Cell Membrane- and Vesicle-Based Bionic Nanodrugs: Applications in Central Nervous System Diseases and Exploration of Nasal–Cerebral Delivery
Abstract
1. Introduction
2. Cell Membrane-Based Bionic Nanodrugs
2.1. Types of Cell Membranes
2.1.1. Red Blood Cell Membranes
2.1.2. Platelet Membranes
2.1.3. Macrophage Membranes
2.1.4. Neutrophil Membranes
2.1.5. Stem Cell Membranes
2.2. Preparation of Cell Membrane-Modified Nanocarriers
2.2.1. Cell Membrane Extraction and Purification
2.2.2. Membrane–Nanocarrier Fusion
3. Bionic Nanomedicines Based on Cell Membrane-Derived Vesicles
3.1. Extracellular Vesicles (EVs)
3.1.1. Red Blood Cell-Derived Extracellular Vesicles
3.1.2. Platelet-Derived Extracellular Vesicles
3.1.3. Macrophage-Derived Extracellular Vesicles
3.1.4. Neutrophil-Derived Extracellular Vesicles
3.1.5. Stem Cell-Derived Extracellular Vesicles (SC-EVs)
3.1.6. Biogenesis of Extracellular Vesicles
3.1.7. Preparation and Separation Methods for Extracellular Vesicles
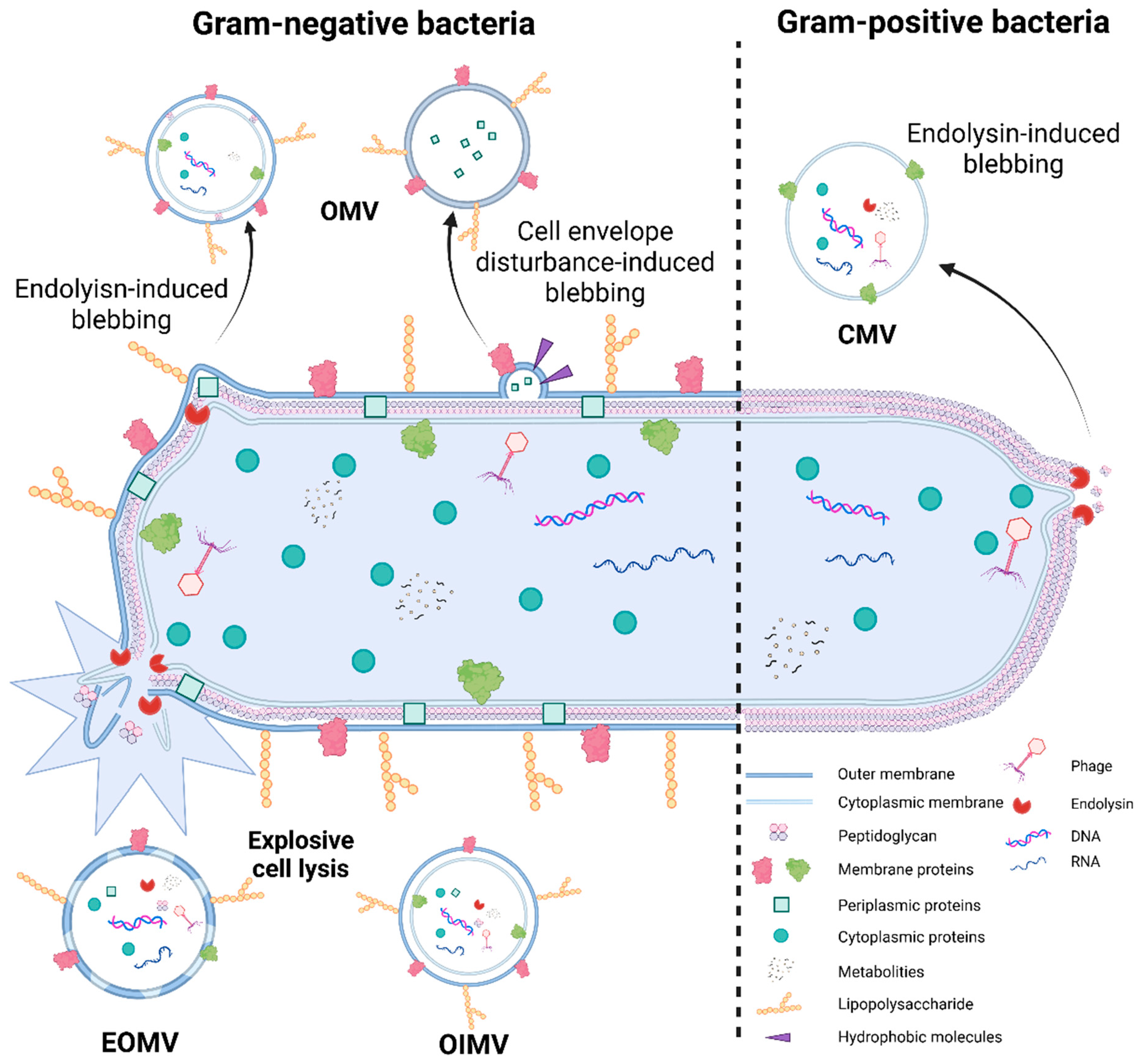
3.2. Bacterial Extracellular Vesicles
3.2.1. Origin of Bacterial Extracellular Vesicles
3.2.2. Mechanisms of Bacterial Extracellular Vesicle Formation
3.2.3. Preparation, Advantages, and Disadvantages of Bacterial Extracellular Vesicles (BEVs)
4. Applications of Bionic Nanodrugs Based on Cell Membranes and Vesicles in CNS Diseases
4.1. Glioblastoma Multiforme
4.2. Ischemic Stroke
4.3. Alzheimer’s Disease
4.4. Parkinson’s Disease
5. Nasal–Cerebral Delivery Routes for Biomimetic Nanodrugs Modified with Cell Membranes and Vesicles
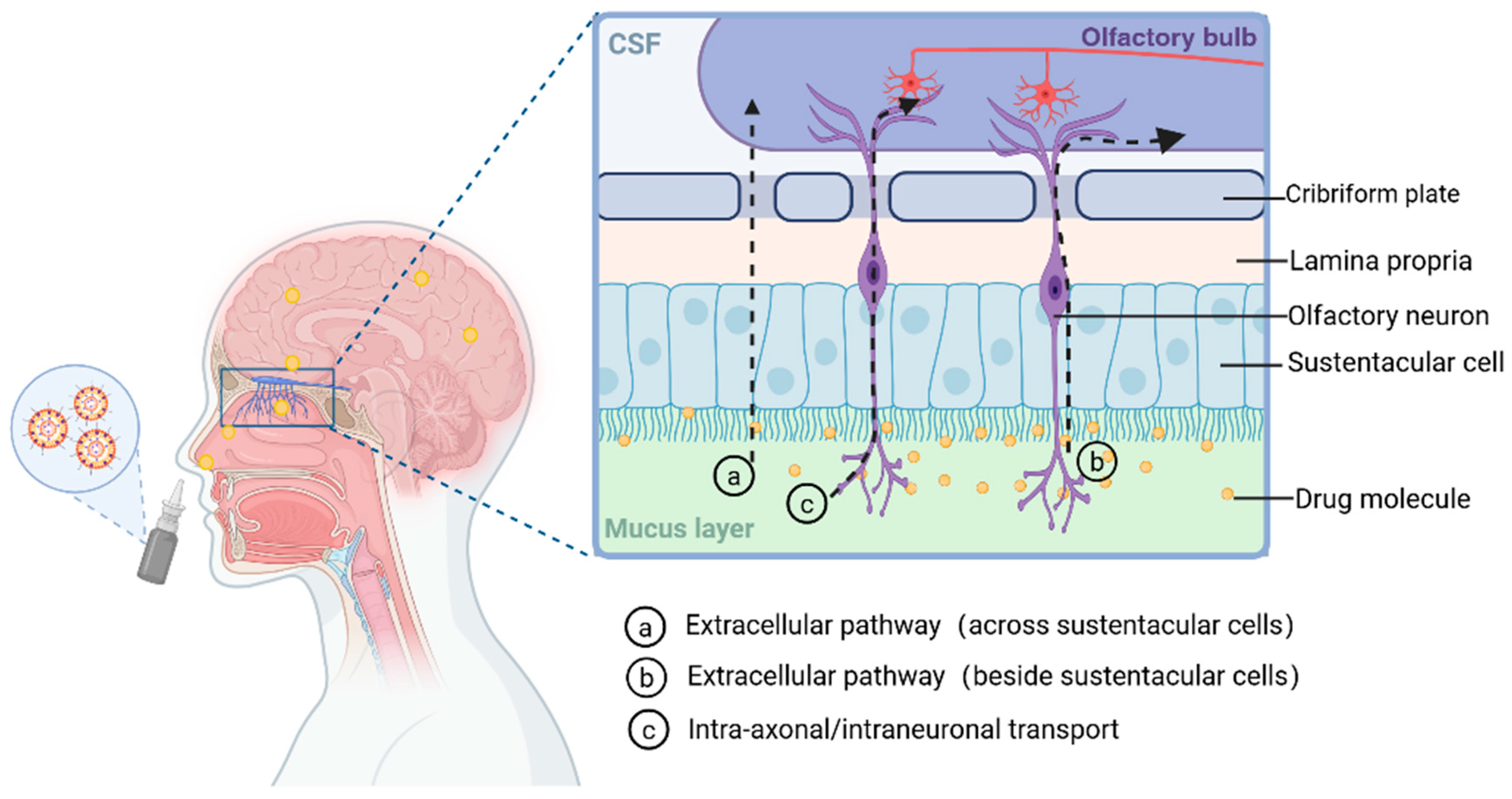
5.1. Physiological Structure of the Nasal Cavity
5.2. Nasal–Cerebral Delivery Routes
5.2.1. Direct Pathway
5.2.2. Indirect Pathways
6. Application of Cell Membrane- and Vesicle-Based Bionic Nanodrugs in the Nasoencephalic Pathway
6.1. Application of Cell Membranes and Membrane Vesicles in Stroke Therapy
6.2. Recent Advances in Engineered Cell Membranes and Exosomes for AD Therapy
6.3. Potential of Exosomes in Nasoencephalic Delivery for PD
6.4. Application of Bionic Cell Membranes in GBM
6.5. Bacterial Exosomes: Emerging Vectors for Naso-Cerebral Delivery
7. Advantages and Challenges of Bionic Drug Delivery Systems Based on Cell Membrane and Vesicle in Nasoencephalic Delivery
7.1. Core Advantages in Nasoencephalic Delivery
7.2. Challenges in Nasal–Cerebral Delivery
8. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, A.Y.L.; Kao, H.K.; Liu, Y.Y.; Loh, C.Y.Y. Engineered extracellular vesicles derived from pluripotent stem cells: A cell-free approach to regenerative medicine. Burn. Trauma 2025, 13, tkaf013. [Google Scholar] [CrossRef]
- GBD 2021 Nervous System Disorders Collaborators. Correction: Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, e9. [Google Scholar] [CrossRef]
- Li, J.; Wei, Y.; Zhang, C.; Bi, R.; Qiu, Y.; Li, Y.; Hu, B. Cell-Membrane-Coated Nanoparticles for Targeted Drug Delivery to the Brain for the Treatment of Neurological Diseases. Pharmaceutics 2023, 15, 621. [Google Scholar] [CrossRef] [PubMed]
- Pandya, J.D.; Musyaju, S.; Modi, H.R.; Okada-Rising, S.L.; Bailey, Z.S.; Scultetus, A.H.; Shear, D.A. Intranasal delivery of mitochondria targeted neuroprotective compounds for traumatic brain injury: Screening based on pharmacological and physiological properties. J. Transl. Med. 2024, 22, 167. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, M.; Ahmed, M.; Osaid, Z.; Hamoudi, R.; Harati, R. Insights into Exosome Transport through the Blood–Brain Barrier and the Potential Therapeutical Applications in Brain Diseases. Pharmaceuticals 2023, 16, 571. [Google Scholar] [CrossRef]
- Choi, H.K.; Chen, M.; Goldston, L.L.; Lee, K.B. Extracellular vesicles as nanotheranostic platforms for targeted neurological disorder interventions. Nano Converg. 2024, 11, 19. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Yi, L.X.; Tan, E.K.; Zhou, Z.D. Passive immunotherapy for Alzheimer’s disease: Challenges & future directions. J. Transl. Med. 2024, 22, 430. [Google Scholar] [CrossRef]
- Liang, Y.; Iqbal, Z.; Lu, J.; Wang, J.; Zhang, H.; Chen, X.; Duan, L.; Xia, J. Cell-derived nanovesicle-mediated drug delivery to the brain: Principles and strategies for vesicle engineering. Mol. Ther. 2023, 31, 1207–1224. [Google Scholar] [CrossRef]
- Cunha, S.; Almeida, H.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Intranasal lipid nanoparticles for the treatment of neurodegenerative diseases. Curr. Pharm. Des. 2017, 23, 6553–6562. [Google Scholar] [CrossRef]
- Cunha, S.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Lipid Nanoparticles for Nasal/Intranasal Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 257–282. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Shen, J.; Duan, X.; Xie, T.; Zhang, X.; Cai, Y.; Pan, J.; Zhang, X.; Sun, X. Advances in locally administered nucleic acid therapeutics. Bioact. Mater. 2025, 49, 218–254. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.U.; Shehzad, A.; Ahmed, M.B.; Lee, Y.S. Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules 2020, 25, 1929. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Q.; Shi, Y.; Cui, M.; Jing, F. Research progress of novel anti-tumor drug formulations. Front. Oncol. 2024, 14, 1507958. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.D.; Salah, Y.A.; Ngowi, E.E.; Zhang, Y.X.; Khattak, S.; Khan, N.H.; Wang, Y.; Li, T.; Guo, Z.H.; Wang, Y.M.; et al. Nanotechnology prospects in brain therapeutics concerning gene-targeting and nose-to-brain administration. iScience 2023, 26, 107321. [Google Scholar] [CrossRef]
- Sun, J.; Han, Y.; Dong, J.; Lv, S.; Zhang, R. Melanin/melanin-like nanoparticles: As a naturally active platform for imaging-guided disease therapy. Mater. Today Bio 2023, 23, 100894. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Z.; Sun, R.; Pan, D.; Du, Q.; Zhou, X.; Chen, Y.; Chen, Y.; Peng, J. Comparison of cell delivery and cell membrane camouflaged PLGA nanoparticles in the delivery of shikonin for colorectal cancer treatment. Colloids Surf. B Biointerfaces 2024, 241, 114017. [Google Scholar] [CrossRef]
