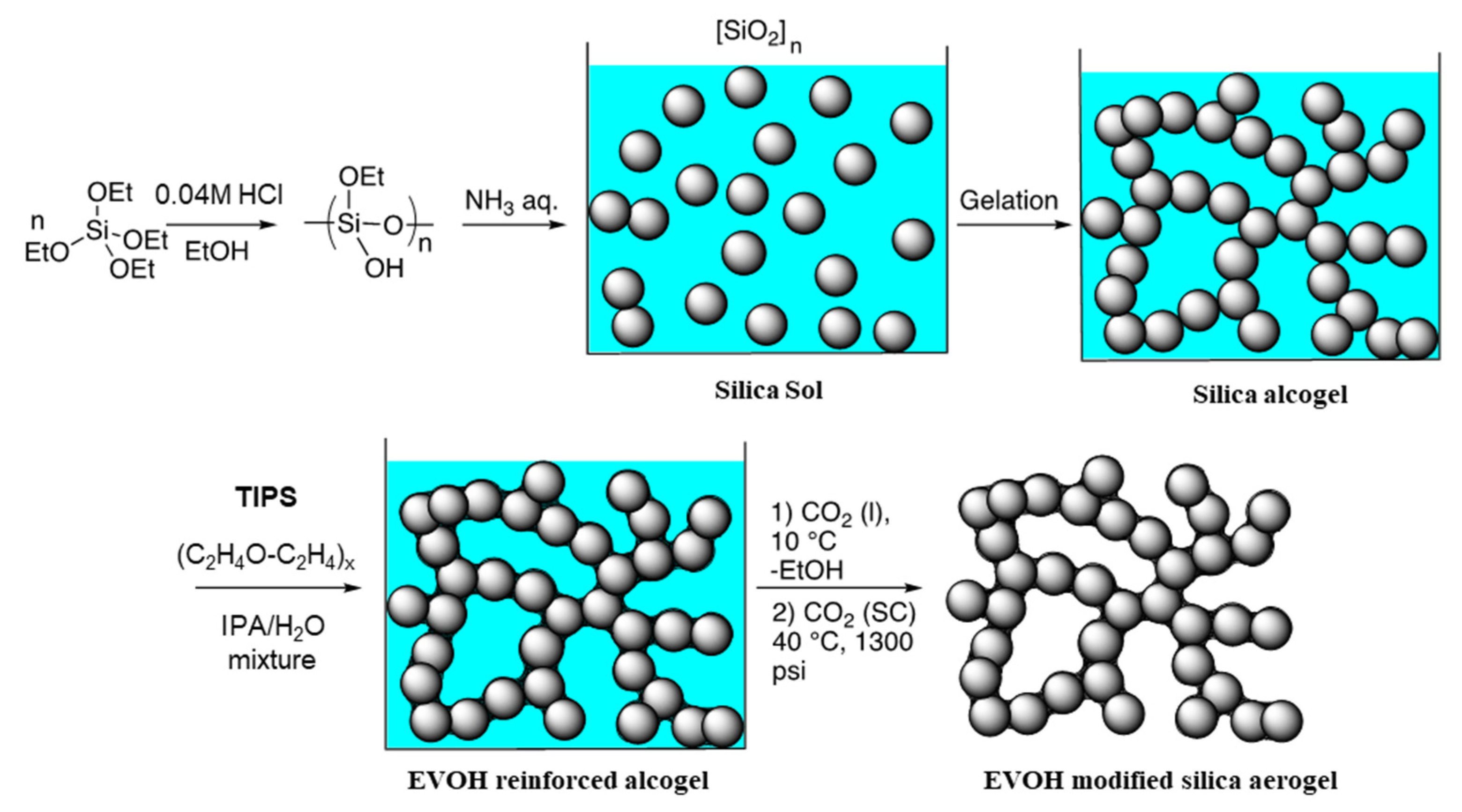
2.1. Formation of EVOH-Modified Aerogels and Basic Properties
2.2. Microstructural Characterization
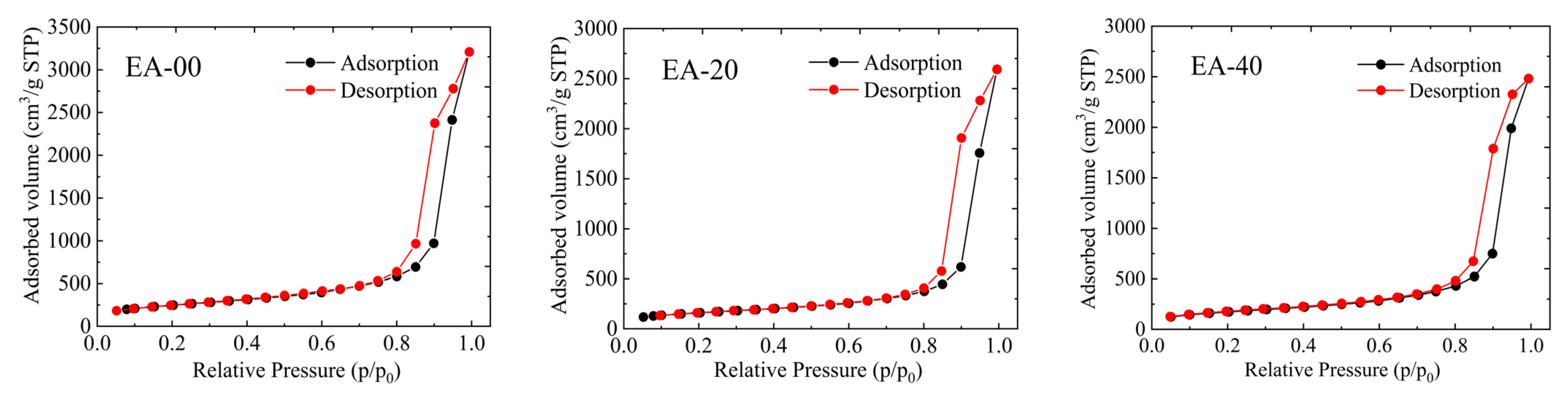
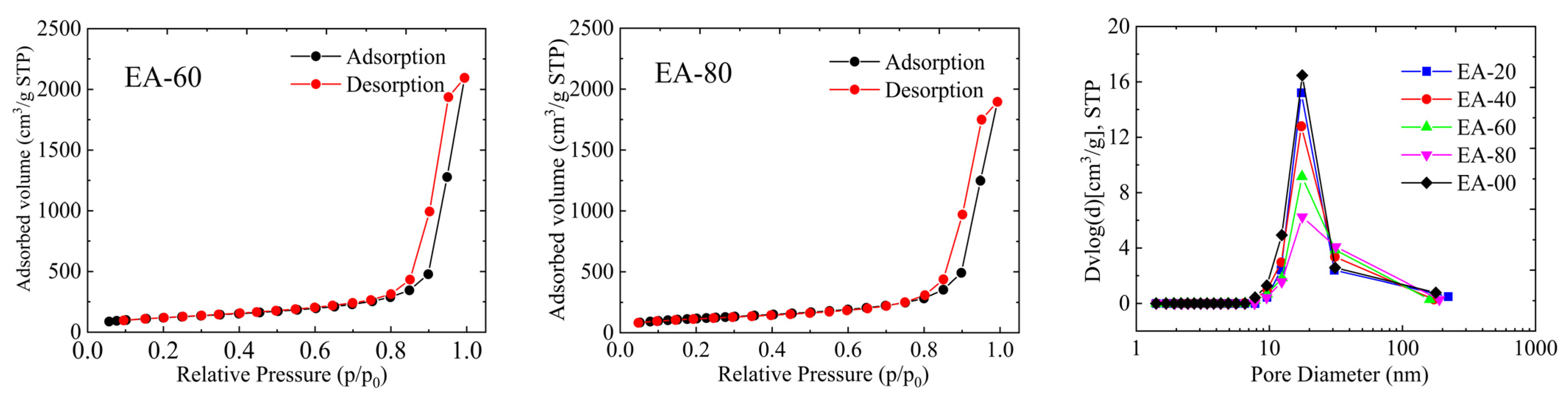
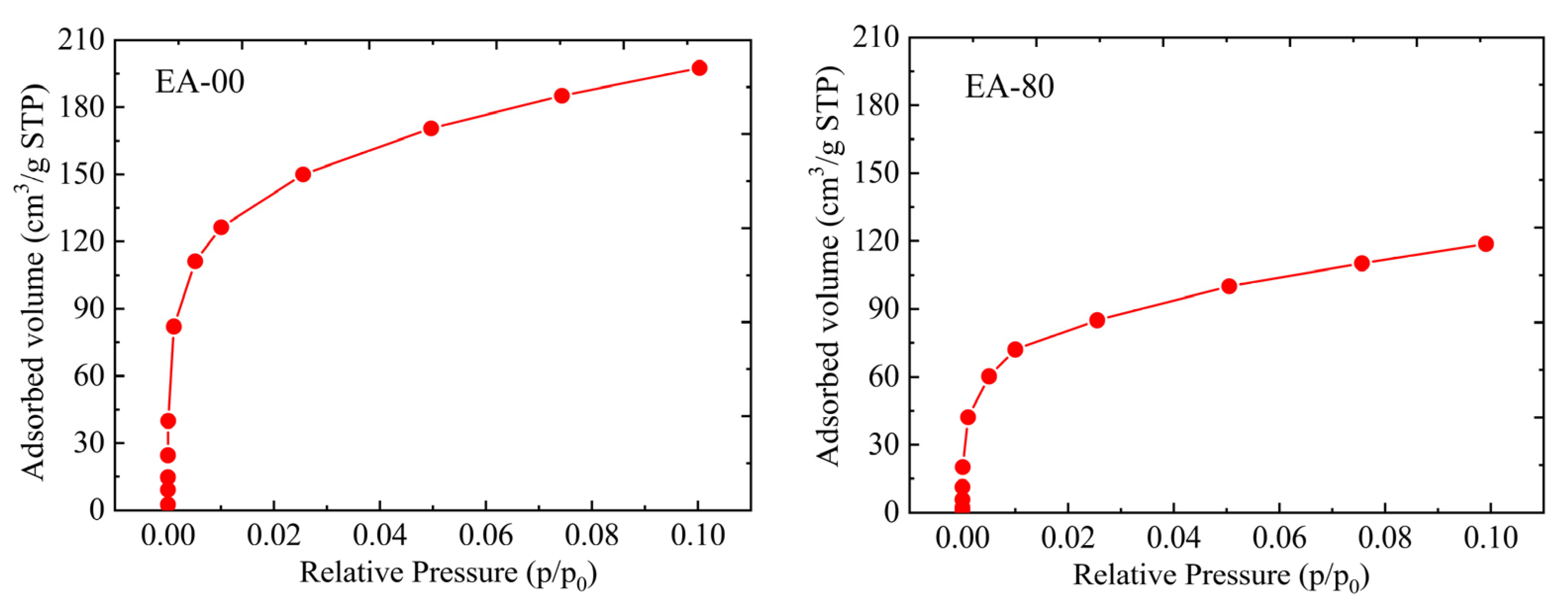
2.3. Pore Structure Analysis
2.4. Thermal Properties
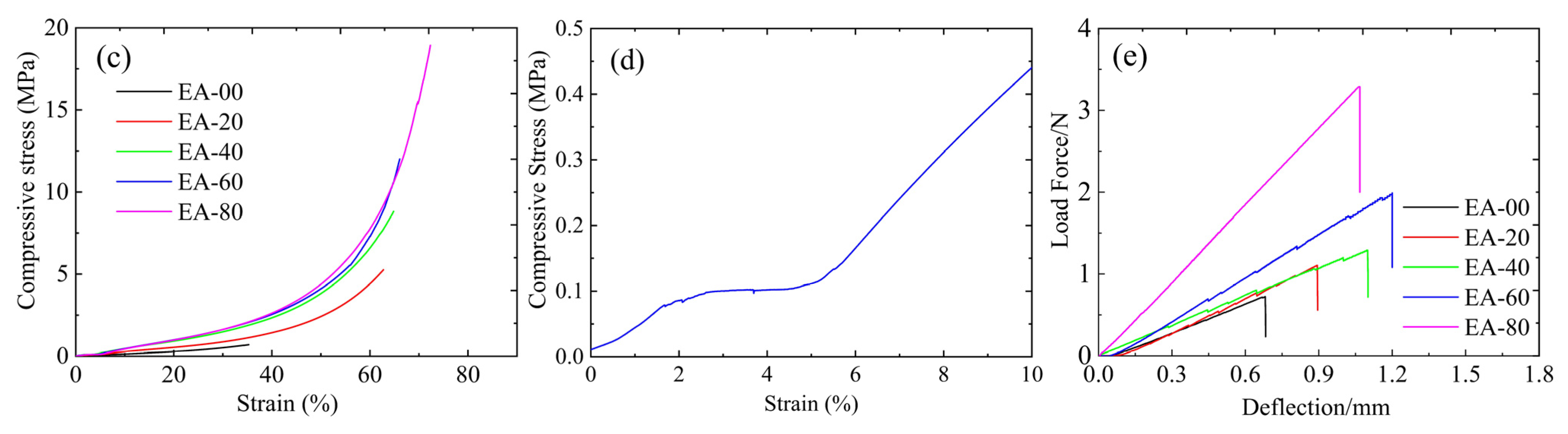
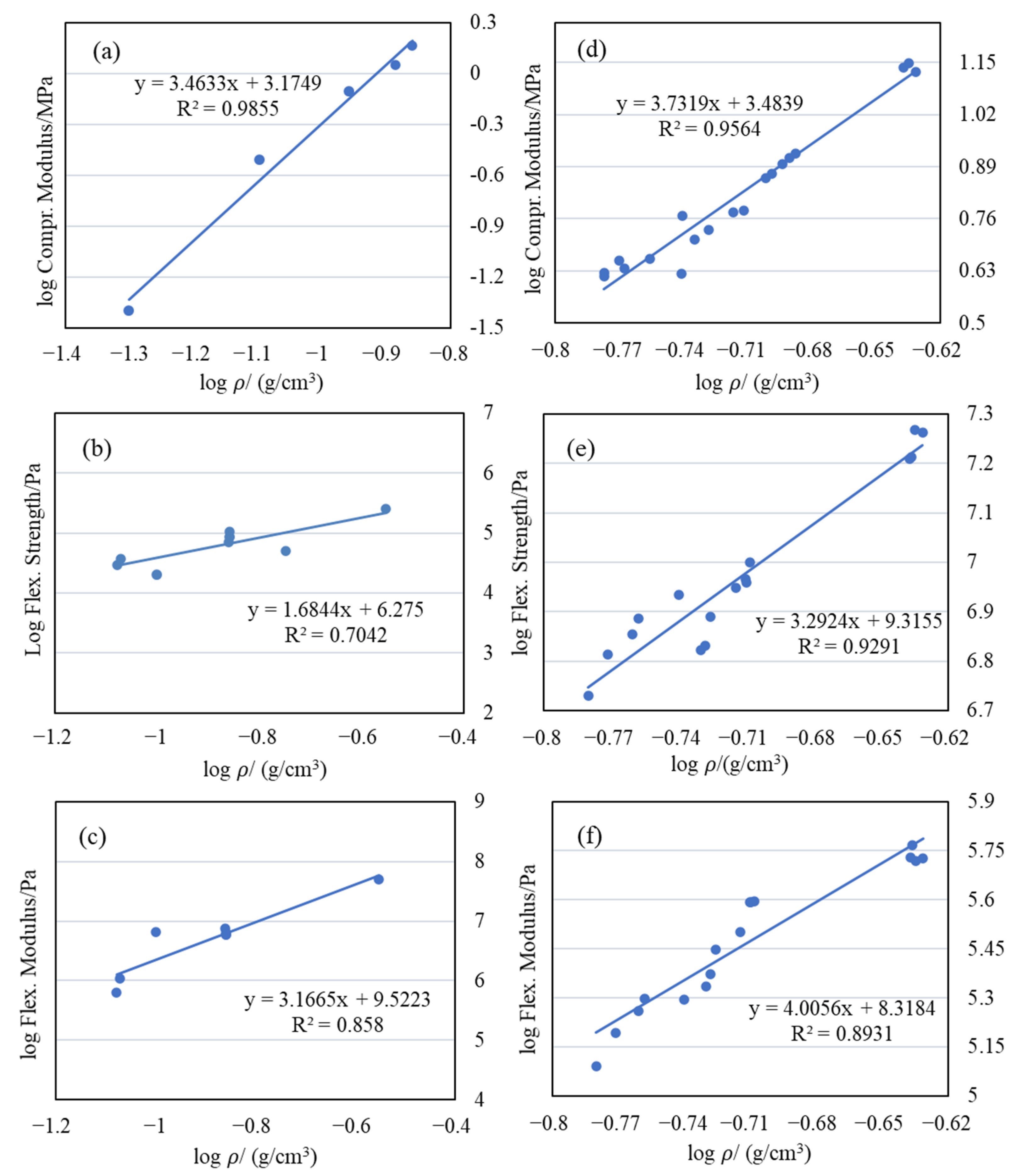
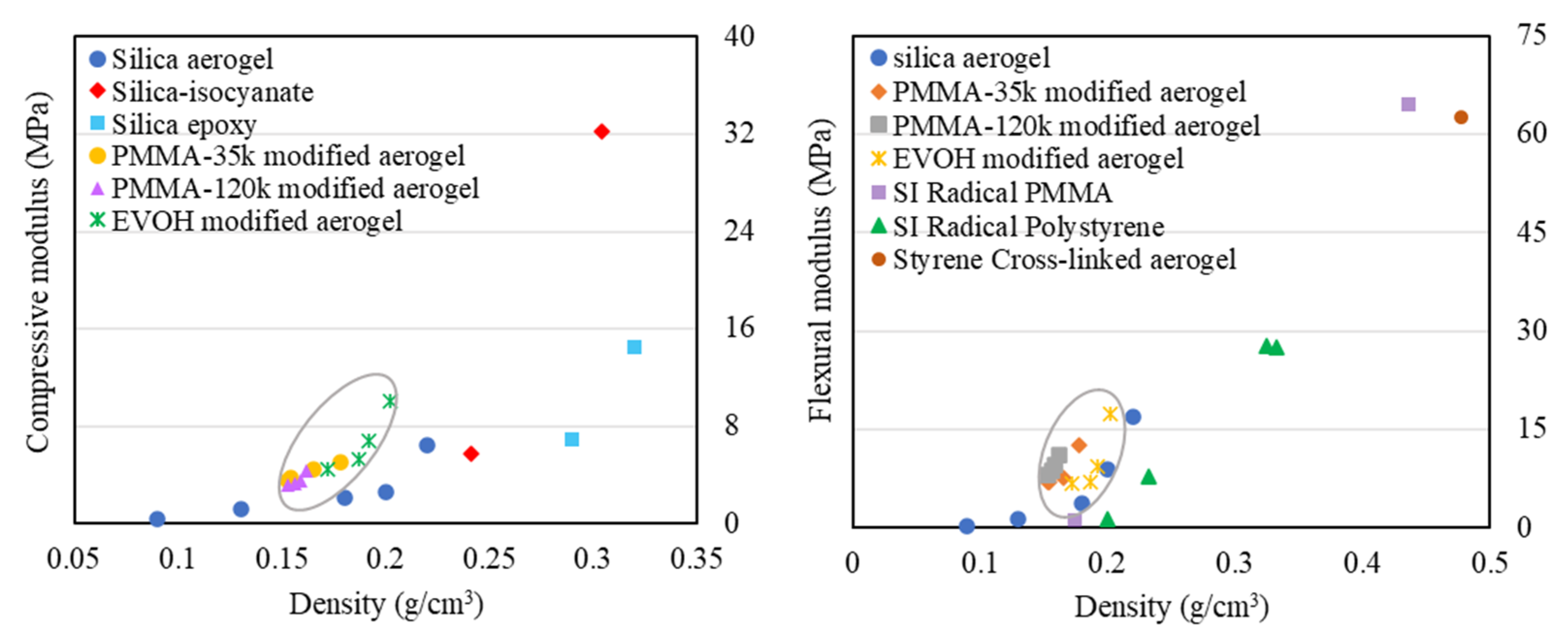
2.5. Mechanical Properties
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of EVOH-Modified Aerogels via TIPS
4.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hüsing, N.; Schubert, U. Aerogels—Airy Materials: Chemistry, Structure, and Properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. (Eds.) Aerogels Handbook; Springer: New York, NY, USA, 2011. [Google Scholar]
- Pollanen, J.; Shirer, K.R.; Blinstein, S.; Davis, J.P.; Choi, H.; Lippman, T.M.; Halperin, W.P.; Lurio, L.B. Globally Anisotropic High Porosity Silica Aerogels. J. Non-Cryst. Solids 2008, 354, 4668–4674. [Google Scholar]
- Venkateswara Rao, A.; Hegde, N.D.; Hirashima, H. Absorption and Desorption of Organic Liquids in Elastic Superhydrophobic Silica Aerogels. J. Colloid Interface Sci. 2007, 305, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, Z.; Lu, Z.; Yang, H.; Yan, Z.; Ji, Y. Investigation on the Pore Structure and Adsorption Capacity of Silica Aerogels Prepared with Different Cations. J. Mater. Sci. 2023, 58, 6602–6617. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Pontinha, A.D.R.; Alves, P.; Santos, P.; Durães, L. Progress in Silica Aerogel-Containing Materials for Buildings’ Thermal Insulation. Constr. Build. Mater. 2021, 286, 122815. [Google Scholar] [CrossRef]
- Chen, Y.X.; Klima, K.M.; Brouwers, H.J.H.; Yu, Q. Effect of Silica Aerogel on Thermal Insulation and Acoustic Absorption of Geopolymer Foam Composites: The Role of Aerogel Particle Size. Compos. Part B 2022, 242, 110048. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Yu, C. Atomic Aerogel Materials (or Single-Atom Aerogels): An Interesting New Paradigm in Materials Science and Catalysis Science. Adv. Mater. 2023, 35, 2211221. [Google Scholar]
