Gelation Performance of HPAM-Cr3+ Gels for Reservoir Profile Control: The Impact of Propagation Distance and Optimization Design
Abstract
1. Introduction
2. Results and Discussion
- (1)
- Definition and Classification of Gel System
- (2)
- Quantitative Relationship Between Injection Concentration and Placement Depth
- (3)
- Design Methodology and Field Application Value
2.1. Characterization of the HPAM-Cr3+ Crosslinking Reaction Product
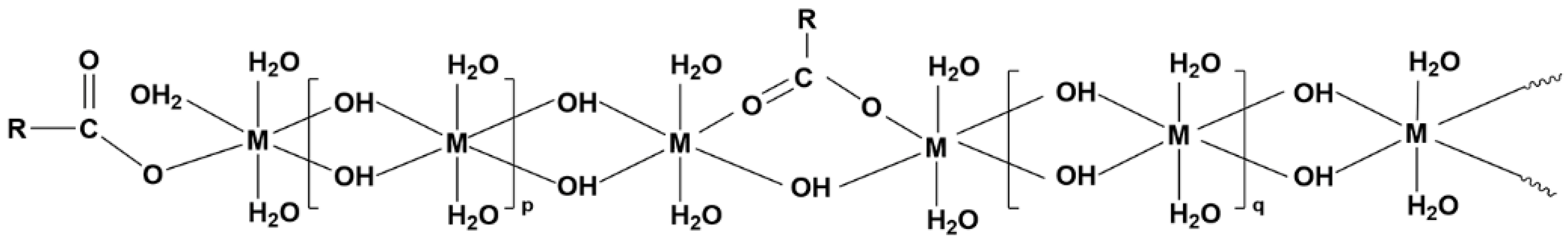
2.1.1. Reaction Mechanism of HPAM-Cr3+ Crosslinking
2.1.2. Characterization of HPAM-Cr3+ Crosslinked Gel Properties
- SSG: This type possesses a moderate crosslinking density, forming a robust three-dimensional network structure. It exhibits excellent long-term stability, maintaining its integrity and water-holding capacity over extended periods.
- SWG: Characterized by a low crosslinking density, SWG forms a weak three-dimensional network. It demonstrates good long-term stability but has a relatively poor water-binding capacity due to its less cohesive structure.
- CDG: CDG exhibits low viscosity and consists of weakly crosslinked HPAM microgels or a small number of HPAM molecules forming gel-like particles without a continuous, bulk three-dimensional network. Despite the lack of a macroscopic network, it shows good long-term stability.
- USG: USG typically appears as a turbid, viscoelastic mass or a coexisting system of water and gel phases resulting from local syneresis or breakdown. It suffers from poor long-term stability, attributed to the continuous shrinkage of the initially formed network over time. This process expels bound water, reduces the effective gel volume, and may eventually lead to complete gel disappearance.
- OCG: Paradoxically, OCG has a viscosity lower than that of an HPAM solution at the same concentration. OCG refers to a formulation system where the HPAM carboxylate-Cr3+ molar ratio falls significantly below the optimal crosslinking window. Under such non-ideal conditions, excess Cr3+ triggers a sharp increase in local crosslinking density, forming highly concentrated ultra-dense coordination nodes. This imbalanced crosslinking mechanism causes spatial collapse of polymer chains, ultimately resulting in the formation of rigid, over-tightened isolated aggregates rather than a continuous and stable three-dimensional network. This structural degradation process severely compromises the gel’s macroscopic plugging capacity and long-term stability, it decreases both bound water and solvation water within the system, leading to pronounced syneresis and high static fluid-loss rate [41].
- SSG: Appears as a homogeneous, transparent viscoelastic solid (Appearance Code 1), with an elastic modulus (G′gel) ≥ 10 Pa and a fluid loss rate (Rw) ≤ 15%.
- SWG: Appears as a homogeneous, transparent viscoelastic solid (Appearance Code 1), with G′gel ≥ G′HPAM(the storage modulus of an HPAM solution at the same concentration as the gel system), G′gel < 10 Pa, and Rw ≥ 15% (where Rw is greater than or equal to the fluid loss rate of the HPAM solution at the same concentration).
- CDG: Appears as a homogeneous, transparent fluid (Appearance Code 1), with G′gel < G′HPAM and Rw ≥ .
- USG: Appears as a turbid viscoelastic solid (Appearance Code 1–2), with G′gel ≥ G′HPAM and Rw ≥ 15%.
