Effect of Sodium Alginate Concentration on the Physicochemical, Structural, Functional Attributes, and Consumer Acceptability of Gel Beads Encapsulating Tangerine Peel (Citrus reticulata Blanco ‘Cho Khun’) Extract
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
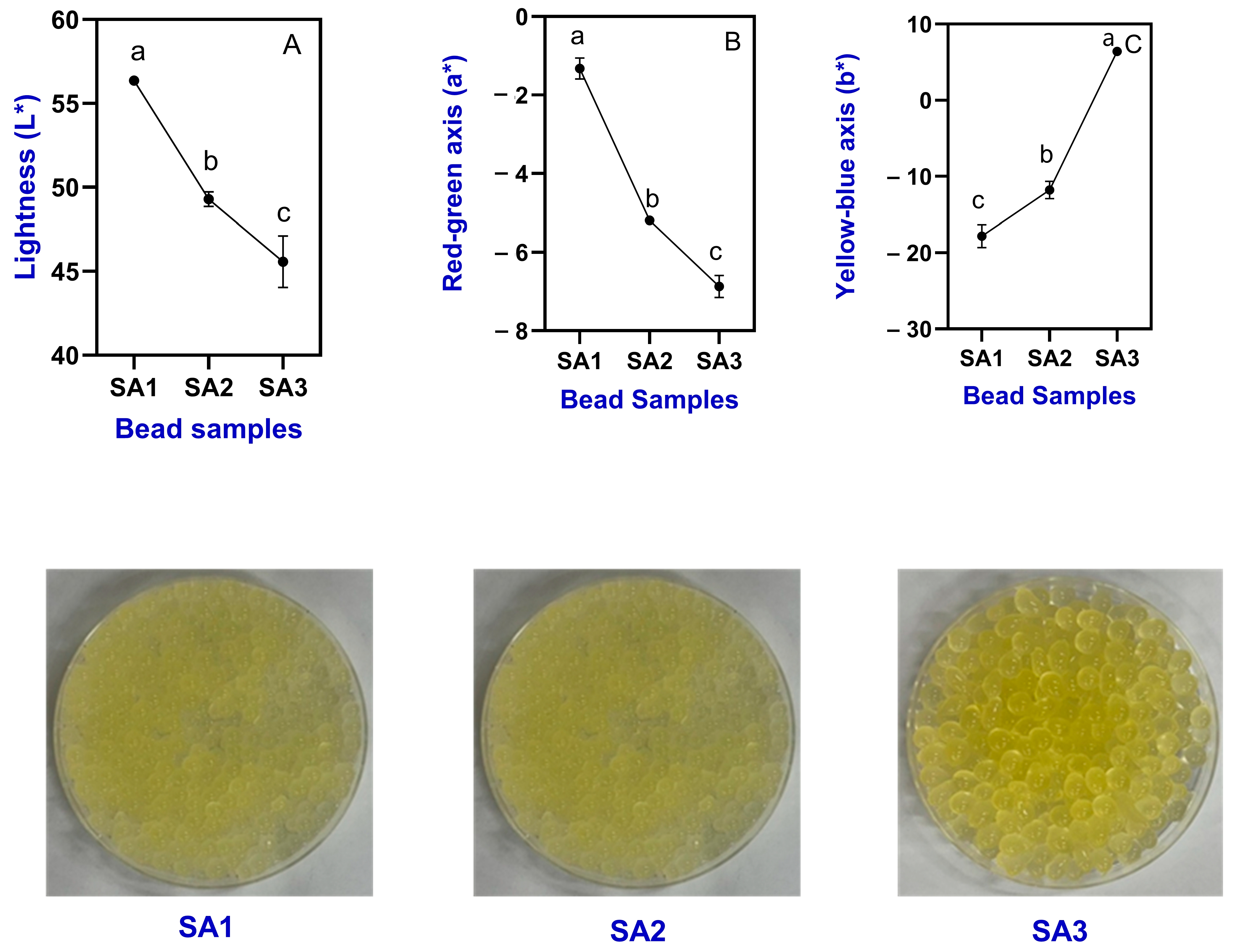
2.1.1. Color Characteristics and Visual Appearance
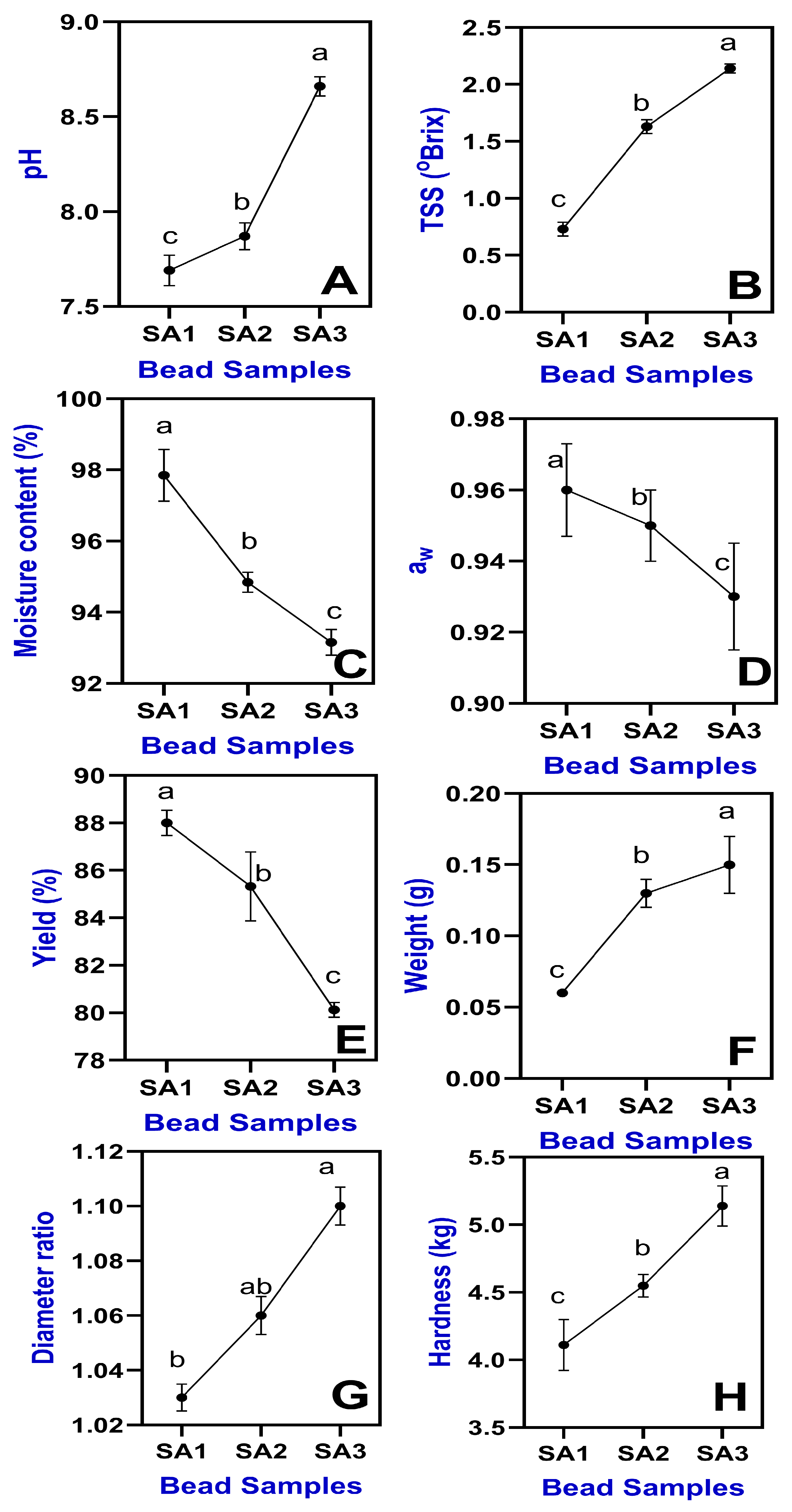
2.1.2. pH and TSS
2.1.3. Moisture Content and Water Activity
2.1.4. Weight and Yield
2.1.5. Diameter Ratio and Hardness
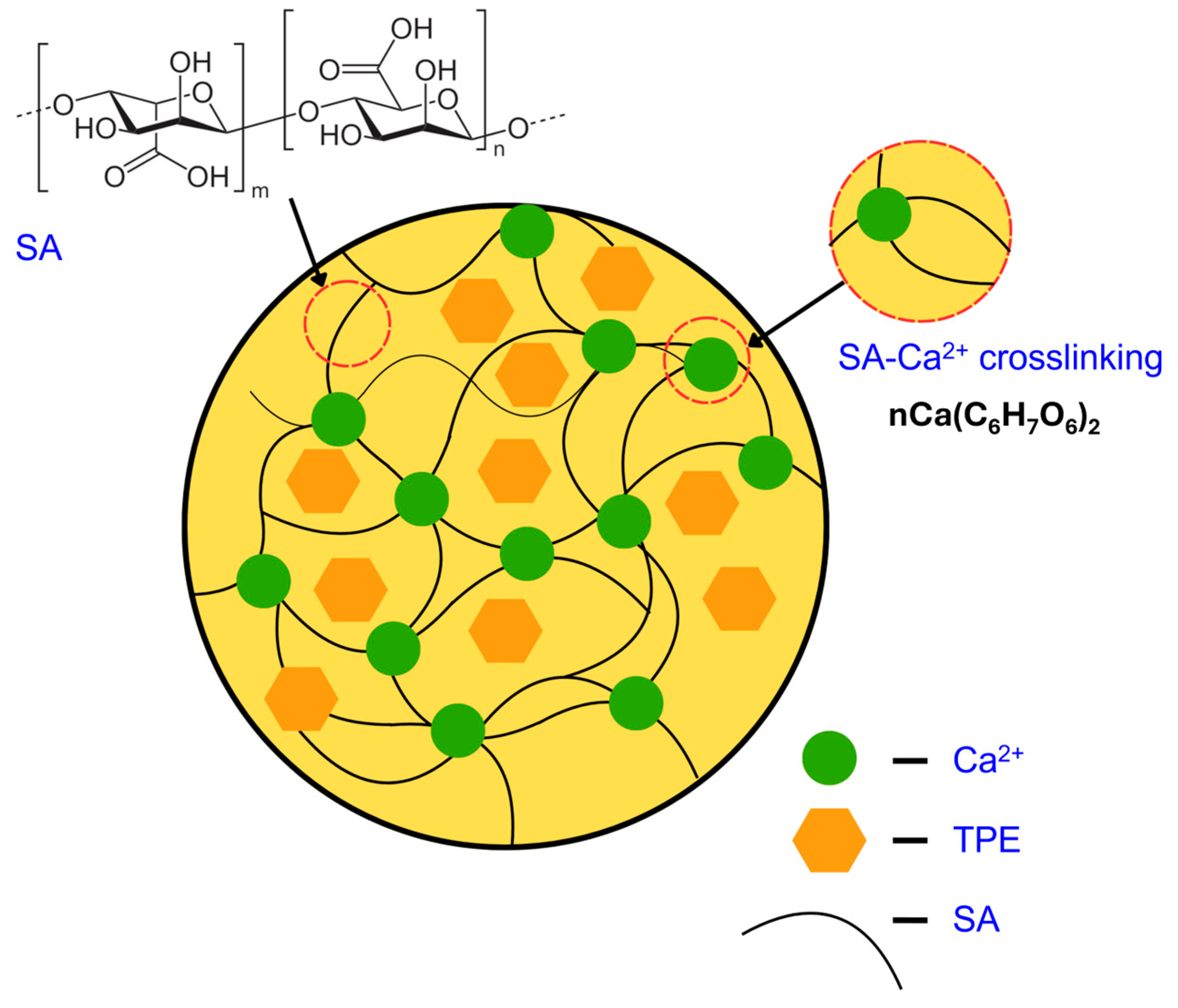
2.2. Structural and Molecular Characteristics
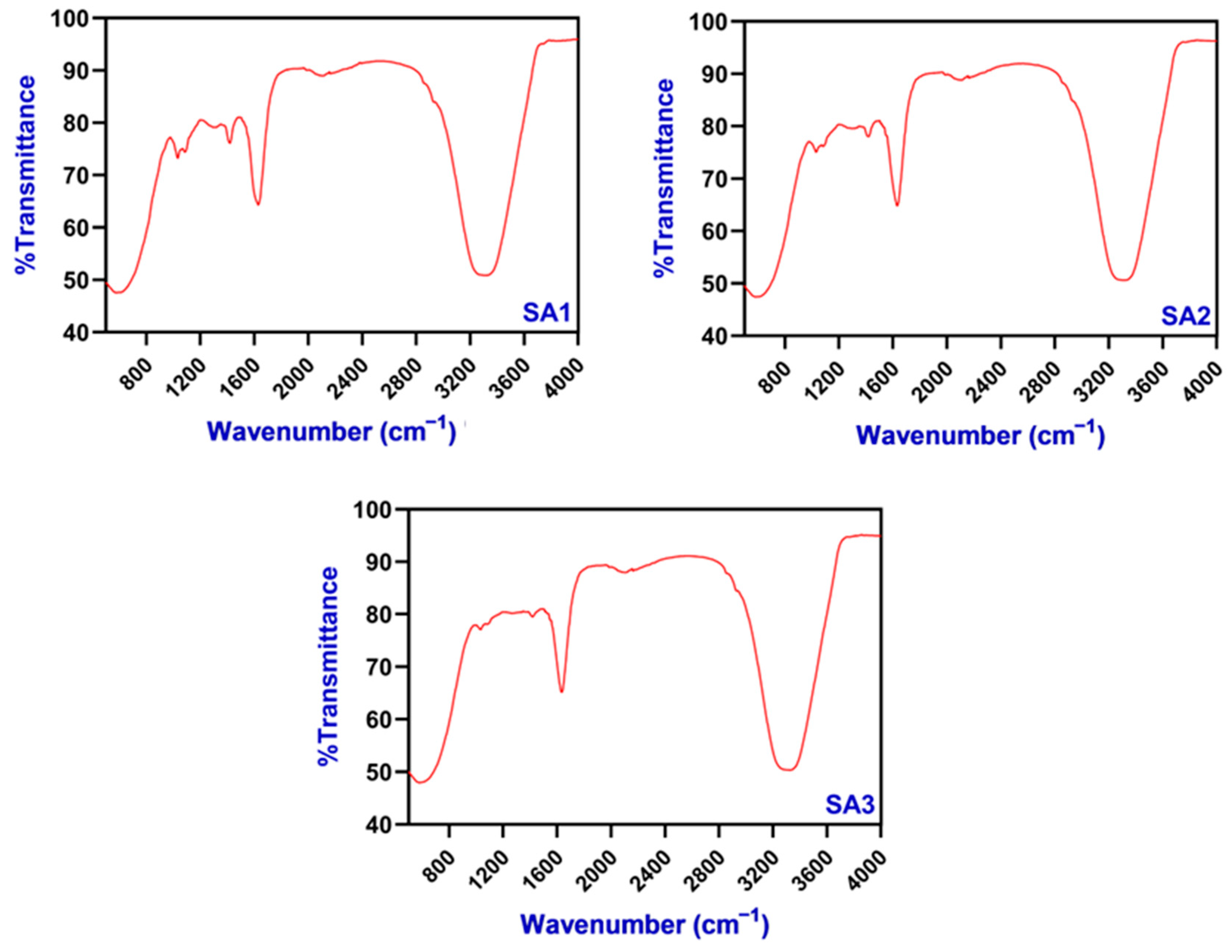
2.2.1. FTIR
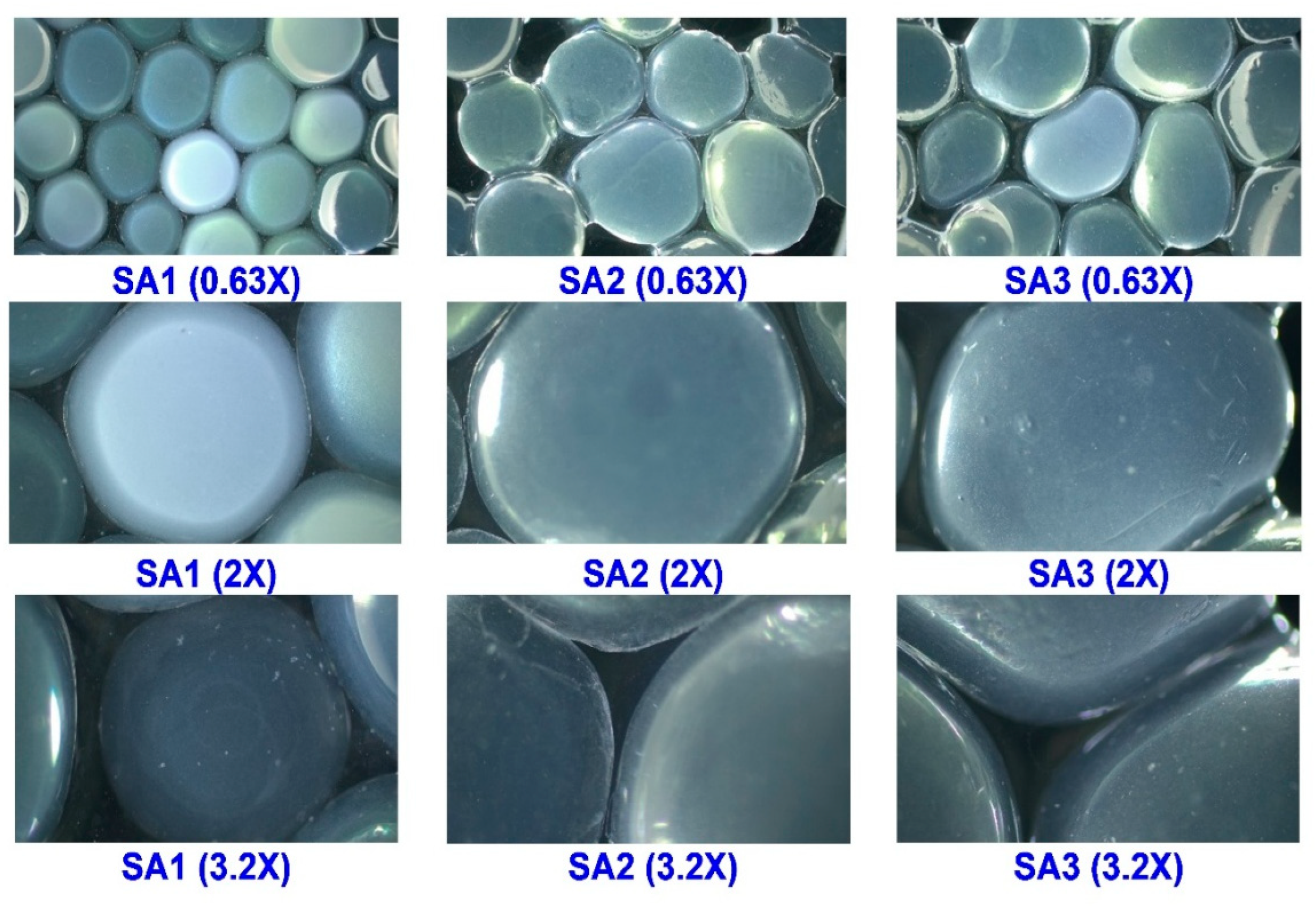
2.2.2. Morphological Observations
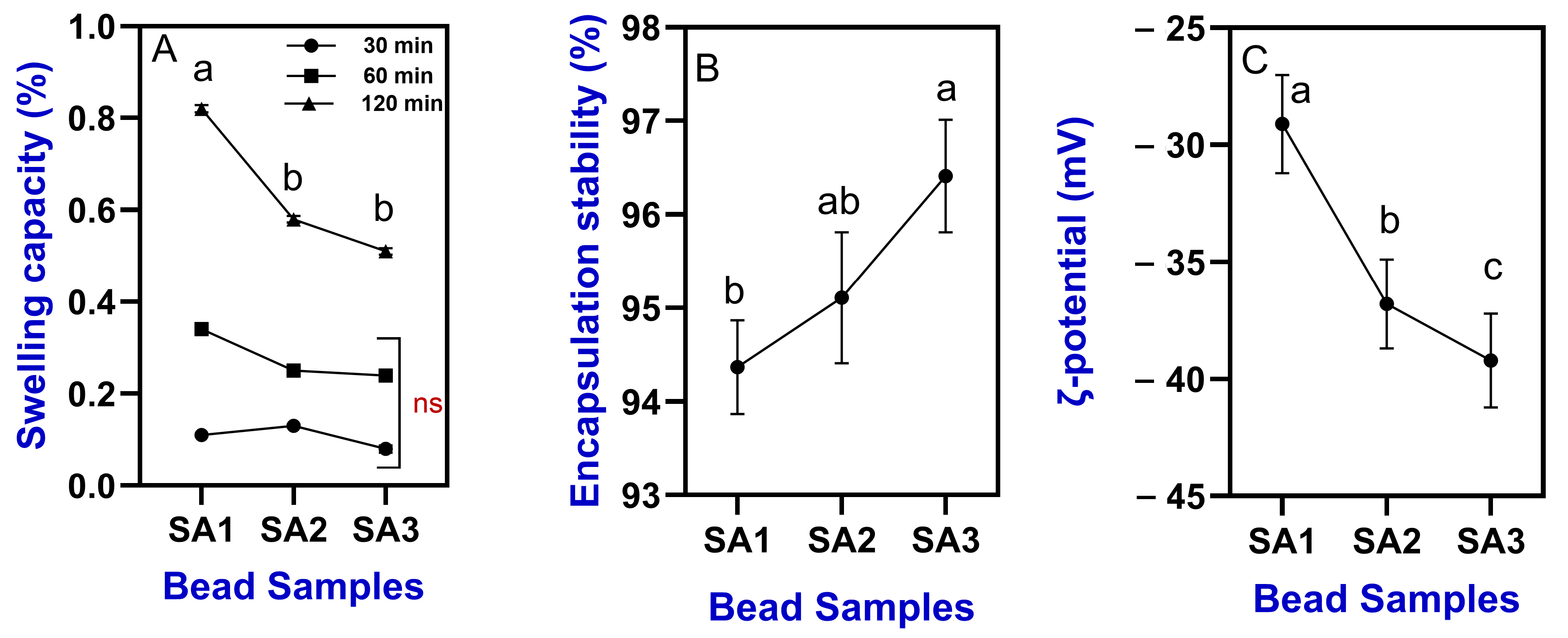
2.2.3. Swelling Capacity and Encapsulation Stability
2.2.4. Zeta (ζ) Potential
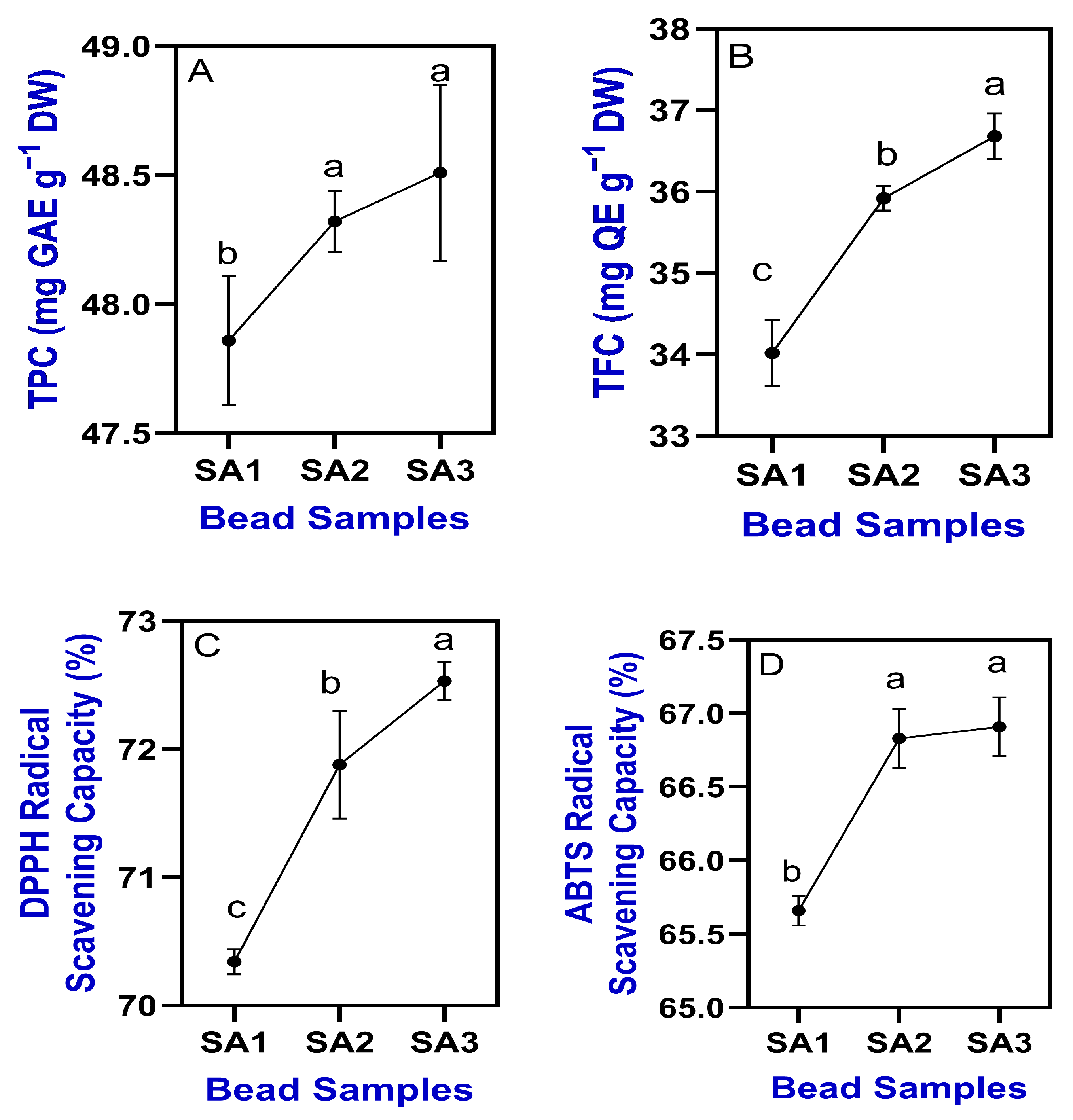
2.3. Phytochemicals and Antioxidant Properties
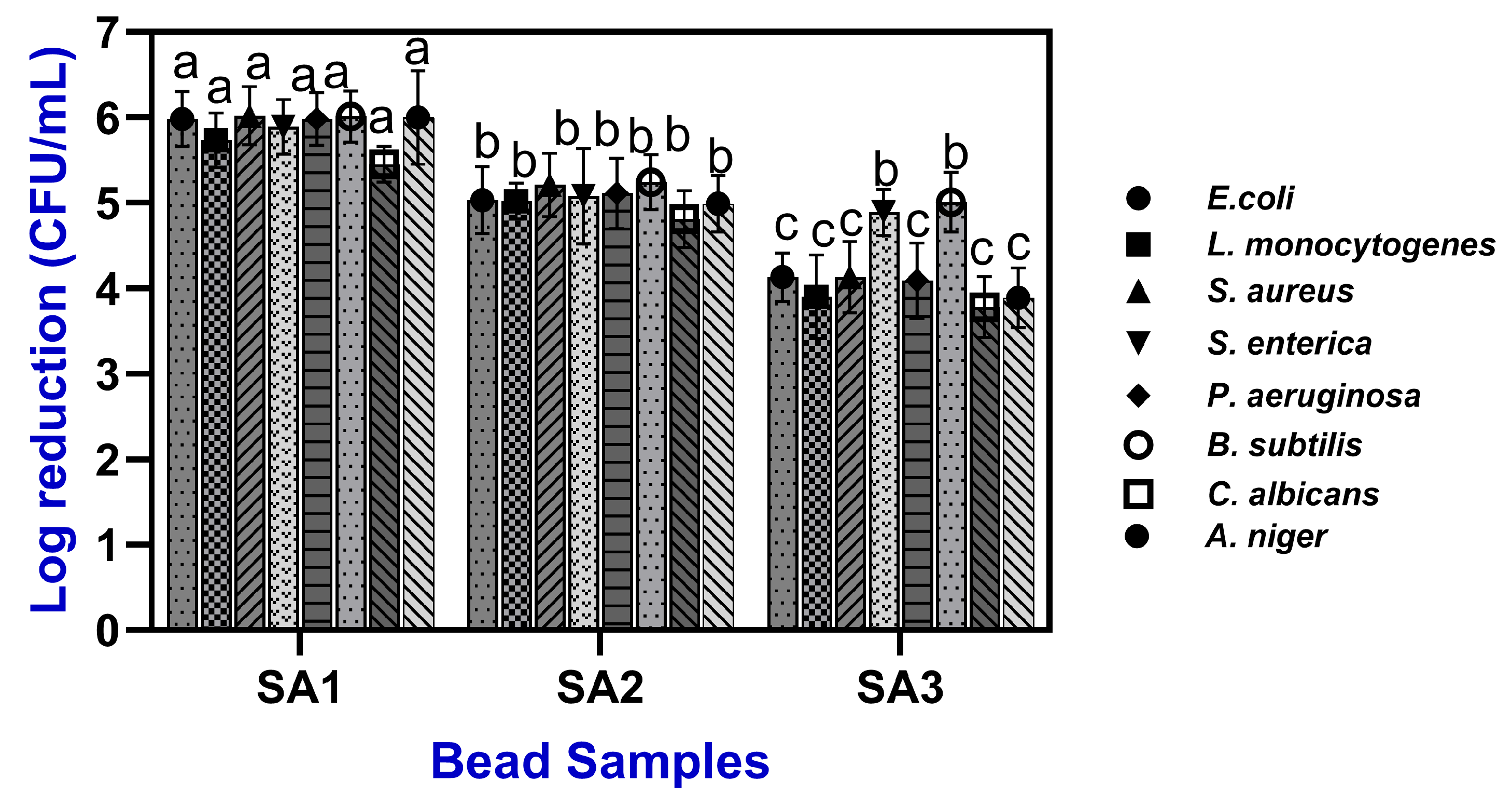
2.4. Antimicrobial Activities
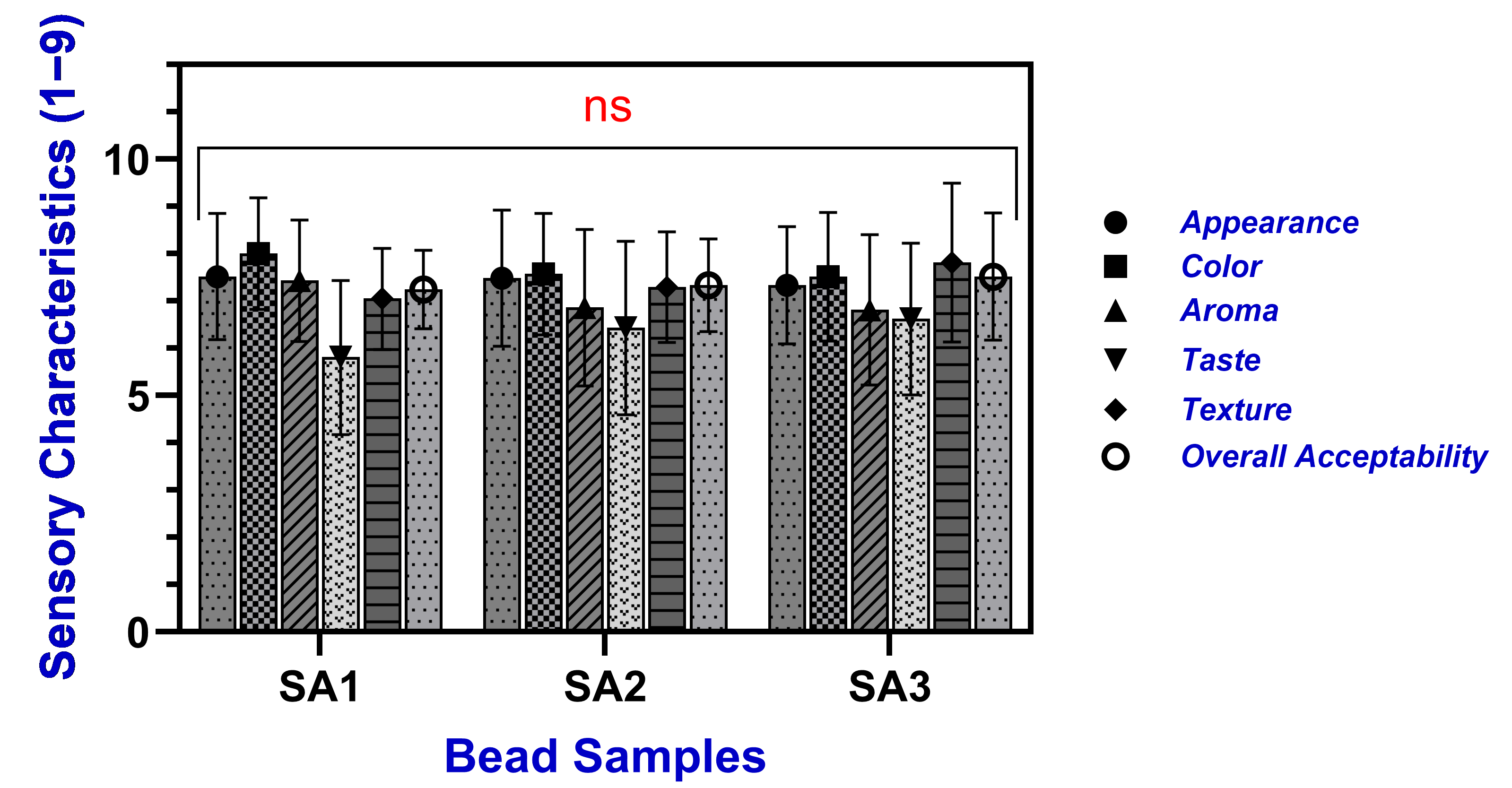
2.5. Sensory Characterization
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
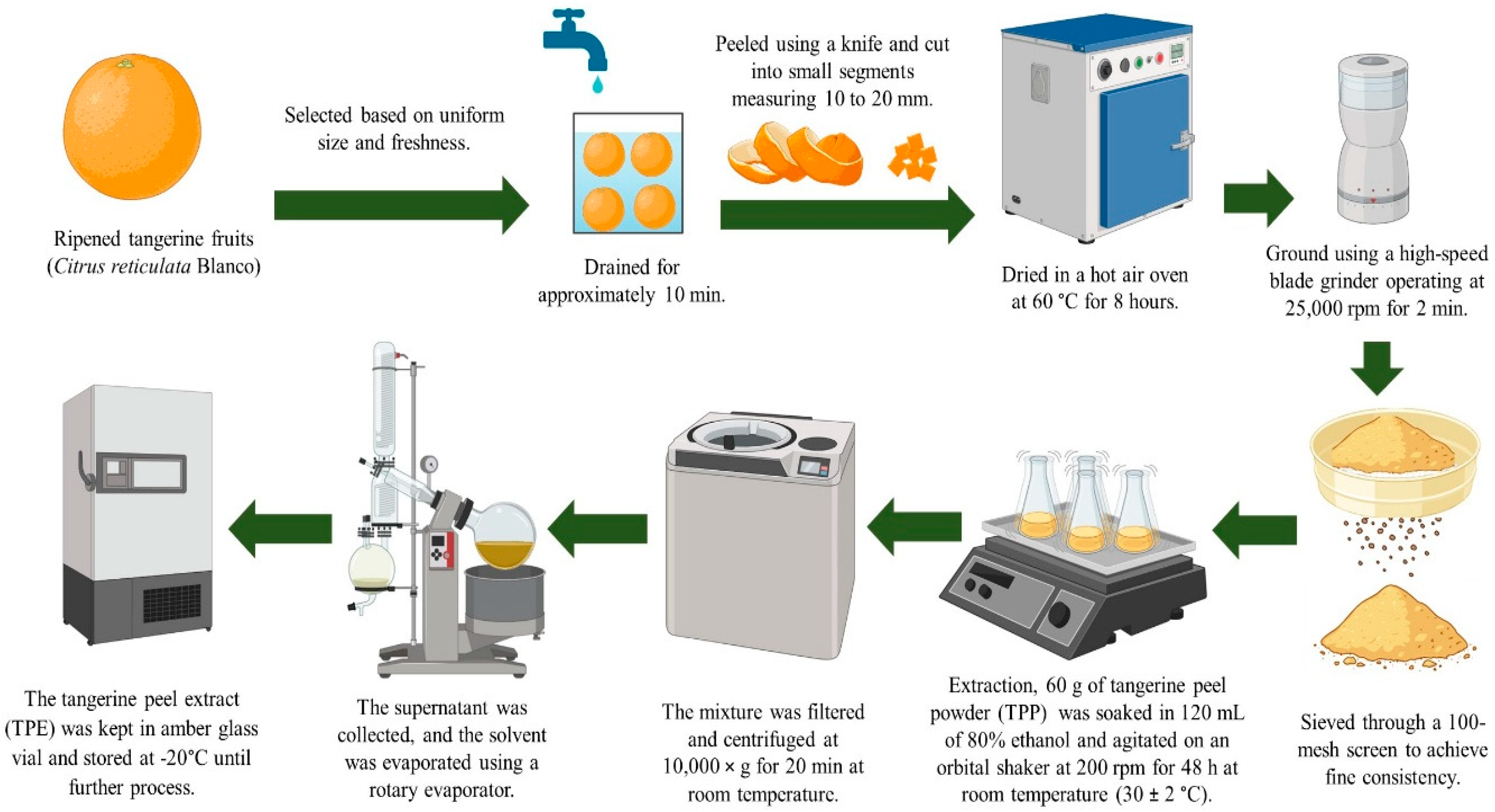
4.2. Preparation of TPE
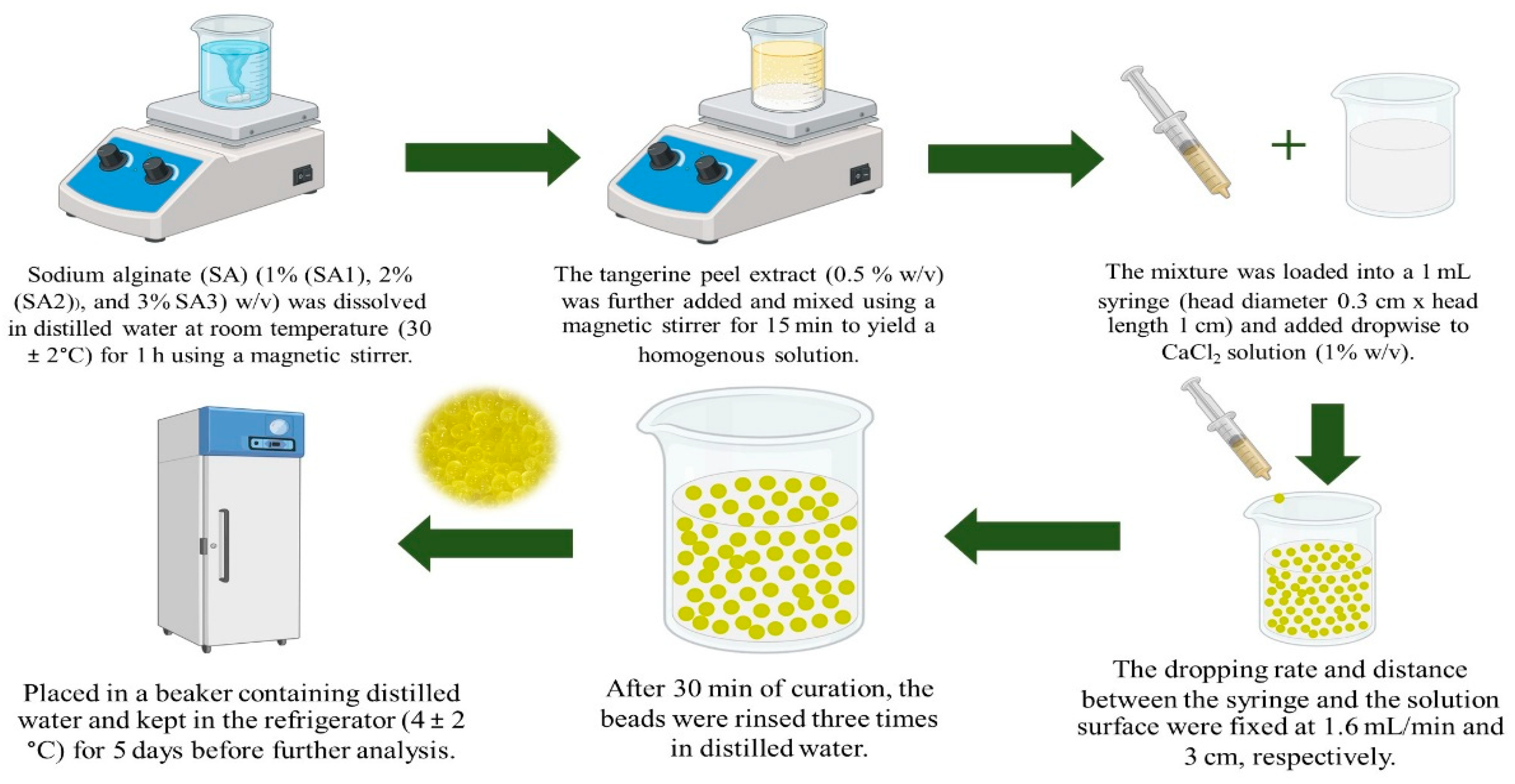
4.3. Preparation of TPEen-SA-Based Gel Beads
4.4. Determination of Physicochemical Properties
4.4.1. Color Characteristics and Visual Appearance
4.4.2. pH and Total Soluble Solids (TSS)
4.4.3. Moisture Content and aw
4.4.4. Weight and Yield
4.4.5. Diameter Ratio and Hardness
4.5. Determination of Structural and Molecular Characteristics
4.5.1. FTIR Spectroscopy
4.5.2. Morphological Observations
4.5.3. Swelling Capacity
4.5.4. Encapsulation Efficiency
4.5.5. ζ-Potential
4.6. Antioxidant Properties
4.6.1. Total Phenolic Content (TPC)
4.6.2. Total Flavonoid Content (TFC)
4.6.3. DPPH Radical Scavenging Activity
4.6.4. ABTS Radical Scavenging Activity
4.7. Antimicrobial Properties
4.8. Sensory Characterization
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a* | Red-green axis |
| ABTS | 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) |
| ATCC | The American Type Culture Collection |
| aw | Water activity |
| b* | Yellow-blue axis |
| BOD | Biochemical oxygen demand |
| CaCl2 | Calcium chloride |
| COO− | Carboxylate |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DW | Dry weight |
| FC | Folin–Ciocalteu |
| FTIR | Fourier transform infrared spectroscopy |
| L* | Lightness |
| MHA | Mueller–Hinton agar |
| MHB | Mueller–Hinton broth |
| PDA | Potato dextrose agar |
| QE | Quercetin equivalent |
| SA | Sodium alginate |
| SA1 | Sodium alginate at concentrations of 1% |
| SA2 | Sodium alginate at concentrations of 2% |
| SA3 | Sodium alginate at concentrations of 3% |
| SDA | Sabouraud dextrose agar |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
| TPCb | The total phenolic content in the disintegrated gel bead supernatant |
| TPCe | The total phenolic content of the TPE solution before the encapsulation |
| TPE | Tangerine peel extract |
| TPEen | TPE encapsulated |
| TPP | Tangerine peel powder |
| TSS | Total soluble solids |
| W0 | The initial weight of the bead samples |
| Wt | The weight of the bead samples at each time interval |
| ζ | Zeta |
References
