Bilayer Biomimetic Scaffolds Loaded with Mesenchymal Stem Cell Secretomes Promote Diabetic Wound Healing
Abstract
1. Introduction
2. Results and Discussion
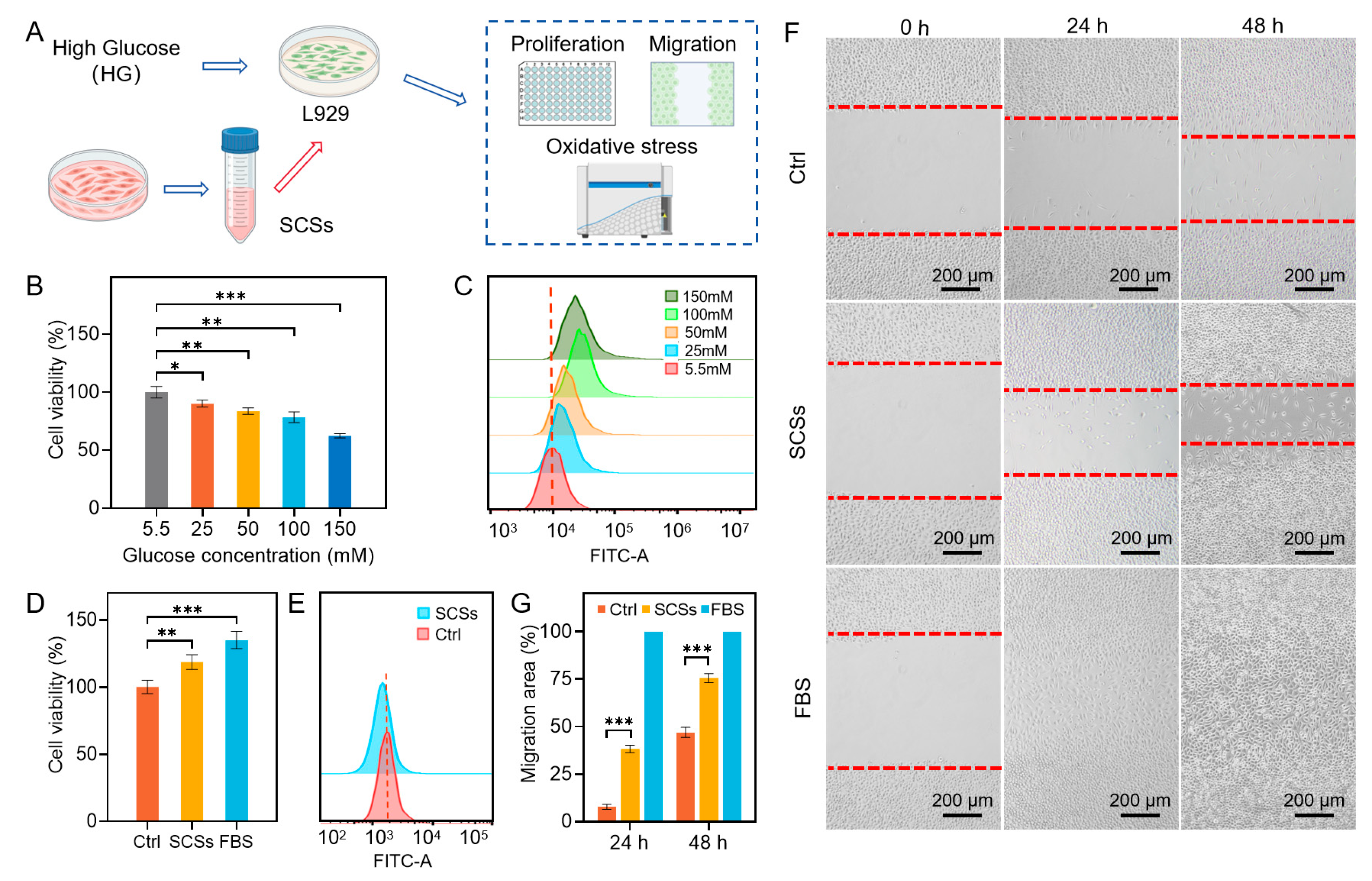
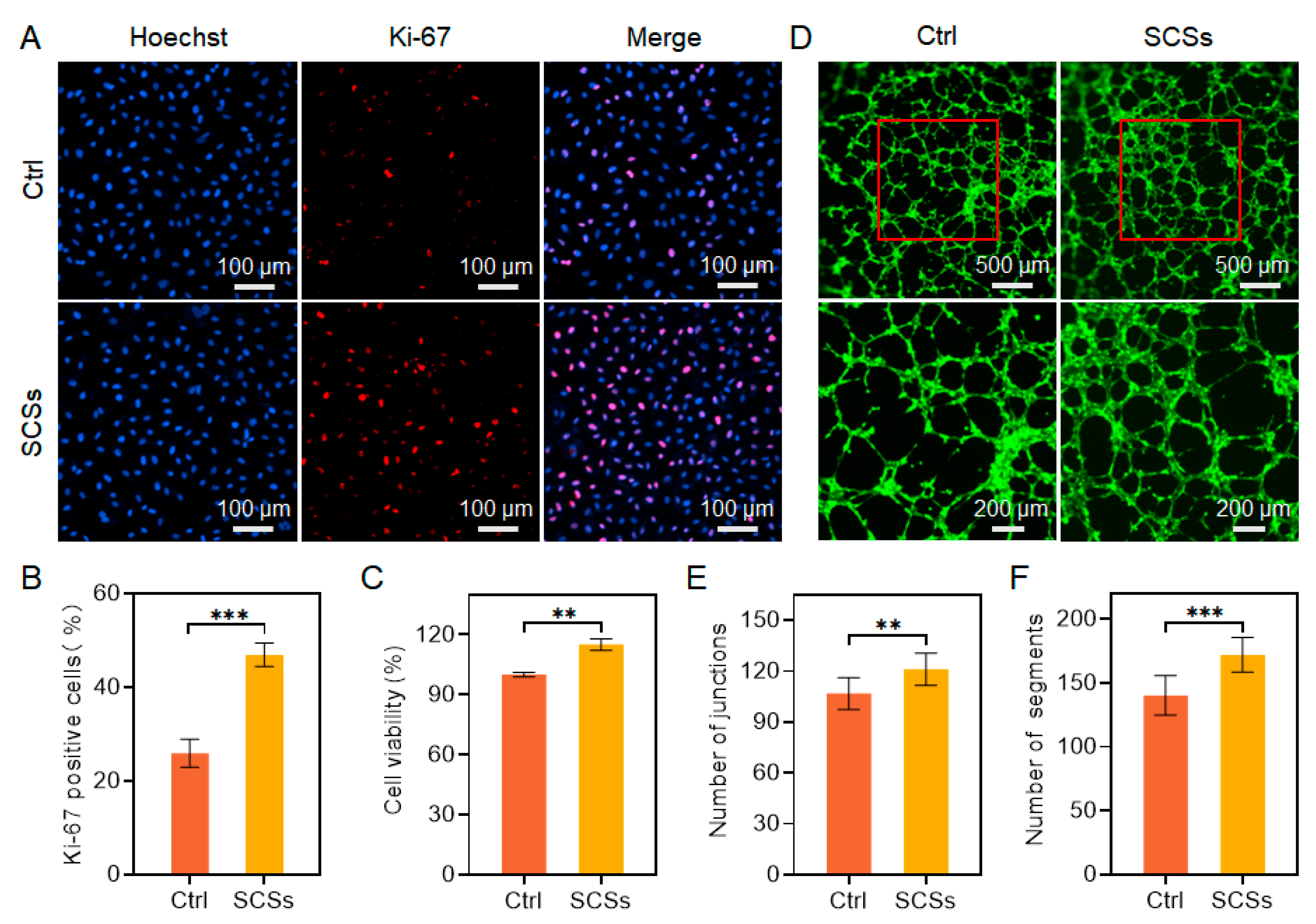
2.1. SCSs Promote Skin Cell Proliferation and Migration and Attenuate Oxidative Stress
2.2. SCSs Stimulate HUVEC Proliferation and Angiogenesis
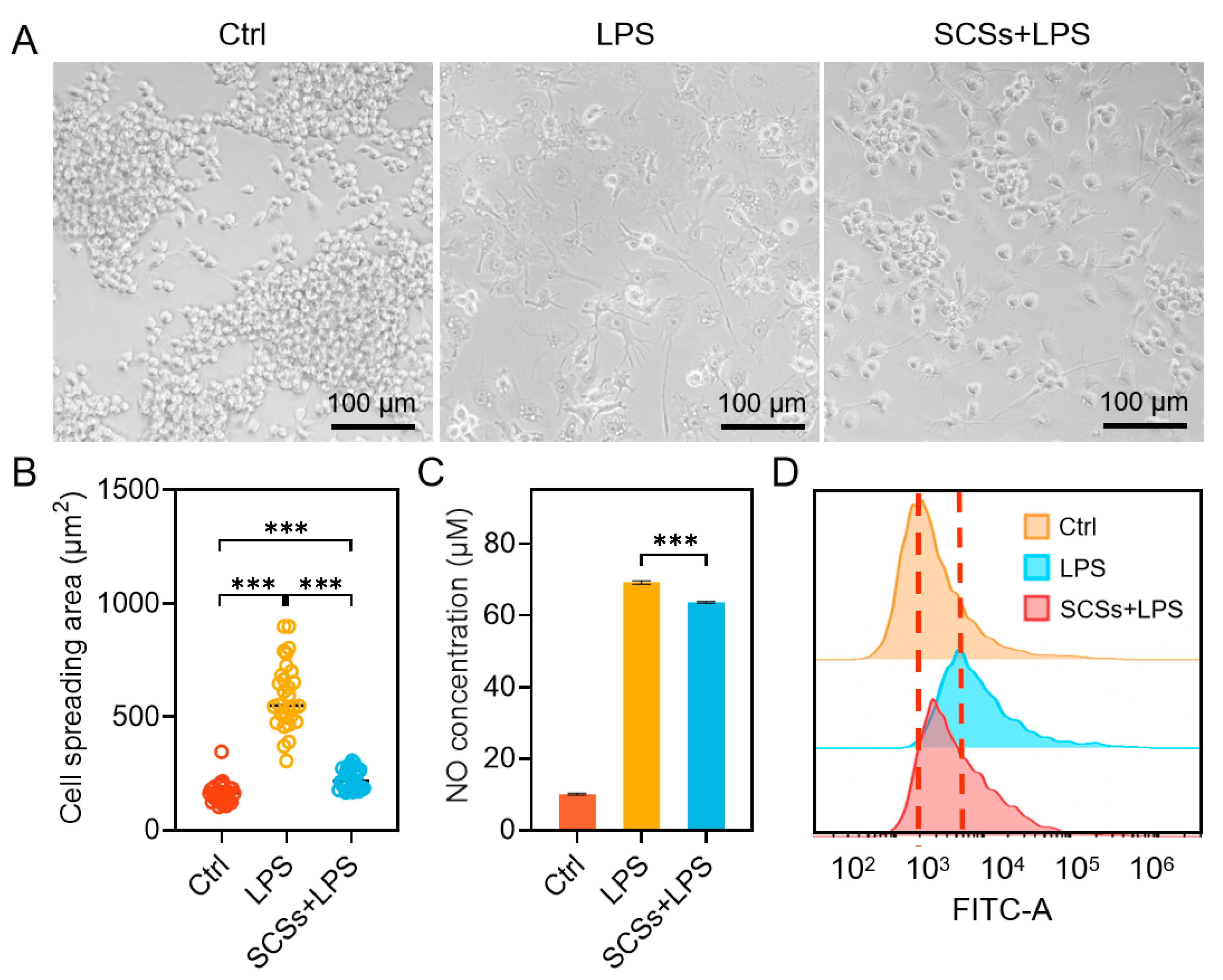
2.3. SCSs Inhibit RAW264.7 Inflammation and Oxidative Stress
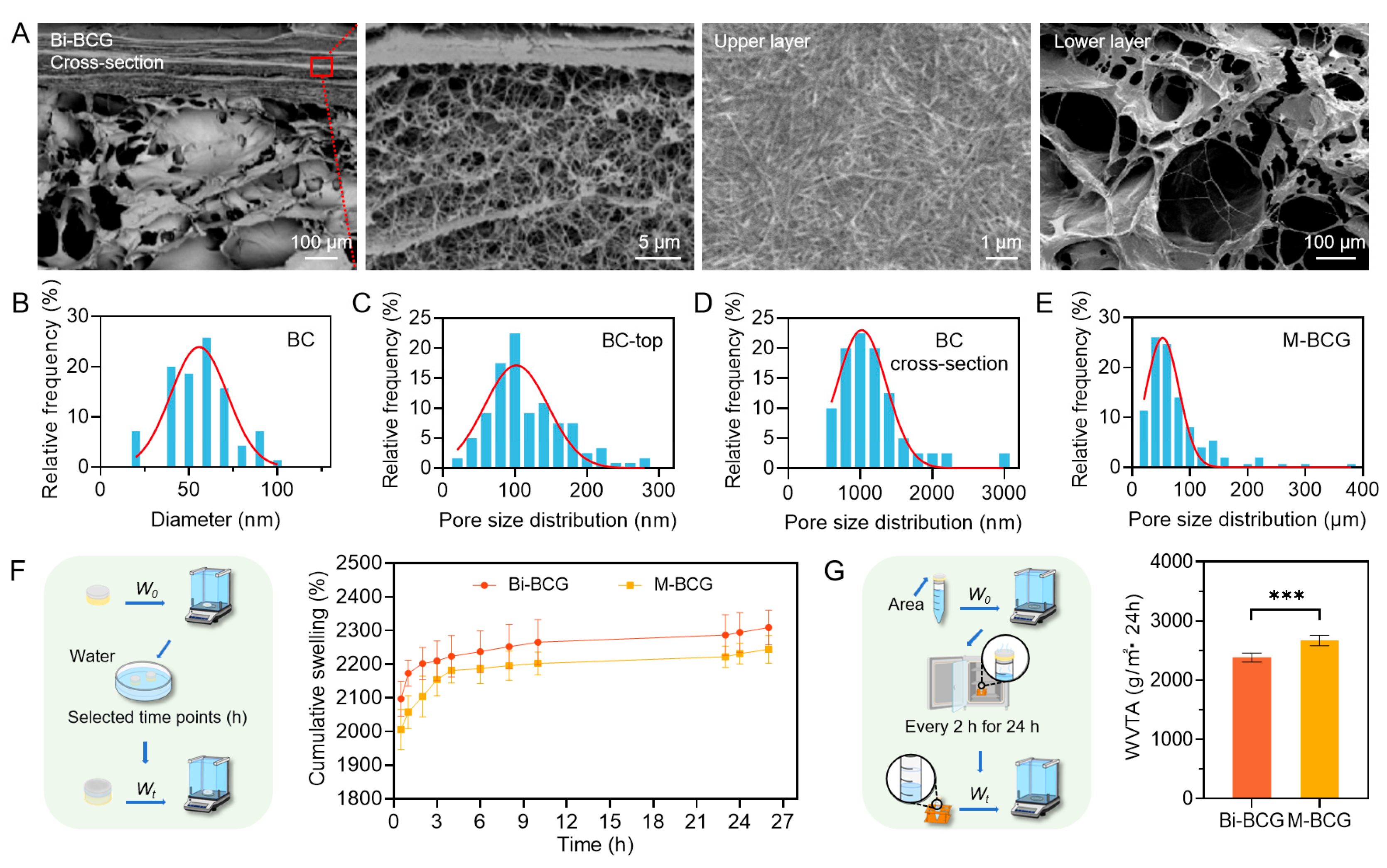
2.4. Fabrication and Characterization of Bi-BCG Scaffolds
2.5. Biocompatibility of Bi-BCG Scaffold
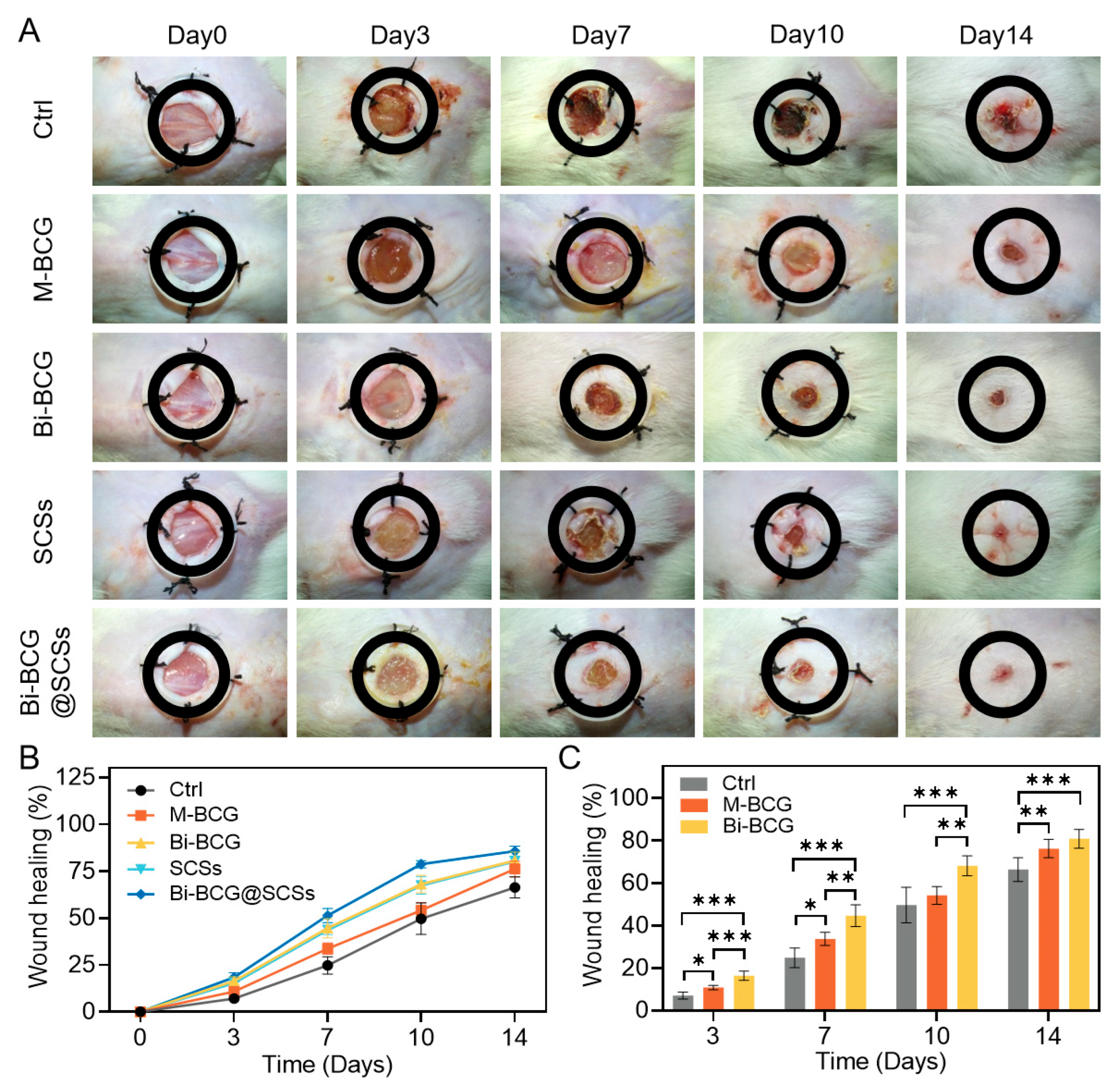
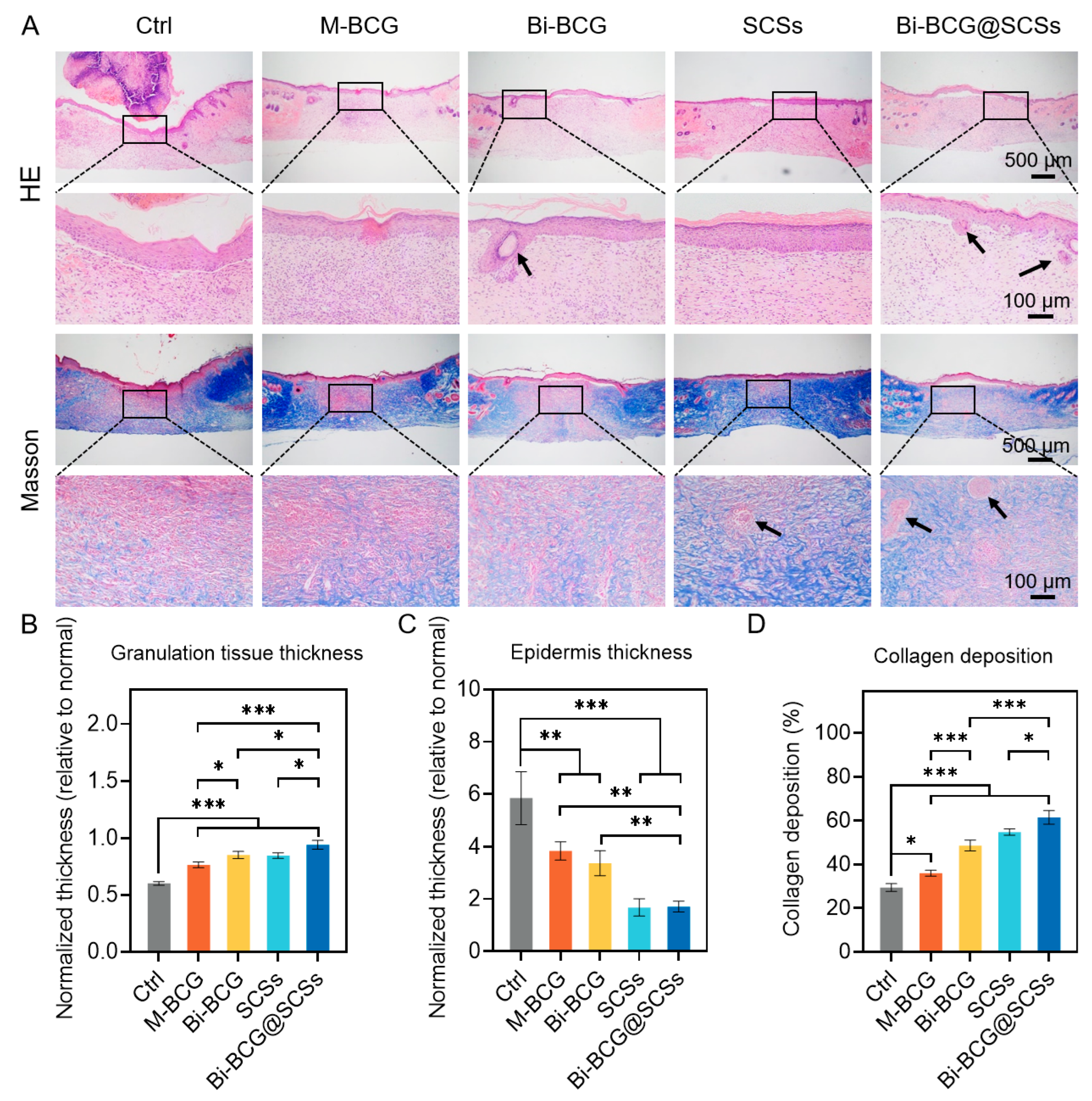
2.6. Accelerating Diabetic Wounds Healing with Bi-BCG Containing SCSs
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Collection of hUCMSC-Conditioned Medium (CM)
4.3. Cell Viability Assay
4.4. Reactive Oxygen Species (ROS) Detection
4.5. Scratch Assay
4.6. Immunofluorescence Staining of Ki-67
4.7. Endothelial Tube Formation Assay
4.8. Nitric Oxide (NO) Production Assay
4.9. Preparation of Bilayer Scaffolds
4.10. SEM Characterization of Hydrogel Structure
4.11. Swelling Behavior
4.12. Water Vapor Transmission Rate (WVTR) Determination
4.13. Cytocompatibility of Bi-BCG Scaffolds
4.14. Diabetic Wound Model
4.15. In Vivo Wound Healing Evaluation
4.16. Histological Analysis
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, F.; Lu, X.; Yang, Y.; Yang, Y.; Li, Y.; Kuai, L.; Li, B.; Dong, H.; Shi, J. Microenvironment-Based Diabetic Foot Ulcer Nanomedicine. Adv. Sci. 2023, 10, e2203308. [Google Scholar] [CrossRef]
- Mohsin, F.; Javaid, S.; Tariq, M.; Mustafa, M. Molecular Immunological Mechanisms of Impaired Wound Healing in Diabetic Foot Ulcers (DFU), Current Therapeutic Strategies and Future Directions. Int. Immunopharmacol. 2024, 139, 112713. [Google Scholar] [CrossRef]
- Sun, Z.; Xiong, H.; Lou, T.; Liu, W.; Xu, Y.; Yu, S.; Wang, H.; Liu, W.; Yang, L.; Zhou, C.; et al. Multifunctional Extracellular Matrix Hydrogel with Self-Healing Properties and Promoting Angiogenesis as an Immunoregulation Platform for Diabetic Wound Healing. Gels 2023, 9, 381. [Google Scholar] [CrossRef]
