Suitability of Polyacrylamide-Based Dosimetric Gel for Proton and Carbon Ion Beam Geometric Characterization
Abstract
1. Introduction
2. Results and Discussion
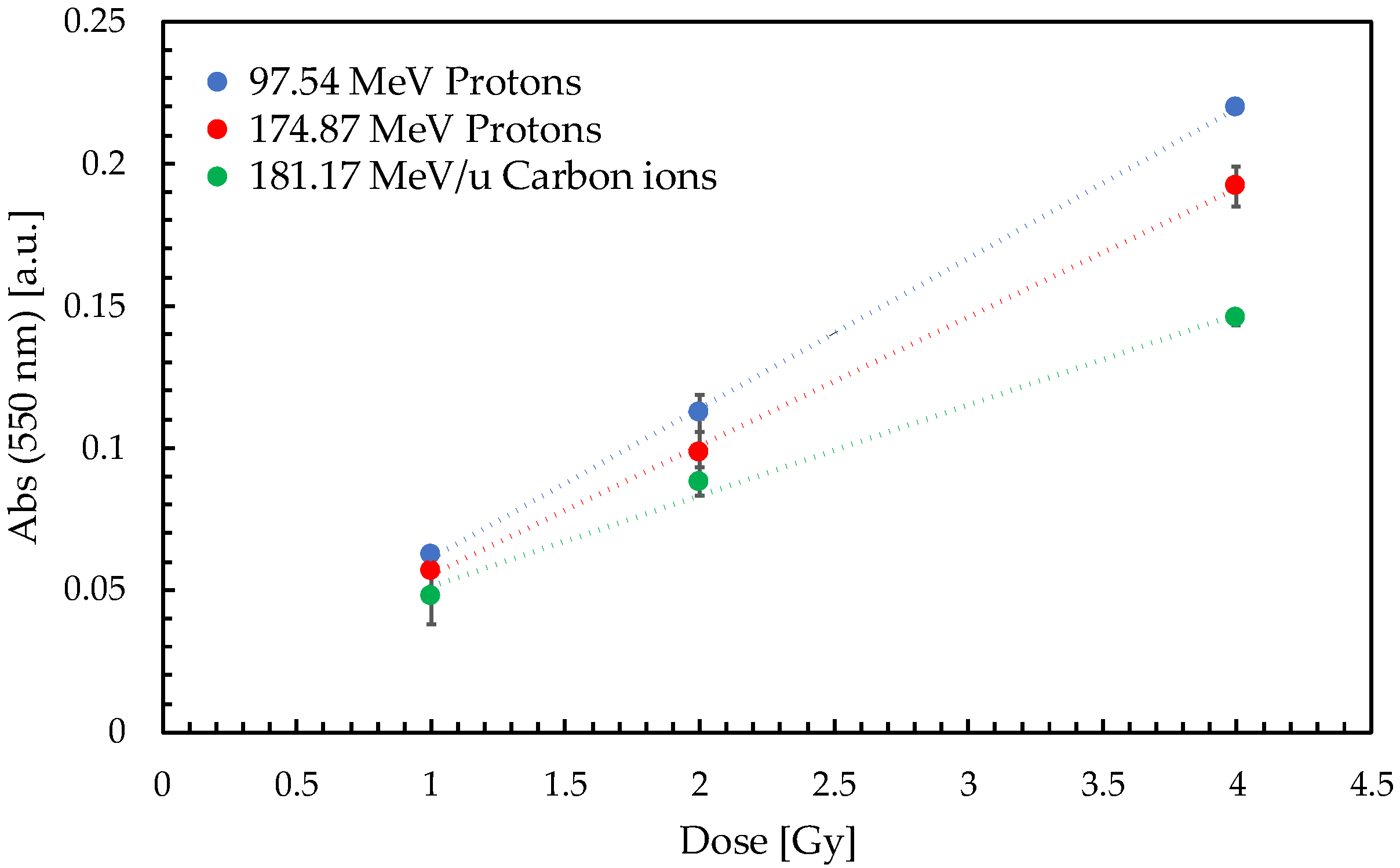
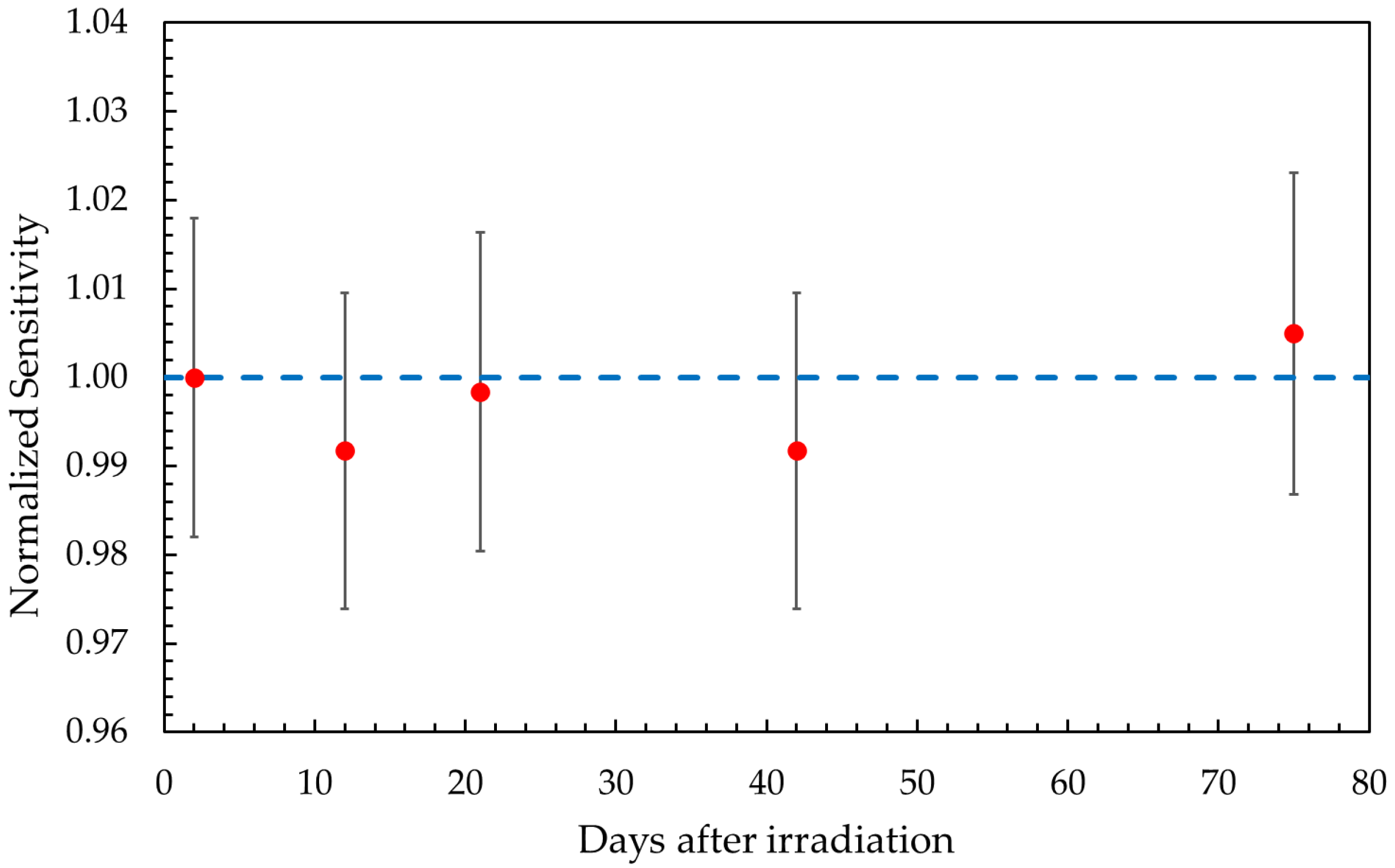
2.1. Gel Dosimeter Response Characterization
2.2. Volumetric Response