- Chen, Z.; Zhu, R.; Li, W.; Luo, D.; Zhang, P.; Xu, C. Optimizing Stability and Efficiency in Low-Content Noble Metal-Doped Aerogel Catalysts for Oxygen Evolution Reaction. Small 2025, 21, 2411579. [Google Scholar]
- Goryunova, K.; Gahramanli, Y.; Gurbanova, R. Adsorption Properties of Silica Aerogel-Based Materials. RSC Adv. 2023, 13, 18207–18216. [Google Scholar] [CrossRef]
- Detcheverry, F.; Kierlik, E.; Rosinberg, M.L.; Tarjus, G. Mechanisms for Gas Adsorption and Desorption in Silica Aerogels: The Effect of Temperature. Langmuir 2004, 20, 8006–8014. [Google Scholar] [CrossRef]
- Jabbari-Gargari, A.; Moghaddas, J.; Hamishehkar, H.; Jafarizadeh-Malmiri, H. Carboxylic Acid Decorated Silica Aerogel Nanostructure as Drug Delivery Carrier. Microporous Mesoporous Mater. 2021, 323, 111220. [Google Scholar] [CrossRef]
- Al-barudi, A.; Sinani, G.; Ulker, Z. Biodegradable Polysaccharide Aerogels Based on Tragacanth and Alginate as Novel Drug Delivery Systems. J. Sol-Gel Sci. Technol. 2024, 109, 748–756. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Ye, W.; Shen, X.; Yuan, X.; Ma, C.; Cao, Y. Performance Regulation of Silica Aerogel Powder Synthesized by a Two-Step Sol-Gel Process with a Fast Ambient Pressure Drying Route. J. Non-Cryst. Solids 2021, 567, 120923. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Ali, A.; Petrik, S.; Coufal, R.; Adach, K.; Fijalkowski, M. Aerogels for Biomedical, Energy and Sensing Applications. Gels 2021, 7, 264. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Liu, Z.; Zhu, C.; Shen, R.; Quan, B.; Yan, X.; Wang, W.; Lu, X.; Qu, J. Interpenetrating Double-Network ANF/MXene-K+ Aerogels Enable Integrated Electromagnetic Interference Shielding, Infrared Camouflage, and Joule Heating in Adaptive Multifunctional Systems. Nano Res. 2025, 18, 94907702. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Gurkovsky, S. Use of Silica Aerogels in Cherenkov Counters. Phys. Part. Nucl. 2008, 39, 78–95. [Google Scholar] [CrossRef]
- Parmenter, K.E.; Milstein, F. Mechanical Properties of Silica Aerogels. J. Non-Cryst. Solids 1998, 223, 179–189. [Google Scholar] [CrossRef]
- Woignier, T.; Reynes, J.; Alaoui, A.H.; Beurroies, I.; Phalippou, J. Different Kinds of Structure in Aerogels: Relationships with the Mechanical Properties. J. Non-Cryst. Solids 1998, 241, 45–52. [Google Scholar] [CrossRef]
