Rational Design of Self-Healing Hydrogel with High Mechanical Strength and Self-Healing Efficiency: A Short Review
Abstract
1. Introduction
2. Evaluation Method of Self-Healing Performance
2.1. Observational Method
2.2. Dynamic Self-Healing Performance Test
2.3. Static Self-Healing Performance Test
3. Classification of Self-Healing Hydrogel
3.1. External-Stimulus-Triggered Self-Healing Hydrogels
3.2. Autonomous Self-Healing Hydrogels
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVA | Polyvinyl alcohol |
| PEG | Polyethylene glycol |
| PDA | Polydopamine |
| PNAGA | Poly N-acryloyl glycinamide |
| GO | Graphene oxide |
| NIPAM | N-isopropylacrylamide |
| PAACA | Poly N-acrylyl-6-aminocaproic acid |
| DAC | 2-dimethylaminoethylacrylate |
| PAM | Polyacrylamide |
| PAA | Polyacrylic |
| TA | Tannic acid |
| CS | Chitosan |
| THF | Tetrahydrofuran |
| SDS | Sodium dodecyl sulfate |
| CNF | Cellulose nanofiber |
References
- Guo, L.; Fu, Z.; Li, H.; Wei, R.; Guo, J.; Wang, H.; Qi, J. Smart Hydrogel: A New Platform for Cancer Therapy. Adv. Colloid Interface 2025, 340, 103470. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, H.; Fortin, D.; Xia, H.; Zhao, Y. Poly(Vinyl Alcohol)–Poly(Ethylene Glycol) Double-Network Hydrogel: A General Approach to Shape Memory and Self-Healing Functionalities. Langmuir 2015, 31, 11709–11716. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Lienemann, P.S.; Lutolf, M.P.; Ehrbar, M. Biomimetic Hydrogels for Controlled Biomolecule Delivery to Augment Bone Regeneration. Adv. Drug Deliver. Rev. 2012, 64, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, J.; Song, J.; Cao, X.; Zhou, B.; Yang, L.; Li, C.; Wang, Y. A Motion-Responsive Injectable Lubricative Hydrogel for Efficient Achilles Tendon Adhesion Prevention. Mater. Today Bio 2025, 30, 101458. [Google Scholar] [CrossRef]
- Li, X.; Su, X. Multifunctional Smart Hydrogels: Potential in Tissue Engineering and Cancer Therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Chen, X.; Cui, Z.; Li, X.; Zhou, Y.; Wang, H.; Sun, R.; Wang, Q. Bioinspired Flexible Kevlar/Hydrogel Composites with Antipuncture and Strain-Sensing Properties for Personal Protective Equipment. ACS Appl. Mater. Interfaces 2024, 16, 45473–45486. