Preparation and Characterization of Chemically Cross-Linked Xanthan/Poly(Vinylalcohol) Hydrogel Films Containing Cerium Oxide Nanoparticles for Potential Application in Removal of Methylene Blue and Crystal Violet Dyes
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of Xn/PVA/CeO2NPs Nanocomposite Films
2.2. Structural Characterization of Nanocomposite Hydrogel Films
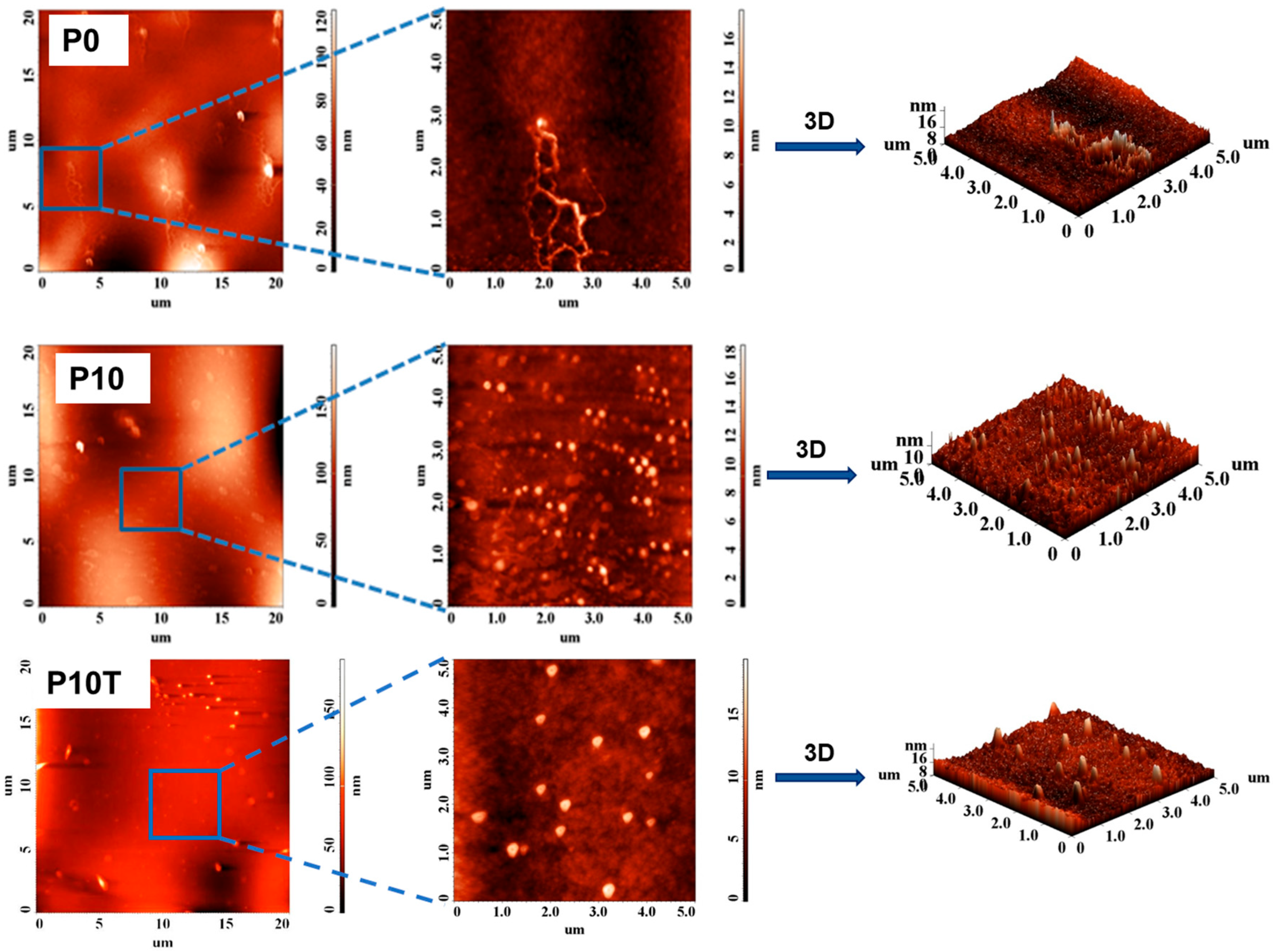
2.3. Morphology of Nanocomposite Hydrogel Films
2.4. Optical Analysis
2.4.1. Optical Properties of Hydrogel Films Without CeO2NPs
2.4.2. Optical Properties of Nanoparticle-Doped Films
2.4.3. Effect of Doping and Thermal Treatment
2.4.4. Urbach Energy Analysis
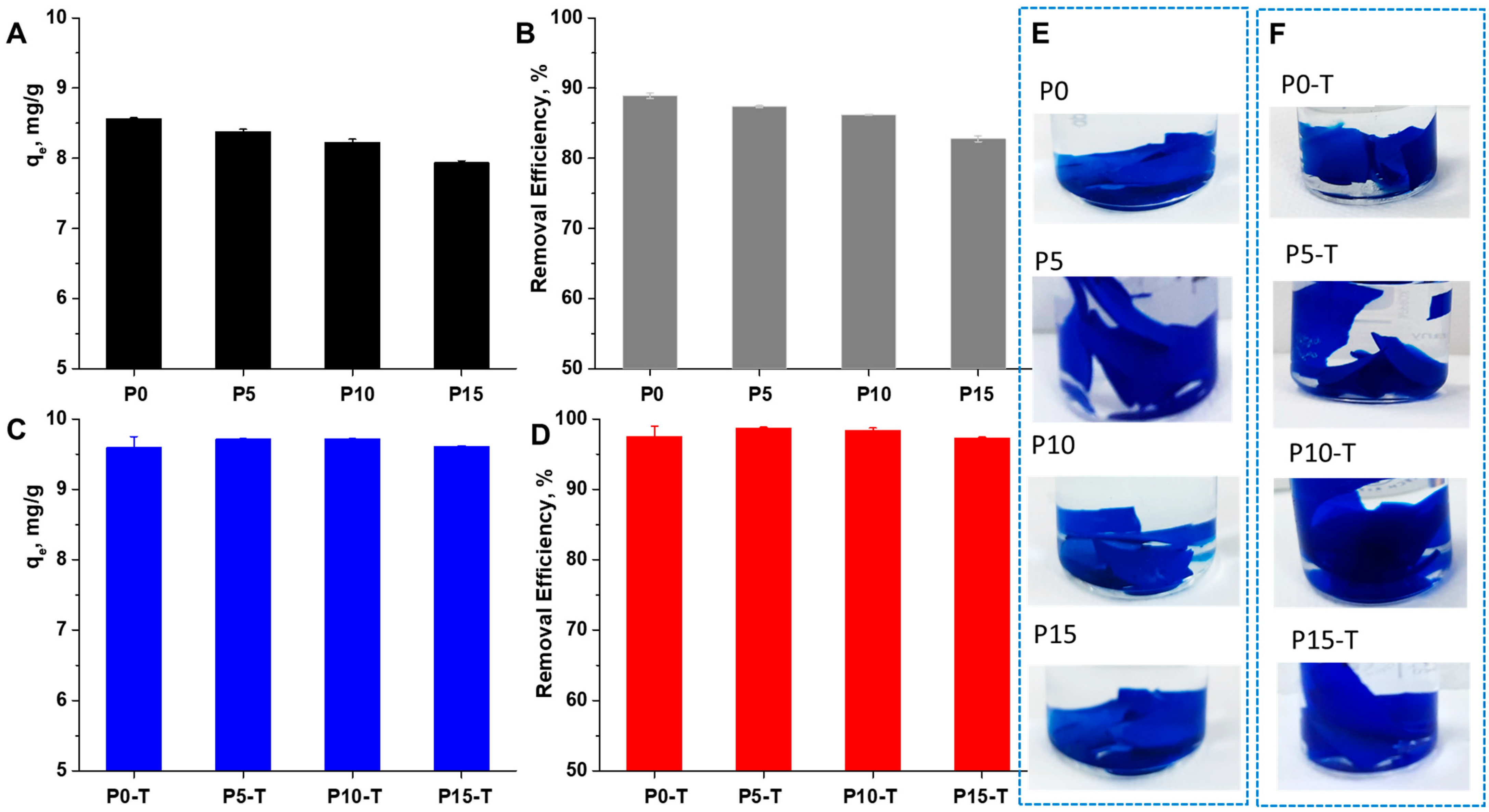
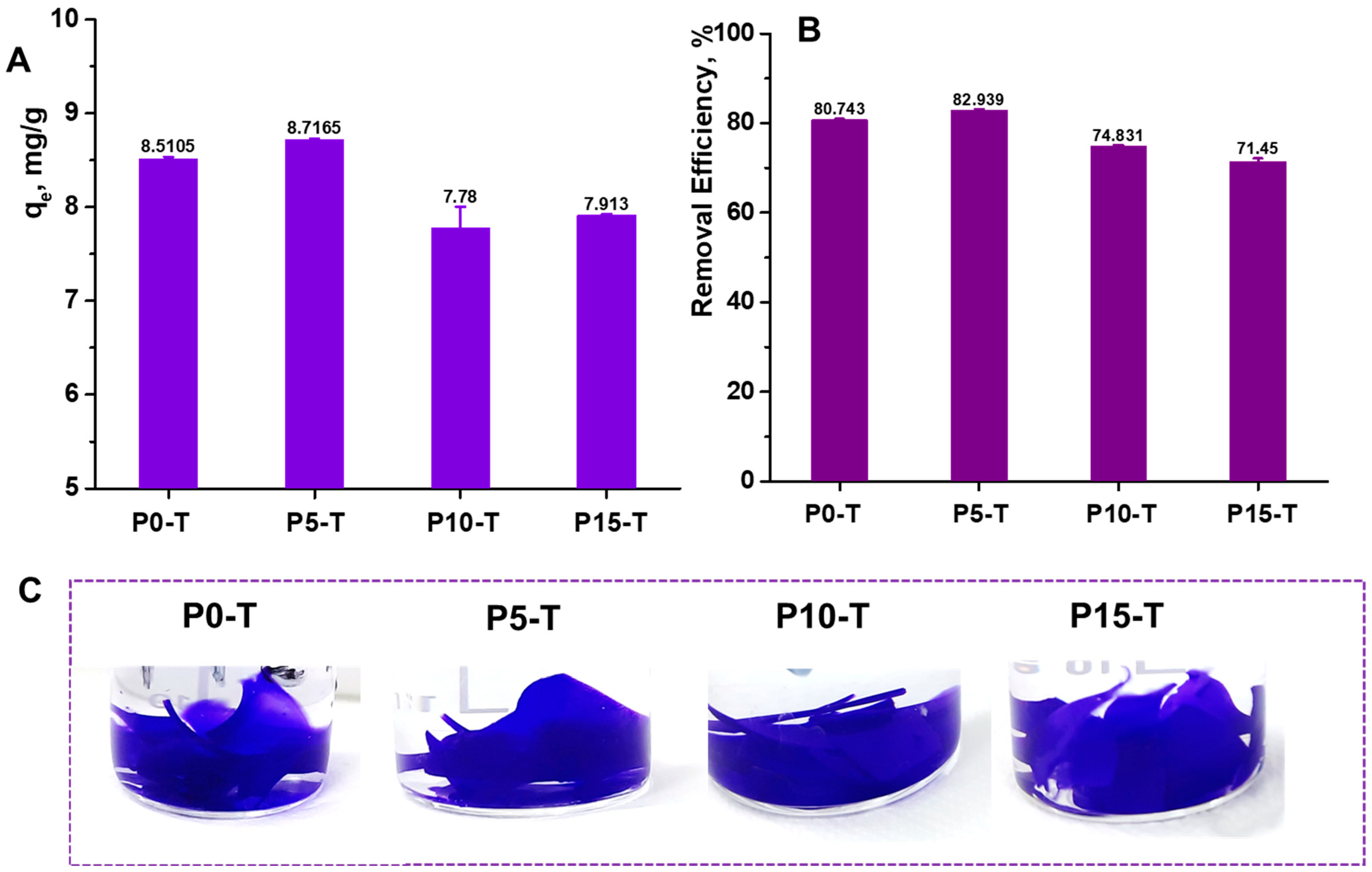
2.5. Sorption of Synthetic Dyes
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Polymeric Films
4.3. Methods of Film Characterization
4.3.1. FTIR Spectroscopy
4.3.2. Swelling Behavior
4.3.3. DSC
4.3.4. SEM
4.3.5. AFM
4.3.6. UV–Vis Spectroscopy
4.4. Sorption Experiments
4.5. Desorption of Dyes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, G.; Thakur, B.; Naushad, M.; Kumar, A.; Stadler, F.J.; Alfadul, S.M.; Mola, G.T. Applications of nanocomposite hydrogels for biomedical engineering and environmental protection. Environ. Chem. Lett. 2018, 16, 113–146. [Google Scholar] [CrossRef]
- Panigrahy, S.K.; Nandha, A.; Chaturvedi, M.; Mishra, P.K. Novel nanocomposites with advanced materials and their role in waste water treatment. Next Sustain. 2024, 4, 100042. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Abdallah, H.M.; Ali, E.A.; Makhado, E.; El-Ghany, N.A.A. Recent developments in conductive polysaccharide adsorbent formulations for environmental remediation: A review. Int. J. Biol. Macromol. 2025, 304, 140915. [Google Scholar] [CrossRef]
- Pandey, S.; Makhado, E.; Kim, S.; Kang, M. Recent developments of polysaccharide based superabsorbent nanocomposite for organic dye contamination removal from wastewater—A review. Environ. Res. 2023, 217, 114909. [Google Scholar] [CrossRef]
- Elella, M.H.A.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; Rehman, A.U.; Yoon, K.R. Xanthan gum-derived materials for applications in environment and eco-friendly materials: A review. J. Environ. Chem. Eng. 2021, 9, 104702. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Alshammari, R.H.; Hasan, I. Synthesis of xanthan gum anchored α-Fe2O3 bionanocomposite material for remediation of Pb (II) contaminated aquatic system. Polymers 2023, 15, 1134. [Google Scholar] [CrossRef]