- Zhong, Z.; Deng, W.; Wu, J.; Shang, H.; Tong, Y.; He, Y.; Huang, Q.; Ba, X.; Chen, Z.; Tang, K. Cell membrane coated nanoparticles as a biomimetic drug delivery platform for enhancing cancer immunotherapy. Nanoscale 2024, 16, 8708–8738. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Tian, X.; Zhang, D.; Cui, H.; Du, W.; Yang, Z.; Li, J.; Li, W.; Xu, J.; et al. Stealth missiles with precision guidance: A novel multifunctional nano-drug delivery system based on biomimetic cell membrane coating technology. Mater. Today Bio 2025, 33, 101922. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Guo, H.; Fang, C.; Yang, Q.; Qin, W.; Wang, H.; Xian, Y.; Yan, X.; Yin, B.; et al. Nanomaterials for stroke diagnosis and treatment. iScience 2024, 27, 111112. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Sha, M.; Ye, Y.; Wang, C. Cell Membrane-Derived Nanovehicles for Targeted Therapy of Ischemic Stroke: From Construction to Application. Pharmaceutics 2023, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, M.; Hadrava Vanova, K.; Uher, O.; Leischner Fialova, J.; Petrlakova, K.; Masarik, M.; Kejík, Z.; Martasek, P.; Pacak, K.; Jakubek, M. Injecting hope: The potential of intratumoral immunotherapy for locally advanced and metastatic cancer. Front. Immunol. 2024, 15, 1479483. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lu, Z.; Wang, C.; Zhu, X.; Yang, Y.; Zhou, Y.; Gong, P. Application and advances of biomimetic membrane materials in central nervous system disorders. J. Nanobiotechnol. 2024, 22, 280. [Google Scholar] [CrossRef]
- Lin, D.; Yang, H.; Liang, X.; Yang, M.; Zhao, Y. The involvement of mitochondria in erythrocyte pathology and diseases: From mechanisms to therapeutic strategies. Clin. Exp. Med. 2025, 25, 144. [Google Scholar] [CrossRef]
- Xie, X.; Wang, H.; Williams, G.R.; Yang, Y.; Zheng, Y.; Wu, J.; Zhu, L.M. Erythrocyte Membrane Cloaked Curcumin-Loaded Nanoparticles for Enhanced Chemotherapy. Pharmaceutics 2019, 11, 429. [Google Scholar] [CrossRef]
- Xu, X.; Li, T.; Jin, K. Bioinspired and Biomimetic Nanomedicines for Targeted Cancer Therapy. Pharmaceutics 2022, 14, 1109. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, Y.; Luo, R.; Zhou, D.; Zheng, F.; Shi, L.; Zhang, H.; Wang, Y.; Xu, X.; Zou, R.; et al. Engineered hybrid cell membrane nanosystems for treating cardiovascular diseases. Mater. Today Bio 2025, 33, 101992. [Google Scholar] [CrossRef]
- Sun, X.; Wang, C.; Gao, M.; Hu, A.; Liu, Z. Remotely Controlled Red Blood Cell Carriers for Cancer Targeting and Near-Infrared Light-Triggered Drug Release in Combined Photothermal–Chemotherapy. Adv. Funct. Mater. 2015, 25, 2386–2394. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Li, T.; Qin, X.; Li, Y.; Shen, X.; Li, S.; Yang, H.; Wu, C.; Zheng, C.; Zhu, J.; You, F.; et al. Cell Membrane Coated-Biomimetic Nanoplatforms Toward Cancer Theranostics. Front. Bioeng. Biotechnol. 2020, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Walker, T.L. The multifaceted role of platelets in mediating brain function. Blood 2022, 140, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.S.; Wu, L.L.; Chen, C.M.; Zheng, H.; Gao, J.; Lu, Z.M.; Li, M. Lipid-hybrid cell-derived biomimetic functional materials: A state-of-the-art multifunctional weapon against tumors. Mater. Today Bio 2023, 22, 100751. [Google Scholar] [CrossRef]
- Avgoustakis, K.; Angelopoulou, A. Biomaterial-Based Responsive Nanomedicines for Targeting Solid Tumor Microenvironments. Pharmaceutics 2024, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Chen, J.; Liu, Y.; Cheng, X.; Yang, F.; Gu, N. Platelet Membrane Biomimetic Magnetic Nanocarriers for Targeted Delivery and in Situ Generation of Nitric Oxide in Early Ischemic Stroke. ACS Nano 2020, 14, 2024–2035. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Y.; Zheng, B.; Zhang, Q.; Zhou, S.; Zhao, L. Finding a needle in a haystack: Functional screening for novel targets in cancer immunology and immunotherapies. Oncogene 2025, 44, 409–426. [Google Scholar] [CrossRef]
- Garmendia Urdalleta, A.; Van Poll, M.; Fahy, N.; Witte-Bouma, J.; Van Wamel, W.; Apachitei, I.; Zadpoor, A.A.; Fratila-Apachitei, L.E.; Farrell, E. The response of human macrophages to 3D printed titanium antibacterial implants does not affect the osteogenic differentiation of hMSCs. Front. Bioeng. Biotechnol. 2023, 11, 1176534. [Google Scholar] [CrossRef]
- Stewart, A.G.; Beart, P.M. Inflammation: Maladies, models, mechanisms and molecules. Br. J. Pharmacol. 2016, 173, 631–634. [Google Scholar] [CrossRef]
- Liu, B.; Yan, W.; Luo, L.; Wu, S.; Wang, Y.; Zhong, Y.; Tang, D.; Maruf, A.; Yan, M.; Zhang, K.; et al. Macrophage membrane camouflaged reactive oxygen species responsive nanomedicine for efficiently inhibiting the vascular intimal hyperplasia. J. Nanobiotechnol. 2021, 19, 374. [Google Scholar] [CrossRef]
- Liu, W.; Zou, Z.; Li, W.; Yang, G.; Zhang, J.; Zhang, Z.; Yao, H. Research status and future perspectives of IL-27 in the treatment of stroke (Review). Int. J. Mol. Med. 2025, 56, 116. [Google Scholar] [CrossRef]
- Kallen, V.; Scherder, R.; Cramer, M.J.; Stam, J.; Johnson, B.; Scherder, E. Neutralizing a Springboard for Inflammation: Physical Activity to Control the Immune Network. Healthcare 2021, 9, 1196. [Google Scholar] [CrossRef]
- Hao, J.; Chen, J.; Wang, M.; Zhao, J.; Wang, J.; Wang, X.; Li, Y.; Tang, H. Neutrophils, as “Trojan horses”, participate in the delivery of therapeutical PLGA nanoparticles into a tumor based on the chemotactic effect. Drug Deliv. 2020, 27, 1–14. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Chen, W.; Hao, T.; Ran, C.; Zhou, Y.; Shen, Y.; You, W.; Wang, T. Smart Thrombosis Care: The Rise of Closed-Loop Diagnosis-to-Treatment Nano Systems. Int. J. Nanomed. 2025, 20, 7851–7868. [Google Scholar] [CrossRef]
- Han, D.; Wang, F.; Qiao, Z.; Wang, B.; Zhang, Y.; Jiang, Q.; Liu, M.; Zhuang, Y.; An, Q.; Bai, Y.; et al. Neutrophil membrane-camouflaged nanoparticles alleviate inflammation and promote angiogenesis in ischemic myocardial injury. Bioact. Mater. 2023, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, M.; Yuan, Y.; Nie, C.; Wei, K.; Zhang, T.; Chen, T.; Chu, X. Neutrophil-Membrane-Coated Biomineralized Metal-Organic Framework Nanoparticles for Atherosclerosis Treatment by Targeting Gene Silencing. ACS Nano 2023, 17, 7721–7732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Nie, D.; Huang, Y.; Yang, Y.; Liu, Q.; Sun, Z.; Jiang, Q.; Ling, Y.; Wen, Y.; Qu, J.; et al. A Magnetic-Responsive Biomimetic Nanosystem Coated with Glioma Stem Cell Membranes Effectively Targets and Eliminates Malignant Gliomas. Biomater. Res. 2024, 28, 0123. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Sipos, F.; Műzes, G. Disagreements in the therapeutic use of mesenchymal stem cell-derived secretome. World J. Stem Cells 2022, 14, 365–371. [Google Scholar] [CrossRef]
- Choi, A.; Javius-Jones, K.; Hong, S.; Park, H. Cell-Based Drug Delivery Systems with Innate Homing Capability as a Novel Nanocarrier Platform. Int. J. Nanomed. 2023, 18, 509–525. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, S.; Liu, J.; Liu, F.; Du, F.; Li, M.; Chen, A.T.; Bao, Y.; Suh, H.W.; Avery, J.; et al. Targeted Drug Delivery to Stroke via Chemotactic Recruitment of Nanoparticles Coated with Membrane of Engineered Neural Stem Cells. Small 2019, 15, e1902011. [Google Scholar] [CrossRef]