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, Y.; Ma, S.; Li, X.; Wang, W.; Chen, X.; Zheng, J.; Fan, Z.; Jiang, Y.; Liao, Y. Multifunctional DNA Hydrogels with Light-Triggered Gas-Therapy and Controlled G-Exos Release for Infected Wound Healing. Bioact. Mater. 2025, 52, 422–437. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Hu, Q. Emerging Self-Regulated Micro/Nano Drug Delivery Devices: A Step Forward towards Intelligent Diagnosis and Therapy. Nano Today 2021, 38, 101127. [Google Scholar] [CrossRef]
- Dou, X.-Q.; Feng, C.-L. Amino Acids and Peptide-Based Supramolecular Hydrogels for Three-Dimensional Cell Culture. Adv. Mater. 2017, 29, 1604062. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lin, S.; Ma, C.; Takaffoli, M.; Fang, N.X.; Zhao, X. Hydraulic Hydrogel Actuators and Robots Optically and Sonically Camouflaged in Water. Nat. Commun. 2017, 8, 14230. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Lin, Y.-J.; Enriquez, E.; Peng, X.-F.; Turng, L.-S. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef]
- Lopez-Silva, T.L.; Leach, D.G.; Azares, A.; Li, I.-C.; Woodside, D.G.; Hartgerink, J.D. Chemical Functionality of Multidomain Peptide Hydrogels Governs Early Host Immune Response. Biomaterials 2020, 231, 119667. [Google Scholar] [CrossRef]
- Lü, S.; Gao, C.; Xu, X.; Bai, X.; Duan, H.; Gao, N.; Feng, C.; Xiong, Y.; Liu, M. Injectable and Self-Healing Carbohydrate-Based Hydrogel for Cell Encapsulation. ACS Appl. Mater. Interfaces 2015, 7, 13029–13037. [Google Scholar] [CrossRef]
- Moutos, F.T.; Freed, L.E.; Guilak, F. A Biomimetic Three-Dimensional Woven Composite Scaffold for Functional Tissue Engineering of Cartilage. Nat. Mater. 2007, 6, 162–167. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-Healing Materials with Microvascular Networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef]
- Sun, C.; Jia, H.; Lei, K.; Zhu, D.; Gao, Y.; Zheng, Z.; Wang, X. Self-Healing Hydrogels with Stimuli Responsiveness Based on Acylhydrazone Bonds. Polymer 2019, 160, 246–253. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of Multiresponsive and Dynamic Chitosan-Based Hydrogels for Controlled Release of Bioactive Molecules. Biomacromolecules 2011, 12, 2894–2901. [Google Scholar] [CrossRef]