- Andrade, A.M.; Barbosa, H.C.; Shah, A.M.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, S.A.; Ramos, F. Citrus by-products: Valuable source of bioactive compounds for food applications. Antioxidants 2022, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Negrea, M.; Cocan, I.; Jianu, C.; Alexa, E.; Berbecea, A.; Poiana, M.; Silivasan, M. Valorization of citrus peel byproducts: A sustainable approach to nutrient-rich jam production. Foods 2025, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Zema, A.D.; Calabro, S.P.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, M.S. Wastewater management in citrus processing industries: An overview of advantages and limits. Water 2019, 11, 2481. [Google Scholar] [CrossRef]
- Alam, P.; Alam, A.; Anwer, M.K.; Alqasoumi, S.I. Quantitative estimation of hesperidin by HPTLC in different varieties of citrus peels. Asian Pac. J. Trop. Biomed. 2014, 4, 262–266. [Google Scholar] [CrossRef]
- Martinidou, E.; Michailidis, M.; Ziogas, V.; Masuero, D.; Angeli, A.; Moysiadis, T.; Martens, S.; Ganopoulos, I.; Molassiotis, A.; Sarrou, E. Comparative evaluation of secondary metabolite chemodiversity of citrus genebank collection in Greece: Can the peel be more than waste? J. Agric. Food Chem. 2024, 72, 9019–9032. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Cronje, P.J.; Landahl, S.; Ortiz, J.O.; Terry, L.A. Rapid methods for extracting and quantifying phenolic compounds in citrus rinds. Food Sci. Nutr. 2016, 4, 4–10. [Google Scholar] [CrossRef]
- Sung, J.; Suh, H.J.; Wamg, Y. Effects of heat treatment of mandarin peel on flavonoid profiles and lipid accumulation in 3T3-L1 adipocytes. J. Food. Drug. Anal. 2019, 27, 729–735. [Google Scholar] [CrossRef]
- Lin, S.; Simal-Gandara, J.; Cao, H.; Xiao, J. The stability and degradation products of polyhydroxy flavonols in boiling water. Curr. Res. Food Sci. 2023, 6, 100509. [Google Scholar] [CrossRef]
- Saini, K.R.; Raamjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, M.G.K.; Keum, Y. Bioactive compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Zhang, L.; Yuan, M.; Zhao, L.; Bai, C.; McClements, D.J. Impacts of hesperidin on whey protein functionality: Interacting mechanism, antioxidant capacity, and emulsion stabilizing effects. Front. Nutr. 2023, 9, 1043095. [Google Scholar] [CrossRef]
- König, A.; Sadova, N.; Dornmayr, M.; Schwarzinger, B.; Neuhauser, C.; Stadlbauer, V.; Wallner, M.; Woischitzschläger, J.; Müller, A.; Tona, R.; et al. Combined acid hydrolysis and fermentation improves bioactivity of citrus flavonoids in vitro and in vivo. Commun Biol. 2023, 6, 1083. [Google Scholar] [CrossRef]
- Rizki, M.I.; Agustina, N.K.A.; Sari, A.K.; Rahmatullah, S.W.; Isnani, N. Characteristics and antioxidant activity of Limau kuit peel (Citrus hystrix) extract with variation of extraction solvent. Med. Lab. Technol. J. 2025, 11, 110–122. [Google Scholar] [CrossRef]