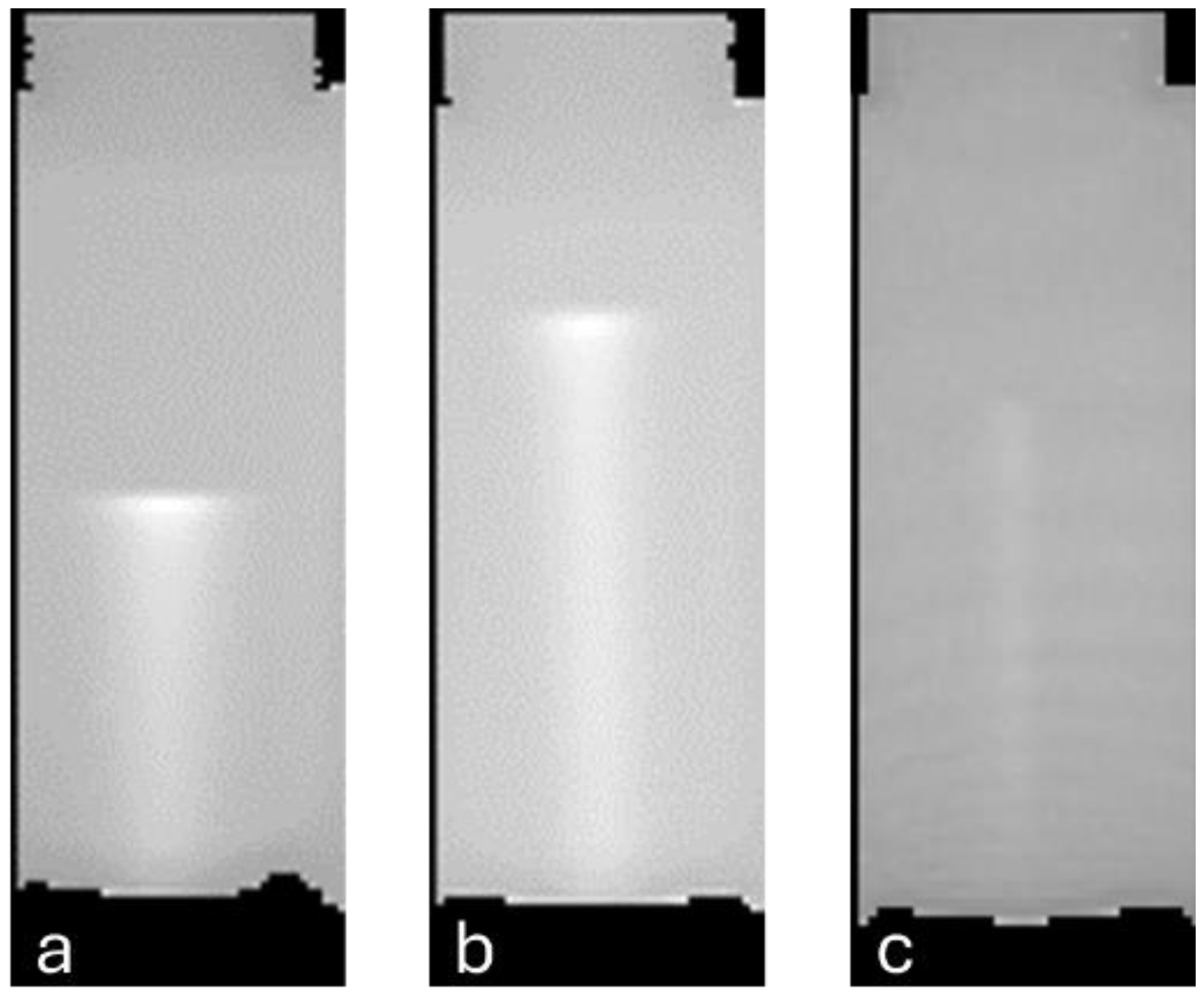
2.2.1. Depth–Dose Profiles
2.2.2. Transverse Dose Distributions
3. Conclusions
4. Materials and Methods
4.1. Gel Dosimeter Preparation
- Dissolve AAm and BIS in ≈55% of the overall deionized water volume while heating at 50 °C and stirring;
- Meanwhile, add porcine skin gelatin to the remaining 45% of water volume while heating at 50 °C and stirring;
- Allow both solutions to cool down to 30 °C while stirring;
- Once cooled, slowly add the AAm/BIS solution to the gelatin one under constant stirring;
- Add p-nitrophenol and THPC dropwise under stirring.
