Effect of Gellan Gum on the Properties of Collagen-HPMC Freeze-Dried Hydrogels for Mucosal Administration
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of the Rheological Behavior of Designed Hydrogels
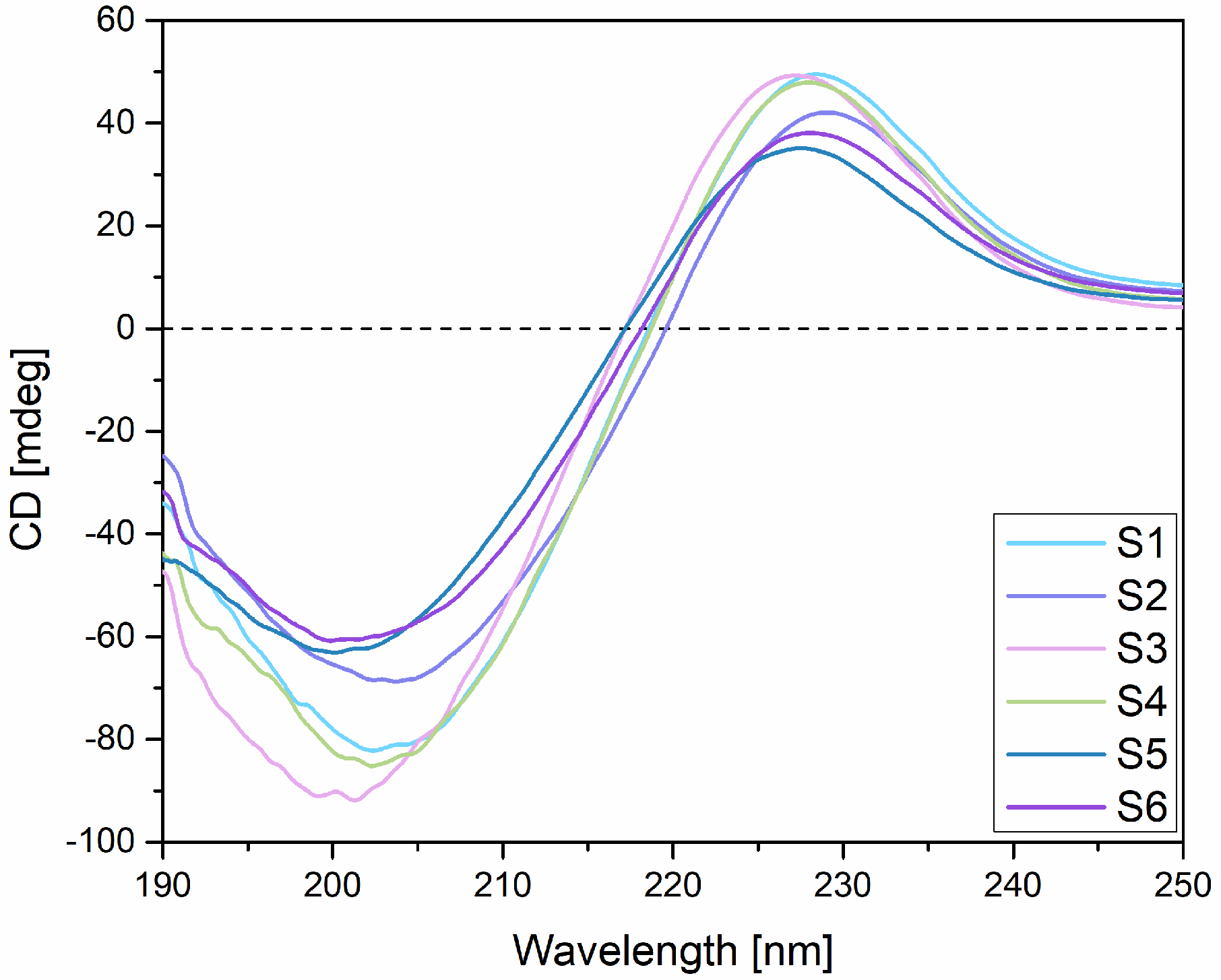
2.2. Circular Dichroism (CD)
2.3. Fourier Transform Infrared (FT-IR) Spectroscopy
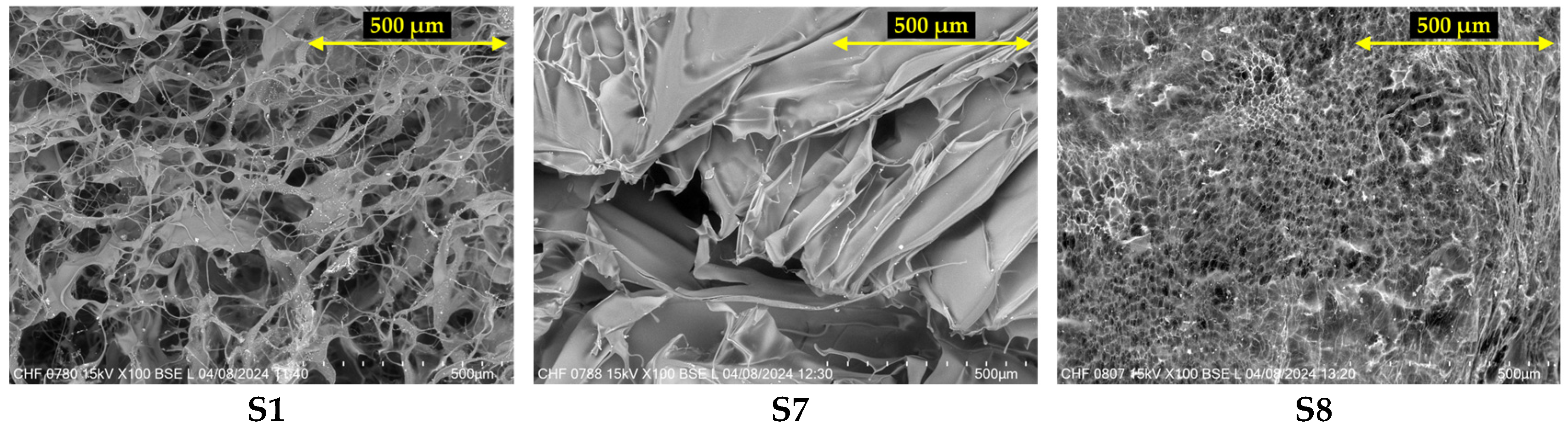
2.4. Scanning Electron Microscopy (SEM)
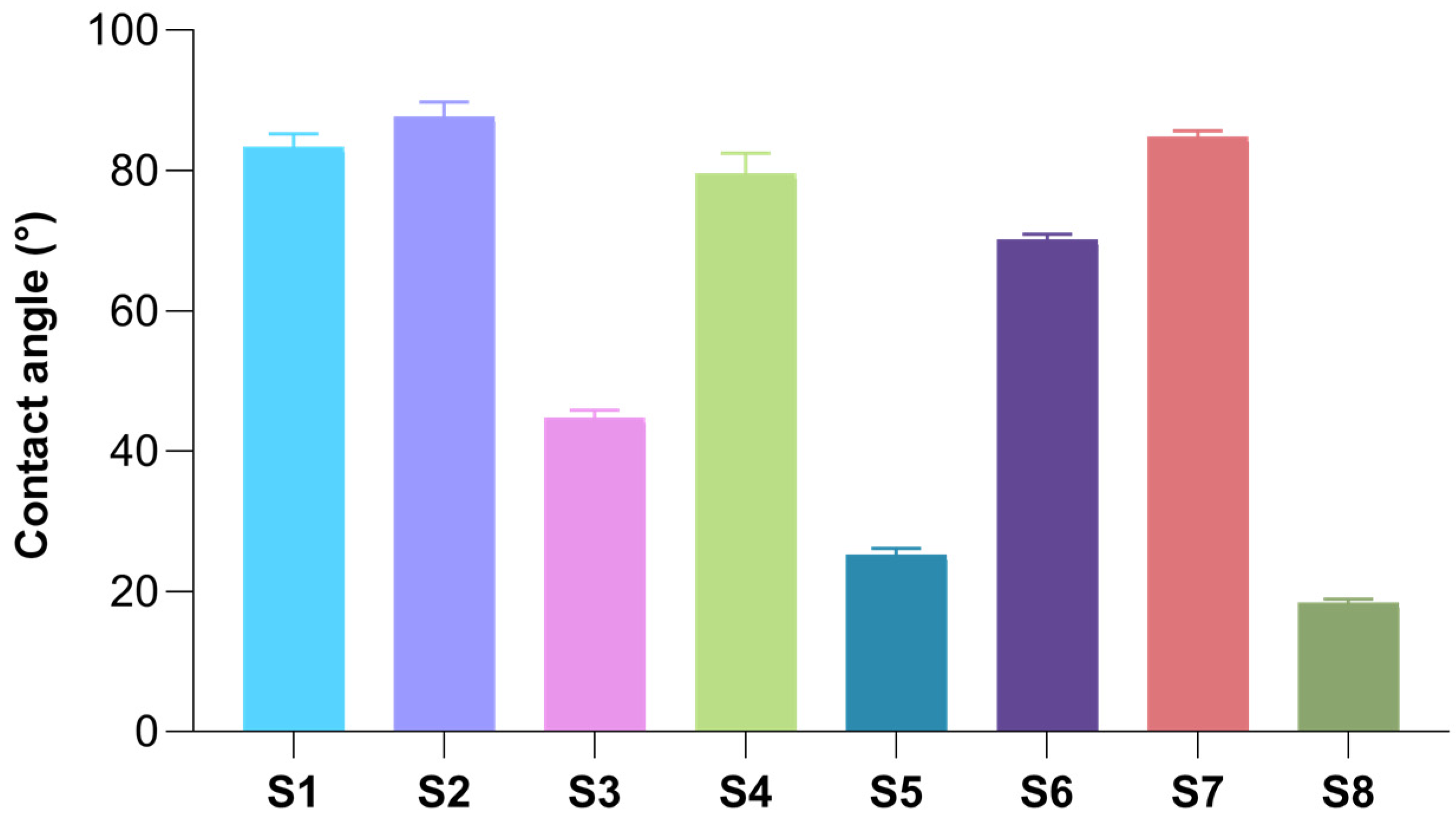
2.5. Surface Wettability of Freeze-Dried Hydrogels
2.6. Swelling Capacity of Freeze-Dried Hydrogels
2.7. Enzymatic Degradation of Freeze-Dried Hydrogels
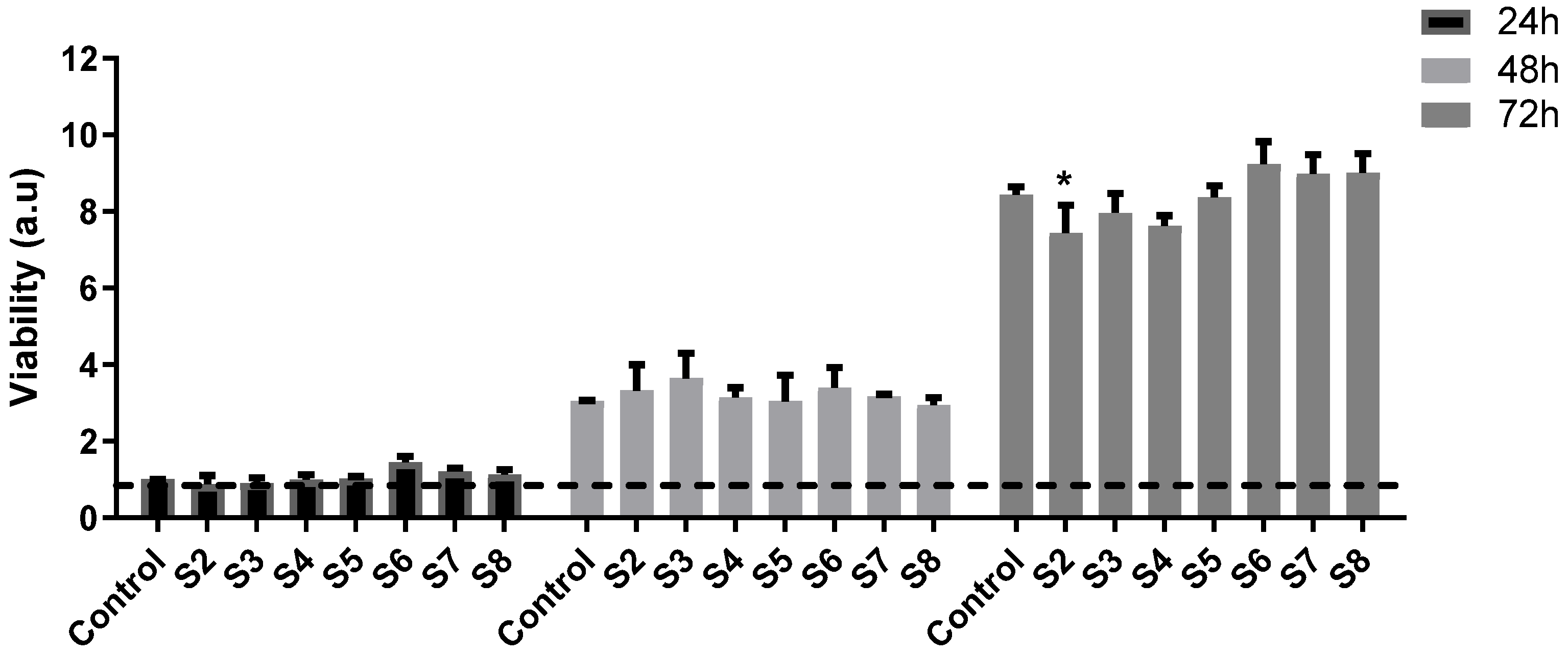
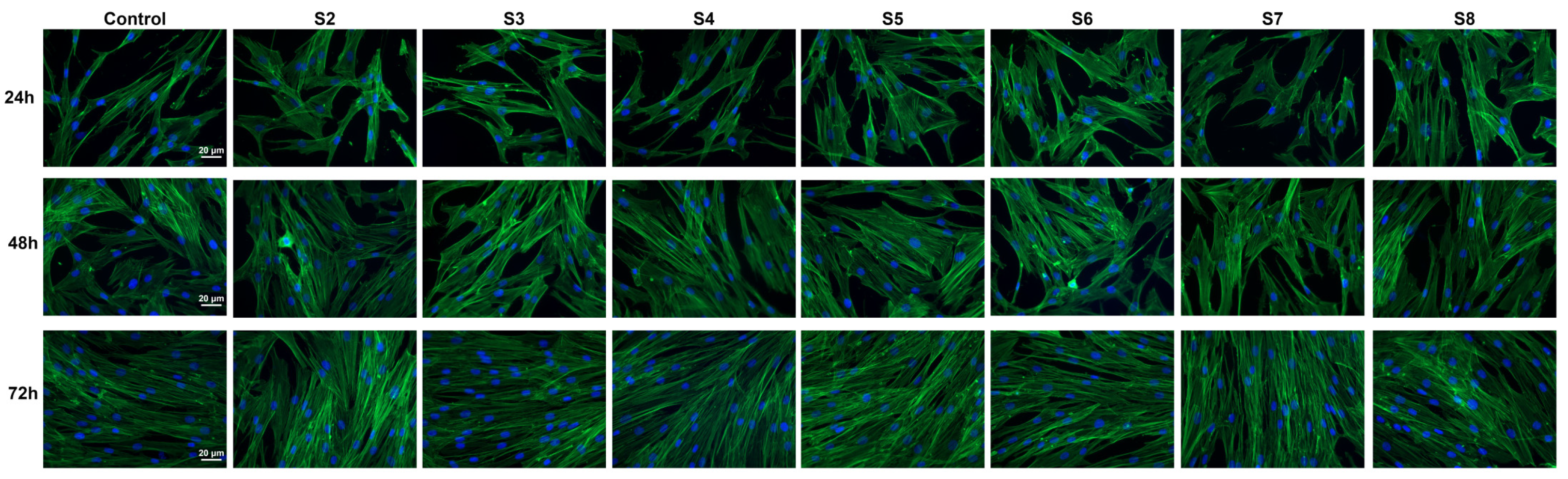
2.8. In Vitro Biocompatibility Assessment
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Individual Hydrogels
4.2.2. Preparation of Freeze-Dried Hydrogels
4.2.3. Evaluation of the Rheological Behavior and Properties of Designed Hydrogels
4.2.4. Circular Dichroism (CD)
4.2.5. Fourier Transform Infrared (FT-IR) Spectroscopy
4.2.6. Scanning Electron Microscopy (SEM)
4.2.7. Surface Wettability of Freeze-Dried Hydrogels
4.2.8. Swelling Capacity of Freeze-Dried Hydrogels
4.2.9. Enzymatic Degradation of Freeze-Dried Hydrogels
4.2.10. In Vitro Biocompatibility Assessment
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brako, F.; Boateng, J. Transmucosal drug delivery: Prospects, challenges, advances, and future directions. Expert Opin. Drug Deliv. 2025, 22, 525–553. [Google Scholar] [CrossRef]
- Goyal, A.K.; Singh, R.; Chauhan, G.; Rath, G. Non-invasive systemic drug delivery through mucosal routes. Artif. Cells Nanomed. Biotechnol. 2018, 46, 539–551. [Google Scholar] [CrossRef]
- Subi, M.T.M.; Selvasudha, N.; Vasanthi, H.R. Vaginal drug delivery system: A promising route of drug administration for local and systemic diseases. Drug Discov. Today 2024, 29, 104012. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; Abdul-Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Carine, T.; Wee Toong, T.; Yi, N.J.; Win Yi, L. Promising Drug Delivery Approaches to Treat Microbial Infections in the Vagina: A Recent Update. Polymers 2020, 13, 26. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Michalak, M.; Bryś, T.; Kudła, M. The Potential of Hydrogel Preparations Containing Plant Materials in Supporting the Treatment of Vaginal and Vulvar Infections—Current State of Knowledge. Polymers 2025, 17, 470. [Google Scholar] [CrossRef]
- De Rosa, N.; Santangelo, F.; Todisco, C.; Dequerquis, F.; Santangelo, C. Collagen-Based Ovule Therapy Reduces Inflammation and Improve Cervical Epithelialization in Patients with Fungal, Viral, and Bacterial Cervico-Vaginitis. Medicina 2023, 59, 1490. [Google Scholar] [CrossRef]
- Naumova, I.; Castelo-Branco, C. Current treatment options for postmenopausal vaginal atrophy. Int. J. Womens Health 2018, 10, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Major, I.; McConville, C. Vaginal drug delivery for the localised treatment of cervical cancer. Drug Deliv. Transl. Res. 2017, 7, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, X.; Wang, H.; Zhou, J.; Lin, X.; Yang, P. Vagina, a promising route for drug delivery. J. Drug Deliv. Sci. Technol. 2024, 93, 105397. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Jadach, B.; Tatarek, A.; Gadziński, P.; Falana, A.; Gralińska, K.; Ekert, M.; Puri, V.; Wrotyńska-Barczyńska, J.; et al. Recent Advances in Polymer-Based Vaginal Drug Delivery Systems. Pharmaceutics 2021, 13, 884. [Google Scholar] [CrossRef]