- Farooq, T.; Rehman, K.; Hameed, A.; Akash, M.S.H. Stem Cell Therapy and Type 1 Diabetes Mellitus: Treatment Strategies and Future Perspectives. Adv. Exp. Med. Biol. 2019, 1084, 95–107. [Google Scholar] [CrossRef]
- Rhew, S.-Y.; Park, S.-M.; Li, Q.; An, J.-H.; Chae, H.-K.; Lee, J.-H.; Ahn, J.-O.; Song, W.-J.; Youn, H.-Y. Efficacy and Safety of Allogenic Canine Adipose Tissue-Derived Mesenchymal Stem Cell Therapy for Insulin-Dependent Diabetes Mellitus in Four Dogs: A Pilot Study. J. Vet. Med. Sci. 2021, 83, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Wein, S.; Jung, S.A.; Al Enezy-Ulbrich, M.A.; Reicher, L.; Rütten, S.; Kühnel, M.; Jonigk, D.; Jahnen-Dechent, W.; Pich, A.; Neuss, S. Impact of Fibrin Gel Architecture on Hepatocyte Growth Factor Release and Its Role in Modulating Cell Behavior for Tissue Regeneration. Gels 2024, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Ni, X.; Xie, Y.; Chen, L.; Cai, B.; Lin, Q.; Ke, R.; Huang, T.; Shan, X.; Wang, B. Research Progress and Application Prospect of Adipose-Derived Stem Cell Secretome in Diabetes Foot Ulcers Healing. Stem Cell Res. Ther. 2024, 15, 279. [Google Scholar] [CrossRef]
- Bormann, D.; Gugerell, A.; Ankersmit, H.J.; Mildner, M. Therapeutic Application of Cell Secretomes in Cutaneous Wound Healing. J. Investig. Dermatol. 2023, 143, 893–912. [Google Scholar] [CrossRef]
- Li, G.; Ni, R.; Shi, Z.; Mao, H.; Luo, Y.; Cao, X.; Zhang, C.; Liu, Y.; Jin, Z.; Chen, J.; et al. Enhancing BMSC Chondrogenesis with a Dynamic Viscoelastic Hyaluronan Hydrogel Loaded with Kartogenin for Cartilage Repair. Int. J. Biol. Macromol. 2025, 312, 144042. [Google Scholar] [CrossRef]
- Hermann, M.; Peddi, A.; Gerhards, A.; Schmid, R.; Schmitz, D.; Arkudas, A.; Weisbach, V.; Horch, R.E.; Kengelbach-Weigand, A. Secretome of Adipose-Derived Stem Cells Cultured in Platelet Lysate Improves Migration and Viability of Keratinocytes. Int. J. Mol. Sci. 2023, 24, 3522. [Google Scholar] [CrossRef]