4.2. Irradiation
4.3. Dosimeter Analysis
4.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, P.; O’Sullivan, B. Radiotherapy Quality Assurance: Time for Everyone to Take It Seriously. Eur. J. Cancer 2003, 39, 423–429. [Google Scholar] [CrossRef]
- De Deene, Y. Gel Dosimetry for the Dose Verification of Intensity Modulated Radiotherapy Treatments. Z. Med. Phys. 2002, 12, 77–88. [Google Scholar] [CrossRef]
- Devic, S. Radiochromic Film Dosimetry: Past, Present, and Future. Phys. Medica 2011, 27, 122–134. [Google Scholar] [CrossRef]
- Low, D.A.; Moran, J.M.; Dempsey, J.F.; Dong, L.; Oldham, M. Dosimetry Tools and Techniques for IMRT. Med. Phys. 2011, 38, 1313–1338. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer Gel Dosimetry. Phys. Med. Biol. 2010, 55, R1. [Google Scholar] [CrossRef]
- Magugliani, G.; Marranconi, M.; Liosi, G.M.; Locatelli, F.; Gambirasio, A.; Trombetta, L.; Hertsyk, V.; Torri, V.; Galluccio, F.; Macerata, E.; et al. Pilot Scale Validation Campaign of Gel Dosimetry for Pre-Treatment Quality Assurance in Stereotactic Radiotherapy. Phys. Medica 2023, 114, 103158. [Google Scholar] [CrossRef]
- Rossi, S. Hadron Therapy Achievements and Challenges: The CNAO Experience. Physics 2022, 4, 229–257. [Google Scholar] [CrossRef]
- Thariat, J.; Valable, S.; Laurent, C.; Haghdoost, S.; Pérès, E.A.; Bernaudin, M.; Sichel, F.; Lesueur, P.; Césaire, M.; Petit, E.; et al. Hadrontherapy Interactions in Molecular and Cellular Biology. Int. J. Mol. Sci. 2020, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Braccini, S. Scientific and Technological Development of Hadrontherapy. In Proceedings of the Astroparticle, Particle and Space Physics, Detectors and Medical Physics Applications—Proceedings of the 11th Conference, Como, Italy, 5–9 October 2009; pp. 598–609. [Google Scholar]
- Gustavsson, H.; Bäck, S.Å.J.; Medin, J.; Grusell, E.; Olsson, L.E. Linear Energy Transfer Dependence of a Normoxic Polymer Gel Dosimeter Investigated Using Proton Beam Absorbed Dose Measurements. Phys. Med. Biol. 2004, 49, 3847–3855. [Google Scholar] [CrossRef] [PubMed]
- Hillbrand, M.; Landry, G.; Ebert, S.; Dedes, G.; Pappas, E.; Kalaitzakis, G.; Kurz, C.; Würl, M.; Englbrecht, F.; Dietrich, O.; et al. Gel Dosimetry for Three Dimensional Proton Range Measurements in Anthropomorphic Geometries. Z. Med. Phys. 2019, 29, 162–172. [Google Scholar] [CrossRef]
- Jirasek, A.; Hilts, M.; McAuley, K.B. Polymer Gel Dosimeters with Enhanced Sensitivity for Use in X-Ray CT Polymer Gel Dosimetry. Phys. Med. Biol. 2010, 55, 5269. [Google Scholar] [CrossRef] [PubMed]