- Parale, V.G.; Kim, T.; Choi, H.; Phadtare, V.D.; Dhavale, R.P.; Kanamori, K.; Park, H.-H. Mechanically Strengthened Aerogels through Multiscale, Multicompositional, and Multidimensional Approaches: A Review. Adv. Mater. 2024, 36, 2307772. [Google Scholar] [CrossRef] [PubMed]
- Babiarczuk, B.; Lewandowski, D.; Kierzek, K.; Detyna, J.; Jones, W.; Kaleta, J.; Krzak, J. Mechanical Properties of Silica Aerogels Controlled by Synthesis Parameters. J. Non-Cryst. Solids 2023, 606, 122171. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The Evolution of ‘Sol–Gel’ Chemistry as a Technique for Materials Synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Ahmad, S.; Sheikh, J.N. Silica Centered Aerogels as Advanced Functional Material and Their Applications: A Review. J. Non-Cryst. Solids 2023, 611, 122322. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Bhagat, S.D.; Hirashima, H.; Pajonk, G.M. Synthesis of Flexible Silica Aerogels Using Methyltrimethoxysilane (MTMS) Precursor. J. Colloid Interface Sci. 2006, 300, 279–285. [Google Scholar] [CrossRef]
- Linhares, T.; de Amorim, M.T.P.; Durães, L. Silica Aerogel Composites with Embedded Fibres: A Review on Their Preparation, Properties and Applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Anthony, D.B.; Nguyen, S.N.; Qian, H.; Xu, S.; Shaw, C.M.D.; Greenhalgh, E.S.; Bismarck, A.; Shaffer, M.S.P. Silica Aerogel Infused Hierarchical Glass Fiber Polymer Composites. Compos. Commun. 2023, 39, 101531. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering Strong Silica Aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Wang, L.; Lian, W.; Yin, B.; Liu, X.; Tang, S. Silica Nanowires-Reinforced Silica Aerogels with Outstanding Thermal Insulation, Thermal Stability and Mechanical Properties. Ceram. Int. 2024, 50, 6693–6702. [Google Scholar] [CrossRef]
- Yang, M.; Si, Q.; Tang, G.; Sheng, Q.; Guo, L.; Yang, R.; Li, N.; Zhang, H.; Gao, Q.; Peng, F. High-Temperature Resistance, Lightweight, and Thermally Insulating Silica Aerogel via Doping Hollow Silica Nanoparticles. ACS Appl. Nano Mater. 2025, 8, 8845–8854. [Google Scholar] [CrossRef]