- De Miera, B.S.; Cañadas, R.; González-Miquel, M.; González, E.J. Recovery of phenolic compounds from orange peel waste by conventional and assisted extraction techniques using sustainable solvents. Front. Biosci. 2023, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizade-Shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive compound encapsulation: Characteristics, applications in food systems, and implications for human health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Pérez-Pérez, V.; Jiménez-Martínez, C.; González-Escobar, J.L.; Corzo-Ríos, L.J. Exploring the impact of encapsulation on the stability and bioactivity of peptides extracted from botanical sources: Trends and opportunities. Front. Chem. 2024, 12, 1423500. [Google Scholar] [CrossRef] [PubMed]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, properties and applications of alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Kavand, A.; Noverraz, F.; Gerber-Lemaire, S. Recent advances in alginate-based hydrogels for cell transplantation applications. Pharmaceutics 2024, 16, 469. [Google Scholar] [CrossRef]
- Milivojević, M.; Popović, A.; Pajić-Lijaković, I.; Šoštarić, I.; Kolašinac, S.; Stevanović, Z.D. Alginate gel-based carriers for encapsulation of carotenoids: On challenges and applications. Gels 2023, 9, 620. [Google Scholar] [CrossRef]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef]
- Wongverawattanakul, C.; Suklaew, P.O.; Chusak, C.; Adisakwattana, S.; Thilavech, T. Encapsulation of Mesona chinensis benth extract in alginate beads enhances the stability and antioxidant activity of polyphenols under simulated gastrointestinal digestion. Foods 2022, 11, 2378. [Google Scholar] [CrossRef]
- Qiu, K.; Huang, Y.; Anselmo, A.C. Polymer and crosslinker content influences performance of encapsulated live biotherapeutic products. Cell. Mol. Bioeng. 2021, 14, 487–499. [Google Scholar] [CrossRef]
- Abbasiliasi, S.; Shun, T.J.; Ibrahim, T.A.T.; Ismail, N.; Ariff, A.B.; Mokhtar, N.K.; Mustafa, S. Use of sodium alginate in the preparation of gelatin-based hard capsule shells and their evaluation in vitro. RSC Adv. 2019, 9, 16147–16157. [Google Scholar] [CrossRef]
- Colin, C.; Akpo, E.; Perrin, A.; Cornu, D.; Cambedouzou, J. Encapsulation in alginates hydrogels and controlled release: An overview. Molecules 2024, 29, 2515. [Google Scholar] [CrossRef]
- Lai, J.; Azad, A.K.; Sulaiman, W.M.A.W.; Kumarasamy, V.; Subramaniyan, V.; Alshehade, S.A. Alginate-based encapsulation fabrication technique for drug delivery: An updated review of particle type, formulation technique, pharmaceutical ingredient, and targeted delivery system. Pharmaceutics 2024, 16, 370. [Google Scholar] [CrossRef]
- Ma, W.-L.; Mou, C.-L.; Chen, S.-H.; Li, Y.-D.; Deng, H.-B. A mild method for encapsulation of citral in monodispersed alginate microcapsules. Polymers 2022, 14, 1165. [Google Scholar] [CrossRef]
- Dhasmana, A.; Preetam, S.; Malik, S.; Jadon, V.S.; Joshi, N.; Bhandari, G.; Gupta, S.; Mishra, R.; Rustagi, S.; Samal, S.K. Revitalizing elixir with orange peel amplification of alginate fish oil beads for enhanced anti-aging efficacy. Sci. Rep. 2024, 14, 20404. [Google Scholar] [CrossRef]
- Julaeha, E.; Puspita, W.R.; Permadi, N.; Harja, A.; Nurjanah, S.; Wahyudi, T.; Al-Anshori, J. Optimization of Citrus aurantifolia peel extract encapsulation in alginate-gelatin hydrogel microbeads for antibacterial wound dressing applications. Carbohydr. Polym. Technol. Appl. 2024, 7, 100406. [Google Scholar] [CrossRef]