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-based biomaterials for pharmaceutical and biomedical applications: A focus on topical drug administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Remiro, P.F.R.; Nagahara, M.H.T.; Azoubel, R.A.; Franz-Montan, M.; d’Ávila, M.A.; Moraes, Â.M. Polymeric Biomaterials for Topical Drug Delivery in the Oral Cavity: Advances on Devices and Manufacturing Technologies. Pharmaceutics 2022, 15, 12. [Google Scholar] [CrossRef]
- Fernandes, A.I.; Jozala, A.F. Polymers Enhancing Bioavailability in Drug Delivery. Pharmaceutics 2022, 14, 2199. [Google Scholar] [CrossRef]
- Jawadi, Z.; Yang, C.; Haidar, Z.S.; Santa Maria, P.L.; Massa, S. Bio-Inspired Muco-Adhesive Polymers for Drug Delivery Applications. Polymers 2022, 14, 5459. [Google Scholar] [CrossRef] [PubMed]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Bradley, M.; Patrojanasophon, P. Maleimide-functionalized carboxymethyl cellulose: A novel mucoadhesive polymer for transmucosal drug delivery. Carbohydr. Polym. 2022, 288, 119368. [Google Scholar] [CrossRef] [PubMed]
- Partenhauser, A.; Bernkop-Schnürch, A. Mucoadhesive polymers in the treatment of dry X syndrome. Drug Discov. Today 2016, 21, 1051–1062. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- He, Q.; Feng, T.; Xie, Y.; Swamiappan, S.; Zhou, Y.; Zhou, Y.; Zhou, H.; Peng, X. Recent Advances in the Development and Application of Cell-Loaded Collagen Scaffolds. Int. J. Mol. Sci. 2025, 26, 4009. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Albu Kaya, M.G.; Titorencu, I.; Dinu-Pîrvu, C.E.; Marin, M.M.; Roșca, A.-M.; Popa, L.; Anuța, V.; Antoniac, A.; Chelaru, C.; et al. Design and evaluation of new wound dressings based on collagen-cellulose derivatives. Mater. Des. 2023, 236, 112469. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Zhou, N.; Liu, Y.D.; Zhang, Y.; Gu, T.W.; Peng, L.H. Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial. Pharmaceutics 2023, 15, 1443. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Albu, M.G.; Popa, L.; Moisescu, S. Response surface methodology and Taguchi approach to assess the combined effect of formulation factors on minocycline delivery from collagen sponges. Pharmazie 2013, 68, 340–348. [Google Scholar]
- Tihan, G.T.; Rău, I.; Zgârian, R.G.; Ungureanu, C.; Barbaresso, R.C.; Kaya, M.G.A.; Dinu-Pîrvu, C.; Ghica, M.V. Oxytetracycline versus Doxycycline Collagen Sponges Designed as Potential Carrier Supports in Biomedical Applications. Pharmaceutics 2019, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Jadach, B.; Mielcarek, Z.; Osmałek, T. Use of Collagen in Cosmetic Products. Curr. Issues Mol. Biol. 2024, 46, 2043–2070. [Google Scholar] [CrossRef]