- Faizan, M.; Qayyum, M.A.; Javed, M.; Fatima, M.; Bahadur, A.; Iqbal, S.; Mahmood, S.; Alsalhi, S.A.; Althobiti, R.A.; Alzahrani, E.; et al. Development of a novel pH-responsive PVA/GO-Glu/TiO2 nanocomposite hydrogel for efficient degradation of organic pollutants. Opt. Mater. 2024, 150, 115337. [Google Scholar] [CrossRef]
- Santoso, S.P.; Angkawijaya, A.E.; Bundjaja, V.; Hsieh, C.-W.; Go, A.W.; Yuliana, M.; Hsu, H.-Y.; Tran-Nguyen, P.L.; Soetaredjo, F.E.; Ismadji, S. TiO2/guar gum hydrogel composite for adsorption and photodegradation of methylene blue. Int. J. Biol. Macromol. 2021, 193, 721–733. [Google Scholar] [CrossRef]
- Kulal, P.; Badalamoole, V. Modified gum ghatti based hybrid hydrogel nanocomposite as adsorbent material for dye removal from wastewater. Int. J. Biol. Macromol. 2024, 283, 137409. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Fakhiminajafi, B.; Pakdel, P.M.; Azimi, H. Simultaneous elimination of cationic dyes from water media by carboxymethyl cellulose-graft-poly (acrylamide)/magnetic biochar nanocomposite hydrogel adsorbent. Environ. Res. 2025, 273, 121150. [Google Scholar] [CrossRef]
- Salehi, M.M.; Hassanzadeh-Afruzi, F.; Esmailzadeh, F.; Choopani, L.; Rajabi, K.; Kuzekanan, H.N.; Azizi, M.; Yeganeh, F.E.; Demchuk, O.M.; Maleki, A. Chlorpyrifos and diazinon elimination through pAAm-g-XG/HKUST-1@Fe3O4 biopolymer nanoadsorbent hydrogel from wastewater: Preparation, characterization, kinetics and isotherm. Sep. Purif. Technol. 2024, 334, 126097. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Shoghinia, B.; Alizadeh, A.H. A self-assembling hydrogel nanocomposite based on xanthan gum modified with SiO2 NPs and HPAM for improved adsorption of crystal violet cationic dye from aqueous solution. Carbohydr. Polym. 2024, 330, 121819. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Hesarsorkh, A.H.A. Chitosan and silica nanoparticles-modified xanthan gum-derived bio-nanocomposite hydrogel film for efficient uptake of methyl orange acidic dye. Carbohydr. Polym. 2024, 328, 121721. [Google Scholar] [CrossRef]
- Hussain, S.; Salman, M.; Al-Ahmary, K.M.; Ahmed, M. Synthesis of potential adsorbent for removal of malachite green dye using alginate hydrogel nanocomposites. Int. J. Biol. Macromol. 2025, 289, 138816. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Ray, S.S.; Maity, A. Synthesis and characterization of alginate beads encapsulated zinc oxide nanoparticles for bacteria disinfection in water. J. Colloid Interface Sci. 2018, 512, 686–692. [Google Scholar] [CrossRef]
- Malatji, N.; Makhado, E.; Modibane, K.D.; Ramohlola, K.E.; Maponya, T.C.; Monama, G.R.; Hato, M.J. Removal of methylene blue from wastewater using hydrogel nanocomposites: A review. Nanomater. Nanotechnol. 2021, 11, 18479804211039425. [Google Scholar] [CrossRef]