- Dervin, S.; Lang, Y.; Perova, T.; Hinder, S.H.; Pillai, S.C. Graphene Oxide Reinforced High Surface Area Silica Aerogels. J. Non-Cryst. Solids 2017, 465, 31–38. [Google Scholar] [CrossRef]
- Wu, X.; Man, J.; Liu, S.; Huang, S.; Lu, J.; Tai, J.; Zhong, Y.; Shen, X.; Cui, S.; Chen, X. Isocyanate-Crosslinked Silica Aerogel Monolith with Low Thermal Conductivity and Much Enhanced Mechanical Properties: Fabrication and Analysis of Forming Mechanisms. Ceram. Int. 2021, 47, 26668–26677. [Google Scholar] [CrossRef]
- Duan, Y.; Jana, S.C.; Lama, B.; Espe, M.P. Self-Crosslinkable Poly(Urethane Urea)-Reinforced Silica Aerogels. RSC Adv. 2015, 5, 71551–71558. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Fabrizio, E.F.; Ilhan, F.; Dass, A.; Leventis, N. Crosslinking Amine-Modified Silica Aerogels with Epoxies: Mechanically Strong Lightweight Porous Materials. Chem. Mater. 2005, 17, 1085–1098. [Google Scholar] [CrossRef]
- Ilhan, F.; Fabrizio, E.F.; McCorkle, L.; Scheiman, D.A.; Dass, A.; Palczer, A.; Meador, M.B.; Johnston, J.C.; Leventis, N. Hydrophobic Monolithic Aerogels by Nanocasting Polystyrene on Amine-Modified Silica. J. Mater. Chem. 2006, 16, 3046–3054. [Google Scholar] [CrossRef]
- Boday, D.J.; Loy, D.A. Strengthening Silica Aerogels with Surface Initiated ATRP Cross-Linked Poly(Methyl Methacrylate). J. Non-Cryst. Solids. 2015, 427, 114–119. [Google Scholar] [CrossRef]
- Boday, D.J.; Stover, R.J.; Muriithi, B.; Keller, M.W.; Wertz, J.T.; Defriend Obrey, K.A.; Loy, D.A. Strong, Low-Density Nanocomposites by Chemical Vapor Deposition and Polymerization of Cyanoacrylates on Aminated Silica Aerogels. ACS Appl. Mater. Interfaces 2009, 1, 1364–1369. [Google Scholar] [CrossRef]
- Jia, H.; Mu, M.; Hou, Y.; Pan, Y.; Liu, C.; Shen, C.; Liu, X. Template-Thermally Induced Phase Separation-Assisted Microporous Regulation in Poly(Lactic Acid) Aerogel for Sustainable Radiative Cooling. Biomacromolecules 2025, 26, 1184–1194. [Google Scholar] [CrossRef]
- Wang, X.; Jana, S.C. Synergistic Hybrid Organic–Inorganic Aerogels. ACS Appl. Mater. Interfaces 2013, 5, 6423–6429. [Google Scholar] [CrossRef]
- Yolsal, U.; Horton, T.A.R.; Wang, M.; Shaver, M.P. Polymer-Supported Lewis Acids and Bases: Synthesis and Applications. Prog. Polym. Sci. 2020, 111, 101313. [Google Scholar] [CrossRef]