- Photiou, L.; Lin, M.J.; Dubin, D.P.; Lenskaya, V.; Khorasani, H. Review of non-invasive vulvovaginal rejuvenation. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 716–726. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xue, L.; Gong, G.; Pan, J.; Wang, X.; Zhang, Y.; Guo, J.; Qin, L. Collagen-based materials in reproductive medicine and engineered reproductive tissues. J. Leather Sci. Eng. 2022, 4, 3. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Janorkar, A.V. Improvements in mechanical properties of collagen-based scaffolds for tissue engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100253. [Google Scholar] [CrossRef]
- Shapiro, R.L.; DeLong, K.; Zulfiqar, F.; Carter, D.; Better, M.; Ensign, L.M. In vitro and ex vivo models for evaluating vaginal drug delivery systems. Adv. Drug Deliv. Rev. 2022, 191, 114543. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Mady, O.Y.; Dewedar, O.; Abdine, N.; Zaytoon, H.; Haggag, Y. Bioadhesive behaviors of HPMC E5: Comparative analysis of various techniques, histological and human radiological evidence. Sci. Rep. 2024, 14, 1840. [Google Scholar] [CrossRef]
- Hernández-González, M.E.; Rodríguez-González, C.A.; Valencia-Gómez, L.E.; Hernández-Paz, J.F.; Jiménez-Vega, F.; Salcedo, M.; Olivas-Armendáriz, I. Characterization of HPMC and PEG 400 Mucoadhesive Film Loaded with Retinyl Palmitate and Ketorolac for Intravaginal Administration. Int. J. Mol. Sci. 2024, 25, 12692. [Google Scholar] [CrossRef]
- Long, Y.; Zhao, X.; Liu, S.; Chen, M.; Liu, B.; Ge, J.; Jia, Y.-G.; Ren, L. Collagen–Hydroxypropyl Methylcellulose Membranes for Corneal Regeneration. ACS Omega 2018, 3, 1269–1275. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, M.; Li, G. Preparation and characterization of collagen/hydroxypropyl methylcellulose (HPMC) blend film. Carbohydr. Polym. 2015, 119, 194–201. [Google Scholar] [CrossRef]
- Luca, I.; Albu Kaya, M.G.; Titorencu, I.; Dinu-Pîrvu, C.E.; Marin, M.M.; Popa, L.; Rosca, A.M.; Antoniac, A.; Anuta, V.; Prisada, R.M.; et al. Influence of Mucoadhesive Polymers on Physicochemical Features and Biocompatibility of Collagen Wafers. ACS Polym. Au 2025, 5, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent Advances in the Development of In Situ Gelling Drug Delivery Systems for Non-Parenteral Administration Routes. Pharmaceutics 2020, 12, 859. [Google Scholar] [CrossRef]
- Abdl Aali, R.A.; Al-Sahlany, S.T. Gellan Gum as a Unique Microbial Polysaccharide: Its Characteristics, Synthesis, and Current Application Trends. Gels 2024, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Mydin, R.B.S.M.N.; Alam, M.; Raman, S.; Parumasivam, T.; Shanker, K.; Mohd Noor, S.N.F.; Kamaruddin, A.F.; Navanita, S.K.; Hadi, M.A.; Vemireddy, B.G.R.; et al. Chapter 1—An overview of gellan gum sources, properties, and its targeted applications. In Application of Gellan Gum as a Biomedical Polymer; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 1–19. [Google Scholar]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B.; Nayak, A.K.; Hasnain, M.S. Chapter 6—Gellan gum in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 145–186. [Google Scholar]