- Jiang, X.; Zeng, F.; Yang, X.; Jian, C.; Zhang, L.; Yu, A.; Lu, A. Injectable Self-Healing Cellulose Hydrogel Based on Host-Guest Interactions and Acylhydrazone Bonds for Sustained Cancer Therapy. Acta Biomater. 2022, 141, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, J.H.; Du, X.J.; Xu, F.; Zrinyi, M.; Osada, Y.; Li, F.; Chen, Y.M. Dextran-Based Self-Healing Hydrogels Formed by Reversible Diels-Alder Reaction under Physiological Conditions. Macromol. Rapid Comm. 2013, 34, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Inoue, M.; Matsuda, M.; Taguchi, T. Quick Self-Healing and Thermo-Reversible Liposome Gel. Colloid. Surface. B 2010, 82, 196–202. [Google Scholar] [CrossRef]
- Taylor, D.L.; in het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2016, 13, 1601916. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yu, C.; Denman, R.J.; Zhang, W. Recent Advances in Dynamic Covalent Chemistry. Chem. Soc. Rev. 2013, 42, 6634. [Google Scholar] [CrossRef]
- Feng, Z.; Zuo, H.; Hu, J.; Gao, W.; Yu, B.; Ning, N.; Tian, M.; Zhang, L. Mussel-Inspired Highly Stretchable, Tough Nanocomposite Hydrogel with Self-Healable and Near-Infrared Actuated Performance. Ind. Eng. Chem. Res. 2019, 59, 166–174. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Fei, G.; Xia, H. Polydopamine Particles Reinforced Poly(Vinyl Alcohol) Hydrogel with NIR Light Triggered Shape Memory and Self-Healing Capability. Macromol. Rapid Comm. 2017, 38, 1700421. [Google Scholar] [CrossRef]
- Ryplida, B.; Lee, K.D.; In, I.; In, I. Light-Induced Swelling-Responsive Conductive, Adhesive, and Stretchable Wireless Film Hydrogel as Electronic Artificial Skin. Adv. Funct. Mater. 2019, 29, 1903209. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Y.; Lu, X.; Wang, K.; Wang, Z.; Zhang, H. Polydopamine Nanoparticles Modulating Stimuli-Responsive PNIPAM Hydrogels with Cell/Tissue Adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, M.; Yan, B.; Li, Y.; Lan, J.; Shi, L.; Ran, R. Polydopamine/Polystyrene Nanocomposite Double-Layer Strain Sensor Hydrogel with Mechanical, Self-Healing, Adhesive and Conductive Properties. Mat. Sci. Eng. C 2020, 109, 110567. [Google Scholar] [CrossRef]
- Yang, L.; Lu, X.; Wang, Z.; Xia, H. Diels–Alder Dynamic Crosslinked Polyurethane/Polydopamine Composites with NIR Triggered Self-Healing Function. Polym. Chem. 2018, 9, 2166–2172. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Ren, J.; Qu, X. 3D Graphene Oxide-Polymer Hydrogel: Near-Infrared Light-Triggered Active Scaffold for Reversible Cell Capture and On-Demand Release. Adv. Mater. 2013, 25, 6737–6743. [Google Scholar] [CrossRef]