- Ma, H.; Wang, B.; Qi, J.; Pan, Y.; Chen, C. Fabrication of Mechanically Strong Silica Aerogels with the Thermally Induced Phase Separation (TIPS) Method of Poly(Methyl Methacrylate). Materials 2023, 16, 3778. [Google Scholar] [CrossRef]
- Wang, G.; Yoshikawa, H.; Tamiya, E.; Uyama, H. Mesoporous Poly(Ethylene-Co-Vinyl Alcohol) Monolith Captured with Silver Nanoparticles as a SERS Substrate: Facile Fabrication and Ultra-High Sensitivity. RSC Adv. 2015, 5, 48835–48841. [Google Scholar] [CrossRef]
- Wang, G.; Uyama, H. Reactive Poly(Ethylene-Co-Vinyl Alcohol) Monoliths with Tunable Pore Morphology for Enzyme Immobilization. Colloid Polym. Sci. 2015, 293, 2429–2435. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An Overview on Silica Aerogels Synthesis and Different Mechanical Reinforcing Strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Bhagat, S.D. Synthesis and Physical Properties of TEOS-Based Silica Aerogels Prepared by Two Step (Acid–Base) Sol–Gel Process. Solid State Sci. 2004, 6, 945–952. [Google Scholar] [CrossRef]
- Woignier, T.; Primera, J.; Alaoui, A.; Etienne, P.; Despestis, F.; Calas-Etienne, S. Mechanical Properties and Brittle Behavior of Silica Aerogels. Gels 2015, 1, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Mulik, S.; Sotiriou-Leventis, C.; Churu, G.; Lu, H.; Leventis, N. Cross-Linking 3D Assemblies of Nanoparticles into Mechanically Strong Aerogels by Surface-Initiated Free-Radical Polymerization. Chem. Mater. 2008, 20, 5035–5046. [Google Scholar] [CrossRef]
- Wu, G.; Wang, J.; Shen, J.; Zhang, Q.; Zhou, B.; Deng, Z.; Fan, B.; Zhou, D.; Zhang, F. Strengthening Mechanism of Porous Silica Films Derived by Two-Step Catalysis. J. Phys. D Appl. Phys. 2001, 34, 1301–1307. [Google Scholar] [CrossRef]
- Zu, G.; Shimizu, T.; Kanamori, K.; Zhu, Y.; Maeno, A.; Kaji, H.; Shen, J.; Nakanishi, K. Transparent, Superflexible Doubly Cross-Linked Polyvinylpolymethylsiloxane Aerogel Superinsulators via Ambient Pressure Drying. ACS Nano 2018, 12, 521–532. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Zhang, G.; Dass, A.; Rawashdeh, A.M.M.; Thomas, J.; Counsil, J.A.; Sotiriou-Leventis, C.; Fabrizio, E.F.; Ilhan, F.; Vassilaras, P.; Scheiman, D.A. Isocyanate-Crosslinked Silica Aerogel Monoliths: Preparation and Characterization. J. Non-Cryst. Solids 2004, 350, 152–164. [Google Scholar] [CrossRef]