- Rathore, B.S.; Perumal, P.; Singh, G.P.; Rathore, R.; Rawal, M.K.; Jadoun, S.; Chauhan, N.P.S. Polyaniline, chitosan and metal oxide based nanocomposites: Synthesis, characterization, and their water remediation applications. J. Mol. Struct. 2025, 1337, 142157. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S. A study on the adsorption of methylene blue onto gum ghatti/TiO2 nanoparticles-based hydrogel nanocomposite. Int. J. Biol. Macromol. 2016, 88, 66–80. [Google Scholar] [CrossRef]
- Makhado, E.; Motshabi, B.R.; Allouss, D.; Ramohlola, K.E.; Modibane, K.D.; Hato, M.J.; Jugade, R.M.; Shaik, F.; Pandey, S. Development of a ghatti gum/poly(acrylic acid)/TiO2 hydrogel nanocomposite for malachite green adsorption from aqueous media: Statistical optimization using response surface methodology. Chemosphere 2022, 306, 135524. [Google Scholar] [CrossRef]
- Rahman, O.; Ali, A.; Husain, A.; Khan, S.A.; Tariq, M.; Urooj, S.; Mihet-Popa, L.; Khan, Q. Investigation of CeO2 nanoparticles on the performance enhancement of insulating oils. Heliyon 2023, 9, e19264. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, C.; Wan, Y.; Majid, M.; Abbas, S.Q.; Wang, Y. In vitro and in vivo evaluation of alginate hydrogel based wound dressing loaded with green chemistry cerium oxide nanoparticles. Front. Chem. 2023, 11, 1298808. [Google Scholar] [CrossRef]
- Garg, D.; Matai, I.; Garg, A.; Sachdev, A. Tragacanth hydrogel integrated CeO2@rGO nanocomposite as reusable photocatalysts for organic dye degradation. ChemistrySelect 2020, 5, 10663–10672. [Google Scholar] [CrossRef]
- Melnikova, N.; Sheferov, I.; Panteleev, D.; Emasheva, A.; Druzhkova, I.; Ignatova, N.; Mishchenko, T.; Vedunova, M. Design and study of nanoceria modified by 5-fluorouracil for gel and polymer dermal film preparation. Pharmaceuticals 2023, 16, 1082. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Reukov, V.V.; Yakimansky, A.V.; Krasnopeeva, E.L.; Ivanova, O.S.; Popov, A.L.; Ivanov, V.K. CeO2 nanoparticle-containing polymers for biomedical applications: A review. Polymers 2021, 13, 924. [Google Scholar] [CrossRef]
- Raja, I.S.; Fathima, N.N. Gelatin−cerium oxide nanocomposite for enhanced excisional wound healing. ACS Appl. Bio Mater. 2018, 1, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yang, X.; Fang, M.; Tian, J.; Zhang, S.; Lu, L.; Zhou, C.; Xu, C.; Qi, Y.; Li, L. Photothermal effective CeO2NPs combined in thermosensitive hydrogels with enhanced antibacterial, antioxidant and vascularization performance to accelerate infected diabetic wound healing. Regen. Biomater. 2023, 10, rbad072. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, B.; Gihar, S.; Kumar, D. Review on emerging trends and challenges in the modification of xanthan gum for various applications. Carbohydr. Res. 2024, 538, 109070. [Google Scholar] [CrossRef]