- Kaur, J.; Thakran, A.; Naqvi, S. Recent advances in cell membrane-based biomimetic delivery systems for Parkinson’s disease: Perspectives and challenges. Asian J. Pharm. Sci. 2025, 20, 101060. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nanomicro Lett. 2019, 11, 100. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Meng, N.; Guo, H.; Wu, S.; Wang, X.; Li, J.; Wang, H.; Jiang, K.; Xie, C.; et al. Platelet membrane-functionalized nanoparticles with improved targeting ability and lower hemorrhagic risk for thrombolysis therapy. J. Control. Release 2020, 328, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zang, J.; Zhao, Z.; Zhang, Q.; Chen, S. The Advances of Neutrophil-Derived Effective Drug Delivery Systems: A Key Review of Managing Tumors and Inflammation. Int. J. Nanomed. 2021, 16, 7663–7681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, W.; Wu, J.; Shen, Y.; Xu, B.; Chen, Z.; Sun, Y. Application of Biomimetic Cell Membrane-Coated Nanocarriers in Cardiovascular Diseases. Int. J. Nanomed. 2025, 20, 8249–8289. [Google Scholar] [CrossRef]
- Maia, R.F.; Vaziri, A.S.; Shahbazi, M.A.; Santos, H.A. Artificial cells and biomimicry cells: A rising star in the fight against cancer. Mater. Today Bio 2025, 32, 101723. [Google Scholar] [CrossRef]
- Yuan, S.; Hu, D.; Gao, D.; Butch, C.J.; Wang, Y.; Zheng, H.; Sheng, Z. Recent advances of engineering cell membranes for nanomedicine delivery across the blood–brain barrier. J. Nanobiotechnol. 2025, 23, 493. [Google Scholar] [CrossRef]
- Li, W.; Cheng, J.; He, F.; Zhang, P.; Zhang, N.; Wang, J.; Song, Q.; Hou, Y.; Gan, Z. Cell membrane-based nanomaterials for theranostics of central nervous system diseases. J. Nanobiotechnol. 2023, 21, 276. [Google Scholar] [CrossRef]
- Chugh, V.; Vijaya Krishna, K.; Pandit, A. Cell Membrane-Coated Mimics: A Methodological Approach for Fabrication, Characterization for Therapeutic Applications, and Challenges for Clinical Translation. ACS Nano 2021, 15, 17080–17123. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, X.; Yin, S.; Yang, Y.; Liu, T.; Wang, K. Biomimetic recombinant of red blood cell membranes for improved photothermal therapy. J. Nanobiotechnol. 2021, 19, 213. [Google Scholar] [CrossRef]
- Quach, A.; Ferrante, A. The Application of Dextran Sedimentation as an Initial Step in Neutrophil Purification Promotes Their Stimulation, due to the Presence of Monocytes. J. Immunol. Res. 2017, 2017, 1254792. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, S. Research update on cell membrane camouflaged nanoparticles for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 944518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Q.; Zhu, Y.; Pei, M.; Wang, K.; Qu, X.; Zhang, Y.; Gao, J.; Qin, H. Targeting inorganic nanoparticles to tumors using biological membrane-coated technology. MedComm 2022, 3, e192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Cao, D.; Guo, Y.; Wu, D.; Yang, M.; Wang, H.; Shao, X.; Li, Y.; Liang, Y. Overcoming Biological Barriers in Cancer Therapy: Cell Membrane-Based Nanocarrier Strategies for Precision Delivery. Int. J. Nanomed. 2025, 20, 3113–3145. [Google Scholar] [CrossRef]
- Guido, C.; Maiorano, G.; Cortese, B.; D’Amone, S.; Palamà, I.E. Biomimetic Nanocarriers for Cancer Target Therapy. Bioengineering 2020, 7, 111. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, T.; Li, N.; Gao, J. Cell membrane-based biomimetic vehicles for effective central nervous system target delivery: Insights and challenges. J. Control. Release 2023, 360, 169–184. [Google Scholar] [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Luo, R.; Liu, M.; Tan, T.; Yang, Q.; Wang, Y.; Men, L.; Zhao, L.; Zhang, H.; Wang, S.; Xie, T.; et al. Emerging Significance and Therapeutic Potential of Extracellular vesicles. Int. J. Biol. Sci. 2021, 17, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, X.; Kong, Q.; He, H.; Sun, J.; Qiu, W.; Zhang, L.; Yang, M. Extracellular Vesicle Preparation and Analysis: A State-of-the-Art Review. Adv. Sci. 2024, 11, e2401069. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ma, Y.; Wu, M.; Chen, Z.; Zhang, L.; Lu, J. Recent progress in aptamer-based microfluidics for the detection of circulating tumor cells and extracellular vesicles. J. Pharm. Anal. 2023, 13, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Liu, M.; Tao, X.; Yin, X.; Shen, C.; Wang, X. Emerging Trends in the Application of Extracellular Vesicles as Novel Oral Delivery Vehicles for Therapeutics in Inflammatory Diseases. Int. J. Nanomed. 2024, 19, 8573–8601. [Google Scholar]
- Tong, L.; Zhang, S.; Huang, R.; Yi, H.; Wang, J.W. Extracellular vesicles as a novel photosensitive drug delivery system for enhanced photodynamic therapy. Front. Bioeng. Biotechnol. 2022, 10, 1032318. [Google Scholar] [CrossRef]
- Su, X.; Wang, H.; Li, Q.; Chen, Z. Extracellular Vesicles: A Review of Their Therapeutic Potentials, Sources, Biodistribution, and Administration Routes. Int. J. Nanomed. 2025, 20, 3175–3199. [Google Scholar] [CrossRef]
- Ma, S.R.; Xia, H.F.; Gong, P.; Yu, Z.L. Red Blood Cell-Derived Extracellular Vesicles: An Overview of Current Research Progress, Challenges, and Opportunities. Biomedicines 2023, 11, 2798. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef]
- Yang, L.; Huang, S.; Zhang, Z.; Liu, Z.; Zhang, L. Roles and Applications of Red Blood Cell-Derived Extracellular Vesicles in Health and Diseases. Int. J. Mol. Sci. 2022, 23, 5927. [Google Scholar] [CrossRef]
- Lam, B.W.S.; Tan, M.; Gao, C.; Pham, T.T.; Tran, L.T.N.; Nguyen, L.N.; Sidik, H.; Lim, M.B.H.; Le, A.H.; Nguyen, T.T.T.; et al. Extracellular Vesicles Administered via Intrathecal Injection Mediate Safe Delivery of Nucleic Acids to the Central Nervous System for Gene Therapy. J. Extracell. Vesicles 2025, 14, e70116. [Google Scholar] [CrossRef]
- Shao, X.; Yan, C.; Wang, C.; Wang, C.; Cao, Y.; Zhou, Y.; Guan, P.; Hu, X.; Zhu, W.; Ding, S. Advanced nanomaterials for modulating Alzheimer’s related amyloid aggregation. Nanoscale Adv. 2022, 5, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Salybekov, A.A.; Kunikeyev, A.D.; Kobayashi, S.; Asahara, T. Latest Advances in Endothelial Progenitor Cell-Derived Extracellular Vesicles Translation to the Clinic. Front. Cardiovasc. Med. 2021, 8, 734562. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Le, A.H.; Dang, C.P.; Chong, S.Y.; Do, D.V.; Peng, B.; Jayasinghe, M.K.; Ong, H.B.; Hoang, D.V.; Louise, R.A.; et al. Endocytosis of red blood cell extracellular vesicles by macrophages leads to cytoplasmic heme release and prevents foam cell formation in atherosclerosis. J. Extracell. Vesicles 2023, 12, e12354. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Dong, J.F.; Zhang, J. Roles and therapeutic potential of different extracellular vesicle subtypes on traumatic brain injury. Cell Commun. Signal 2023, 21, 211. [Google Scholar] [CrossRef]