- Boday, D.J.; Keng, P.Y.; Muriithi, B.; Pyun, J.; Loy, D.A. Mechanically Reinforced Silica Aerogel nanocomposites via Surface Initiated Atom Transfer Radical Polymerizations. J. Mater. Chem. 2010, 20, 6863–6865. [Google Scholar] [CrossRef]
- Van Bommel, M.; Engelsen, C.D.; Van Miltenburg, J. A Thermoporometry Study of Fumed Silica/Aerogel Composites. J. Porous Mater. 1997, 4, 143–150. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore Size Distribution Analysis of Microporous Carbons: A Density Functional Theory Approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Olivier, J.P.; Conklin, W.B.; Szombathely, M.V. Determination of Pore Size Distribution from Density Functional Theory: A Comparison of Nitrogen and Argon Results. In Studies in Surface Science and Catalysis; Rouquerol, J., Rodríguez-Reinoso, F., Sing, K.S.W., Unger, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 87, pp. 81–89. [Google Scholar]
- Ravikovitch, P.I.; Domhnaill, S.C.O.; Neimark, A.V.; Schueth, F.; Unger, K.K. Capillary Hysteresis in Nanopores: Theoretical and Experimental Studies of Nitrogen Adsorption on MCM-41. Langmuir 1995, 11, 4765–4772. [Google Scholar] [CrossRef]
- Nguyen, C.; Do, D.D. The Dubinin–Radushkevich Equation and the Underlying Microscopic Adsorption Description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; McCarver, P.M.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Fractal Multiscale Nanoporous Polyurethanes: Flexible to Extremely Rigid Aerogels from Multifunctional Small Molecules. Chem. Mater. 2013, 25, 3205–3224. [Google Scholar] [CrossRef]
- Bang, A.; Buback, C.; Sotiriou-Leventis, C.; Leventis, N. Flexible Aerogels from Hyperbranched Polyurethanes: Probing the Role of Molecular Rigidity with Poly(Urethane Acrylates) Versus Poly(Urethane Norbornenes). Chem. Mater. 2014, 26, 6979–6993. [Google Scholar] [CrossRef]
- Diascorn, N.; Calas, S.; Sallée, H.; Achard, P.; Rigacci, A. Polyurethane Aerogels Synthesis for Thermal Insulation—Textural, Thermal and Mechanical Properties. J. Supercrit. Fluids 2015, 106, 76–84. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, Q.; Wang, L.; Cao, J.; Song, J.; Song, L.; Zhang, Y. Recent Advances in the Synthesis and Application of Graphene Aerogel and Silica Aerogel for Environment and Energy Storage: A Review. J. Environ. Manag. 2025, 377, 124668. [Google Scholar] [CrossRef]