- Mostaghimi, M.; Majdinasab, M.; Golmakani, M.T.; Hadian, M.; Hosseini, S.M.H. Development and characterization of antimicrobial alginate hydrogel beads filled with cinnamon essential oil nanoemulsion. J. Biomater. Sci. Polym. Ed. 2023, 34, 2144–2160. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, B.; Sharma, S. Hydrogels for potential food application: Effect of sodium alginate and calcium chloride on physical and morphological properties. Pharm. Innov. J. 2018, 7, 142–148. [Google Scholar]
- Xiao, Y.; Wang, L.; Zhang, X.; Ren, Y.; Wang, J.; Niu, B.; Li, W. Preparation and characterization of silica-coated sodium alginate hydrogel beads and the delivery of curcumin. J. Biomater. Sci. Polym. Ed. 2024, 35, 2153–2169. [Google Scholar] [CrossRef]
- Kowalski, G.; Witczak, M.; Kuterasiński, Ł. Structure effects on swelling properties of hydrogels based on sodium alginate and acrylic polymers. Molecules 2024, 29, 1937. [Google Scholar] [CrossRef]
- Stachowiak, N.; Kowalonek, J.; Kozlowska, J.; Burkowska-But, A. Stability studies, biodegradation tests, and mechanical properties of sodium alginate and gellan gum beads containing surfactant. Polymers 2023, 15, 2568. [Google Scholar] [CrossRef]
- Chuang, J.J.; Huang, Y.Y.; Lo, S.H.; Hsu, T.F.; Huang, W.Y.; Huang, S.L.; Lin, Y.S. Effects of pH on the shape of alginate particles and its release behavior. Int. J. Polym. Sci. 2017, 2017, 3902704. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; de Claville Christiansen, J. Swelling of homogeneous alginate gels with multi-stimuli sensitivity. Int. J. Mol. Sci. 2023, 24, 5064. [Google Scholar] [CrossRef]
- Makarova, A.O.; Derkach, S.R.; Khair, T.; Kazantseva, M.A.; Zuev, Y.F.; Zueva, O.S. Ion-induced polysaccharide gelation: Peculiarities of alginate egg-box association with different divalent cations. Polymers 2023, 15, 1243. [Google Scholar] [CrossRef]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and characteristics of alginate microparticles for food, pharmaceutical and cosmetic applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Agles, A.A.; Bourg, I.C. Structure and dynamics of water in polysaccharide (alginate) solutions and gels explained by the core–shell model. Biomacromolecules 2024, 25, 6403–6415. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic Gajic, I.M.; Milovanovic, M.G.; Zerajic, S.; Gajic, D.G. Optimization of ultrasound-assisted extraction and encapsulation of antioxidants from orange peels in alginate-chitosan microparticles. Antioxidants 2022, 11, 297. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-based hydrogels and scaffolds for biomedical applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef]
- Bennacef, C.; Desobry, S.; Jasniewski, J.; Leclerc, S.; Probst, L.; Desobry-Banon, S. Influence of alginate properties and calcium chloride concentration on alginate bead reticulation and size: A phenomenological approach. Polymers 2023, 15, 4163. [Google Scholar] [CrossRef] [PubMed]
- Moya, M.L.; Morley, M.; Khanna, O.; Opara, E.C.; Brey, E.M. Stability of alginate microbead properties in vitro. J. Mater. Sci. Mater. Med. 2012, 23, 903–912. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Katakam, P.; Khan, A. Development and characterization of calcium-alginate beads of apigenin: In vitro antitumor, antibacterial, and antioxidant activities. Mar. Drugs 2021, 19, 467. [Google Scholar] [CrossRef]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.L.; Marian, E. Sodium alginate—Natural microencapsulation material of polymeric microparticles. Int. J. Mol. Sci. 2022, 23, 12108. [Google Scholar] [CrossRef]