- Lalebeigi, F.; Alimohamadi, A.; Afarin, S.; Aliabadi, H.A.M.; Mahdavi, M.; Farahbakhshpour, F.; Hashemiaval, N.; Khandani, K.K.; Eivazzadeh-Keihan, R.; Maleki, A. Recent advances on biomedical applications of gellan gum: A review. Carbohydr. Polym. 2024, 334, 122008. [Google Scholar] [CrossRef]
- Patel, P.; Patel, P. Formulation and evaluation of clindamycin HCL in situ gel for vaginal application. Int. J. Pharm. Investig. 2015, 5, 50–56. [Google Scholar] [CrossRef]
- Permana, A.D.; Asri, R.M.; Amir, M.N.; Himawan, A.; Arjuna, A.; Juniarti, N.; Utami, R.N.; Mardikasari, S.A. Development of Thermoresponsive Hydrogels with Mucoadhesion Properties Loaded with Metronidazole Gel-Flakes for Improved Bacterial Vaginosis Treatment. Pharmaceutics 2023, 15, 1529. [Google Scholar] [CrossRef] [PubMed]
- Jalil, A.; Asim, M.H.; Le, N.N.; Laffleur, F.; Matuszczak, B.; Tribus, M.; Bernkop-Schnürch, A. S-protected gellan gum: Decisive approach towards mucoadhesive antimicrobial vaginal films. Int. J. Biol. Macromol. 2019, 130, 148–157. [Google Scholar] [CrossRef]
- Pahwa, R.; Ahuja, M. Nanocellulose-gellan cross-linked scaffolds for vaginal delivery of fluconazole. Int. J. Biol. Macromol. 2023, 229, 668–683. [Google Scholar] [CrossRef]
- Biswal, S.; Parmanik, A.; Das, D.; Sahoo, R.N.; Nayak, A.K. Gellan gum-based in-situ gel formulations for ocular drug delivery: A practical approach. Int. J. Biol. Macromol. 2025, 290, 138979. [Google Scholar] [CrossRef]
- Pahwa, R.; Sharma, R.; Ahuja, M. Chapter 22—Gellan gum–based ocular formulations. In Application of Gellan Gum as a Biomedical Polymer; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 435–455. [Google Scholar]
- Jelkmann, M.; Leichner, C.; Zaichik, S.; Laffleur, F.; Bernkop-Schnürch, A. A gellan gum derivative as in-situ gelling cationic polymer for nasal drug delivery. Int. J. Biol. Macromol. 2020, 158, 1037–1046. [Google Scholar] [CrossRef]
- Sipos, B.; Budai-Szűcs, M.; Katona, G.; Csóka, I. Gellan Gum-Based In Situ Hydrogels for Nasal Delivery of Polymeric Micelles Loaded with Risperidone. Gels 2025, 11, 404. [Google Scholar] [CrossRef]
- Li, A.; Khan, I.N.; Khan, I.U.; Yousaf, A.M.; Shahzad, Y. Gellan Gum-Based Bilayer Mucoadhesive Films Loaded with Moxifloxacin Hydrochloride and Clove Oil for Possible Treatment of Periodontitis. Drug Des. Dev. Ther. 2021, 15, 3937–3952. [Google Scholar] [CrossRef]
- Prezotti, F.G.; Siedle, I.; Boni, F.I.; Chorilli, M.; Müller, I.; Cury, B.S.F. Mucoadhesive films based on gellan gum/pectin blends as potential platform for buccal drug delivery. Pharm. Dev. Technol. 2020, 25, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Osmari, B.F.; Giuliani, L.M.; Reolon, J.B.; Rigo, G.V.; Tasca, T.; Cruz, L. Gellan gum-based hydrogel containing nanocapsules for vaginal indole-3-carbinol delivery in trichomoniasis treatment. Eur. J. Pharm. Sci. 2020, 151, 105379. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, R.; Wu, Y.; Xu, L. Advances in tissue engineering of gellan gum-based hydrogels. Carbohydr. Polym. 2024, 324, 121484. [Google Scholar] [CrossRef] [PubMed]