- Ahangar, P.; Mills, S.J.; Smith, L.E.; Strudwick, X.L.; Ting, A.E.; Vaes, B.; Cowin, A.J. Human Multipotent Adult Progenitor Cell-Conditioned Medium Improves Wound Healing through Modulating Inflammation and Angiogenesis in Mice. Stem Cell Res. Ther. 2020, 11, 299. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, K.; Wang, M.; He, Z.; Yu, B.; Wang, X.; Pan, X.; Luo, Y.; Xu, S.; Lau, J.T.Y.; et al. VEGF-FGF Signaling Activates Quiescent CD63+ Liver Stem Cells to Proliferate and Differentiate. Adv. Sci. 2024, 11, e2308711. [Google Scholar] [CrossRef]
- Smolinská, V.; Boháč, M.; Danišovič, Ľ. Current Status of the Applications of Conditioned Media Derived from Mesenchymal Stem Cells for Regenerative Medicine. Physiol. Res. 2023, 72, S233–S245. [Google Scholar] [CrossRef]
- Wang, B.; Pang, M.; Song, Y.; Wang, H.; Qi, P.; Bai, S.; Lei, X.; Wei, S.; Zong, Z.; Lin, S.; et al. Human Fetal Mesenchymal Stem Cells Secretome Promotes Scarless Diabetic Wound Healing through Heat-Shock Protein Family. Bioeng. Transl. Med. 2023, 8, e10354. [Google Scholar] [CrossRef]
- Shoma Suresh, K.; Bhat, S.; Guru, B.R.; Muttigi, M.S.; Seetharam, R.N. A Nanocomposite Hydrogel Delivery System for Mesenchymal Stromal Cell Secretome. Stem Cell Res. Ther. 2020, 11, 205. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Fiorica, C.; Carreca, A.P.; Iannolo, G.; Pitarresi, G.; Amico, G.; Giammona, G.; Conaldi, P.G.; Chinnici, C.M. Modulating the Release of Bioactive Molecules of Human Mesenchymal Stromal Cell Secretome: Heparinization of Hyaluronic Acid-Based Hydrogels. Int. J. Pharm. 2024, 653, 123904. [Google Scholar] [CrossRef]
- Guo, K.; Li, G.; Yu, Q.; Yang, Y.; Liu, H.; Zhao, Y.; Huang, Y.; Zhang, H.; Li, W. Injectable Hyaluronate-Based Hydrogel with a Dynamic/Covalent Dual-Crosslinked Architecture for Bone Tissue Engineering: Enhancing Osteogenesis and Immune Regulation. Int. J. Biol. Macromol. 2024, 282, 137249. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhou, J.; He, Z.; Zhang, L.; Du, F.; Nie, M.; Zhou, Y.; Hao, H.; Zhang, L.; Yu, S.; et al. In Situ-Formed Fibrin Hydrogel Scaffold Loaded With Human Umbilical Cord Mesenchymal Stem Cells Promotes Skin Wound Healing. Cell Transpl. 2023, 32, 9636897231156215. [Google Scholar] [CrossRef]