- Liu, L.; Deng, Q.J. Role of platelet-derived extracellular vesicles in traumatic brain injury-induced coagulopathy and inflammation. Neural Regen. Res. 2022, 17, 2102–2107. [Google Scholar] [CrossRef]
- Corvigno, S.; Johnson, A.M.; Wong, K.K.; Cho, M.S.; Afshar-Kharghan, V.; Menter, D.G.; Sood, A.K. Novel Markers for Liquid Biopsies in Cancer Management: Circulating Platelets and Extracellular Vesicles. Mol. Cancer Ther. 2022, 21, 1067–1075. [Google Scholar] [CrossRef]
- Nyam-Erdene, A.; Nebie, O.; Delila, L.; Buée, L.; Devos, D.; Chou, S.Y.; Blum, D.; Burnouf, T. Characterization and Chromatographic Isolation of Platelet Extracellular Vesicles from Human Platelet Lysates for Applications in Neuroregenerative Medicine. ACS Biomater. Sci. Eng. 2021, 7, 5823–5835. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-derived extracellular vesicles: Diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020, 11, 924. [Google Scholar] [CrossRef]
- Zhang, C.; Li, D.; Hu, H.; Wang, Z.; An, J.; Gao, Z.; Zhang, K.; Mei, X.; Wu, C.; Tian, H. Engineered extracellular vesicles derived from primary M2 macrophages with anti-inflammatory and neuroprotective properties for the treatment of spinal cord injury. J. Nanobiotechnol. 2021, 19, 373. [Google Scholar] [CrossRef]
- Song, Y.; Hu, J.; Ma, C.; Liu, H.; Li, Z.; Yang, Y. Macrophage-Derived Exosomes as Advanced Therapeutics for Inflammation: Current Progress and Future Perspectives. Int. J. Nanomed. 2024, 19, 1597–1627. [Google Scholar] [CrossRef]
- Jung, I.; Shin, S.; Baek, M.C.; Yea, K. Modification of immune cell-derived exosomes for enhanced cancer immunotherapy: Current advances and therapeutic applications. Exp. Mol. Med. 2024, 56, 19–31. [Google Scholar] [CrossRef]
- Youn, Y.J.; Shrestha, S.; Lee, Y.B.; Kim, J.K.; Lee, J.H.; Hur, K.; Mali, N.M.; Nam, S.W.; Kim, S.H.; Lee, S.; et al. Neutrophil-derived trail is a proinflammatory subtype of neutrophil-derived extracellular vesicles. Theranostics 2021, 11, 2770–2787. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liao, L.; Dai, J.; Mazhar, M.; Yang, G.; Wang, H.; Dechsupa, N.; Wang, L. Mesenchymal stem cell-derived extracellular vesicles/exosome: A promising therapeutic strategy for intracerebral hemorrhage. Regen. Ther. 2023, 22, 181–190. [Google Scholar] [CrossRef]
- Go, V.; Sarikaya, D.; Zhou, Y.; Bowley, B.G.E.; Pessina, M.A.; Rosene, D.L.; Zhang, Z.G.; Chopp, M.; Finklestein, S.P.; Medalla, M.; et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells enhance myelin maintenance after cortical injury in aged rhesus monkeys. Exp. Neurol. 2021, 337, 113540. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xu, Q.; Chen, X.; Deng, X.; Chen, N.; Kou, M.T.; Huang, Y.; Guo, J.; Xiao, Z.; Wang, J. Biomimetic nanomaterials in myocardial infarction treatment: Harnessing bionic strategies for advanced therapeutics. Mater. Today Bio 2024, 25, 100957. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Zhou, N.; Zhou, Z.; Fu, Z.; Guo, R.; Akogo, H.Y.; Yang, J.; Yu, M.; Jiang, Y.; Lan, S.; et al. Intranasal administration of stem cell derivatives for the treatment of AD animal models: A systematic review and meta-analysis. Stem Cell Res. Ther. 2025, 16, 409. [Google Scholar] [CrossRef]
- Calvo, B.; Schembri-Wismayer, P.; Durán-Alonso, M.B. Age-Related Neurodegenerative Diseases: A Stem Cell’s Perspective. Cells 2025, 14, 347. [Google Scholar] [CrossRef]
- Ding, M.; Shen, Y.; Wang, P.; Xie, Z.; Xu, S.; Zhu, Z.; Wang, Y.; Lyu, Y.; Wang, D.; Xu, L.; et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 2165–2177. [Google Scholar] [CrossRef]
- Kim, K.M.; Meng, Q.; Perez de Acha, O.; Mustapic, M.; Cheng, A.; Eren, E.; Kundu, G.; Piao, Y.; Munk, R.; Wood, W.H., III; et al. Mitochondrial RNA in Alzheimer’s Disease Circulating Extracellular Vesicles. Front. Cell Dev. Biol. 2020, 8, 581882. [Google Scholar] [CrossRef]
- Corbeil, D.; Santos, M.F.; Karbanová, J.; Kurth, T.; Rappa, G.; Lorico, A. Uptake and Fate of Extracellular Membrane Vesicles: Nucleoplasmic Reticulum-Associated Late Endosomes as a New Gate to Intercellular Communication. Cells 2020, 9, 1931. [Google Scholar] [CrossRef]
- Małys, M.S.; Aigner, C.; Schulz, S.M.; Schachner, H.; Rees, A.J.; Kain, R. Isolation of Small Extracellular Vesicles from Human Sera. Int. J. Mol. Sci. 2021, 22, 4653. [Google Scholar] [CrossRef]
- Li, M.; Fang, F.; Sun, M.; Zhang, Y.; Hu, M.; Zhang, J. Extracellular vesicles as bioactive nanotherapeutics: An emerging paradigm for regenerative medicine. Theranostics 2022, 12, 4879–4903. [Google Scholar] [CrossRef]
- Shpigelman, J.; Lao, F.S.; Yao, S.; Li, C.; Saito, T.; Sato-Kaneko, F.; Nolan, J.P.; Shukla, N.M.; Pu, M.; Messer, K.; et al. Generation and Application of a Reporter Cell Line for the Quantitative Screen of Extracellular Vesicle Release. Front. Pharmacol. 2021, 12, 668609. [Google Scholar] [CrossRef]
- Mentkowski, K.I.; Snitzer, J.D.; Rusnak, S.; Lang, J.K. Therapeutic Potential of Engineered Extracellular Vesicles. AAPS J. 2018, 20, 50. [Google Scholar] [CrossRef]
- Bian, S.; Liu, H. Isolation and identification methods of extracellular vesicles. J. N. Med. 2019, 50, 658–662. [Google Scholar] [CrossRef]
- Tran, H.L.; Zheng, W.; Issadore, D.A.; Im, H.; Cho, Y.K.; Zhang, Y.; Liu, D.; Liu, Y.; Li, B.; Liu, F.; et al. Extracellular Vesicles for Clinical Diagnostics: From Bulk Measurements to Single-Vesicle Analysis. ACS Nano 2025, 19, 28021–28109. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Zou, W.; Xu, P.; Cheng, F.; Zhang, E.Y.; Zhang-Chen, A.; Kleiboeker, S.; Qiu, J. Identification of AXL as a co-receptor for human parvovirus B19 infection of human erythroid progenitors. Sci. Adv. 2023, 9, eade0869. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef]
- Liu, J.; Shen, T.; Zhang, Y.; Wei, X.; Bao, Y.; Ai, R.; Gan, S.; Wang, D.; Lai, X.; Zhao, L.; et al. Cell dehydration enables massive production of engineered membrane vesicles with therapeutic functions. J. Extracell. Vesicles 2024, 13, e12483. [Google Scholar] [CrossRef]
- Sun, B.; Sawant, H.; Borthakur, A.; Bihl, J.C. Emerging therapeutic role of gut microbial extracellular vesicles in neurological disorders. Front. Neurosci. 2023, 17, 1241418. [Google Scholar] [CrossRef]