- Dodi, G.; Sabau, R.E.; Crețu, B.E.B.; Gardikiotis, I. Exploring the Antioxidant Potential of Gellan and Guar Gums in Wound Healing. Pharmaceutics 2023, 15, 2152. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, C.; Lai, R.C.; Su, K.; Zhang, F.; Wang, D.-a. An improved injectable polysaccharide hydrogel: Modified gellan gum for long-term cartilage regenerationin vitro. J. Mater. Chem. 2009, 19, 1968–1977. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Gardel, L.S.; Rada, T.; Martins, L.; Gomes, M.E.; Reis, R.L. Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects. J. Orthop. Res. 2010, 28, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Silva-Correia, J.; Oliveira, J.M.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J. Tissue Eng. Regen. Med. 2011, 5, e97–e107. [Google Scholar] [CrossRef]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Pandit, A.; Biggs, M.J. Nanocellulose reinforced gellan-gum hydrogels as potential biological substitutes for annulus fibrosus tissue regeneration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Alheib, O.; da Silva, L.P.; da Silva Morais, A.; Mesquita, K.A.; Pirraco, R.P.; Reis, R.L.; Correlo, V.M. Injectable laminin-biofunctionalized gellan gum hydrogels loaded with myoblasts for skeletal muscle regeneration. Acta Biomater. 2022, 143, 282–294. [Google Scholar] [CrossRef]
- Liu, H.; Li, K.; Guo, B.; Yuan, Y.; Ruan, Z.; Long, H.; Zhu, J.; Zhu, Y.; Chen, C. Engineering an injectable gellan gum-based hydrogel with osteogenesis and angiogenesis for bone regeneration. Tissue Cell 2024, 86, 102279. [Google Scholar] [CrossRef]
- Feketshane, Z.; Alven, S.; Aderibigbe, B.A. Gellan Gum in Wound Dressing Scaffolds. Polymers 2022, 14, 4098. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, Z.; Zulkifli, M.F.; Masimen, M.A.A.; Ismail, W.I.W.; Sharifudin, M.A.; Amin, K.A.M. Investigating the efficacy of gellan gum hydrogel films infused with Acacia stingless bee honey in wound healing. Int. J. Biol. Macromol. 2025, 296, 139753. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ismail, S.; Hamed, S.; El-Shishtawy, E.M. Butoconazole nitrate vaginal sponge: Drug release and antifungal efficacy. J. Drug Deliv. Sci. Technol. 2018, 48, 274–287. [Google Scholar] [CrossRef]
- Furst, T.; Piette, M.; Lechanteur, A.; Evrard, B.; Piel, G. Mucoadhesive cellulosic derivative sponges as drug delivery system for vaginal application. Eur. J. Pharm. Biopharm. 2015, 95, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hashempur, M.H.; Radmanesh, A.; Karami, F.; Zomorodian, K.; Amirzadeh, N.; Shenavari, S.; Zareshahrabadi, Z. Fabrication of a sponge-like protein based hydrogel incorporating fluconazole against Candida species as a potential treatment for vulvovaginal candidiasis infection. Sci. Rep. 2025, 15, 24364. [Google Scholar] [CrossRef]
- Brandão Reolon, J.; Hammerschmitt, B.; Sari, M.H.; Luna Lazo, R.E.; de Fátima, A.; Capeletti, M.; Rigo, M.; Bonini, J.S.; Abaide, A.; Pontarolo, R.; et al. Predictive Modeling of Rheological Behavior in Semisolid Pharmaceutical Formulations Using Computational Tools. Braz. Arch. Biol. Technol. 2024, 67, e24240050. [Google Scholar] [CrossRef]