- Zhang, C.; Grossier, R.; Candoni, N.; Veesler, S. Preparation of alginate hydrogel microparticles by gelation introducing cross-linkers using droplet-based microfluidics: A review of methods. Biomater. Res. 2021, 25, 41. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Sandolo, C.; Perin, D.; Coviello, T.; Lapasin, R.; Grassi, G. Structural characterization of calcium alginate matrices by means of mechanical and release tests. Molecules 2009, 14, 3003–3017. [Google Scholar] [CrossRef]
- Jeong, C.; Kim, S.; Lee, C.; Cho, S.; Kim, S.B. Changes in the physical properties of calcium alginate gel beads under a wide range of gelation temperature conditions. Foods 2020, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Belattmania, Z.; Kaidi, S.; El Atouani, S.; Katif, C.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B.; Vasconcelos, V. Isolation and FTIR-ATR and 1H NMR characterization of alginates from the main alginophyte species of the atlantic coast of Morocco. Molecules 2020, 25, 4335. [Google Scholar] [CrossRef]
- Helmiyati; Aprilliza, M. Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012019. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Cinti, F.; Toto, E.; Santonicola, M.G. Synthesis and characterization of alginate gel beads with embedded zeolite structures as carriers of hydrophobic curcumin. Gels 2023, 9, 714. [Google Scholar] [CrossRef]
- Khorshidian, N.; Mahboubi, A.; Kalantari, N.; Hosseini, H.; Yousefi, M.; Arab, M.; de Cruz, A.G.; Mortazavian, A.M.; Mahdavi, F.S. Chitosan-coated alginate microcapsules loaded with herbal galactagogue extract: Formulation optimization and characterization. Iran J. Pharm. Res. 2019, 18, 1180. [Google Scholar] [CrossRef]
- Stanisławska, N.; Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Makarewicz, M.; Krzan, M. Formation and investigation of physicochemical and microbiological properties of biocomposite films containing turmeric extract nano/microcapsules. Polymers 2023, 15, 919. [Google Scholar] [CrossRef] [PubMed]
- Galogahi, F.M.; Tran, D.T.; Ouyang, L.; Nguyen, N.T. Generation of oil-in-water emulsion-core sodium alginate beads. Macromol. Chem. Phys. 2025, 226, e00227. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Rokhati, N.; Djaeni, M.; Kumoro, A.C.; Purwati, D.; Hakiim, A.; Ashianti, A.D.; Utomo, D.P. Effect of cross-linking agents on sodium alginate-based quercetin beads: Physicochemical properties and controlled release kinetics. Food Res. 2024, 8, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Schuster, E.; Eckardt, J.; Hermansson, A.M.; Larsson, A.; Lorén, N.; Altskär, A.; Ström, A. Microstructural, mechanical and mass transport properties of isotropic and capillary alginate gels. Soft Matter 2014, 10, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Besiri, I.N.; Goudoulas, T.B.; Fattahi, E.; Becker, T. Experimental advances in the real-time recording of cross-linking alginate in situ gelation: A Review. Polymers 2023, 15, 2875. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and characterization of alginate hydrogels with high water-retaining capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef]