- Jiang, M.; Jiang, X.; Li, H.; Zhang, C.; Zhang, Z.; Wu, C.; Zhang, J.; Hu, J.; Zhang, J. The Role of Mesenchymal Stem Cell-Derived EVs in Diabetic Wound Healing. Front. Immunol. 2023, 14, 1136098. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, A.G.; Karakeçili, A.; Topuz, B.; Batur, B.; Onar, O.; Elçiğir, M.E.; Oto, Ç.; Hanifehnezhad, A.; Orhan, K. Tri-Layered Bioactive Cutaneous Scaffold with Integrated Bioactive Metal Organic Frameworks to Promote Full-Thickness Infected Diabetic Wound Healing: Multifaceted Therapeutic Strategies. Adv. Healthc. Mater. 2025, 14, e2500356. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, J.; Chen, H.; Zou, Y.; Wang, Y.; Zhou, C.; Tong, L.; Wang, P.; Liu, T.; Liang, J.; et al. Porous Gradient Hydrogel Promotes Skin Regeneration by Angiogenesis. J. Colloid Interface Sci. 2024, 671, 312–324. [Google Scholar] [CrossRef]
- Xia, Y.; Yan, S.; Wei, H.; Zhang, H.; Hou, K.; Chen, G.; Cao, R.; Zhu, M. Multifunctional Porous Bilayer Artificial Skin for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 34578–34590. [Google Scholar] [CrossRef] [PubMed]
- Ijaola, A.O.; Akamo, D.O.; Damiri, F.; Akisin, C.J.; Bamidele, E.A.; Ajiboye, E.G.; Berrada, M.; Onyenokwe, V.O.; Yang, S.-Y.; Asmatulu, E. Polymeric Biomaterials for Wound Healing Applications: A Comprehensive Review. J. Biomater. Sci. Polym. Ed. 2022, 33, 1998–2050. [Google Scholar] [CrossRef]
- Asadi, N.; Mehdipour, A.; Ghorbani, M.; Mesgari-Abbasi, M.; Akbarzadeh, A.; Davaran, S. A Novel Multifunctional Bilayer Scaffold Based on Chitosan Nanofiber/Alginate-Gelatin Methacrylate Hydrogel for Full-Thickness Wound Healing. Int. J. Biol. Macromol. 2021, 193, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Suo, D.; Xu, T.; Zhao, S.; Xu, X.; Bei, H.-P.; Wong, K.K.-Y.; Li, Q.; Zheng, Z.; Li, B.; et al. Sprayable Biomimetic Double Mask with Rapid Autophasing and Hierarchical Programming for Scarless Wound Healing. Sci. Adv. 2024, 10, eado9479. [Google Scholar] [CrossRef]