2.2. Diagnostic Chart for HPAM-Cr3+ Crosslinked Reaction Products
2.3. Factors Influencing the Performance of HPAM-Cr3+ Crosslinking Reaction Products
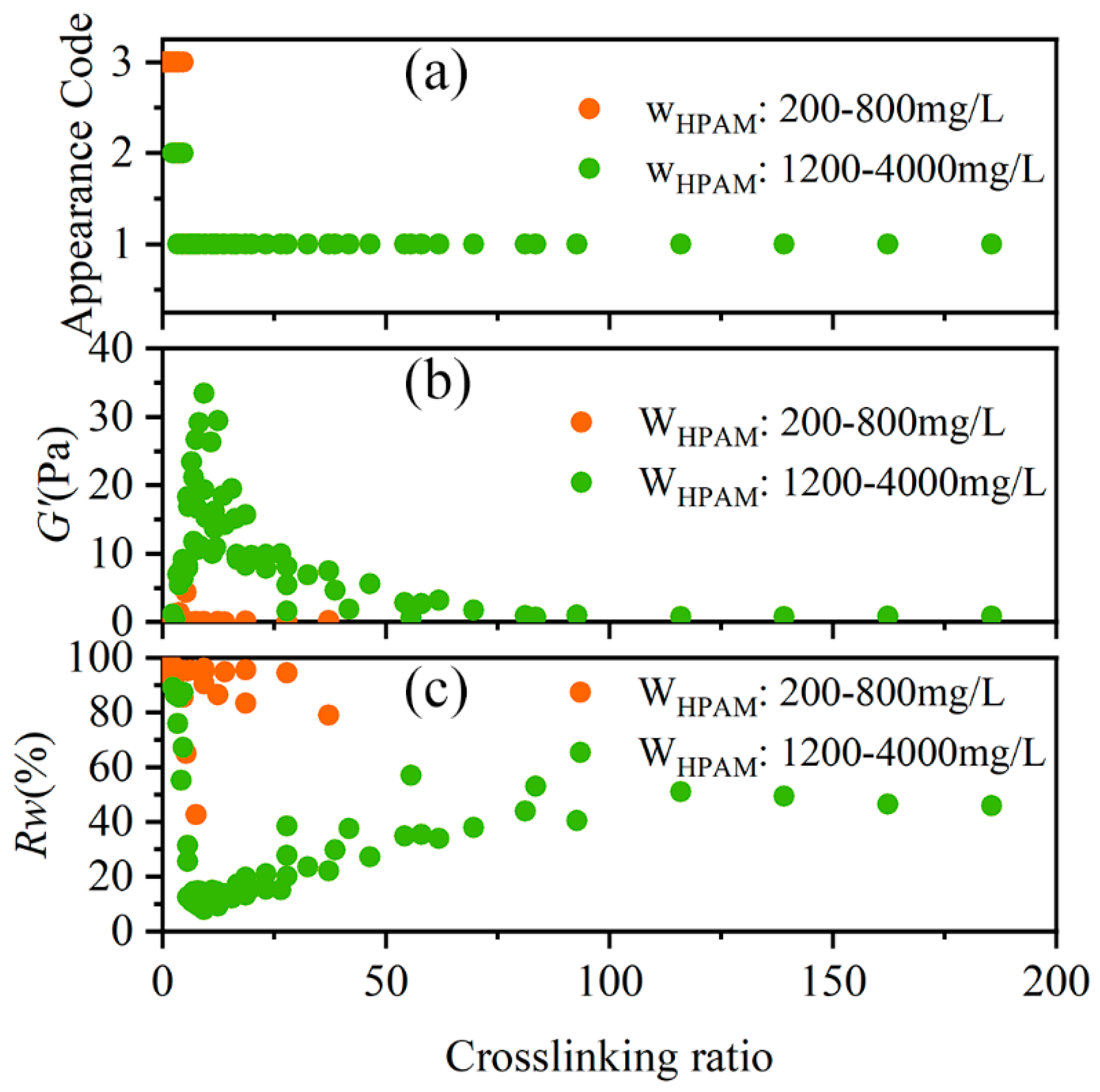
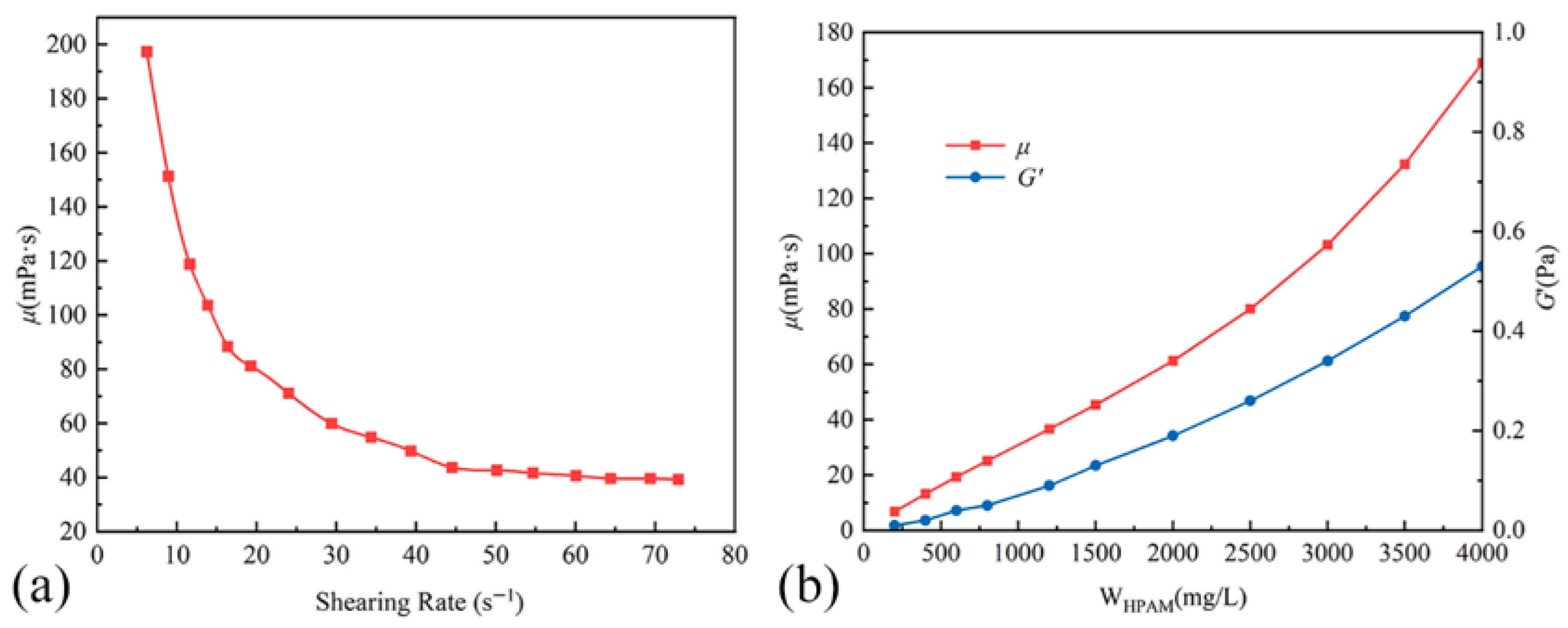
2.3.1. Effects of HPAM Concentration
2.3.2. Effects of Crosslinking Ratio
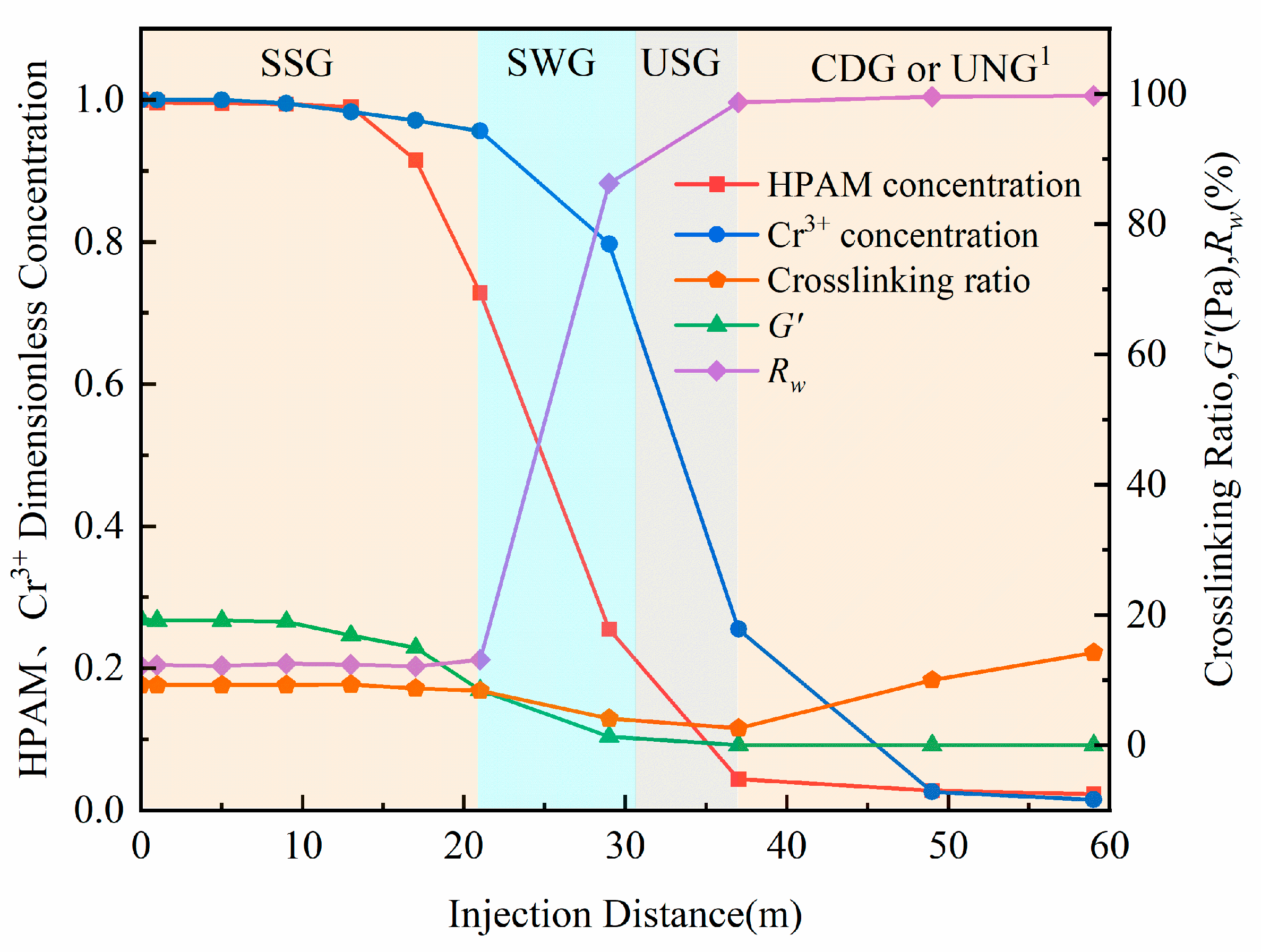
2.4. Effect of Propagation Distance on Gelation Performance
2.5. Study on Gel Dosage Design Methodology
2.5.1. Optimization Method for HPAM Concentration
2.5.2. Optimization Method for Cr3+ Concentration
3. Conclusions
- A comprehensive gel characterization methodology was established by integrating visual inspection, rheological parameters, and long-term stability, enabling accurate classification of gels into five distinct categories: SSG, SMG, CDG, UMG, and OWG. This methodology was applied to combine contour maps of visual appearance, storage modulus (G′), and water loss rate, generating a morphology distribution map for HPAM-Cr3+ crosslinking reaction products. This map provides a critical basis for selecting appropriate formulations for the HPAM-Cr3+ gel system.
- The factors influencing the performance of HPAM-Cr3+ crosslinking reaction products were systematically investigated, identifying HPAM concentration, crosslinking ratio, and system propagation distance as key parameters. Results demonstrated that no gel formation occurs at HPAM concentrations below 800 mg/L, while concentrations above 2500 mg/L effectively inhibit over-crosslinking. The crosslinking ratio range for forming SSG was determined to be 5.56 to 18.68, with an optimal value of 9.27. Sand-pack flow experiments over 60 m revealed that stable SSG forms within 21 m of propagation, SMG forms between 21–34 m, and no coherent gel forms beyond 34 m. This indicates that only the first 35% of the designed treatment distance develops effective SSG for plugging.