- Zhan, L.; Lin, Z.; Li, W.; Qin, Y.; Sun, Q.; Ji, N.; Xie, F. The construction of sodium alginate/carboxymethyl chitosan microcapsules as the physical barrier to reduce corn starch digestion. Foods 2024, 13, 1355. [Google Scholar] [CrossRef]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Papain entrapment in alginate beads for stability improvement and site-specific delivery: Physicochemical characterization and factorial optimization using neural network modeling. AAPS Pharm. Sci. Tech. 2005, 6, 31. [Google Scholar] [CrossRef]
- Essifi, K.; Brahmi, M.; Berraaouan, D.; Ed-Daoui, A.; El Bachiri, A.; Fauconnier, M.L.; Tahani, A. Influence of sodium alginate concentration on microcapsules properties foreseeing the protection and controlled release of bioactive substances. J. Chem. 2021, 2021, 5531479. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lattekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Aziz, S.N.; Badawy, A.A.; Nessem, D.I.; Abd El Malak, N.S.; Naguib, M.J. Chitosan-coated alginate (CCA) nanoparticles for augmentation of topical antihistaminic activity of diphenhydramine: In-vitro optimization, skin histopathology and pharmacodynamic studies with in vitro/in vivo correlation. Drug. Dev. Ind. Pharm. 2023, 49, 316–327. [Google Scholar] [CrossRef]
- Sepúlveda-Rivas, S.; Fritz, H.F.; Valenzuela, C.; Santiviago, C.A.; Morales, J.O. Development of novel EE/alginate polyelectrolyte complex nanoparticles for lysozyme delivery: Physicochemical properties and in vitro safety. Pharmaceutics 2019, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.K.; Boateng, J.S. Enhancing stability and mucoadhesive properties of chitosan nanoparticles by surface modification with sodium alginate and polyethylene glycol for potential oral mucosa vaccine delivery. Mar. Drugs 2022, 20, 156. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Awad, M.; Al-Farraj, A.S.; Al-Turki, A.M. Stability and dynamic aggregation of bare and stabilized zero-valent iron nanoparticles under variable solution chemistry. Nanomaterials 2020, 10, 192. [Google Scholar] [CrossRef]
- Chen, G.L.; Cai, H.Y.; Chen, J.P.; Li, R.; Zhong, S.Y.; Jia, X.J.; Liu, X.F.; Song, B.B. Chitosan/alginate nanoparticles for the enhanced oral antithrombotic activity of clam heparinoid from the clam Coelomactra antiquata. Mar. Drugs 2022, 20, 136. [Google Scholar] [CrossRef]
- Hassani, A.; Mahmood, S.; Enezei, H.H.; Hussain, S.A.; Hamad, H.A.; Aldoghachi, A.F.; Hagar, A.; Doolaanea, A.A.; Ibrahim, W.N. Formulation, characterization and biological activity screening of sodium alginate-gum arabic nanoparticles loaded with curcumin. Molecules 2020, 25, 2244. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, P.; Wang, F.; Chen, H.; Chen, L.; Hu, Y.; Liu, Y. Integrated HS-GC–IMS and UPLC-Q-Orbitrap HRMS-based metabolomics revealed the characteristics and differential volatile and nonvolatile metabolites of different citrus peels. Curr. Res. Food Sci. 2024, 8, 100755. [Google Scholar] [CrossRef]
- Feng, Q.; Fan, B.; He, Y.C. Antibacterial, antioxidant, Cr (VI) adsorption and dye adsorption effects of biochar-based silver nanoparticles-sodium alginate-tannic acid composite gel beads. Int. J. Biol. Macromol. 2024, 271, 132453. [Google Scholar] [CrossRef]