- Maryanski, M.J.; Schulz, R.J.; Ibbott, G.S.; Gatenby, J.C.; Xie, J.; Horton, D.; Gore, J.C. Magnetic Resonance Imaging of Radiation Dose Distributions Using a Polymer-Gel Dosimeter. Phys. Med. Biol. 1994, 39, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Behrens, R.; Zutz, H.; Busse, J.; Mather, M.L.; Whittaker, A.K.; Baldock, C. Ultrasound Evaluation of Polymer Gel Dosimeters. Phys. Med. Biol. 2002, 47, 1449. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Geraily, G.; Abtahi, S.M.M. A Systematic Review of Clinical Applications of Polymer Gel Dosimeters in Radiotherapy. Appl. Radiat. Isot. 2019, 143, 47–59. [Google Scholar] [CrossRef]
- Brown, S.; Venning, A.; De Deene, Y.; Vial, P.; Oliver, L.; Adamovics, J.; Baldock, C. Radiological Properties of the PRESAGE and PAGAT Polymer Dosimeters. Appl. Radiat. Isot. 2008, 66, 1970–1974. [Google Scholar] [CrossRef]
- Deene, Y. De Essential Characteristics of Polymer Gel Dosimeters. J. Phys. Conf. Ser. 2004, 3, 34–57. [Google Scholar] [CrossRef]
- De Deene, Y.; Venning, A.; Hurley, C.; Healy, B.J.; Baldock, C. Dose-Response Stability and Integrity of the Dose Distribution of Various Polymer Gel Dosimeters. Phys. Med. Biol. 2002, 47, 2459. [Google Scholar] [CrossRef]
- Venning, A.J.; Brindha, S.; Hill, B.; Baldock, C. Preliminary Study of a Normoxic PAG Gel Dosimeter with Tetrakis (Hydroxymethyl) Phosphonium Chloride as an Anti-Oxidant. J. Phys. Conf. Ser. 2004, 3, 155. [Google Scholar] [CrossRef]
- Jirasek, A.I.; Duzenli, C. Effects of Crosslinker Fraction in Polymer Gel Dosimeters Using FT Raman Spectroscopy. Phys. Med. Biol. 2001, 46, 1949–1961. [Google Scholar] [CrossRef]
- McAuley, K.B.; Nasr, A.T. Fundamentals of Gel Dosimeters. J. Phys. Conf. Ser. 2013, 444, 012001. [Google Scholar] [CrossRef]
- Jirasek, A.; Hilts, M.; Shaw, C.; Baxter, P. Investigation of Tetrakis Hydroxymethyl Phosphonium Chloride as an Antioxidant for Use in X-Ray Computed Tomography Polyacrylamide Gel Dosimetry. Phys. Med. Biol. 2006, 51, 1891–1906. [Google Scholar] [CrossRef] [PubMed]
- Magugliani, G.; Liosi, G.M.; Marranconi, M.; Micotti, E.; Caprioli, M.; Gambirasio, A.; Locatelli, F.; Macerata, E.; Mossini, E.; Salmoiraghi, P.; et al. Practical Role of Polymerization Inhibitors in Polymer Gel Dosimeters. Nuovo C. Della Soc. Ital. Di Fis. C 2020, 43, 1–10. [Google Scholar] [CrossRef]
- Lepage, M.; Jordan, K. 3D Dosimetry Fundamentals: Gels and Plastics. J. Phys. Conf. Ser. 2010, 250, 253–261. [Google Scholar] [CrossRef]
- Gambarini, G.; Carrara, M.; Mariani, M.; Pirola, L.; Tomatis, S.; Valente, M.; Vanossi, E. Optical Analysis of Gel Dosimeters: Comparison of Fricke and Normoxic Polymer Gels. Nucl. Instrum. Methods Phys. Res. B 2007, 263, 191–195. [Google Scholar] [CrossRef]
- Gore, J.C.; Kang, Y.S. Measurement of Radiation Dose Distributions by Nuclear Magnetic Resonance (NMR) Imaging. Phys. Med. Biol. 1984, 29, 1189–1197. [Google Scholar] [CrossRef]