- Bashir, Y.; Khan, A.U. The interplay between the gut-brain axis and the microbiome: A perspective on psychiatric and neurodegenerative disorders. Front. Neurosci. 2022, 16, 1030694. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.C.P.; Jiao, M.; Xingxuan, C.; Wei, J. Extracellular vesicles derived from host and gut microbiota as promising nanocarriers for targeted therapy in osteoporosis and osteoarthritis. Front. Pharmacol. 2023, 13, 1051134. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, Z.; Wang, Q.; Wang, M.; Ming, Y.; Chen, W.; Huang, Y.; Tang, Z.; Huang, M.; Liu, H.; et al. Opportunities and challenges of bacterial extracellular vesicles in regenerative medicine. J. Nanobiotechnol. 2025, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Li, Q.; Chen, X.; Onyango, S.; Xie, J.; Nie, S. Bacterial extracellular vesicles: Vital contributors to physiology from bacteria to host. Microbiol. Res. 2024, 284, 127733. [Google Scholar] [CrossRef]
- Dai, K.; Liao, B.; Huang, X.; Liu, Q. Consistency in bacterial extracellular vesicle production: Key to their application in human health. Extracell. Vesicles Circ. Nucleic Acids 2025, 6, 1–20. [Google Scholar] [CrossRef]
- Sandanusova, M.; Turkova, K.; Pechackova, E.; Kotoucek, J.; Roudnicky, P.; Sindelar, M.; Kubala, L.; Ambrozova, G. Growth phase matters: Boosting immunity via Lacticasebacillus-derived membrane vesicles and their interactions with TLR2 pathways. J. Extracell. Biol. 2024, 3, e169. [Google Scholar] [CrossRef]
- Mantella, V.; Bienz, S.; Brigger, F.; Baulier, E.; Ramus, M.; Zoratto, N.; Honrath, S.; Naresh, K.; Sander, S.; Dengjel, J.; et al. Isolation of bacterial extracellular vesicles from raw samples using a portable microstructured electrochemical device. Drug Deliv. Transl. Res. 2025, in press. [Google Scholar] [CrossRef]
- Wang, S.; Luo, J.; Wang, H.; Chen, T.; Sun, J.; Xi, Q.; Zhang, Y. Extracellular Vesicles: A Crucial Player in the Intestinal Microenvironment and Beyond. Int. J. Mol. Sci. 2024, 25, 3478. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.; Xiang, X.; Hao, C.; Ma, D. Roles of bacterial extracellular vesicles in systemic diseases. Front. Microbiol. 2023, 14, 1258860. [Google Scholar] [CrossRef]
- Zhu, Z.; Antenucci, F.; Villumsen, K.R.; Bojesen, A.M. Bacterial Outer Membrane Vesicles as a Versatile Tool in Vaccine Research and the Fight against Antimicrobial Resistance. mBio 2021, 12, e0170721. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, J. Influences of bacterial extracellular vesicles on macrophage immune functions. Front. Cell. Infect. Microbiol. 2024, 14, 1411196. [Google Scholar] [CrossRef]
- Xie, J.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R.E. Bacterial extracellular vesicles: An emerging avenue to tackle diseases. Trends Microbiol. 2023, 31, 1206–1224. [Google Scholar] [CrossRef]
- Xie, J.; Li, Q.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R.E. The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. 2022, 40, 1173–1194. [Google Scholar] [CrossRef]
- De Langhe, N.; Van Dorpe, S.; Guilbert, N.; Vander Cruyssen, A.; Roux, Q.; Deville, S.; Dedeyne, S.; Tummers, P.; Denys, H.; Vandekerckhove, L.; et al. Mapping bacterial extracellular vesicle research: Insights, best practices and knowledge gaps. Nat. Commun. 2024, 15, 9410. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Kalluri, R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene 2020, 39, 6951–6960. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Liu, S.; Xing, B. Bacteria extracellular vesicle as nanopharmaceuticals for versatile biomedical potential. Nano Converg. 2024, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zou, Y.; Feng, C.; Lee, A.; Yin, J.; Chung, R.; Park, J.B.; Rizos, H.; Tao, W.; Zheng, M.; et al. Charge Conversional Biomimetic Nanocomplexes as a Multifunctional Platform for Boosting Orthotopic Glioblastoma RNAi Therapy. Nano Lett. 2020, 20, 1637–1646. [Google Scholar] [CrossRef]
- Yin, T.; Fan, Q.; Hu, F.; Ma, X.; Yin, Y.; Wang, B.; Kuang, L.; Hu, X.; Xu, B.; Wang, Y. Engineered Macrophage-Membrane-Coated Nanoparticles with Enhanced PD-1 Expression Induce Immunomodulation for a Synergistic and Targeted Antiglioblastoma Activity. Nano Lett. 2022, 22, 6606–6614. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Li, Z.; Peng, Y.; Tan, H.; Wang, C.; Huang, G.; Li, W.; Ma, G.; Wei, W. Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Signal Transduct. Target. Ther. 2022, 7, 74. [Google Scholar] [CrossRef]
- You, H.; Zhang, S.; Zhang, Y.; Chen, Q.; Wu, Y.; Zhou, Z.; Zhao, Z.; Su, B.; Li, X.; Guo, Y.; et al. Engineered Bacterial Outer Membrane Vesicles-Based Doxorubicin and CD47-siRNA Co-Delivery Nanoplatform Overcomes Immune Resistance to Potentiate the Immunotherapy of Glioblastoma. Adv. Mater. 2025, 37, e2418053. [Google Scholar] [CrossRef]
- Lv, W.; Xu, J.; Wang, X.; Li, X.; Xu, Q.; Xin, H. Bioengineered Boronic Ester Modified Dextran Polymer Nanoparticles as Reactive Oxygen Species Responsive Nanocarrier for Ischemic Stroke Treatment. ACS Nano 2018, 12, 5417–5426. [Google Scholar] [CrossRef]
- Quan, X.; Han, Y.; Lu, P.; Ding, Y.; Wang, Q.; Li, Y.; Wei, J.; Huang, Q.; Wang, R.; Zhao, Y. Annexin V-Modified Platelet-Biomimetic Nanomedicine for Targeted Therapy of Acute Ischemic Stroke. Adv. Healthc. Mater. 2022, 11, e2200416. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Tang, L.; Zhang, Y.; Ma, X.; Yin, Y.; Kuang, L.; Fan, Q.; Wang, B.; Hu, X.; Yin, T.; et al. A Homing Peptide Modified Neutrophil Membrane Biomimetic Nanoparticles in Response to ROS/inflammatory Microenvironment for Precise Targeting Treatment of Ischemic Stroke. Adv. Funct. Mater. 2024, 34, 2309167. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Z.; Huang, X.; Xue, J.; Zhang, S.; Guo, X.; Zhou, S. Bacteria-Derived Outer-Membrane Vesicles Hitchhike Neutrophils to Enhance Ischemic Stroke Therapy. Adv. Mater. 2023, 35, e2301779. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, Z.; Yang, Z.; Zhang, Y.; Chen, H.; Yang, X.; Fang, X.; Zhu, Y.; Zhang, J.; Ouyang, F.; et al. Lactobacillus plantarum-derived extracellular vesicles protect against ischemic brain injury via the microRNA-101a-3p/c-Fos/TGF-β axis. Pharmacol. Res. 2022, 182, 106332. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Ye, C.; Tian, C.; Zhao, D.; Li, H.; Sun, Z.; Miao, Y.; Zhang, Q.; Wang, J.; Dou, Y. Engineered macrophage-biomimetic versatile nanoantidotes for inflammation-targeted therapy against Alzheimer?s disease by neurotoxin neutralization and immune recognition suppression. Bioact. Mater. 2023, 26, 337–352. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Zhang, H.; Zhao, C.; Du, X.; Ren, J.; Qu, X. Biomimetic engineering of a neuroinflammation-targeted MOF nanozyme scaffolded with photo-trigger released CO for the treatment of Alzheimer’s disease. Chem. Sci. 2024, 15, 13201–13208. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Gutiérrez-Mercado, Y.K.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.L.; Vázquez-Méndez, E.; Padilla-Camberos, E.; Canales-Aguirre, A.A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [CrossRef]