- Jing, Q.; Ma, Y.; He, J.; Ren, Z. Highly stable, mechanically enhanced, and easy-to-collect Sodium alginate/NZVI-rGO gel beads for efficient removal of Cr (VI). Polymers 2023, 15, 3764. [Google Scholar] [CrossRef] [PubMed]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.M.; Perković, G.; Bucić-Kojić, A. Physicochemical characterization and evaluation of gastrointestinal in vitro behavior of alginate-based microbeads with encapsulated grape pomace extracts. Pharmaceutics 2023, 15, 980. [Google Scholar] [CrossRef]
- Flamminii, F.; Paciulli, M.; Di Michele, A.; Littardi, P.; Carini, E.; Chiavaro, E.; Pittia, P.; Di Mattia, C.D. Alginate-based microparticles structured with different biopolymers and enriched with a phenolic-rich olive leaves extract: A physico-chemical characterization. Curr. Res. Food Sci. 2021, 4, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Sabry, B.A.; Badr, A.N.; Mohammed, D.M.; Desoukey, M.A.; Farouk, A. Validating the protective role of orange and tangerine peel extracts foramending food safety against microorganisms’ contamination using molecular docking. Heliyon 2024, 10, e27737. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El-Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Al hamza Hasoon, B.A.; Mahmood, B.S.; Mohamed, E.A.; Jabir, M.S.; Jawad, K.H.; Hussein, N.N.; Sulaiman, G.M.; Dewir, Y.H.; Mendler-Drienyovszki, N. Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments. Green Process. Synth. 2024, 13, 20240126. [Google Scholar] [CrossRef]
- Rijo, P.; Matias, D.; Fernandes, A.S.; Simões, M.F.; Nicolai, M.; Reis, C.P. Antimicrobial plant extracts encapsulated into polymeric beads for potential application on the skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Powell, L.C.; Pritchard, M.F.; Khan, S.; Craine, K.M.; Onsøyen, E.; Rye, P.D.; et al. Alginate oligosaccharides inhibit fungal cell growth and potentiate the activity of antifungals against Candida and Aspergillus spp. PLoS ONE 2014, 9, e112518. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.R.; Sosa, N.; López, T.A.; Quintanilla-Carvajal, M.X.; Perullini, M.; Santagapita, P.R. Bioaccessibility assay, antioxidant activity and consumer-oriented sensory analysis of Beta vulgaris by-product encapsulated in Ca (II)-alginate beads for different foods. Food Chem. Mol. Sci. 2022, 5, 100140. [Google Scholar] [CrossRef]
- Sobri, A.M.; Seow, L.J.; Issara, U.; Ab Rahman, N.A.; Huda, N.; Seow, E.K. Effect of sodium alginate on the physicochemical and sensory properties of vegan surimi. Canrea J. Food Technol. Nutr. Culin. J. 2023, 6, 142–153. [Google Scholar] [CrossRef]
- Stribiţcaia, E.; Krop, E.M.; Lewin, R.; Holmes, M.; Sarkar, A. Tribology and rheology of bead-layered hydrogels: Influence of bead size on sensory perception. Food Hydrocoll. 2020, 104, 105692. [Google Scholar] [CrossRef]
- Farahani, Z.K.; Mousavi, M.; Ardebili, S.M.S.; Bakhoda, H. Modification of sodium alginate by octenyl succinic anhydride to fabricate beads for encapsulating jujube extract. Curr. Res. Food Sci. 2022, 5, 157–166. [Google Scholar] [CrossRef]
- Uzokboev, S.; Akhmadbekov, K.; Nuritdinova, R.N.; Tawfik, S.M.; Lee, Y.I. Unveiling the potential of alginate-based nanomaterials in sensing technology and smart delivery applications. Beilstein J. Nanotechnol. 2024, 15, 1077–1104. [Google Scholar] [CrossRef] [PubMed]