- Heufelder, J.; Stiefel, S.; Pfaender, M.; Lüdemann, L.; Grebe, G.; Heese, J. Use of BANG® Polymer Gel for Dose Measurements in a 68 MeV Proton Beam. Med. Phys. 2003, 30, 1235–1240. [Google Scholar] [CrossRef]
- Jirasek, A.; Duzenli, C. Relative Effectiveness of Polyacrylamide Gel Dosimeters Applied to Proton Beams: Fourier Transform Raman Observations and Track Structure Calculations. Med. Phys. 2002, 29, 569–577. [Google Scholar] [CrossRef]
- Mirandola, A.; Molinelli, S.; Vilches Freixas, G.; Mairani, A.; Gallio, E.; Panizza, D.; Russo, S.; Ciocca, M.; Donetti, M.; Magro, G.; et al. Dosimetric Commissioning and Quality Assurance of Scanned Ion Beams at the Italian National Center for Oncological Hadrontherapy. Med. Phys. 2015, 42, 5287–5300. [Google Scholar] [CrossRef]
- Abtahi, S.M. Characteristics of a Novel Polymer Gel Dosimeter Formula for MRI Scanning: Dosimetry, Toxicity and Temporal Stability of Response. Phys. Medica 2016, 32, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- McJury, M.; Oldham, M.; Leach, M.O.; Webb, S. Dynamics of Polymerization in Polyacrylamide Gel (PAG) Dosimeters: (I) Ageing and Long-Term Stability. Phys. Med. Biol. 1999, 44, 1863–1873. [Google Scholar] [CrossRef]
- Valdetaro, L.B.; Høye, E.M.; Skyt, P.S.; Petersen, J.B.B.; Balling, P.; Muren, L.P. Empirical Quenching Correction in Radiochromic Silicone-Based Three-Dimensional Dosimetry of Spot-Scanning Proton Therapy. Phys. Imaging Radiat. Oncol. 2021, 18, 11–18. [Google Scholar] [CrossRef]
- Lopatiuk-Tirpak, O.; Su, Z.; Li, Z.; Zeidan, O.A.; Meeks, S.L.; Maryanski, M.J. Direct Response to Proton Beam Linear Energy Transfer (LET) in a Novel Polymer Gel Dosimeter Formulation. Technol. Cancer Res. Treat. 2012, 11, 441–445. [Google Scholar] [CrossRef]
- De Deene, Y.; Hanselaer, P.; De Wagter, C.; Achten, E.; De Neve, W. An Investigation of the Chemical Stability of a Monomer/Polymer Gel Dosimeter. Phys. Med. Biol. 2000, 45, 859–878. [Google Scholar] [CrossRef]
- IAEA TRS 398 Absorbed Dose Determination in External Beam Radiotherapy. At. Energy 2000, 81, 592–593. [CrossRef]
- De Deene, Y. Review of Quantitative MRI Principles for Gel Dosimetry. J. Phys. Conf. Ser. 2009, 164, 012033. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Mark Haacke, E. Principles, Techniques, and Applications of T2*-Based MR Imaging and Its Special Applications. Radiographics 2009, 29, 1433–1449. [Google Scholar] [CrossRef]
- Rocha, F.S.; Gomes, A.J.; Lunardi, C.N.; Kaliaguine, S.; Patience, G.S. Experimental Methods in Chemical Engineering: Ultraviolet Visible Spectroscopy—UV-Vis. Can. J. Chem. Eng. 2018, 96, 2512–2517. [Google Scholar] [CrossRef]
- De Deene, Y.; Baldock, C. Optimization of Multiple Spin-Echo Sequences for 3D Polymer Gel Dosimetry. Phys. Med. Biol. 2002, 47, 3117–3141. [Google Scholar] [CrossRef] [PubMed]