- Rao, K.M.; Yeo, D.; Kim, E.; Rao, K.S.V.K.; Chandra, M.R.G.S.; Han, S.S. Xanthan gum and chitosan polyelectrolyte hydrogels with self-reinforcement of Zn+2 for wound dressing applications. Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134550. [Google Scholar] [CrossRef]
- Ali, A.; Bairagi, S.; Ganie, S.A.; Ahmed, S. Polysaccharides and proteins based bionanocomposites as smart packaging materials: From fabrication to food packaging applications a review. Int. J. Biol. Macromol. 2023, 252, 126534. [Google Scholar] [CrossRef]
- Kashaudhan, K.; Pande, P.P.; Sharma, J.; Shankar, R.; Nath, A. Modified xanthan gum (natural polymeric material) and its derivative for wastewater treatment: A review. Environ. Prog. Sustain. Energy 2024, 43, e14419. [Google Scholar] [CrossRef]
- Raschip, I.E.; Paduraru-Mocanu, O.M.; Nita, L.E.; Dinu, M.V. Antibacterial porous xanthan-based films containing flavoring agents evaluated by near infrared chemical imaging technique. J. Appl. Polym. Sci. 2020, 137, e49111. [Google Scholar] [CrossRef]
- Raschip, I.E.; Fifere, N.; Varganici, C.-D.; Dinu, M.V. Development of antioxidant and antimicrobial xanthan-based cryogels with tuned porous morphology and controlled swelling features. Int. J. Biol. Macromol. 2020, 156, 608–620. [Google Scholar] [CrossRef]
- Raschip, I.E.; Platon, I.-V.; Fifere, N.; Darie-Nita, R.-N.; Aprotosoaie, A.C.; Dinu, M.V. Stabilization of anthocyanins in xanthan-based systems for synergistic cryogels with enhanced physicochemical and biological properties for visual freshness monitoring of Prussian carp (Carassius gibelio). Food Hydrocoll. 2025, 168, 111566. [Google Scholar] [CrossRef]
- Raschip, I.E.; Fifere, N.; Lazar, M.M.; Hitruc, G.-E.; Dinu, M.V. Ice-templated and cross-linked xanthan-based hydrogels: Towards tailor-made properties. Gels 2023, 9, 528. [Google Scholar] [CrossRef]
- Ghorai, S.; Sarkar, A.K.; Panda, A.B.; Pal, S. Effective removal of Congo red dye from aqueous solution using modified xanthan gum/silica hybrid nanocomposite as adsorbent. Bioresour. Technol. 2013, 144, 485–491. [Google Scholar] [CrossRef]
- Ghorai, S.; Sarkar, A.K.; Pal, S. Rapid adsorptive removal of toxic Pb2+ ion from aqueous solution using recyclable, biodegradable nanocomposite derived from templated partially hydrolyzed xanthan gum and nanosilica. Bioresour. Technol. 2014, 170, 578–582. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Application of Xanthan gum/n-acetyl cysteine modified mica bionanocomposite as an adsorbent for the removal of toxic heavy metals. Groundw. Sustain. Dev. 2018, 7, 101–108. [Google Scholar] [CrossRef]