- Choi, H.; Han, H.H.; Parale, V.G.; Kim, T.; Park, W.; Kim, Y.; Kim, J.; Choi, Y.; Bae, Y.-S.; Park, H.-H. Rigid Amine-Incorporated Silica Aerogel for Highly Efficient CO2 Capture and Heavy Metal Removal. Chem. Eng. J. 2024, 483, 149357. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Demilecamps, A.; Zhang, Y.; Brunner, S.; Huber, L.; Tingaut, P.; Rigacci, A.; Budtova, T.; Koebel, M.M. Strong, Thermally Superinsulating Biopolymer–Silica Aerogel Hybrids by Cogelation of Silicic Acid with Pectin. Angew. Chem. Int. Ed. 2015, 54, 14282–14286. [Google Scholar] [CrossRef]
- Katti, A.; Shimpi, N.; Roy, S.; Lu, H.; Fabrizio, E.F.; Dass, A.; Capadona, L.A.; Leventis, N. Chemical, Physical, and Mechanical Characterization of Isocyanate Cross-Linked Amine-Modified Silica Aerogels. Chem. Mater. 2006, 18, 285–296. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Capadona, L.A.; McCorkle, L.; Papadopoulos, D.S.; Leventis, N. Structure-Property Relationships in Porous 3D Nanostructures as a Function of Preparation Conditions: Isocyanate Cross-Linked Silica Aerogels. Chem. Mater. 2007, 19, 2247–2260. [Google Scholar] [CrossRef]
- ASTM D695-23; Standard Test Method for Compressive Properties of Rigid Plastics. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM C1684-18; Standard Test Method for Flexural Strength of Advanced Ceramics at Ambient Temperature—Cylindrical Rod Strength. ASTM International: West Conshohocken, PA, USA, 2023.



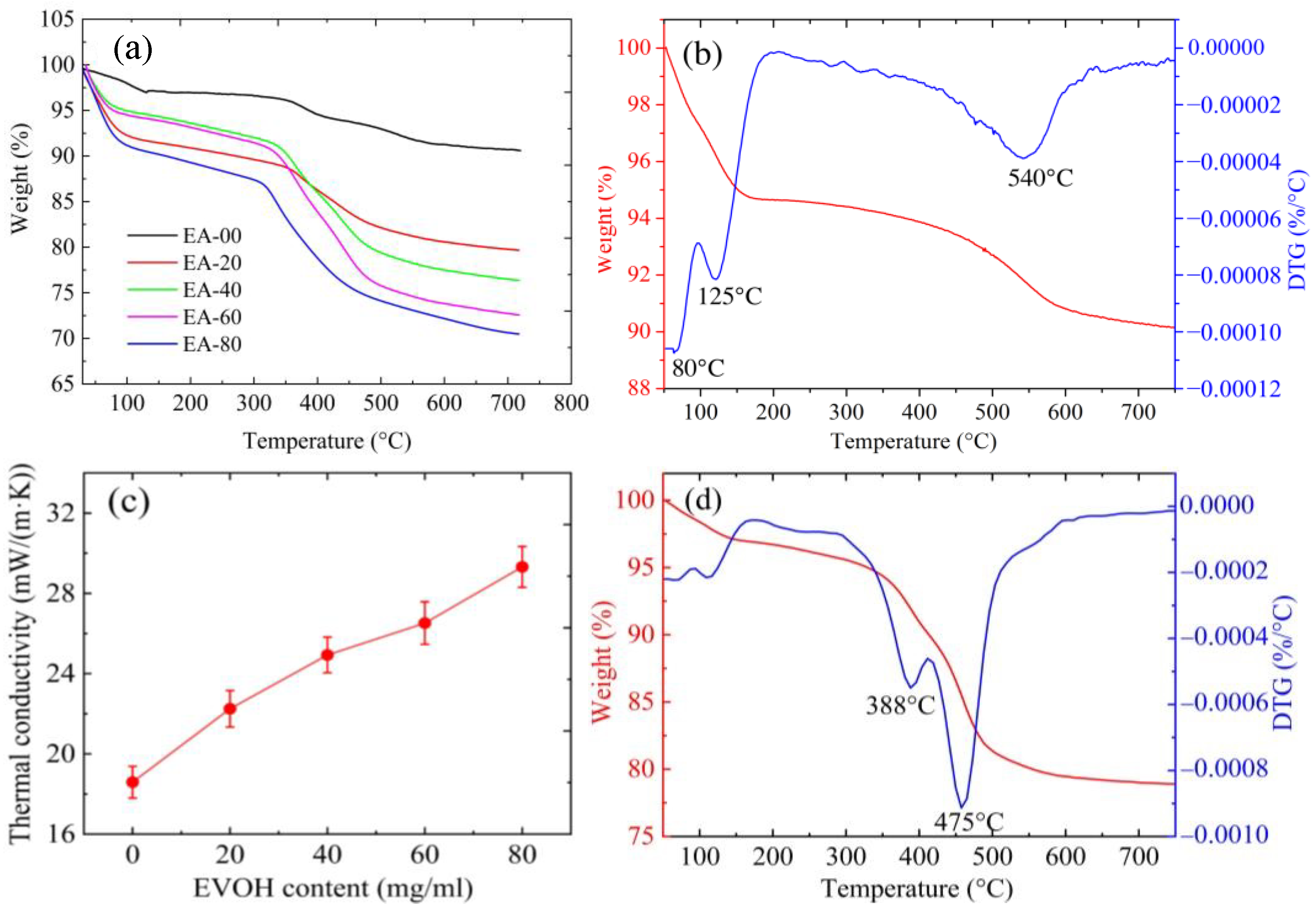

| Samples * | Bulk Density (g/cm3) | Surface Area (m2/g) | Porosity (%) | Linear Shrinkage (%) | EVOH Content TGA (g/g) | Mass Loss TGA (%) |
|---|---|---|---|---|---|---|
| EA-00 | 0.136 ± 0.002 | 874 | 93.7 | 8.6 | 0 | 9.0 |
| EA-20 | 0.170 ± 0.004 | 618 | 91.6 | 8.2 | 0.09 | 21.1 |
| EA-40 | 0.183 ± 0.005 | 568 | 89.5 | 7.6 | 0.145 | 23.6 |
| EA-60 | 0.192 ± 0.001 | 429 | 89.1 | 7.3 | 0.152 | 27.4 |
| EA-80 | 0.200 ± 0.001 | 401 | 86.8 | 6.9 | 0.176 | 29.5 |
| Samples | SBET (m2/g) | SNLDFT (m2/g) | SDR (m2/g) | Vtotal (cm3/g) | Vpore,NLDFT (cm3/g) | Vpore,DR (cm3/g) | Dpore,BJH (nm) |