- das Neves, J.; da Silva, M.V.; Gonçalves, M.P.; Amaral, M.H.; Bahia, M.F. Rheological properties of vaginal hydrophilic polymer gels. Curr. Drug Deliv. 2009, 6, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Hîrjău, M.; Lupuleasa, D.; Dinu-Pîrvu, C.-E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Fernández, M.A.; Aguilar, E. Essential Guide to Hydrogel Rheology in Extrusion 3D Printing: How to Measure It and Why It Matters? Gels 2023, 9, 517. [Google Scholar] [CrossRef]
- Yu, T.; Malcolm, K.; Woolfson, D.; Jones, D.S.; Andrews, G.P. Vaginal gel drug delivery systems: Understanding rheological characteristics and performance. Expert Opin. Drug Deliv. 2011, 8, 1309–1322. [Google Scholar] [CrossRef]
- Wang, K.; Cao, R.; Dong, H. Diversity of Collagen Proteins and Their Biomedical Applications in Drug Delivery. Appl. Sci. 2025, 15, 6472. [Google Scholar] [CrossRef]
- Kirkness, M.W.; Lehmann, K.; Forde, N.R. Mechanics and structural stability of the collagen triple helix. Curr. Opin. Chem. Biol. 2019, 53, 98–105. [Google Scholar] [CrossRef]
- Wosicka-Frąckowiak, H.; Poniedziałek, K.; Woźny, S.; Kuprianowicz, M.; Nyga, M.; Jadach, B.; Milanowski, B. Collagen and Its Derivatives Serving Biomedical Purposes: A Review. Polymers 2024, 16, 2668. [Google Scholar] [CrossRef] [PubMed]
- Fields, G.B. The collagen triple-helix: Correlation of conformation with biological activities. Connect. Tissue Res. 1995, 31, 235–243. [Google Scholar] [CrossRef]
- Malcor, J.D.; Mallein-Gerin, F. Biomaterial functionalization with triple-helical peptides for tissue engineering. Acta Biomater. 2022, 148, 1–21. [Google Scholar] [CrossRef]
- Marin, M.M.; Kaya, M.; Vlăsceanu, G.; Ghitman, J.; Radu, I.-C.; Iovu, H. The Effect of Crosslinking Agents on the Properties of Type II Collagen Biomaterials. Mater. Plast. 2021, 57, 166–180. [Google Scholar] [CrossRef]
- Tronci, G.; Russell, S.J.; Wood, D.J. Photo-active collagen systems with controlled triple helix architecture. J. Mater. Chem. B 2013, 1, 3705–3715. [Google Scholar] [CrossRef]
- Fathima, N.; Devi, R.; Rekha, K.; Dhathathreyan, A. Collagen-curcumin interaction—A physico-chemical study. J. Chem. Sci. 2009, 121, 509–514. [Google Scholar] [CrossRef]
- Gao, J.; Ning, C.; Wang, M.; Wei, M.; Ren, Y.; Li, W. Structural, antioxidant activity, and stability studies of jellyfish collagen peptide–calcium chelates. Food Chem. X 2024, 23, 101706. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, S.; Shen, L.; Li, G. Factors affecting thermal stability of collagen from the aspects of extraction, processing and modification. J. Leather Sci. Eng. 2020, 2, 19. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Jiang, H.; Kong, Y.; Song, L.; Liu, J.; Wang, Z. A Thermostable Type I Collagen from Swim Bladder of Silver Carp (Hypophthalmichthys molitrix). Mar. Drugs 2023, 21, 280. [Google Scholar] [CrossRef] [PubMed]
- Furqan, M.; Iqbal, F.; Tulain, R. Microwave radiation induced synthesis of hydroxypropyl methylcellulose-graft-(polyvinylalcohal-co-acrylic acid) polymeric network and its in vitro evaluation. Acta Pol. Pharm.-Drug Res. 2017, 74, 527–541. [Google Scholar]