- Li, S.; Ren, Y.; Xiao, X.; Chen, Y.; Zhao, W.; Liu, H.; Ding, Z.; Xu, Y.; Xin, H.; Guo, Y. Enhanced neuroprotection in Parkinson’s disease by neutrophil-biomimetic nanovesicles through autophagy inhibition and apoptosis antagonism. Chem. Eng. J. 2025, 521, 166832. [Google Scholar] [CrossRef]
- Pouyan, A.; Ghorbanlo, M.; Eslami, M.; Jahanshahi, M.; Ziaei, E.; Salami, A.; Mokhtari, K.; Shahpasand, K.; Farahani, N.; Meybodi, T.E.; et al. Glioblastoma multiforme: Insights into pathogenesis, key signaling pathways, and therapeutic strategies. Mol. Cancer 2025, 24, 58. [Google Scholar] [CrossRef]
- Liu, J.; Yang, F.; Hu, J.; Zhang, X. Nanoparticles for efficient drug delivery and drug resistance in glioma: New perspectives. CNS Neurosci. Ther. 2024, 30, e14715. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, G.; Tang, X.; Tong, A.; Zhou, L. Immunotherapy of glioblastoma: Recent advances and future prospects. Hum. Vaccines Immunother. 2022, 18, 2055417. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Wang, S.; Kroemer, G.; Penninger, J.M.; Uversky, V.N.; Pratico, D.; Henninger, N.; Reiter, R.J.; Bruno, A.; Joshipura, K.; et al. Targeting autophagy in ischemic stroke: From molecular mechanisms to clinical therapeutics. Pharmacol. Ther. 2021, 225, 107848. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. Jama 2021, 325, 1088–1098. [Google Scholar] [CrossRef]
- Fukuta, T.; Oku, N.; Kogure, K. Application and Utility of Liposomal Neuroprotective Agents and Biomimetic Nanoparticles for the Treatment of Ischemic Stroke. Pharmaceutics 2022, 14, 361. [Google Scholar] [CrossRef]
- Ji, P.; Xu, Q.; Li, J.; Wang, Z.; Mao, W.; Yan, P. Advances in nanoparticle-based therapeutics for ischemic stroke: Enhancing drug delivery and efficacy. Biomed. Pharmacother. 2024, 180, 117564. [Google Scholar] [CrossRef]
- Migliavacca, M.; Correa-Paz, C.; Pérez-Mato, M.; Bielawski, P.B.; Zhang, I.; Marie, P.; Hervella, P.; Rubio, M.; Maysinger, D.; Vivien, D.; et al. Thrombolytic therapy based on lyophilized platelet-derived nanocarriers for ischemic stroke. J. Nanobiotechnol. 2024, 22, 10. [Google Scholar] [CrossRef]
- Ruan, H.; Li, Y.; Zheng, D.; Deng, L.; Chen, G.; Zhang, X.; Tang, Y.; Cui, W. Engineered extracellular vesicles for ischemic stroke treatment. Innovation 2023, 4, 100394. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Ferrari, C.; Sorbi, S. The complexity of Alzheimer’s disease: An evolving puzzle. Physiol. Rev. 2021, 101, 1047–1081. [Google Scholar] [CrossRef]
- Alcolea, D.; Beeri, M.S.; Rojas, J.C.; Gardner, R.C.; Lleó, A. Blood Biomarkers in Neurodegenerative Diseases: Implications for the Clinical Neurologist. Neurology 2023, 101, 172–180. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef]
- Ye, Y.; Gao, M.; Shi, W.; Gao, Y.; Li, Y.; Yang, W.; Zheng, X.; Lu, X. The immunomodulatory effects of mesenchymal stem cell-derived extracellular vesicles in Alzheimer’s disease. Front. Immunol. 2024, 14, 1325530. [Google Scholar] [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Vijiaratnam, N.; Simuni, T.; Bandmann, O.; Morris, H.R.; Foltynie, T. Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol. 2021, 20, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Gattoni, M.F.; Gobbo, S.; Feroldi, S.; Salvatore, A.; Navarro, J.; Sorbi, S.; Saibene, F.L. Identification of Cognitive Training for Individuals with Parkinson’s Disease: A Systematic Review. Brain Sci. 2025, 15, 61. [Google Scholar] [CrossRef]
- Izco, M.; Blesa, J.; Schleef, M.; Schmeer, M.; Porcari, R.; Al-Shawi, R.; Ellmerich, S.; de Toro, M.; Gardiner, C.; Seow, Y.; et al. Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology. Mol. Ther. 2019, 27, 2111–2122. [Google Scholar] [CrossRef]
- Sun, K.; Zheng, X.; Jin, H.; Yu, F.; Zhao, W. Exosomes as CNS Drug Delivery Tools and Their Applications. Pharmaceutics 2022, 14, 2252. [Google Scholar] [CrossRef]
- Wang, P.; Lan, G.; Xu, B.; Yu, Z.; Tian, C.; Lei, X.; Meissner, W.G.; Feng, T.; Yang, Y.; Zhang, J. α-Synuclein-carrying astrocytic extracellular vesicles in Parkinson pathogenesis and diagnosis. Transl. Neurodegener. 2023, 12, 40. [Google Scholar] [CrossRef]
- Hou, J.J.; Li, W.W.; Wang, X.L.; Ma, A.H.; Qin, Y.H. Efficacy of extracellular vesicles as a cell-free therapy in colitis: A systematic review and meta-analysis of animal studies. Front. Pharmacol. 2023, 14, 1260134. [Google Scholar] [CrossRef]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Kisku, A.; Nishad, A.; Agrawal, S.; Paliwal, R.; Datusalia, A.K.; Gupta, G.; Singh, S.K.; Dua, K.; Sulakhiya, K. Recent developments in intranasal drug delivery of nanomedicines for the treatment of neuropsychiatric disorders. Front. Med. 2024, 11, 1463976. [Google Scholar] [CrossRef]
- Marcello, E.; Chiono, V. Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting. Int. J. Mol. Sci. 2023, 24, 3390. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, X.; Yu, S.; Gong, G.; Shu, H. Research progress in brain-targeted nasal drug delivery. Front. Aging Neurosci. 2024, 15, 1341295. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulos, C.; Witt, S.T.; Haller, S.; Dizdar, N.; Zachrisson, H.; Engström, M.; Larsson, E.M. A study of neural activity and functional connectivity within the olfactory brain network in Parkinson’s disease. Neuroimage Clin. 2019, 23, 101946. [Google Scholar] [CrossRef]
- Tapia-Arellano, A.; Cabrera, P.; Cortés-Adasme, E.; Riveros, A.; Hassan, N.; Kogan, M.J. Tau- and α-synuclein-targeted gold nanoparticles: Applications, opportunities, and future outlooks in the diagnosis and therapy of neurodegenerative diseases. J. Nanobiotechnol. 2024, 22, 248. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Huang, Y.; Ma, Y.; Song, Q.; Chen, H.; Jiang, G.; Gao, X. Intranasal drug delivery: The interaction between nanoparticles and the nose-to-brain pathway. Adv. Drug Deliv. Rev. 2024, 207, 115196. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Selvaraj, K.; Gowthamarajan, K.; Karri, V.V.S.R. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef]