- Feng, L.; Shi, W.; Chen, Q.; Cheng, H.; Bao, J.; Jiang, C.; Zhao, W.; Zhao, C. Smart Asymmetric Hydrogel with Integrated Multi-Functions of NIR-Triggered Tunable Adhesion, Self-Deformation, and Bacterial Eradication. Adv. Healthc. Mater. 2021, 10, e2100784. [Google Scholar] [CrossRef]
- Górska, A.; Dorożyński, P.; Węglarz, W.P.; Jasiński, K.; Kurek, M.; Jachowicz, R.; Klaja, J.; Kulinowski, P. Spatiotemporal Characterization of Hydration Process of Asymmetric Polymeric Wound Dressings for Decubitus Ulcers. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 843–853. [Google Scholar] [CrossRef]
- Gea, S.; Putra, I.B.; Lindarto, D.; Pasaribu, K.M.; Saraswati, Y.; Karina, M.; Goei, R.; Tok, A.I.Y. Bacterial Cellulose Impregnated with Andaliman (Zanthoxylum acanthopodium) Microencapsulation as Diabetic Wound Dressing. Int. J. Biol. Macromol. 2023, 253, 126572. [Google Scholar] [CrossRef]
- He, W.; Wu, J.; Xu, J.; Mosselhy, D.A.; Zheng, Y.; Yang, S. Bacterial Cellulose: Functional Modification and Wound Healing Applications. Adv. Wound Care 2021, 10, 623–640. [Google Scholar] [CrossRef]
- Raut, M.P.; Asare, E.; Syed Mohamed, S.M.D.; Amadi, E.N.; Roy, I. Bacterial Cellulose-Based Blends and Composites: Versatile Biomaterials for Tissue Engineering Applications. Int. J. Mol. Sci. 2023, 24, 986. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Morowvat, M.H.; Niknezhad, S.V.; Baghbadorani, M.A.; Michálek, M.; Chen, S.; Nemati, M.M.; Negahdaripour, M.; Heidari, R.; Azadi, A.; et al. A Photocrosslinkable and Hemostatic Bilayer Wound Dressing Based on Gelatin Methacrylate Hydrogel and Polyvinyl Alcohol Foam for Skin Regeneration. Int. J. Biol. Macromol. 2024, 266, 131231. [Google Scholar] [CrossRef]
- Somasekharan, L.T.; Raju, R.; Kumar, S.; Geevarghese, R.; Nair, R.P.; Kasoju, N.; Bhatt, A. Biofabrication of Skin Tissue Constructs Using Alginate, Gelatin and Diethylaminoethyl Cellulose Bioink. Int. J. Biol. Macromol. 2021, 189, 398–409. [Google Scholar] [CrossRef]