- An optimized design method for gel dosage was developed based on this research. The method determines the optimal gel volume by calculating the crosslinking system throughput at the target fluid diversion interface and referencing the gel morphology distribution map. Furthermore, calculation formulas were provided to determine the required initial concentrations of HPAM and Cr3+ in the crosslinking system to achieve optimal plugging performance at the target location. This provides a straightforward and effective approach for the precise design of in-depth conformance control agents in oil reservoirs.
- In light of the distance thresholds identified of this study, future studies should prioritize gel chemistries with enhanced shear resistance and reduced rock adsorption so that the concentration–ratio state remains within the SSG domain along extended flow paths. A second direction is time-programmed or protected crosslinkers to delay gelation until arrival while enabling rapid post-arrival strength build-up. Finally, embedding shear- and adsorption-corrections into our diagnostic chart via a coupled transport–rheology–adsorption model will allow prediction of an effective propagation distance under realistic velocity profiles and guide field-scale optimization.
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Organic Chromium Crosslinker
4.3. Preparation of the HPAM-Cr3+ Crosslinked System
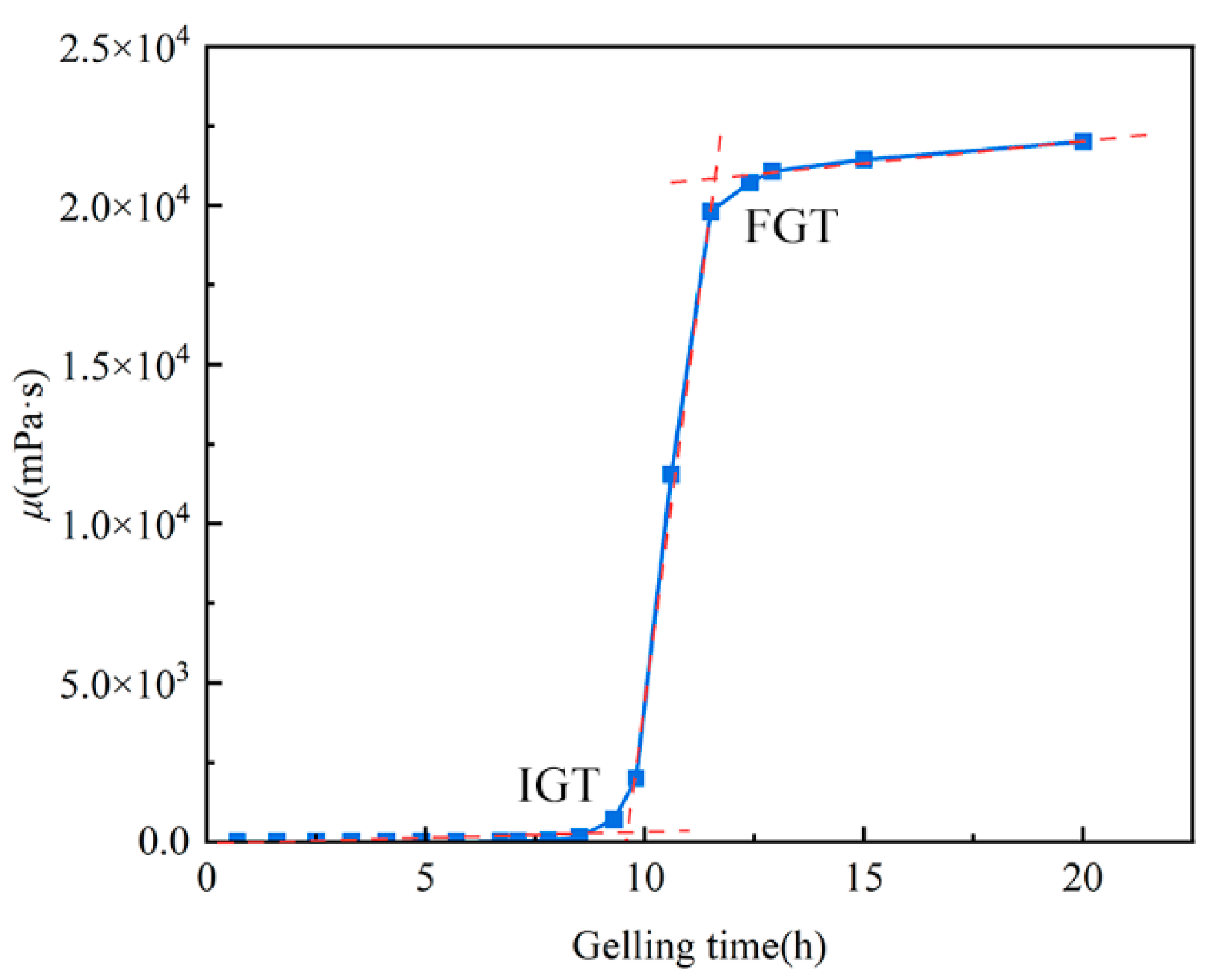
4.4. Determination of Gelation Time
4.5. Determination of Gel Storage Modulus
4.6. Determination of Gel Static Fluid Loss Rate
4.7. Determination of HPAM and Cr3+ Concentrations
4.7.1. Determination of HPAM Concentration
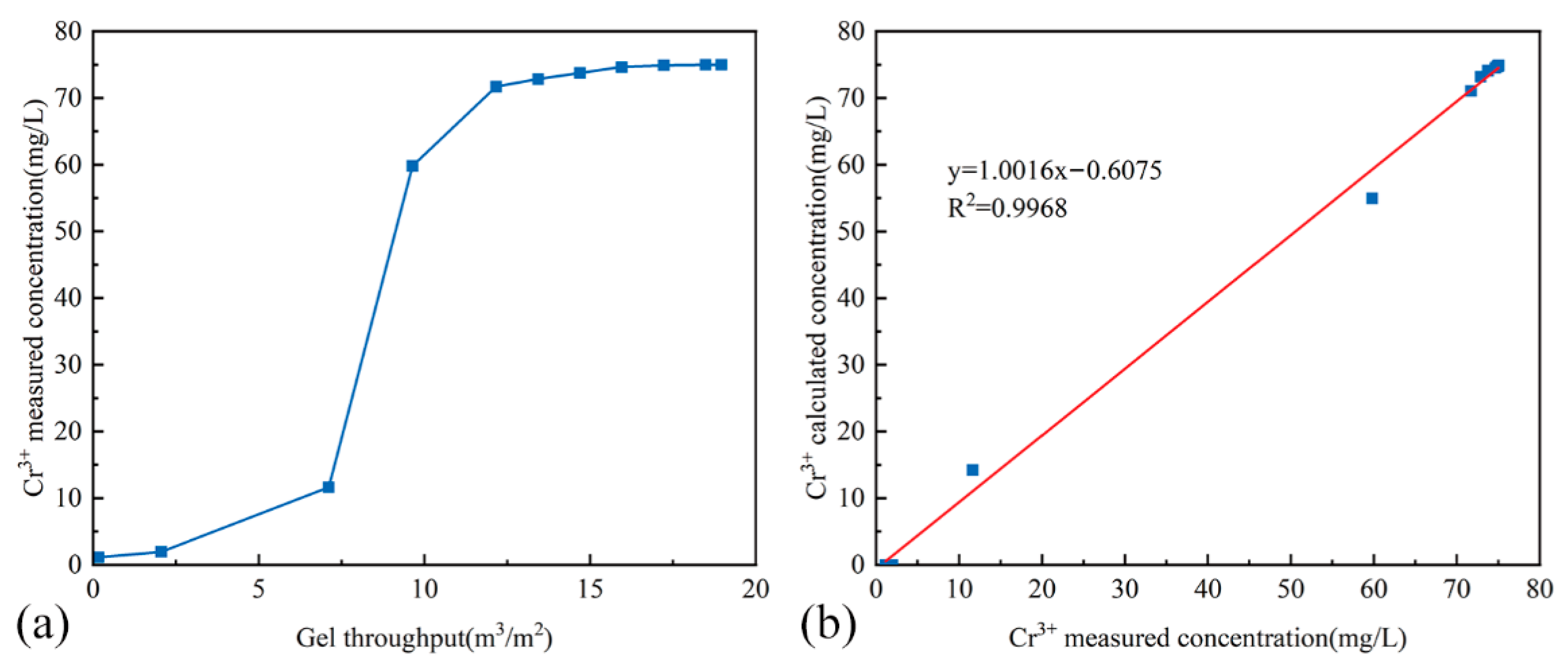
4.7.2. Determination of Cr3+ Concentration
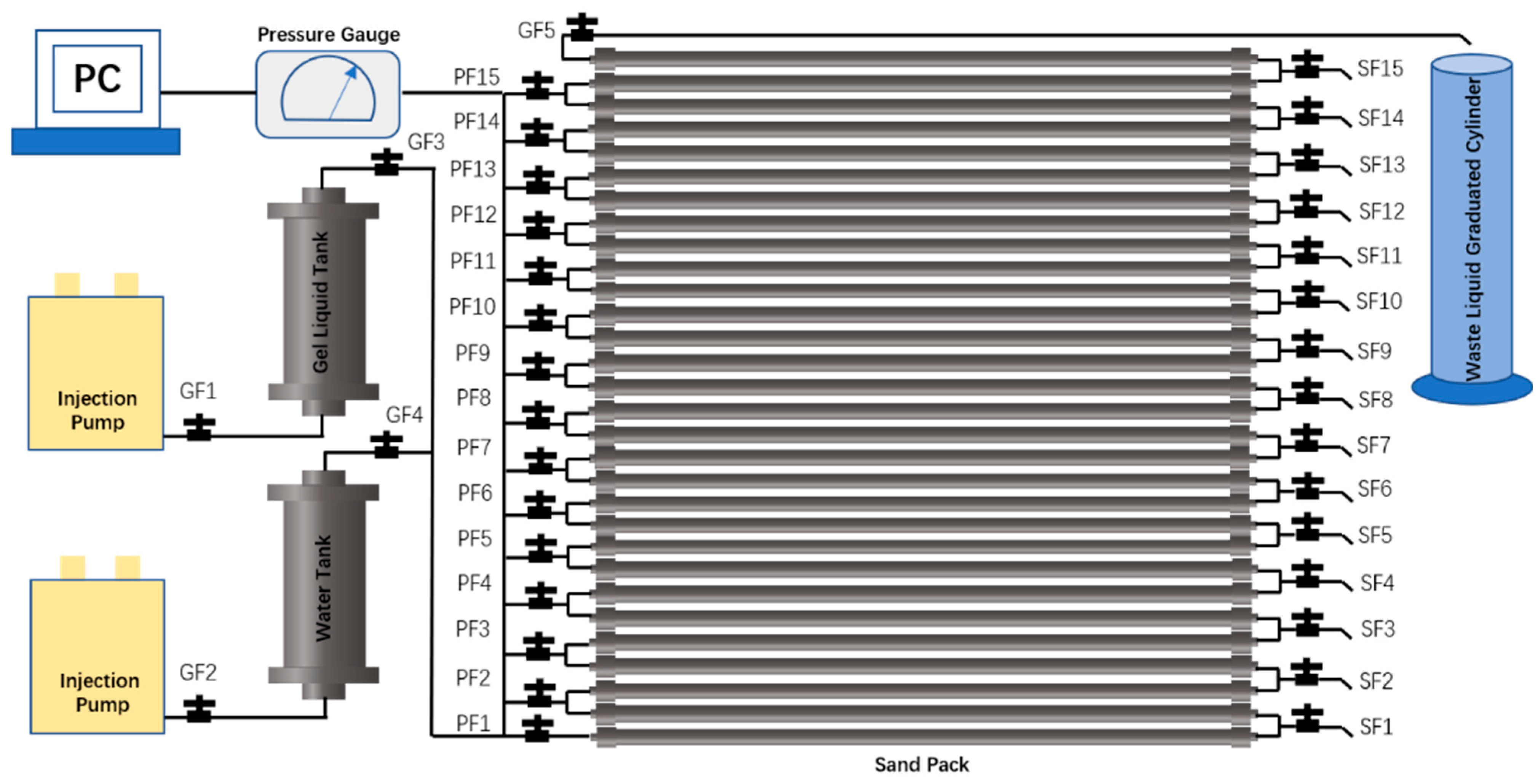
4.8. Propagation Experiment of the Crosslinked System in Sand-Pack
- The sand-pack array and injection/production system were assembled inside a constant-temperature chamber according to the experimental flowchart Figure 11, with all valves initially closed. The chamber temperature was set to 60 °C. Valves GF3, PF1, GF5, and GF4 were then opened sequentially.