- Kurapati, R.; Raichur, A.M. Near-Infrared Light-Responsive Graphene Oxide Composite Multilayer Capsules: A Novel Route for Remote Controlled Drug Delivery. Chem. Commun. 2013, 49, 734–736. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Yu, M.; Hou, M.; Gong, G.; Tan, H.; Li, N.; Xu, J. NIR Light-Induced Rapid Self-Healing Hydrogel toward Multifunctional Applications in Sensing. Nano Energy 2023, 107, 108119. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Hansen, M.R.; Birkedal, H. Metals & Polymers in the Mix: Fine-Tuning the Mechanical Properties & Color of Self-Healing Mussel-Inspired Hydrogels. J. Mater. Chem. B 2014, 2, 8292–8297. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.-P.; Wang, P.; Yu, S.-H. Stretchable and Self-Healing Graphene Oxide–Polymer Composite Hydrogels: A Dual-Network Design. Chem. Mater. 2013, 25, 3357–3362. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Chen, H.; Yan, H.-L.; Huang, L.; Yang, J.; Zheng, J. A Novel Design Strategy for Fully Physically Linked Double Network Hydrogels with Tough, Fatigue Resistant, and Self-Healing Properties. Adv. Funct. Mater. 2015, 25, 1598–1607. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Jin, Z. Tough, Swelling-Resistant, Self-Healing, and Adhesive Dual-Cross-Linked Hydrogels Based on Polymer–Tannic Acid Multiple Hydrogen Bonds. Macromolecules 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Pan, C.; Liu, L.; Chen, Q.; Zhang, Q.; Guo, G. Tough, Stretchable, Compressive Novel Polymer/Graphene Oxide Nanocomposite Hydrogels with Excellent Self-Healing Performance. ACS Appl. Mater. Interfaces 2017, 9, 38052–38061. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, G.; He, C.; Wang, H. Self-Healing in Tough Graphene Oxide Composite Hydrogels. Macromol. Rapid Comm. 2013, 34, 1002–1007. [Google Scholar] [CrossRef]