- Pasini, C.; Re, F.; Trenta, F.; Russo, D.; Sartore, L. Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration. Gels 2024, 10, 426. [Google Scholar] [CrossRef]
- Shan, Y.; Li, C.; Wu, Y.; Li, Q.; Liao, J. Hybrid Cellulose Nanocrystal/Alginate/Gelatin Scaffold with Improved Mechanical Properties and Guided Wound Healing. RSC Adv. 2019, 9, 22966–22979. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Song, Q.; Jiang, Z.; Li, N.; Liu, P.; Liu, L.; Tang, M.; Cheng, G. Anti-Inflammatory Effects of Three-Dimensional Graphene Foams Cultured with Microglial Cells. Biomaterials 2014, 35, 6930–6940. [Google Scholar] [CrossRef]
- Vizely, K.; Wagner, K.T.; Mandla, S.; Gustafson, D.; Fish, J.E.; Radisic, M. Angiopoietin-1 Derived Peptide Hydrogel Promotes Molecular Hallmarks of Regeneration and Wound Healing in Dermal Fibroblasts. iScience 2023, 26, 105984. [Google Scholar] [CrossRef]
- Ren, H.; Su, P.; Zhao, F.; Zhang, Q.; Huang, X.; He, C.; Wu, Q.; Wang, Z.; Ma, J.; Wang, Z. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote Skin Wound Healing in Diabetic Mice by Regulating Epidermal Autophagy. Burn. Trauma 2024, 12, tkae001. [Google Scholar] [CrossRef]
- Feng, Z.-H.; Chen, J.; Yuan, P.-T.; Ji, Z.-Y.; Tao, S.-Y.; Zheng, L.; Wei, X.-A.; Zheng, Z.-Y.; Zheng, B.-J.; Chen, B.; et al. Urolithin A Promotes Angiogenesis and Tissue Regeneration in a Full-Thickness Cutaneous Wound Model. Front. Pharmacol. 2022, 13, 806284. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Tan, T.-W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Izadi, R.; Hejazi, S.H.; Bahramikia, S. Alternative Viewpoint against Diabetic Wound Based on Stem Cell Secretome That Can Mediated Angiogenesis and Reduce Inflammation. Arch. Dermatol. Res. 2023, 316, 28. [Google Scholar] [CrossRef]