- Rassu, G.; Sorrenti, M.; Catenacci, L.; Pavan, B.; Ferraro, L.; Gavini, E.; Bonferoni, M.C.; Giunchedi, P.; Dalpiaz, A. Versatile Nasal Application of Cyclodextrins: Excipients and/or Actives? Pharmaceutics 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Dalaqua, M.; do Nascimento, F.B.P.; Miura, L.K.; Reis, F.; Garcia, M.R.T.; Barbosa Júnior, A.A. Magnetic resonance imaging of the cranial nerves in congenital, traumatic, and vascular diseases: A pictorial essay. Radiol. Bras. 2021, 54, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in brain targeting drug delivery system by nasal route. J. Control. Release 2017, 268, 364–389. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.D.; Duarte, J.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. Nanosystems for Brain Targeting of Antipsychotic Drugs: An Update on the Most Promising Nanocarriers for Increased Bioavailability and Therapeutic Efficacy. Pharmaceutics 2023, 15, 678. [Google Scholar] [CrossRef]
- Pan, J.; Fu, Y.; Yang, P.; Li, W.; Luo, Z.; Zhang, A.; Du, J.; Mei, F.; Liu, F.; Qi, S.; et al. The Cerebral Lymphatic System: Function, Controversies, and Therapeutic Approaches for Central Nervous System Diseases. Cell. Mol. Neurobiol. 2025, 45, 80. [Google Scholar] [CrossRef]
- Xu, J.; Tao, J.; Wang, J. Design and Application in Delivery System of Intranasal Antidepressants. Front. Bioeng. Biotechnol. 2020, 8, 626882. [Google Scholar] [CrossRef]
- Maher, R.; Moreno-Borrallo, A.; Jindal, D.; Mai, B.T.; Ruiz-Hernandez, E.; Harkin, A. Intranasal Polymeric and Lipid-Based Nanocarriers for CNS Drug Delivery. Pharmaceutics 2023, 15, 746. [Google Scholar] [CrossRef]
- Chung, S.; Peters, J.M.; Detyniecki, K.; Tatum, W.; Rabinowicz, A.L.; Carrazana, E. The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters. Epilepsy Behav. Rep. 2023, 21, 100581. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Zhang, M.; Zhang, J.; Kang, N.; Zheng, L.; Ding, Z. The Optimization Design of Macrophage Membrane Camouflaging Liposomes for Alleviating Ischemic Stroke Injury through Intranasal Delivery. Int. J. Mol. Sci. 2024, 25, 2927. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, M.; Zhang, J.; Kang, N.; Zheng, L.; Ding, Z. Targeted Delivery of Macrophage Membrane Biomimetic Liposomes Through Intranasal Administration for Treatment of Ischemic Stroke. Int. J. Nanomed. 2024, 19, 6177–6199. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V.; Lee, J.Y.; D’Egidio, F.; de Kalbermatten, M.; Garitaonandia, I.; Guzman, R. Nose-to-brain delivery of stem cells in stroke: The role of extracellular vesicles. Stem Cells Transl. Med. 2024, 13, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Sun, C.; Gao, X.; Bi, W.; Chen, H.; Ren, W.; Wang, L.; Luo, E.; Xing, C.; Du, H. Engineered Stem Cell Membrane-Coated Nanodrugs for Targeted Therapy of Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2025, 17, 47497–47511. [Google Scholar] [CrossRef]
- Xie, X.; Song, Q.; Dai, C.; Cui, S.; Tang, R.; Li, S.; Chang, J.; Li, P.; Wang, J.; Li, J.; et al. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer’s disease: A phase I/II clinical trial. Gen. Psychiatry 2023, 36, e101143. [Google Scholar] [CrossRef]
- Madhu, L.N.; Kodali, M.; Upadhya, R.; Rao, S.; Somayaji, Y.; Attaluri, S.; Shuai, B.; Kirmani, M.; Gupta, S.; Maness, N.; et al. Extracellular vesicles from human-induced pluripotent stem cell-derived neural stem cells alleviate proinflammatory cascades within disease-associated microglia in Alzheimer’s disease. J. Extracell. Vesicles 2024, 13, e12519. [Google Scholar] [CrossRef]
- Saha, P.; Kathuria, H.; Pandey, M.M. Intranasal nanotherapeutics for brain targeting and clinical studies in Parkinson’s disease. J. Control. Release 2023, 358, 293–318. [Google Scholar] [CrossRef]
- Narbute, K.; Piļipenko, V.; Pupure, J.; Dzirkale, Z.; Jonavičė, U.; Tunaitis, V.; Kriaučiūnaitė, K.; Jarmalavičiūtė, A.; Jansone, B.; Kluša, V.; et al. Intranasal Administration of Extracellular Vesicles Derived from Human Teeth Stem Cells Improves Motor Symptoms and Normalizes Tyrosine Hydroxylase Expression in the Substantia Nigra and Striatum of the 6-Hydroxydopamine-Treated Rats. Stem Cells Transl. Med. 2019, 8, 490–499. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Leite, C.M.; Moreno, N.S.; Miranda, R.R.; Pincela Lins, P.M.; Rodero, C.F.; de Oliveira Junior, E.; Lima, E.M.; Reis, R.M.; Zucolotto, V. Nose-to-Brain Delivery of Biomimetic Nanoparticles for Glioblastoma Targeted Therapy. ACS Appl. Mater. Interfaces 2025, 17, 484–499. [Google Scholar] [CrossRef]
- Shen, H.; Aggarwal, N.; Cui, B.; Foo, G.W.; He, Y.; Srivastava, S.K.; Li, S.; Seah, M.Z.X.; Wun, K.S.; Ling, H.; et al. Engineered commensals for targeted nose-to-brain drug delivery. Cell 2025, 188, 1545–1562.e16. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Zhang, D.; Cui, H.; Tian, X.; Du, W.; Yang, Z.; Wan, D.; Qiu, Z.; Liu, C.; et al. Precision-Guided Stealth Missiles in Biomedicine: Biological Carrier-Mediated Nanomedicine Hitchhiking Strategy. Adv. Sci. 2025, 12, e2504672. [Google Scholar] [CrossRef]
- Dong, X.; Gao, J.; Su, Y.; Wang, Z. Nanomedicine for Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7600. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, F.; Xu, W.; Qian, H. Engineered Extracellular Vesicles as a Targeted Delivery Platform for Precision Therapy. Tissue Eng. Regen. Med. 2023, 20, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.M.; Pinky, P.P.; Manickam, D.S. Molecular engineering of extracellular vesicles for drug delivery: Strategies, challenges, and perspectives. J. Control. Release 2025, 386, 114068. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Han, Y.; Hu, Y.; Geng, Z.; Su, J. Bacterial extracellular vesicles-based therapeutic strategies for bone and soft tissue tumors therapy. Theranostics 2022, 12, 6576–6594. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, M.; Zhang, J.; Maincent, P.; Xia, X.; Wu, W. Updated Progress of Nanocarrier-Based Intranasal Drug Delivery Systems for Treatment of Brain Diseases. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 433–467. [Google Scholar] [CrossRef]
- Yang, T.; Zhai, J.; Hu, D.; Yang, R.; Wang, G.; Li, Y.; Liang, G. “Targeting Design” of Nanoparticles in Tumor Therapy. Pharmaceutics 2022, 14, 1919. [Google Scholar] [CrossRef]
- Liu, B.D.; Akbar, R.; Oliverio, A.; Thapa, K.; Wang, X.; Fan, G.C. Bacterial Extracellular Vesicles in the Regulation of Inflammatory Response and Host-Microbe Interactions. Shock 2024, 61, 175–188. [Google Scholar] [CrossRef]
- Piunti, C.; Cimetta, E. Microfluidic approaches for producing lipid-based nanoparticles for drug delivery applications. Biophys. Rev. 2023, 4, 031304. [Google Scholar] [CrossRef]


| Cell Membrane Type | Core Biological Advantages | Potential Applications in CNS Diseases | Main Drawbacks |
|---|---|---|---|
| Red Blood Cell Membrane (RBC Membrane) | Immune Evasion and Long Circulation | As a long-acting drug delivery platform, it achieves targeted delivery to brain lesions through surface modification [28,29,30]. | Surface modification may induce hemolysis [53]. |