- Njuguna, D.G.; Schönherr, H. Smart and regeneratable Xanthan gum hydrogel adsorbents for selective removal of cationic dyes. J. Environ. Chem. Eng. 2022, 10, 107620. [Google Scholar] [CrossRef]
- Mittal, H.; Parashar, V.; Mishra, S.B.; Mishra, A.K. Fe3O4 MNPs and gum xanthan based hydrogels nanocomposites for the efficient capture of malachite green from aqueous solution. Chem. Eng. J. 2014, 255, 471–482. [Google Scholar] [CrossRef]
- Taktak, F.F.; Ozyaranlar, E. Semi-interpenetrating network based on xanthan gum-cl-2-(N-morpholinoethyl methacrylate)/titanium oxide for the single and binary removal of cationic dyes from water. Int. J. Biol. Macromol. 2022, 221, 238–255. [Google Scholar] [CrossRef]
- Mesgari, M.; Matin, M.M.; Goharshadi, E.K.; Mashreghi, M. Biogenesis of bacterial cellulose/xanthan/CeO2NPs composite films for active food packaging. Int. J. Biol. Macromol. 2024, 273, 133091. [Google Scholar] [CrossRef]
- Orhan, B.; Karadeniz, D.; Kalaycıoglu, Z.; Kaygusuz, H.; Torlak, E.; Erim, F.B. Foam-based antibacterial hydrogel composed of carboxymethyl cellulose/polyvinyl alcohol/cerium oxide nanoparticles for potential wound dressing. Int. J. Biol. Macromol. 2025, 291, 138924. [Google Scholar] [CrossRef]
- Fifere, N.; Airinei, A.; Dobromir, M.; Sacarescu, L.; Dunca, S.I. Revealing the effect of synthesis conditions on the structural, optical, and antibacterial properties of cerium oxide nanoparticles. Nanomaterials 2021, 11, 2596. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Petri, D.F.S. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydr. Polym. 2013, 92, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Romasanta, L.J.; D’Alençon, L.; Kirchner, S.; Pradère, C.; Leng, J. Thin coatings of cerium oxide nanoparticles with anti-reflective properties. Appl. Sci. 2019, 9, 3886. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV−Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Fís. 2007, 53, 18–22. [Google Scholar]
- Harynski, Ł.; Olejnik, A.; Grochowska, K.; Siuzdak, K. A facile method for Tauc exponent and corresponding electronic transitions determination in semiconductors directly from UV–Vis spectroscopy data. Opt. Mater. 2022, 127, 112205. [Google Scholar] [CrossRef]
- Sharma, J.; Singh, G.; Thakur, A.; Saini, G.S.S.; Goyal, N.; Tripathi, S.K. Preparation and characterization of SnSe nanocrystalline thin films. J. Optoelectron. Adv. Mater. 2005, 7, 2085–2094. [Google Scholar]