- Li, S.; Ding, X.; Yan, X.; Qian, J.; Tan, Q. ceAF Ameliorates Diabetic Wound Healing by Alleviating Inflammation and Oxidative Stress via TLR4/NF-κB and Nrf2 Pathways. J. Diabetes Res. 2023, 2023, 2422303. [Google Scholar] [CrossRef]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage Polarization and Diabetic Wound Healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Vishwakarma, N.K.; Kavimandan, G.; Prakash, A.; Mahto, S.K. Freeze-Thaw-Induced Physically Cross-Linked Superabsorbent Polyvinyl Alcohol/Soy Protein Isolate Hydrogels for Skin Wound Dressing: In Vitro and In Vivo Characterization. ACS Appl. Mater. Interfaces 2022, 14, 14033–14048. [Google Scholar] [CrossRef]
- Shafique, M.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Mahmood, A.; Abbasi, M.; Aziz, H.C.; et al. Bio-Functional Hydrogel Membranes Loaded with Chitosan Nanoparticles for Accelerated Wound Healing. Int. J. Biol. Macromol. 2021, 170, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef] [PubMed]
- Prado-Yupanqui, J.W.; Ramírez-Orrego, L.; Cortez, D.; Vera-Ponce, V.J.; Chenet, S.M.; Tejedo, J.R.; Tapia-Limonchi, R. The Hidden Power of the Secretome: Therapeutic Potential on Wound Healing and Cell-Free Regenerative Medicine-A Systematic Review. Int. J. Mol. Sci. 2025, 26, 1926. [Google Scholar] [CrossRef]
- González-González, A.; García-Sánchez, D.; Dotta, M.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Mesenchymal Stem Cells Secretome: The Cornerstone of Cell-Free Regenerative Medicine. World J. Stem Cells 2020, 12, 1529–1552. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Sierra-Sánchez, Á.; Sanchez-Diaz, M.; Quiñones-Vico, M.I.; Sanabria-de-la-Torre, R.; Martinez-Lopez, A.; Arias-Santiago, S. Mesenchymal Stromal Cell-Conditioned Medium for Skin Diseases: A Systematic Review. Front. Cell Dev. Biol. 2021, 9, 654210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Zhang, Y.; Zhao, Y.; Shen, J.; Du, F.; Chen, Y.; Li, M.; Wu, X.; Chen, M.; et al. Bilayer Hydrogel with a Protective Film and a Regenerative Hydrogel for Effective Diabetic Wound Treatment. Biomater. Sci. 2024, 12, 5036–5051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nie, J.; Xu, H.; Qiu, Y.; Li, X.; Gu, W.; Tang, G.; Luo, J. Fluorescent Microspheres for One-Photon and Two-Photon Imaging of Mesenchymal Stem Cells. J. Mater. Chem. B 2017, 5, 7809–7818. [Google Scholar] [CrossRef]