- Verma, A.; Dixit, R.; Singh, U.; Soni, S.; Mishra, A.; Bansal, A.; Pandit, J. Preparation and Characterization of Gellan-Chitosan Polyelectrolyte Complex Beads. Lat. Am. J. Pharm. 2011, 30, 1186–1195. [Google Scholar]
- Shakeri, M.-s.; Naji-Tabasi, S. Characterization and optimization of gellan gum production by natural Sphingomonas sp. SM2. LWT 2024, 200, 116164. [Google Scholar] [CrossRef]
- Vranceanu, M.; Şaban, R.; Antoniac, I.; Albu, M.; Miculescu, F. Development and characterization of novel porous collagen based biocomposite for bone tissue regeneration. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2012, 74, 145–156. [Google Scholar]
- Szychlinska, M.A.; Calabrese, G.; Ravalli, S.; Dolcimascolo, A.; Castrogiovanni, P.; Fabbi, C.; Puglisi, C.; Lauretta, G.; Di Rosa, M.; Castorina, A.; et al. Evaluation of a Cell-Free Collagen Type I-Based Scaffold for Articular Cartilage Regeneration in an Orthotopic Rat Model. Materials 2020, 13, 2369. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Shahidi-Noghabi, M.; Dovom, A. Investigating the fabrication and functional properties of new composite hydrogels containing gellan/alginate/xanthan gum. J. Sol-Gel Sci. Technol. 2023, 105, 1–13. [Google Scholar] [CrossRef]
- Rupenthal, I.; Green, C.; Alany, R. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: Physicochemical characterisation and in vitro release. Int. J. Pharm. 2011, 411, 69–77. [Google Scholar] [CrossRef]
- Rojewska, M.; Olejniczak-Rabinek, M.; Bartkowiak, A.; Snela, A.; Prochaska, K.; Lulek, J. The wettability and swelling of selected mucoadhesive polymers in simulated saliva and vaginal fluids. Colloids Surf. B Biointerfaces 2017, 156, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.; Ghica, M.V.; Albu, M.G.; Ortan, A.; Dinu-Pîrvu, C.E. Hysteresis of contact angle. Dynamic wettability studies of collagen and doxycycline porous matrices crosslinked with tannic acid. Dig. J. Nanomater. Biostruct. 2013, 8, 937–943. [Google Scholar]
- Rohan, L.C.; Sassi, A.B. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009, 11, 78–87. [Google Scholar] [CrossRef]
- Adnane, M.; Meade, K.G.; O’Farrelly, C. Cervico-vaginal mucus (CVM)—An accessible source of immunologically informative biomolecules. Vet. Res. Commun. 2018, 42, 255–263. [Google Scholar] [CrossRef]
- Tietz, K.; Klein, S. Simulated Genital Tract Fluids and Their Applicability in Drug Release/Dissolution Testing of Vaginal Dosage Forms. Dissolut. Technol. 2018, 25, 40–51. [Google Scholar] [CrossRef]
- Carbinatto, F.M.; de Castro, A.D.; Evangelista, R.C.; Cury, B.S.F. Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian J. Pharm. Sci. 2014, 9, 27–34. [Google Scholar] [CrossRef]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. 3-Biopolymer Composites With High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics; Sadasivuni, K.K., Ponnamma, D., Kim, J., Cabibihan, J.J., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. [Google Scholar]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Wojasiński, M.; Dąbrowska, J.; Krzyżowska, M.; Nowicka, M.; Ciach, T.; Winnicka, K. Chitosan-poly(ethylene oxide) nanofibrous mat as a vaginal platform for tenofovir disoproxyl fumarate—The effect of vaginal pH on drug carrier performance. Int. J. Biol. Macromol. 2022, 222, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Teodora Tihan, G.; Ungureanu, C.; Constantin Barbaresso, R.; Gabriela Zgârian, R.; Rău, I.; Meghea, A.; Georgiana Albu, M.; Violeta Ghica, M. Chloramphenicol collagen sponges for local drug delivery in dentistry. Comptes Rendus Chim. 2015, 18, 986–992. [Google Scholar] [CrossRef]