- Calvache-Muñoz, J.; Prado, F.A.; Rodríguez-Páez, J.E. Cerium oxide nanoparticles: Synthesis, characterization and tentative mechanism of particle formation. Coll. Surf. A 2017, 529, 146–159. [Google Scholar] [CrossRef]
- Nadjia, L.; Abdelkader, E.; Naceur, B.; Ahmed, B. CeO2 nanoscale particles: Synthesis, characterization and photocatalytic activity under UVA light irradiation. J. Rare Earths 2018, 36, 575–587. [Google Scholar] [CrossRef]
- Portillo, M.C.; Moreno, O.P.; Mora-Ramírez, M.A.; Santiesteban, H.J.; Avendaño, C.B.; Bernal, Y.P. Optical and structural analysis of the charge transfer of Ce3++ e−→Ce4+ ion in the cerium oxide (CeO2). Optik 2021, 248, 168178. [Google Scholar] [CrossRef]
- Skorodumova, N.V.; Ahuja, R.; Simak, S.I.; Abrikosov, I.A.; Johansson, B.; Lundqvist, B.I. Electronic, bonding, and optical properties of CeO2 and Ce2O3 from first principles. Phys. Rev. B 2001, 64, 115108. [Google Scholar] [CrossRef]
- Đorđevic, M.P.; Vukoje, I.; Lazic, V.; Đorđevic, V.; Sredojevic, D.; Dostanic, J.; Loncarevic, D.; Ahrenkiel, S.P.; Belic, M.R.; Nedeljkovic, J.M. Electronic structure of surface complexes between CeO2 and benzene derivatives: A comparative experimental and DFT study. Mater. Chem. Phy. 2019, 236, 121816. [Google Scholar] [CrossRef]
- Marabelli, F.; Wachter, P. Covalent insulator CeO2: Optical reflectivity measurements. Phys. Rev. B 1987, 36, 1238–1243. [Google Scholar] [CrossRef]
- Cresi, J.S.P.; Di Mario, L.; Catone, D.; Martelli, F.; Paladini, A.; Turchini, S.; D’Addato, S.; Luches, P.; O’Keeffe, P. Ultrafast formation of small polarons and the optical gap in CeO2. J. Phys. Chem. Lett. 2020, 11, 5686–5691. [Google Scholar] [CrossRef] [PubMed]
- Imhof, S.; Thränhardt, A. Phonon-assisted transitions and optical gain in indirect semiconductors. Phys. Rev. B 2010, 82, 085303. [Google Scholar] [CrossRef]
- Hemalatha, K.S.; Rukmani, K. Synthesis, characterization and optical properties of polyvinyl alcohol–cerium oxide nanocomposite films. RSC Adv. 2016, 6, 74354–74366. [Google Scholar] [CrossRef]
- Leonhardt, A.; de la Rosa, C.J.L.; Nuytten, T.; Banszerus, L.; Sergeant, S.; Mootheri, V.K.; Taniguchi, T.; Watanabe, K.; Stampfer, C.; Huyghebaert, C.; et al. Use of the indirect photoluminescence peak as an optical probe of interface defectivity in MoS2. Adv. Mater. Interfaces 2020, 7, 2000413. [Google Scholar] [CrossRef]
- Kabir, H.; Nasrin, R.; Rahman, M.M.; Bhuiyan, A.H. Heat treatment effect on the structural, morphological, and optical properties of plasma polymerized furan-2-carbaldehyde thin films. Results Phys. 2020, 16, 103014. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Akl, A.A. Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattices Microstruct. 2016, 89, 153–169. [Google Scholar] [CrossRef]