- Formation water was injected at 10 mL/min for 3 PV. Subsequently, valves GF4 and GF5 were closed while valves GF1 and GF2 were opened.
- The crosslinked system was injected at 10 mL/min for 1 PV. Valve GF3 was then closed, and a 50 mL sample was collected through valve SF1 before closing it. Additional 50 mL samples were sequentially collected from valves SF2, SF3, SF4, SF5, SF6, SF8, SF10, SF13, and the production outlet. The corresponding average distances from the injection point were 1 m, 5 m, 9 m, 13 m, 17 m, 21 m, 29 m, 41 m, 53 m, and 59 m, respectively.
- For chemical analysis, 1 mL of each sample was diluted to 50 mL with deionized water in a volumetric flask. HPAM concentration was determined by starch-cadmium iodide spectrophotometry, while chromium ion concentration was measured using iCAP -7000 ICP-OES (Thermo Scientific, Waltham, MA, USA).
- From each original sample, 40 mL was divided equally into two aliquots. After gelation at 60 °C, the G′ and Rw of the formed gels were measured separately for each aliquot.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPAM | Partially Hydrolyzed Polyacrylamide |
| SSG | Stable Strong Gel |
| SWG | Stable Weak Gel |
| CDG | Colloidal Dispersion Gel |
| USG | Unstable Gel |
| OCG | Over-crosslinked Gel |
| UGF | Un-gelled Fluid |
| IGT | Initial Gelation Time |
| FGT | Final Gelation Time |
| G′ | Storage modulus |
| Rw | Water loss rate |
References
- Hao, J.; Yuan, Y.; Zhang, H.; Zhou, L.; Wang, Y. Experimental Study on the Influence of Waterflooding Heterogeneity upon Water-injection Development Effect in Low Permeability Reservoir. Fresenius Environ. Balletin 2019, 28, 7548–7555. [Google Scholar]
- Morgan, A.; Ampomah, W.; Grigg, R.; Wang, S.; Czarnota, R. Mechanisms of Waterflood Inefficiency: Analysis of Geological, Petrophysical and Reservoir History, a Field Case Study of FWU (East Section). Energies 2024, 17, 1565. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Z.; Sun, Q.; Zhang, L.; Zhang, X. Comprehensive evaluation of waterflooding front in low-permeability reservoir. Energy Sci. Eng. 2021, 9, 1394–1408. [Google Scholar] [CrossRef]
- Morgan, A.; Ampomah, W.; Grigg, R.; Czarnota, R.; Wang, S. A comprehensive experimental study on the mechanisms of waterflood inefficiency in a Morrowan sandstone reservoir. Sci. Rep. 2025, 15, 27255. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Huang, G. Flow Mechanism of Partially Cross-Linked Polyacrylamide in Porous Media. J. Dispers. Sci. Technol. 2014, 35, 1270–1277. [Google Scholar] [CrossRef]
- Ke, C.; Sun, W.; Li, Y.; Hu, J.; Lu, G.; Zheng, X.; Zhang, Q.; Zhang, X. Polymer-Assisted Microbial-Enhanced Oil Recovery. Energy Fuels 2018, 32, 5885–5892. [Google Scholar] [CrossRef]
- Xie, K.; Su, C.; Liu, C.; Mei, J.; Yu, H.; He, X.; Lu, X. Profile change rule during Cr3+ polymer weak gel flooding and related improving method. Lithol. Reserv. 2022, 34, 160–170. [Google Scholar]
- Sun, X.; Bai, B.; Alhuraishawy, A.K.; Zhu, D. Understanding the Plugging Performance of HPAM-Cr (III) Polymer Gel for CO2 Conformance Control. SPE J. 2021, 26, 3109–3118. [Google Scholar] [CrossRef]
- Karimi, S.; Esmaeilzadeh, F.; Mowla, D. Identification and selection of a stable gel polymer to control or reduce water production in gas condensate fields. J. Nat. Gas Sci. Eng. 2014, 21, 940–950. [Google Scholar] [CrossRef]
- Telin, A.; Lenchenkova, L.; Yakubov, R.; Poteshkina, K.; Krisanova, P.; Filatov, A.; Stefantsev, A. Application of hydrogels and hydrocarbon-based gels in oil production processes and well drilling. Gels 2023, 9, 609. [Google Scholar] [CrossRef]
- Vargas-Vasquez, S.M.; Romero-Zeron, L.B.; MacMillan, B. Effect of Cr(III) acetate concentration on the 1H NMR behavior of HPAm/Cr(III) acetate gels. Int. J. Polym. Anal. Charact. 2008, 13, 163–179. [Google Scholar] [CrossRef]
- Karimi, S.; Kazemi, S.; Kazemi, N. Syneresis measurement of the HPAM-Cr (III) gel polymer at different conditions: An experimental investigation. J. Nat. Gas Sci. Eng. 2016, 34, 1027–1033. [Google Scholar] [CrossRef]
- Han, J.; Sun, J.; Lv, K.; Yang, J.; Li, Y. Polymer gels used in oil-gas drilling and production engineering. Gels 2022, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wu, Q.; Ding, L.; Guo, H.; Zhao, A. Preparation and rheological Evaluation of a thixotropic polymer gel for water shutoff in fractured tight reservoirs. J. Nat. Gas Sci. Eng. 2022, 208, 109542. [Google Scholar] [CrossRef]