- He, X.; Zhang, C.; Wang, M.; Zhang, Y.; Liu, L.; Yang, W. An Electrically and Mechanically Autonomic Self-Healing Hybrid Hydrogel with Tough and Thermoplastic Properties. ACS Appl. Mater. Interfaces 2017, 9, 11134–11143. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Chen, L.; Yin, Y.; Zhou, J.; Liu, M. Anti-Freezing, Conductive Self-Healing Organohydrogels with Stable Strain-Sensitivity at Subzero Temperatures. Angew. Chem. Int. Edit. 2017, 56, 14159–14163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, Z.; Wang, Y.-T.; Guo, L.; Yin, C.; Zhang, X.; Hao, J.; Zhang, G.; Chen, L. Eco-Friendly, Self-Healing Hydrogels for Adhesive and Elastic Strain Sensors, Circuit Repairing, and Flexible Electronic Devices. Macromolecules 2019, 52, 2531–2541. [Google Scholar] [CrossRef]
- Azevedo, S.; Costa, A.M.S.; Andersen, A.; Choi, I.S.; Birkedal, H.; Mano, J.F. Bioinspired Ultratough Hydrogel with Fast Recovery, Self-Healing, Injectability and Cytocompatibility. Adv. Mater. 2017, 29, 1700759. [Google Scholar] [CrossRef]
- Li, Z.; Lu, W.; Ngai, T.; Le, X.; Zheng, J.; Zhao, N.; Huang, Y.; Wen, X.; Zhang, J.; Chen, T. Mussel-Inspired Multifunctional Supramolecular Hydrogels with Self-Healing, Shape Memory and Adhesive Properties. Polym. Chem. 2016, 7, 5343–5346. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-Molecule Mechanics of Mussel Adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef]
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties. Chem. Mater. 2018, 30, 3110–3121. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Zhao, Y.; Long, S.; Zheng, J. Dual Physically Crosslinked Double Network Hydrogels with High Toughness and Self-Healing Properties. Soft Matter 2017, 13, 911–920. [Google Scholar] [CrossRef]
- Shao, C.; Chang, H.; Wang, M.; Xu, F.; Yang, J. High-Strength, Tough, and Self-Healing Nanocomposite Physical Hydrogels Based on the Synergistic Effects of Dynamic Hydrogen Bond and Dual Coordination Bonds. ACS Appl. Mater. Interfaces 2017, 9, 28305–28318. [Google Scholar] [CrossRef]
- Saleem, M.; Abdullah, R.; Ali, A.; Park, B.J.; Choi, E.H.; Hong, I.S.; Lee, K.H. Facile Synthesis, Cytotoxicity and Bioimaging of Fe3+ Selective Fluorescent Chemosensor. Bioorgan. Med. Chem. 2014, 22, 2045–2051. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.; Wang, K.; Fang, L.; Zhang, H.; Tang, Y.; Ding, Y.; Weng, L.-T.; Xu, J.; Weng, J.; et al. Tough, Self-Healable and Tissue-Adhesive Hydrogel with Tunable Multifunctionality. NPG Asia Mater. 2017, 9, e372. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, W.; Li, H.; Yu, X.; Ding, S.; Wu, C. An Autonomous Self-Healing Hydrogel with High Polydopamine Content for Improved Tensile Strength. J. Mater. Sci. 2020, 55, 17255–17265. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, H.; Zhao, Y. Poly(Vinyl Alcohol) Hydrogel Can Autonomously Self-Heal. ACS Macro Lett. 2012, 1, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, C.; Yu, X.; Li, H.; Ding, S.; Zhang, W. Biocompatible Autonomic Self-Healing PVA-TA Hydrogel with High Mechanical Strength. Macromol. Chem. Phys. 2021, 222, 2100061. [Google Scholar] [CrossRef]
- Yu, X.; Huang, J.; Wu, C.; Zhang, W. Biocompatible Autonomous Self-Healing PVA-CS/TA Hydrogels Based on Hydrogen Bonding and Electrostatic Interaction. Sci. Rep. 2025, 15, 1893. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Lang, C.; Qiao, S.; An, G.; Fan, X.; Zhao, L.; Hou, C.; Liu, J. Enzyme-Regulated Fast Self-Healing of a Pillararene-Based Hydrogel. Biomacromolecules 2017, 18, 1885–1892. [Google Scholar] [CrossRef]
- Chen, W.-P.; Hao, D.-Z.; Hao, W.-J.; Guo, X.-L.; Jiang, L. Hydrogel with Ultrafast Self-Healing Property Both in Air and Underwater. Acs Appl. Mater. Interfaces 2018, 10, 1258–1265. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, Y.; Zhu, Y.; Hao, L.; Chen, Y.; An, G.; Wu, H.; Shi, X.; Mao, C. A Rapidly Self-Healing Host-Guest Supramolecular Hydrogel with High Mechanical Strength and Excellent Biocompatibility. Angew. Chem. Int. Edit. 2018, 57, 9008–9012. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, S.; Sun, W.; Liu, X.; Fu, S.; Tong, Z. Notch Insensitive and Self-Healing PNIPAm-PAM-Clay Nanocomposite Hydrogels. Soft Matter 2014, 10, 3506. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Xu, X.; Yue, Y.; Mei, C.; Huang, C.; Jiang, S.; Wu, Q.; Han, J. Nanocellulose-Mediated Electroconductive Self-Healing Hydrogels with High Strength, Plasticity, Viscoelasticity, Stretchability, and Biocompatibility toward Multifunctional Applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, G.; Feng, X.; Liu, H.; Li, F.; Wang, M.; Li, H. Room-Temperature Self-Healing Tough Nanocomposite Hydrogel Crosslinked by Zirconium Hydroxide Nanoparticles. Compos. Sci. Technol. 2017, 140, 54–62. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, G.; Zeng, X.; Li, J.; Li, G.; Huang, W.; Sun, R.; Wong, C. High-Strength, Tough, Fatigue Resistant, and Self-Healing Hydrogel Based on Dual Physically Cross-Linked Network. ACS Appl. Mater. Interfaces 2016, 8, 24030–24037. [Google Scholar] [CrossRef]