| Platelet Membrane | Natural Targeting Ability | For thrombus-targeted therapy of IS, drugs are accurately delivered to the lesion site [35]. | The cycle time is shorter than that of the red blood cell membrane [54]. |
| Macrophage Membrane | Inflammatory Targeting and Immune Regulation | It treats neuroinflammation-related diseases to achieve precise localization of lesions and immune regulation [38,39]. | Weak active targeting capability [55]. |
| Neutrophil Membrane | Chemotactic Migration Ability | It treats acute or chronic neuroinflammation and delivers drugs efficiently to the inflammatory area [41,43]. | Organ toxicity [56]. |
| Stem Cell Membrane | BBB Crossing and Precise Homing | It treats neurodegenerative diseases, improving the efficiency of drug entry into the brain and the enrichment degree at the lesion site [50,52]. | Low specificity [53]. |
| Cell Membrane-Modified Nanocarriers | Extracellular Vesicles (EVs) | Bacterial Extracellular Vesicles (BEVs) | |
|---|---|---|---|
| Nature | Artificially camouflaged core–shell structure | Naturally occurring carriers secreted by cells | Naturally occurring carriers secreted by bacteria |
| Source | Membranes extracted from intact cells [20] | Vesicles secreted by living eukaryotic cells [69] | Shed from or secreted by bacterial outer membranes [114] |
| Preparation | Physical disruption and fusion with high controllability [24] | Cell culture and purification with limited yield | Bacterial culture and purification, enabling easy large-scale production [123] |
| Advantages | Adjustable nanocores with diverse functions | Carry endogenous molecules with inherent biological activity [79] | Possess pathogen-associated molecular patterns (PAMPs) on the surface, providing efficient immunostimulation |
| Limitations | Complex preparation process, which may damage membrane proteins [60] | Difficulty in standardizing yield and purity [102] | Potential toxicity, requiring detoxification or modification [122] |
| Type of Disease | Biomimetic Material | Critical Role | Specific Mechanism |
|---|---|---|---|
| Glioblastoma Multiforme (GBM) | Red Blood Cell Membrane-Mimetic Nanoparticles (Ang-RBCm-CA/siRNA) | Long Circulation and Active Targeting | They utilize the red blood cell membrane to prolong circulation, and after modification with Angiopep-2, they can cross the BBB and target glioma [127]. |
| Macrophage Membrane-Mimetic Nanocarriers (PD-1-MM@PLGA/RAPA) | BBB Penetration and Tumor Accumulation | Engineered macrophage membranes can assist nanoparticles in effectively penetrating the BBB and accumulating in tumor tissues [128]. | |
| M1 Macrophage-Derived Extracellular Vesicles (M1EVs) | Chemotaxis and Immunomodulation | They utilize the chemotactic properties of M1 macrophages to accumulate at the tumor site and regulate immune cells in the tumor microenvironment [129]. | |
| Bacterial Outer Membrane Vesicles (OMVs) | Multifunctionality | They act as a carrier and immune adjuvant, can cross the BBB, and simultaneously enhance antitumor efficacy through photothermal effects and immune activation [130]. | |
| Ischemic Stroke (IS) | Red Blood Cell Membrane Nanoparticles (SHp-RBC-NPs) | Targeting and ROS-Responsive Release | They use red blood cell membranes for encapsulation and are modified with homing peptides to achieve precise targeting of ischemic brain tissue and effectively release drugs based on the ROS microenvironment of the lesion [131]. |
| Platelet Membrane Nanocarriers (APLT-PA) | Thrombus Targeting and Bleeding Risk Reduction | They leverage the natural targeting properties of platelet membranes to precisely deliver the thrombolytic drug tPA to the thrombus site [132]. | |
| Neutrophil Membrane Nanoparticles (SNM-NPs) | Inflammatory Chemotaxis and Oxidative Stress Response | They utilize the chemotaxis of neutrophil membranes and the dual targeting of homing peptides to guide nanoparticles to migrate to the injured area and effectively release drugs in response to ROS [133]. | |
| Bacterial Outer Membrane Vesicles (OMV@PGZ) | Brain Entry and Dual Pathway Inhibition | They utilize the interaction between OMVs and neutrophils to efficiently deliver drugs across the BBB and simultaneously inhibit the inflammatory and ferroptosis pathways [134]. | |
| Lactobacillus plantarum-Derived Extracellular Vesicles (LEVs) | Neurorepair | They regulate downstream signaling pathways by upregulating miRNAs, improve neuronal apoptosis, and promote neural repair [135]. | |
| Alzheimer’s Disease (AD) | Engineered Macrophage Membrane Nanocarriers (OT-Lipo@M) | BBB Crossing and Inflammatory Targeting | They utilize the phagocytosis resistance and pro-inflammatory tropism of macrophage membranes to actively cross the BBB and target inflammatory foci in the AD brain [136]. |
| Neutrophil Membrane Nanoparticles (Neu-MOF/Fla) | BBB Crossing and Multifunctional Synergy | They cross the BBB by utilizing the chemotaxis of neutrophil membranes, while the nanozyme scavenges ROS and releases anti-inflammatory factors to achieve combined therapy [137]. | |
| Mesenchymal Stem Cell-Derived Extracellular Vesicles (MSC-EVs) | Endogenous Therapy | The EVs themselves possess the capabilities of immunomodulation, neuroprotection, and promotion of neurogenesis [138]. | |
| Parkinson’s Disease (PD) | Neutrophil Membrane-Mimetic Vesicles (Neutro/miR-188-3p) | BBB Crossing and Gene Therapy | They efficiently cross the BBB by utilizing the chemotaxis of neutrophil membranes, release miRNAs to regulate specific signaling pathways, and inhibit neuronal apoptosis [139]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, F.; Hou, R.; Han, B.; Fang, X. Cell Membrane- and Vesicle-Based Bionic Nanodrugs: Applications in Central Nervous System Diseases and Exploration of Nasal–Cerebral Delivery. Gels 2025, 11, 846. https://doi.org/10.3390/gels11110846
Ding F, Hou R, Han B, Fang X. Cell Membrane- and Vesicle-Based Bionic Nanodrugs: Applications in Central Nervous System Diseases and Exploration of Nasal–Cerebral Delivery. Gels. 2025; 11(11):846. https://doi.org/10.3390/gels11110846
Chicago/Turabian StyleDing, Fan, Runzhe Hou, Bing Han, and Xuexun Fang. 2025. "Cell Membrane- and Vesicle-Based Bionic Nanodrugs: Applications in Central Nervous System Diseases and Exploration of Nasal–Cerebral Delivery" Gels 11, no. 11: 846. https://doi.org/10.3390/gels11110846
APA StyleDing, F., Hou, R., Han, B., & Fang, X. (2025). Cell Membrane- and Vesicle-Based Bionic Nanodrugs: Applications in Central Nervous System Diseases and Exploration of Nasal–Cerebral Delivery. Gels, 11(11), 846. https://doi.org/10.3390/gels11110846