- De Deene, Y.; Van De Walle, R.; Achten, E.; De Wagter, C. Mathematical Analysis and Experimental Investigation of Noise in Quantitative Magnetic Resonance Imaging Applied in Polymer Gel Dosimetry. Signal Process. 1998, 70, 85–101. [Google Scholar] [CrossRef]
- Castriconi, R.; Ciocca, M.; Mirandola, A.; Sini, C.; Broggi, S.; Schwarz, M.; Fracchiolla, F.; Martišíková, M.; Aricò, G.; Mettivier, G.; et al. Dose-Response of EBT3 Radiochromic Films to Proton and Carbon Ion Clinical Beams. Phys. Med. Biol. 2017, 62, 377–393. [Google Scholar] [CrossRef]





| PAGAT | Ion Chamber | Difference | |
|---|---|---|---|
| 97.54 MeV p | 72.4 ± 0.7 mm | 72.2 ± 0.2 mm | 0.2 mm |
| 118.20 MeV p | 104.5 ± 0.7 mm | 103.9 ± 0.2 mm | 0.6 mm |
| 208.58 MeV/u C | 94.0 ± 0.7 mm | 93.4 ± 0.2 mm | 0.6 mm |
| FWHM | ||||
|---|---|---|---|---|
| Depth | PAGAT | Gafchromic | Difference | |
| 97.54 MeV p | d = 20 mm | 15.9 mm | 15.8 mm | 0.1 mm |
| d = 35 mm | 16.1 mm | 16.9 mm | −0.8 mm | |
| d = 66 mm | 17.5 mm | 17.8 mm | −0.3 mm | |
| 118.20 MeV p | d = 20 mm | 13.2 mm | 13.7 mm | −0.5 mm |
| d = 50 mm | 14.0 mm | 15.3 mm | −1.3 mm | |
| d = 96.5 mm | 16.1 mm | 17.5 mm | −1.4 mm | |
| 208.58 MeV/u C | d = 20 mm | 6.4 mm | 6.6 mm | −0.2 mm |
| d = 45 mm | 6.7 mm | 6.8 mm | −0.1 mm | |
| d = 80 mm | 7.5 mm | 7.7 mm | −0.2 mm | |
| Compound | Concentration |
|---|---|
| AAm | 3% wt. |
| BIS | 3% wt. |
| Gelatin | 5% wt. |
| Deionized water | 89% wt. |
| p-nitrophenol | 2.5 ppm |
| THPC | 10 mM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brambilla, R.; Trombetta, L.; Magugliani, G.; Russo, S.; Bazani, A.; Rossi, E.; Mossini, E.; Macerata, E.; Galluccio, F.; Mariani, M.; et al. Suitability of Polyacrylamide-Based Dosimetric Gel for Proton and Carbon Ion Beam Geometric Characterization. Gels 2025, 11, 794. https://doi.org/10.3390/gels11100794
Brambilla R, Trombetta L, Magugliani G, Russo S, Bazani A, Rossi E, Mossini E, Macerata E, Galluccio F, Mariani M, et al. Suitability of Polyacrylamide-Based Dosimetric Gel for Proton and Carbon Ion Beam Geometric Characterization. Gels. 2025; 11(10):794. https://doi.org/10.3390/gels11100794
Chicago/Turabian StyleBrambilla, Riccardo, Luca Trombetta, Gabriele Magugliani, Stefania Russo, Alessia Bazani, Eleonora Rossi, Eros Mossini, Elena Macerata, Francesco Galluccio, Mario Mariani, and et al. 2025. "Suitability of Polyacrylamide-Based Dosimetric Gel for Proton and Carbon Ion Beam Geometric Characterization" Gels 11, no. 10: 794. https://doi.org/10.3390/gels11100794
APA StyleBrambilla, R., Trombetta, L., Magugliani, G., Russo, S., Bazani, A., Rossi, E., Mossini, E., Macerata, E., Galluccio, F., Mariani, M., & Ciocca, M. (2025). Suitability of Polyacrylamide-Based Dosimetric Gel for Proton and Carbon Ion Beam Geometric Characterization. Gels, 11(10), 794. https://doi.org/10.3390/gels11100794