- Wei, L.; Dai, J.; Wang, L. Crosslinking Control Technology by HPAM/Cr3+ Gel System HPAM/Cr(III). Chem. Eng. Oil Gas 2011, 40, 263–265. [Google Scholar]
- Zhang, S.; Wei, F.; Liu, P.; Shao, L.; Li, W. Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff. E-Polymers 2020, 20, 411–422. [Google Scholar] [CrossRef]
- Cheng, H.; Zheng, X.; Wu, Y.; Zhang, J.; Zhao, X.; Li, C. Experimental and numerical investigation on oil displacement mechanism of weak gel in waterflood reservoirs. Gels 2022, 8, 309. [Google Scholar] [CrossRef]
- Gao, S.; Jing, G.; He, S. HPAM/Cr(III) Gelling Fluid STP for Indepth Reservoir Permeability Adjusting. Oilfield Chem. 2004, 21, 48–51. [Google Scholar]
- Ge, L.; Chen, X.; Wang, G.; Zhang, G.; Li, J.; Liu, Y.; Xiao, L.; Wen, Y.; Yuan, W.; Qu, M.; et al. Analysis of the distribution pattern of re-maining oil and development potential after weak gel flooding in the offshore LD oilfield. Gels 2024, 10, 236. [Google Scholar] [CrossRef]
- Wang, K.; Luo, M.; Li, M.; Kang, S.; Li, X.; Pu, C.; Liu, J. Optimization of Hydrolyzed Polyacrylamide/Chromium (III)-Acetate Gel- Plugging Process after Preflush Crosslinker in Fractured Extralow Permeability Reservoir at Moderate Temperature. SPE J. 2023, 28, 683–696. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Zhang, J.; Lv, P.; Shi, S. Dynamic Gelation of HPAM/Cr(III) under Shear in an Agitator and Porous Media. Oil Gas Sci. Technol. 2016, 70, 941–949. [Google Scholar]
- Zhang, L.; Liu, Y.; Wang, Z.; Li, H.; Zhao, Y.; Pan, Y.; Liu, Y.; Yuan, W.; Hou, J. Evaluation of profile control and oil displacement effect of starch gel and Nano-MoS2 combination system in high-temperature heterogenious reservoir. Gels 2024, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Al Brahim, A.; Eriyagama, Y.; Bai, B.; Schuman, T. Feasibility Study of Recrosslinkable Preformed Particle Gels for Natural Gas Injection Profile Control. SPE J. 2023, 28, 1829–1841. [Google Scholar] [CrossRef]
- Sun, X.; Bai, B.; Long, Y.; Wang, Z. A comprehensive review of hydrogel performance under CO2 conditions for conformance control. J. Pet. Sci. Eng. 2020, 185, 106662. [Google Scholar] [CrossRef]
- Al Brahim, A. Investigation of Recrosslinkable Preformed Particle Gels for Natural Gas Conformance Control. Ph.D. Thesis, Missouri University, Columbia, MO, USA, 2023. [Google Scholar]
- Zhao, Y. Enhanced Heavy Oil Recovery by Low Salinity Polymer Flood Combined with Microgel Treatment. Ph.D. Thesis, Missouri University, Columbia, MO, USA, 2021. [Google Scholar]
- Liang, Y.; Jin, C.; Wang, Z.; Guo, Y.; Shi, S.; Fan, L.; Song, D.; Li, N.; Zhang, Y.; Wang, J.; et al. Insights on the penetration and migration of chemically cross-linked systems in porous media. J. Pet. Sci. Eng. 2022, 213, 110374. [Google Scholar] [CrossRef]
- Wilton, R.; Asghari, K. Improving gel performance in fractures: Chromium pre-flush and overload. J. Can. Pet. Technol. 2007, 46, 33–39. [Google Scholar] [CrossRef]
- Qiao, W.; Zhang, G.; Ge, J.; Li, J.; Jiang, P.; Pei, H. Influence study of flue gas on chromium acetate/HPAM gel in flue gas flooding reservoir. Pet. Sci. Technol. 2023, 41, 325–342. [Google Scholar] [CrossRef]
- Shang, X.; Dong, C.; Huang, F.; Xiong, C.; Wei, F. Migration and plugging mechanisms of polymer gel in 3D fractured media. Journal of China University of Petroleum. Ed. Nat. Sci. 2021, 45, 171–178. [Google Scholar]
- Lin, B. Microgel Placement and Plugging Performance in Sand Filled Fracture for Conformance Control. Ph.D. Thesis, Missouri University, Columbia, MO, USA, 2020. [Google Scholar]
- Khadij, N.; Barati, A.; Davarnejad, R. Investigation of kinetic properties of hydrolyzed polyacrylamide crosslinked by transient metal ligand. J. Sci. Ind. Res. 2013, 72, 669–672. [Google Scholar]
- Cai, W.; Huang, R. Slow gelation of titanium(IV) with partially hydrolyzed polyacrylamide. Crosslinking reaction and gel properties. Polym. J. 2001, 33, 330–335. [Google Scholar]
- Abdullah, A.; Dennis, L.; Hamzah, S.; Mohana, S.; Mudasar, Z.; Abdurrashid, H.; Zulkifli, M.; Rabiu, B.; Baker, N.; Ahmad, H. The effects of nanofluid thermophysical properties on enhanced oil recovery in a heterogenous porous media. Case Stud. Chem. Environ. Eng. 2024, 9, 100556. [Google Scholar]
- Zhou, K.; Wu, D.; An, Z. Experimental Study on Matched Particle Size and Elastic Modulus of Preformed Particle Gel for Oil Reservoirs. Gels 2022, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Sui, X.; Xiao, L.; Guo, Z.; Yao, Y.; Xiao, Y.; Chen, G.; Song, K.; Mack, J. Successful field pilot of in-depth colloidal dispersion gel (CDG) technology in Daqing oil field. SPE Reserv. Eval. Eng. 2006, 9, 664–673. [Google Scholar] [CrossRef]