|---|---|---|---|---|---|---|---|
| EA-00 | 874 | 928 | 2352 | 5.055 | 4.713 | 0.870 | 17.73 |
| EA-20 | 618 | 686 | 1833 | 4.019 | 3.442 | 0.651 | 17.35 |
| EA-40 | 568 | 587 | 1487 | 3.753 | 2.987 | 0.475 | 17.49 |
| EA-60 | 429 | 453 | 1196 | 2.943 | 2.528 | 0.425 | 17.88 |
| EA-80 | 401 | 410 | 1049 | 2.742 | 2.176 | 0.406 | 17.33 |
| Samples | Density (g/cm3) | Flex. Strength (MPa) | Flex. Modulus (MPa) | Compr. Strength (MPa) | Compr. Modulus (MPa) |
|---|---|---|---|---|---|
| EA-00 | 0.136 ± 0.002 | 0.098 ± 0.020 | 5.61 ± 0.24 | 0.74 ± 0.17 | 1.78 ± 0.31 |
| EA-20 | 0.172 ± 0.006 | 0.172 ± 0.033 | 6.68 ± 0.97 | 5.73 ± 0.62 | 4.44 ± 0.26 |
| EA-40 | 0.184 ± 0.005 | 0.232 ± 0.036 | 7.09 ± 0.61 | 8.71 ± 0.57 | 5.34 ± 0.75 |
| EA-60 | 0.190 ± 0.002 | 0.374 ± 0.037 | 9.31 ± 0.60 | 14.89 ± 0.76 | 6.83 ± 0.47 |
| EA-80 | 0.201 ± 0.001 | 0.545 ± 0.027 | 17.34 ± 1.24 | 18.37 ± 0.93 | 10.07 ± 0.34 |
| Samples | Slopes of Log Compr. Modulus Versus Log Density |
|---|---|
| Silica aerogel | 3.46 |
| PU-silica composites | 3.70 |
| PU aerogels | 3.62 |
| Pectin–silica composites | 3.82 |
| Cellulose–silica composites | 3.23 |
| EVOH-modified aerogels | 3.73 |
| PMMA-35k-modified aerogels | 4.49 |
| PMMA-120k-modified aerogels | 6.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Wang, B.; Zhang, Y.; Zheng, L. Mechanically Reinforced Silica Aerogels via Thermally Induced Phase Separation of Poly(ethylene-co-vinyl Alcohol). Gels 2025, 11, 870. https://doi.org/10.3390/gels11110870
Ma H, Wang B, Zhang Y, Zheng L. Mechanically Reinforced Silica Aerogels via Thermally Induced Phase Separation of Poly(ethylene-co-vinyl Alcohol). Gels. 2025; 11(11):870. https://doi.org/10.3390/gels11110870
Chicago/Turabian StyleMa, Hainan, Baomin Wang, Yongjun Zhang, and Liquan Zheng. 2025. "Mechanically Reinforced Silica Aerogels via Thermally Induced Phase Separation of Poly(ethylene-co-vinyl Alcohol)" Gels 11, no. 11: 870. https://doi.org/10.3390/gels11110870
APA StyleMa, H., Wang, B., Zhang, Y., & Zheng, L. (2025). Mechanically Reinforced Silica Aerogels via Thermally Induced Phase Separation of Poly(ethylene-co-vinyl Alcohol). Gels, 11(11), 870. https://doi.org/10.3390/gels11110870