- Liu, P.; Cao, B.; Zhou, Y.; Zhang, H.; Wang, C. Human Umbilical Cord-Derived Mesenchymal Stem Cells Alleviate Oxidative Stress-Induced Islet Impairment via the Nrf2/HO-1 Axis. J. Mol. Cell Biol. 2023, 15, mjad035. [Google Scholar] [CrossRef]
- Song, Q.; Pifferi, S.; Shi, L.; Chen, C.; Proietti Zaccaria, R.; Menini, A.; Cao, J.; Zhang, Q.; Torre, V. Textured Nanofibrils Drive Microglial Phenotype. Biomaterials 2020, 257, 120177. [Google Scholar] [CrossRef]
- Shi, B.; Zhu, T.; Luo, Y.; Zhang, X.; Yao, J.; Cao, X.; Zhu, Y.; Miao, H.; Li, L.; Song, Q.; et al. Three-Dimensional Bioprinted Cell-Adaptive Hydrogel with Anisotropic Micropores for Enhancing Skin Wound Healing. Int. J. Biol. Macromol. 2024, 280, 136106. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, F.; Chen, Y.; Li, H.; Zhang, Q.; Ji, Q.; Zou, L.; Wang, Z.; Wu, Z.; Yu, S.; Zhang, H.; et al. Bilayer Biomimetic Scaffolds Loaded with Mesenchymal Stem Cell Secretomes Promote Diabetic Wound Healing. Gels 2025, 11, 845. https://doi.org/10.3390/gels11110845
Shen F, Chen Y, Li H, Zhang Q, Ji Q, Zou L, Wang Z, Wu Z, Yu S, Zhang H, et al. Bilayer Biomimetic Scaffolds Loaded with Mesenchymal Stem Cell Secretomes Promote Diabetic Wound Healing. Gels. 2025; 11(11):845. https://doi.org/10.3390/gels11110845
Chicago/Turabian StyleShen, Fangling, Yiting Chen, Hongwen Li, Qi Zhang, Qixiong Ji, Linyuan Zou, Zhe Wang, Zhengyao Wu, Shengkai Yu, Hua Zhang, and et al. 2025. "Bilayer Biomimetic Scaffolds Loaded with Mesenchymal Stem Cell Secretomes Promote Diabetic Wound Healing" Gels 11, no. 11: 845. https://doi.org/10.3390/gels11110845
APA StyleShen, F., Chen, Y., Li, H., Zhang, Q., Ji, Q., Zou, L., Wang, Z., Wu, Z., Yu, S., Zhang, H., & Song, Q. (2025). Bilayer Biomimetic Scaffolds Loaded with Mesenchymal Stem Cell Secretomes Promote Diabetic Wound Healing. Gels, 11(11), 845. https://doi.org/10.3390/gels11110845