- Ahsaei, Z.; Parsaei, R.; Kalantariasl, A.; Abolmaali, S.; Tamaddon, A. Enhanced oil recovery through controlled surfactant release from thermosensitive polymer-functionalized mesoporous silica: Interfacial tension, wettability, surfactant adsorption, and emulsification performance. Colloids Surf. A Physicochem. Eng. Asp. 2025, 725, 137550. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, F. Crosslinking mechanism of multivalent metal ions with polyacrylamide. J. China Univ. Pet. (Ed. Nat. Sci.) 1992, 3, 32–39. [Google Scholar]
- Allain, C.; Salome, L. Gelation of semidilute polymer solutions by ion complexation: Critical behavior of the rheological properties versus cross-link concentration. Macromolecules 1990, 23, 981–987. [Google Scholar] [CrossRef]
- Klaveness, T.; Ruoff, P. Kinetics of the Crosslinking of Polyacrylamide with Cr (III). Analysis of Possible Mechanisms. J. Phys. Chem. 1994, 98, 10119–10123. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, F.; Jiao, C.; Wan, Y.; Dai, C. Studies on changes and implacement mode of conventional polymer gels in high temperature and high salinity environment. Oilfield Chem. 2004, 21, 244–247. [Google Scholar]
- Purkaple, J.; Summers, L. Evaluation of Commercial Crosslinked Polyacrylamide Gel Systems for Injection Profile Modification. In Proceedings of the SPE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 4–7 April 1988. SPE-17331-MS. [Google Scholar]
- Meister, J. Bulk Gel Strength Tester. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Phoenix, AZ, USA, 9–11 April 1985. SPE-13567-MS. [Google Scholar]
- Park, P.; Sung, W. Polymer translocation induced by adsorption. J. Chem. Phys. 1998, 108, 3013–3018. [Google Scholar] [CrossRef]
- Duan, H. Study on the Crosslinking Mechanism and Dynamics of Gelation of Partially Hydrolyzed Polyacrylamide/Cr(III) Systems. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2002. [Google Scholar]
- Sydansk, R. A new conformance improvement treatment chromium(III) gel technology. In Proceedings of the SPE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 4–7 April 1988. SPE-17329-MS. [Google Scholar]
- Mehdi, M.; Mehmet, E. Laboratory investigation on gelation behavior of Xanthan crosslinked with Borate intended to combat lost circulation. In Proceedings of the SPE Production and Operations Conference and Exhibition, Florence, Italy, 20–22 September 2010. SPE-136094-MS. [Google Scholar]
- Liu, J.; Seright, R. Rheology of gels used for conformance control in fractures. SPE J. 2001, 6, 120–125. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Ge, J.; Jiang, P.; Zhu, X. Experimental research of syneresis mechanism of HPAM/Cr3+ gel. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 96–103. [Google Scholar] [CrossRef]




| Gel Morphology | Visual Appearance | G′gel/Pa | Rw/% | ||
|---|---|---|---|---|---|
| Morphology Description | Morphology Code | Appearance Description | Appearance Code | ||
| Stable Strong Gel | SSG | Homogeneous Transparent Viscoelastic Body | 1 | ≥10 | ≤15 |
| Stable Weak Gel | SWG | Homogeneous Transparent Viscoelastic Body | 1 | ≥G′HPAM, <10 | ≤ Rw ≥ 15 |
| Colloidal Dispersion Gel | CDG | Homogeneous Transparent Fluid | 1 | <G′HPAM | |
| Unstable Gel | USG | Turbid Viscoelastic Body | 1–2 | ≥G′HPAM | ≥15 |
| Over-crosslinked Gel | OCG | Heterogeneous Fluid | 3 | <G′HPAM | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Hu, J.; Wang, X.; Zhang, G. Gelation Performance of HPAM-Cr3+ Gels for Reservoir Profile Control: The Impact of Propagation Distance and Optimization Design. Gels 2025, 11, 872. https://doi.org/10.3390/gels11110872
Li M, Hu J, Wang X, Zhang G. Gelation Performance of HPAM-Cr3+ Gels for Reservoir Profile Control: The Impact of Propagation Distance and Optimization Design. Gels. 2025; 11(11):872. https://doi.org/10.3390/gels11110872
Chicago/Turabian StyleLi, Mengyun, Junjie Hu, Xiang Wang, and Guicai Zhang. 2025. "Gelation Performance of HPAM-Cr3+ Gels for Reservoir Profile Control: The Impact of Propagation Distance and Optimization Design" Gels 11, no. 11: 872. https://doi.org/10.3390/gels11110872
APA StyleLi, M., Hu, J., Wang, X., & Zhang, G. (2025). Gelation Performance of HPAM-Cr3+ Gels for Reservoir Profile Control: The Impact of Propagation Distance and Optimization Design. Gels, 11(11), 872. https://doi.org/10.3390/gels11110872