- Fifere, N.; Airinei, A.; Timpu, D.; Rotaru, A.; Sacarescu, L.; Ursu, L. New insights into structural and magnetic properties of Ce doped ZnO nanoparticles. J. Alloys Compd. 2018, 757, 60–69. [Google Scholar] [CrossRef]
- Pelletier, E.; Viebke, C.; Meadows, J.; Williams, P.A.A. Rheological study of the order–disorder conformational transition of xanthan gum. Biopolymers 2001, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Lazar, M.M.; Dinu, M.V.; Dascalu, I.A.; Nacu, I.; Verestiuc, L. Superelastic chitosan/laponite nanocomposite sponges with tunable functional properties as promising biomaterials for wound management. ACS Appl. Bio Mater. 2025, 8, 7699–7714. [Google Scholar] [CrossRef]
- Raschip, I.E.; Hitruc, G.E.; Vasile, C.; Popescu, M.-C. Effect of the lignin type on the morphology and thermal properties of the xanthan/lignin hydrogels. Int. J. Biol. Macromol. 2013, 54, 230–237. [Google Scholar] [CrossRef]
- Dinu, M.V.; Humelnicu, D.; Lazar, M.M.; Dragan, E.S. Cationic composite cryogels as potential filters for removal of metallic pollutants, anionic dyes, and bacteria from water. ACS Appl. Eng. Mater. 2025, 3, 3271–3284. [Google Scholar] [CrossRef]
- Lazar, M.M.; Ghiorghita, C.-A.; Rusu, D.; Dinu, M.V. Nanocomposite cryogels based on chitosan for efficient removal of a triphenylmethane dye from aqueous systems. Gels 2025, 11, 729. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Lazar, M.M.; Platon, I.-V.; Humelnicu, D.; Doroftei, F.; Dinu, M.V. Feather-weight cryostructured thiourea-chitosan aerogels for highly efficient removal of heavy metal ions and bacterial pathogens. Int. J. Biol. Macromol. 2023, 235, 123910. [Google Scholar] [CrossRef] [PubMed]
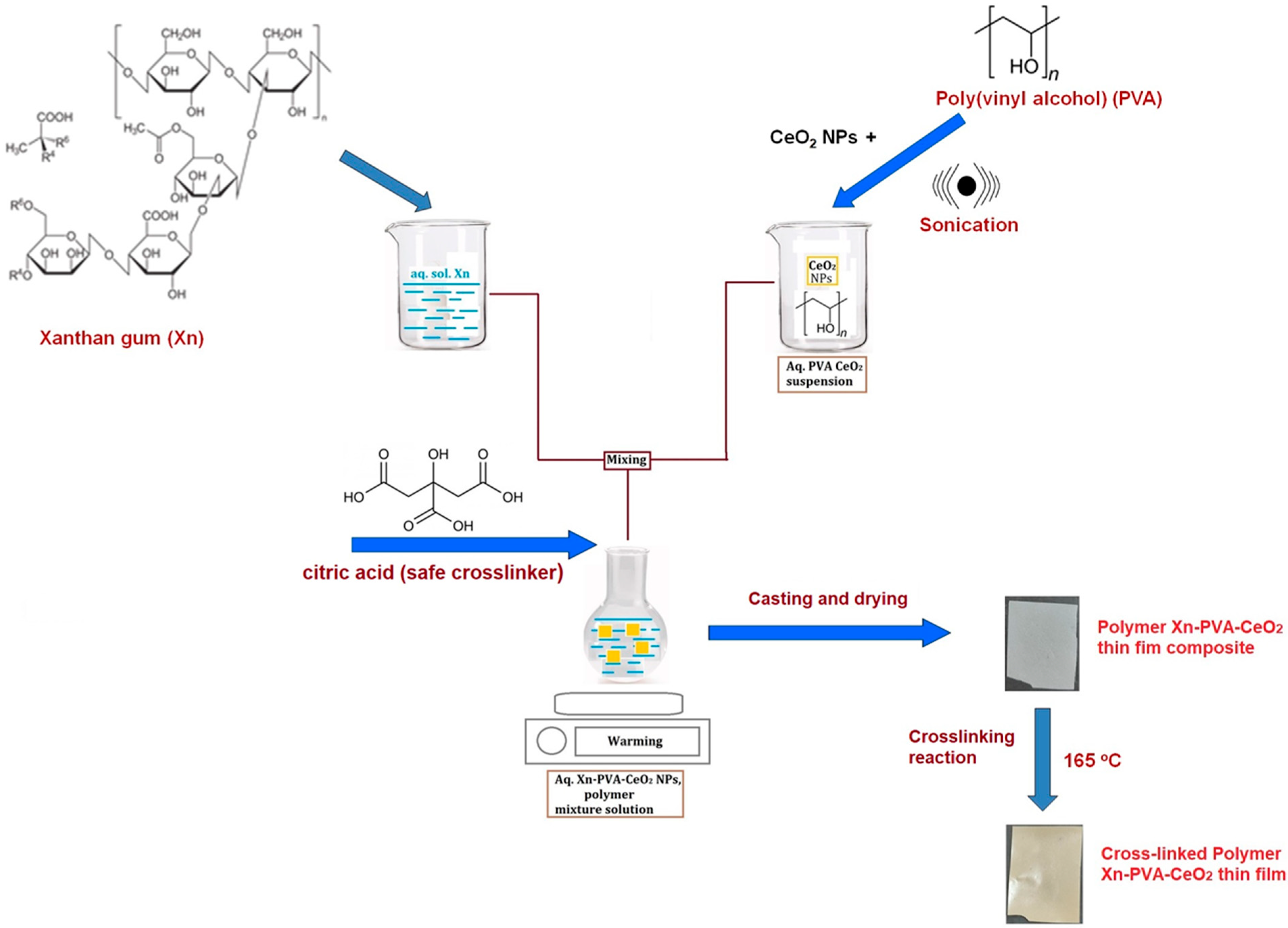
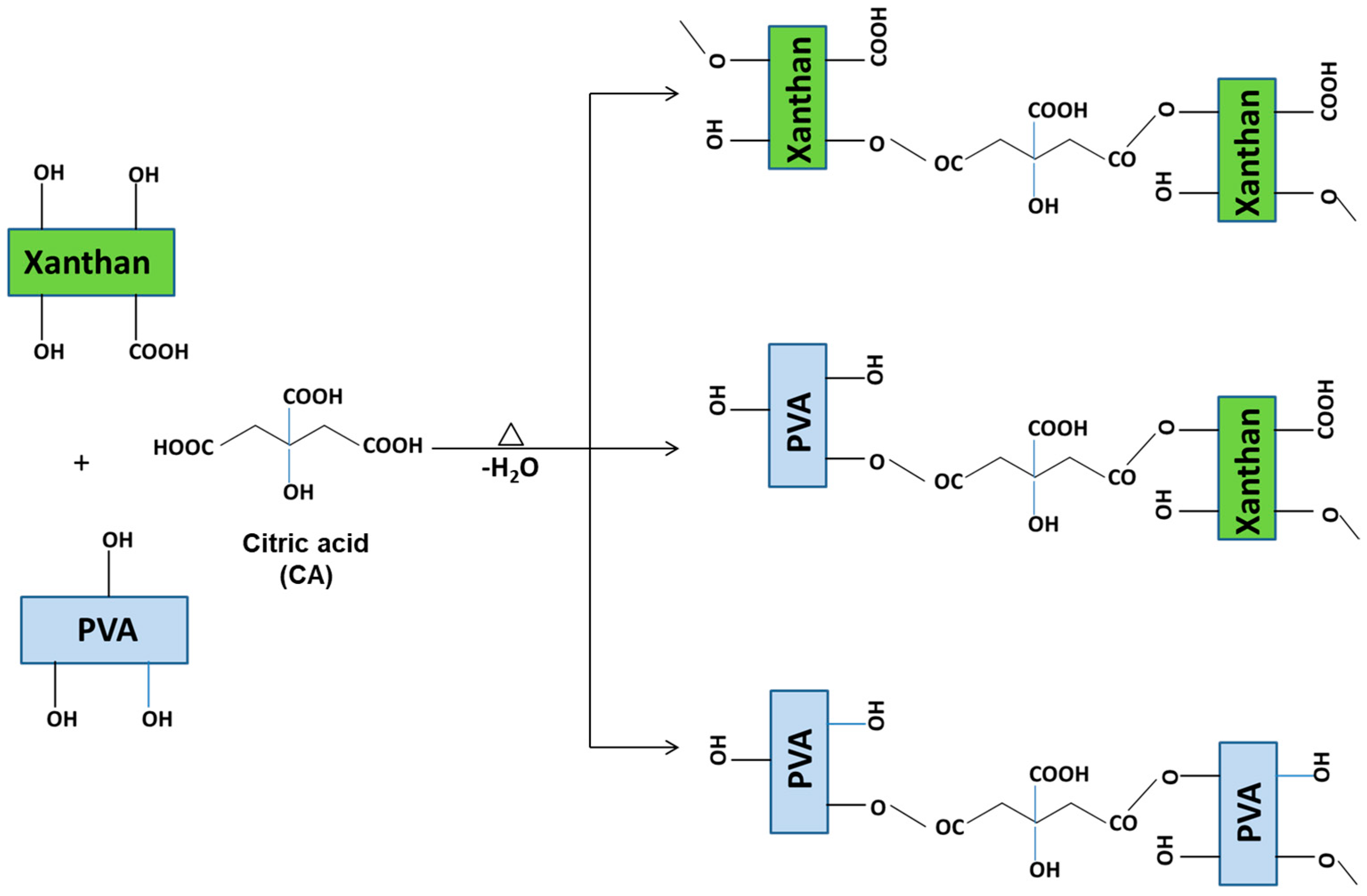
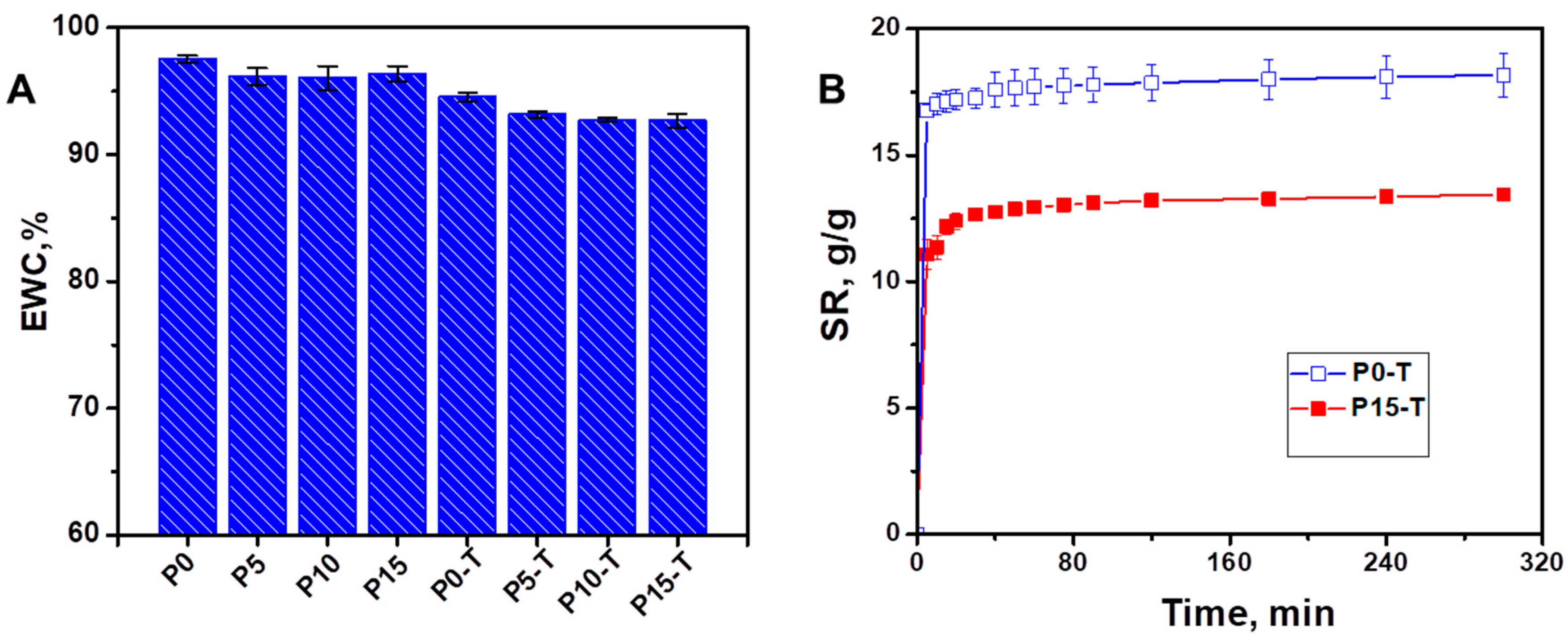
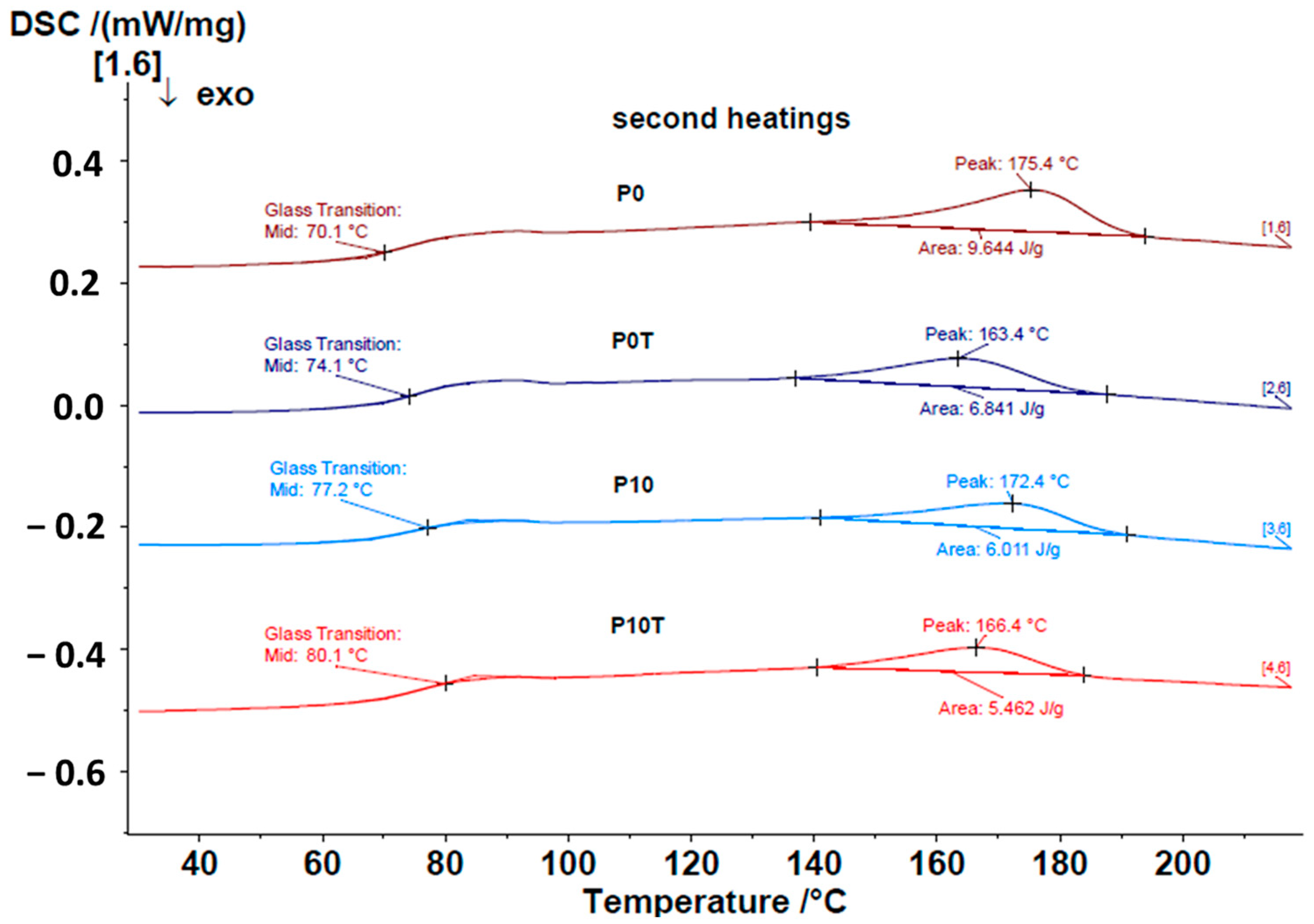

| 1 Sample Code | Xn/PVA % Weight Ratio | % CeO2 NPs | Thermal Treatment, °C |
|---|---|---|---|
| P0 | 50/50 | 0 | - |
| P0-T | 50/50 | 0 | 165 |
| P5 | 50/50 | 5 | - |
| P5-T | 50/50 | 5 | 165 |
| P10 | 50/50 | 10 | - |
| P10-T | 50/50 | 10 | 165 |
| P15 | 50/50 | 15 | - |
| P15-T | 50/50 | 15 | 165 |
| Samples | 5 × 5 µm2 | 10 × 10 µm2 | 20 × 20 µm2 |
|---|---|---|---|
| P0 | 1.473 | 3.267 | 9.401 |
| P10 | 1.822 | 3.961 | 24.367 |
| P10-T | 2.142 | - | 31.987 |
| Samples | (eV) | (meV) | (%) | |||
|---|---|---|---|---|---|---|
| n = 1/2 | n = 2 | |||||
| CeO2 | 3.173 | 4.102 | 2.951 | 4.042 | 282.75 | - |
| P5 | 3.18 | 4.043 | 3 | 4.009 | 259.39 | - |