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The Human Microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef]
- Łaniewski, P.; Herbst-Kralovetz, M.M. Bacterial vaginosis and health-associated bacteria modulate the immunometabolic landscape in 3D model of human cervix. NPJ Biofilms Microbiomes 2021, 7, 88. [Google Scholar] [CrossRef]
- Lithgow, K.V.; Buchholz, V.C.H.; Ku, E.; Konschuh, S.; D’Aubeterre, A.; Sycuro, L.K. Protease activities of vaginal Porphyromonas species disrupt coagulation and extracellular matrix in the cervicovaginal niche. npj Biofilms Microbiomes 2022, 8, 8. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ţuţuianu, R.; Roşca, A.M.; Florea, G.; Prună, V.; Iacomi, D.M.; Rădulescu, L.A.; Neagu, T.P.; Lascăr, I.; Titorencu, I.D. Heterogeneity of human fibroblasts isolated from hypertrophic scar. Rom. J. Morphol. Embryol. 2019, 60, 793–802. [Google Scholar]









| Sample | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|
| m | 3.412 | 3.741 | 5.212 | 4.017 | 6.327 | 4.583 | 12.218 | 10.365 |
| n | 0.68 | 0.62 | 0.75 | 0.67 | 0.71 | 0.56 | 0.35 | 0.69 |
| R2 | 0.9960 | 0.9990 | 0.9990 | 0.9992 | 0.9985 | 0.9999 | 0.9992 | 0.9987 |
| Sample | 1% Collagen Gel, % * | 2% HPMC Gel, % * | 1.2% GG Gel, % * |
|---|---|---|---|
| S1 | 100 | 0 | 0 |
| S2 | 75 | 25 | 0 |
| S3 | 75 | 0 | 25 |
| S4 | 75 | 12.5 | 12.5 |
| S5 | 50 | 0 | 50 |
| S6 | 50 | 25 | 25 |
| S7 | 0 | 100 | 0 |
| S8 | 0 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, I.; Albu Kaya, M.G.; Țuțuianu, R.; Dinu-Pîrvu, C.E.; Marin, M.M.; Popa, L.; Titorencu, I.; Anuța, V.; Ghica, M.V. Effect of Gellan Gum on the Properties of Collagen-HPMC Freeze-Dried Hydrogels for Mucosal Administration. Gels 2025, 11, 793. https://doi.org/10.3390/gels11100793
Luca I, Albu Kaya MG, Țuțuianu R, Dinu-Pîrvu CE, Marin MM, Popa L, Titorencu I, Anuța V, Ghica MV. Effect of Gellan Gum on the Properties of Collagen-HPMC Freeze-Dried Hydrogels for Mucosal Administration. Gels. 2025; 11(10):793. https://doi.org/10.3390/gels11100793
Chicago/Turabian StyleLuca, Ioana, Mădălina Georgiana Albu Kaya, Raluca Țuțuianu, Cristina Elena Dinu-Pîrvu, Maria Minodora Marin, Lăcrămioara Popa, Irina Titorencu, Valentina Anuța, and Mihaela Violeta Ghica. 2025. "Effect of Gellan Gum on the Properties of Collagen-HPMC Freeze-Dried Hydrogels for Mucosal Administration" Gels 11, no. 10: 793. https://doi.org/10.3390/gels11100793
APA StyleLuca, I., Albu Kaya, M. G., Țuțuianu, R., Dinu-Pîrvu, C. E., Marin, M. M., Popa, L., Titorencu, I., Anuța, V., & Ghica, M. V. (2025). Effect of Gellan Gum on the Properties of Collagen-HPMC Freeze-Dried Hydrogels for Mucosal Administration. Gels, 11(10), 793. https://doi.org/10.3390/gels11100793