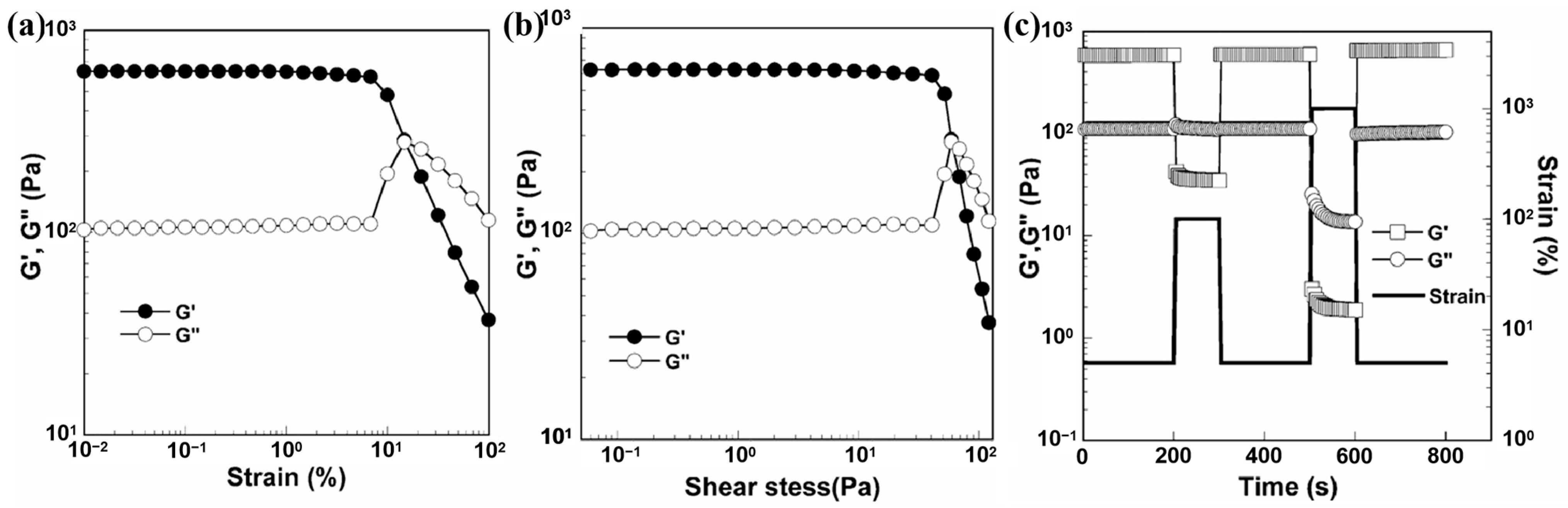
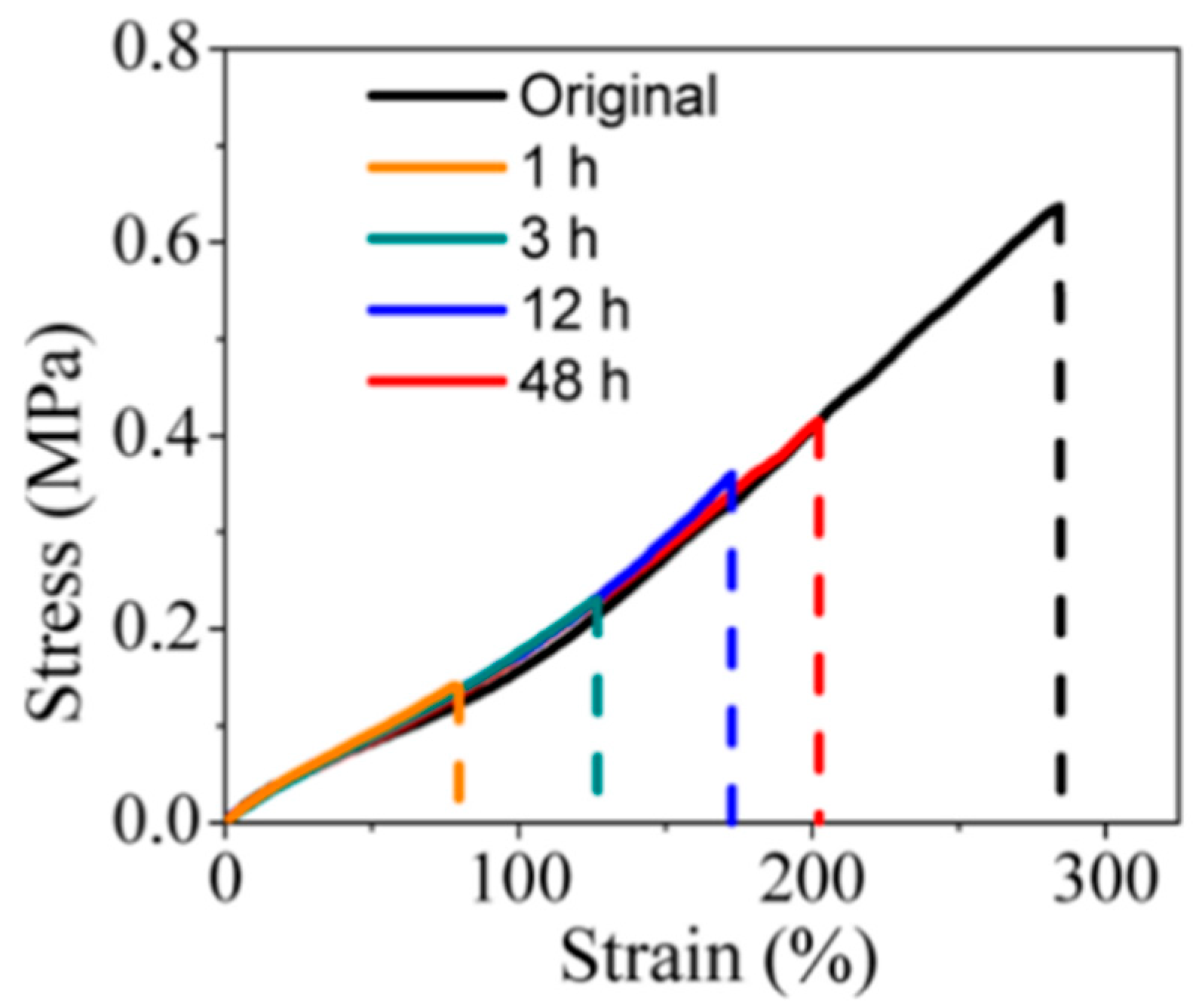
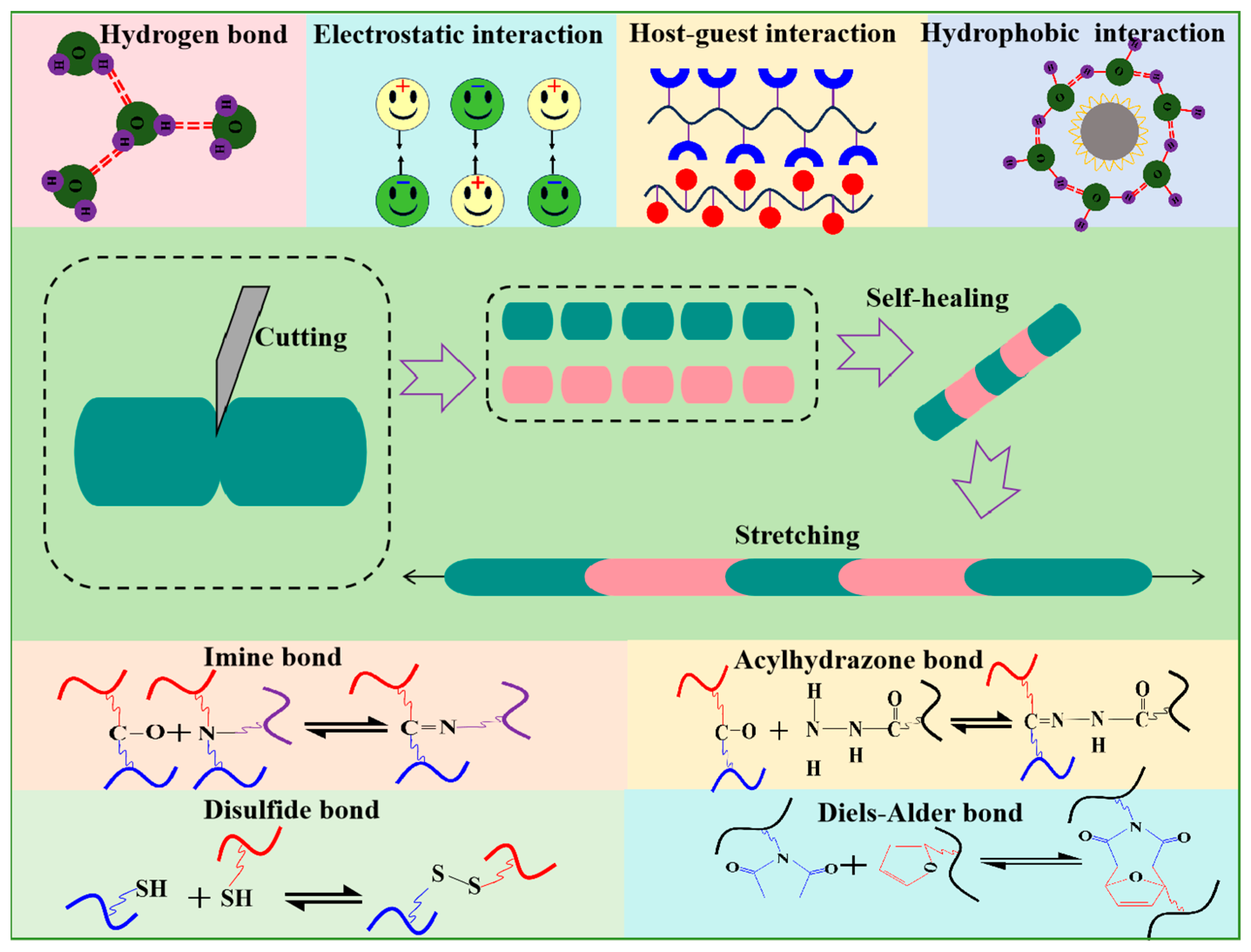
| Method | Characteristics |
|---|---|
| Observational method | Simple and fast, yet not quantitatively evaluable. |
| Dynamic self-healing performance test | Capable of monitoring variations in storage modulus and loss modulus, yet unable to evaluate the tensile strength. |
| Static self-healing performance test | Quantitatively evaluate the self-healing efficiency under 100% destruction to fully understand the recovery situation of the tensile strength. |
| Hydrogel | Tensile Strength | Self-Healing Time | Self-Healing Efficiency | Ref. |
|---|---|---|---|---|
| PDA-PAM | 8 kPa | 2 h | 98% | [54] |
| PDA-talc-PAM | 8.5 kPa | 2 h | 60% | [16] |
| PDA-PAM | 16 kPa | 2 h | 96% | [55] |
| PDA-PGO-PAM | 21 kPa | 24 h | 62% | [27] |
| DF-PEG | 23 kPa | 24 h | 100% | [59] |
| Agarose/PVA | 25 kPa | 10 s | 100% | [60] |
| β-CD-AOI2-A-TEG-Ad | 28 kPa | 1 h | 63% | [61] |
| PNIPAM-PAM-clay | 60 kPa | 150 h | 90% | [62] |
| CNF-PPy/PB | 63 kPa | 20 s | 97% | [63] |
| PVA/PAA | 160 kPa | 12 h | 37% | [64] |
| Zr-NC gel | 195 kPa | 12 h | 75% | [65] |
| PVA-TA | 224 kPa | 2 h | 87% | [57] |
| PVA | 278 kPa | 48 h | 72% | [56] |
| PVA-CS/TA | 447 kPa | 2 h | 84% | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Huang, J.; Yang, F.; Li, J. Rational Design of Self-Healing Hydrogel with High Mechanical Strength and Self-Healing Efficiency: A Short Review. Gels 2025, 11, 807. https://doi.org/10.3390/gels11100807
Yu X, Huang J, Yang F, Li J. Rational Design of Self-Healing Hydrogel with High Mechanical Strength and Self-Healing Efficiency: A Short Review. Gels. 2025; 11(10):807. https://doi.org/10.3390/gels11100807
Chicago/Turabian StyleYu, Xiaogang, Jinxin Huang, Fang Yang, and Jinbo Li. 2025. "Rational Design of Self-Healing Hydrogel with High Mechanical Strength and Self-Healing Efficiency: A Short Review" Gels 11, no. 10: 807. https://doi.org/10.3390/gels11100807
APA StyleYu, X., Huang, J., Yang, F., & Li, J. (2025). Rational Design of Self-Healing Hydrogel with High Mechanical Strength and Self-Healing Efficiency: A Short Review. Gels, 11(10), 807. https://doi.org/10.3390/gels11100807