| P10 | 3.182 | 4.058 | 2.977 | 4.051 | 223.65 | - |
| P15 | 3.153 | 2.912 | 213.90 | - | ||
| P5-T | 3.110 | 3.023 | 505.43 | 94.85 | ||
| P10-T | 3.120 | 3.014 | 345.03 | 54.27 | ||
| P15-T | 3.121 | 3.006 | 326.51 | 52.65 | ||
| 1 Sorbent | Dye Type | Sorbent Dose, g/L | pH | RE% | Reference |
|---|---|---|---|---|---|
| TiO2-embedded guar gum hydrogel | MB | - | 10 | ~93% | [8] |
| Gum ghatti-g-PAMPS/Fe3O4 hydrogel nanocomposite | MB | 10 | 2–10 | ~63.5–78% | [9] |
| CMC-g-PAAm/CL-Fe3O4 | MB | 1.5 | 9 | ~95.01% | [10] |
| HPAM/SiO2@Xn | CV | 20 | 7 | ~95% | [12] |
| TiO2-containing Gum ghatti-cl-PAAm composite hydrogel | MB | - | 7 | ~98% | [18] |
| Cross-linked Acrylate Xn | MB | 1.2 | 11 | >95% | [39] |
| Xn-cl-2-(N-morpholinoethyl methacrylate)/TiO2 hydrogels | MB | 40 | 11 | ~80% | [41] |
| Xn-cl-2-(N-morpholinoethyl methacrylate)/TiO2 hydrogels | CV | 40 | 11 | ~60% | [41] |
| Xn/PVA/CeO2 nanocomposite hydrogel films | MB | 5 | 5.8 | ~97–98% | This study |
| Xn/PVA/CeO2 nanocomposite hydrogel films | CV | 5 | 5.8 | ~71–83% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fifere, N.; Lazar, M.M.; Raschip, I.E.; Airinei, A.; Varganici, C.-D.; Dinu, M.V. Preparation and Characterization of Chemically Cross-Linked Xanthan/Poly(Vinylalcohol) Hydrogel Films Containing Cerium Oxide Nanoparticles for Potential Application in Removal of Methylene Blue and Crystal Violet Dyes. Gels 2025, 11, 809. https://doi.org/10.3390/gels11100809
Fifere N, Lazar MM, Raschip IE, Airinei A, Varganici C-D, Dinu MV. Preparation and Characterization of Chemically Cross-Linked Xanthan/Poly(Vinylalcohol) Hydrogel Films Containing Cerium Oxide Nanoparticles for Potential Application in Removal of Methylene Blue and Crystal Violet Dyes. Gels. 2025; 11(10):809. https://doi.org/10.3390/gels11100809
Chicago/Turabian StyleFifere, Nicusor, Maria Marinela Lazar, Irina Elena Raschip, Anton Airinei, Cristian-Dragos Varganici, and Maria Valentina Dinu. 2025. "Preparation and Characterization of Chemically Cross-Linked Xanthan/Poly(Vinylalcohol) Hydrogel Films Containing Cerium Oxide Nanoparticles for Potential Application in Removal of Methylene Blue and Crystal Violet Dyes" Gels 11, no. 10: 809. https://doi.org/10.3390/gels11100809
APA StyleFifere, N., Lazar, M. M., Raschip, I. E., Airinei, A., Varganici, C.-D., & Dinu, M. V. (2025). Preparation and Characterization of Chemically Cross-Linked Xanthan/Poly(Vinylalcohol) Hydrogel Films Containing Cerium Oxide Nanoparticles for Potential Application in Removal of Methylene Blue and Crystal Violet Dyes. Gels, 11(10), 809. https://doi.org/10.3390/gels11100809