- Sunarharum, W.B.; Kambodji, A.D.; Nur, M. The physical properties of coffee caviar as influenced by sodium alginate concentration and calcium sources. IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 012021. [Google Scholar] [CrossRef]
- Sharma, P.; Dadwal, V.; Rahmatkar, S.N.; Gupta, M.; Singh, D. Flavonoid composition and antioxidant efficacy of citrus peels: An integrated in vitro and in Silico approach toward potential neuroprotective agents. J. Sci. Ind. Res. 2022, 81, 445–454. [Google Scholar] [CrossRef]
- Kamprawet, P.; Vatthanakul, S. Producing passion fruit beads by reverse spherification technique. Thai Sci. Technol. J. 2018, 26 (Suppl. S8), 1381–1393. [Google Scholar]
- Phawaphuthanon, N.; Behnam, S.; Koo, S.Y.; Pan, C.H.; Chung, D. Characterization of core–shell calcium-alginate macrocapsules fabricated by electro-coextrusion. Int. J. Biol. Macromol. 2014, 65, 267–274. [Google Scholar] [CrossRef]
- Gong, R.; Li, C.; Zhu, S.; Zhang, Y.; Du, Y.; Jiang, J. A novel pH-sensitive hydrogel based on dual crosslinked alginate/N-α-glutaric acid chitosan for oral delivery of protein. Carbohydr. Polym. 2011, 85, 869–874. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: London, UK, 2013; Volume 2. [Google Scholar]
- Costanzo, G.; Vitale, E.; Iesce, M.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant properties of pulp, peel and seeds of Phlegrean mandarin (Citrus reticulata Blanco) at different stages of fruit ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Keawpeng, I.; Paulraj, B.; Venkatachalam, K. Antioxidant and antimicrobial properties of mung bean phyto-film combined with longkong pericarp extract and sonication. Membranes 2022, 12, 379. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Wichchukit, S.; O’Mahony, M. The 9-point hedonic scale and hedonic ranking in food science: Some reappraisals and alternatives. J. Sci. Food Agric. 2015, 95, 2167–2178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatachalam, K.; Charoenphun, N.; Nitikornwarakul, C.; Lekjing, S. Effect of Sodium Alginate Concentration on the Physicochemical, Structural, Functional Attributes, and Consumer Acceptability of Gel Beads Encapsulating Tangerine Peel (Citrus reticulata Blanco ‘Cho Khun’) Extract. Gels 2025, 11, 808. https://doi.org/10.3390/gels11100808
Venkatachalam K, Charoenphun N, Nitikornwarakul C, Lekjing S. Effect of Sodium Alginate Concentration on the Physicochemical, Structural, Functional Attributes, and Consumer Acceptability of Gel Beads Encapsulating Tangerine Peel (Citrus reticulata Blanco ‘Cho Khun’) Extract. Gels. 2025; 11(10):808. https://doi.org/10.3390/gels11100808
Chicago/Turabian StyleVenkatachalam, Karthikeyan, Narin Charoenphun, Chawakwan Nitikornwarakul, and Somwang Lekjing. 2025. "Effect of Sodium Alginate Concentration on the Physicochemical, Structural, Functional Attributes, and Consumer Acceptability of Gel Beads Encapsulating Tangerine Peel (Citrus reticulata Blanco ‘Cho Khun’) Extract" Gels 11, no. 10: 808. https://doi.org/10.3390/gels11100808
APA StyleVenkatachalam, K., Charoenphun, N., Nitikornwarakul, C., & Lekjing, S. (2025). Effect of Sodium Alginate Concentration on the Physicochemical, Structural, Functional Attributes, and Consumer Acceptability of Gel Beads Encapsulating Tangerine Peel (Citrus reticulata Blanco ‘Cho Khun’) Extract. Gels, 11(10), 808. https://doi.org/10.3390/gels11100808







