Study on Carboxymethylation Modification of Konjac Gum and Its Effect in Drilling Fluid and Fracturing Fluid
Abstract
1. Introduction
2. Results and Discussion
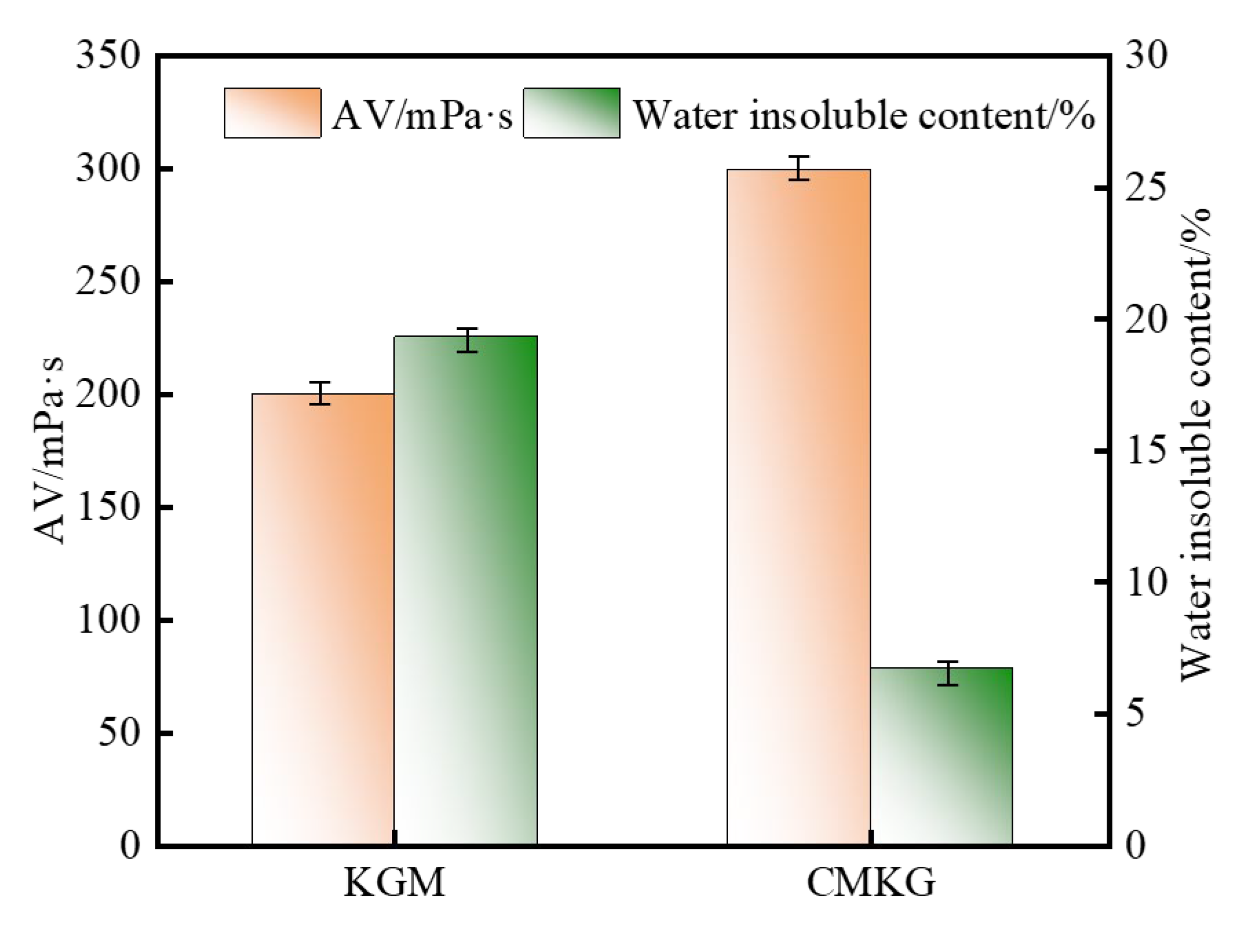
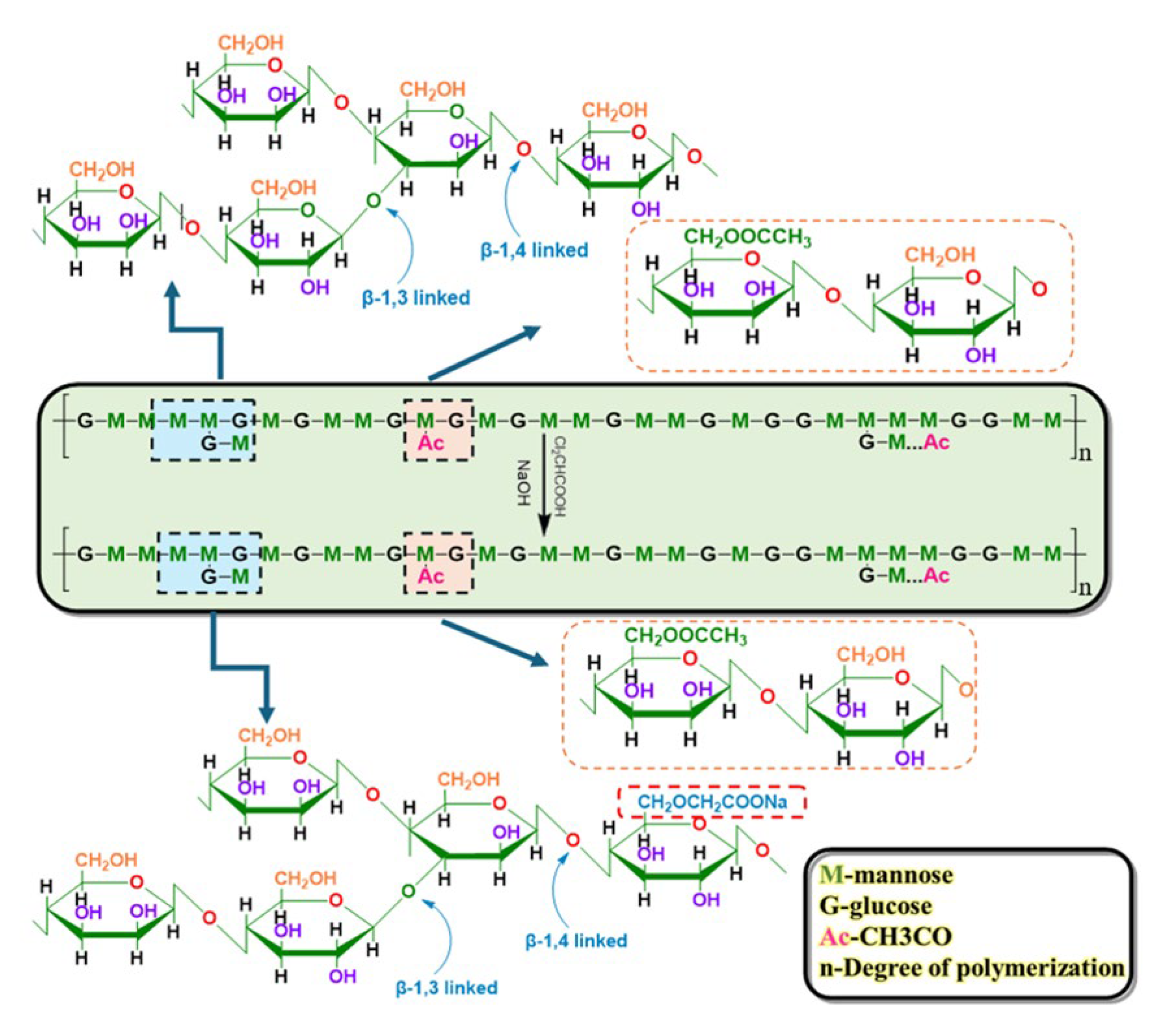
2.1. Modification of KGMs
2.2. Characterization Results
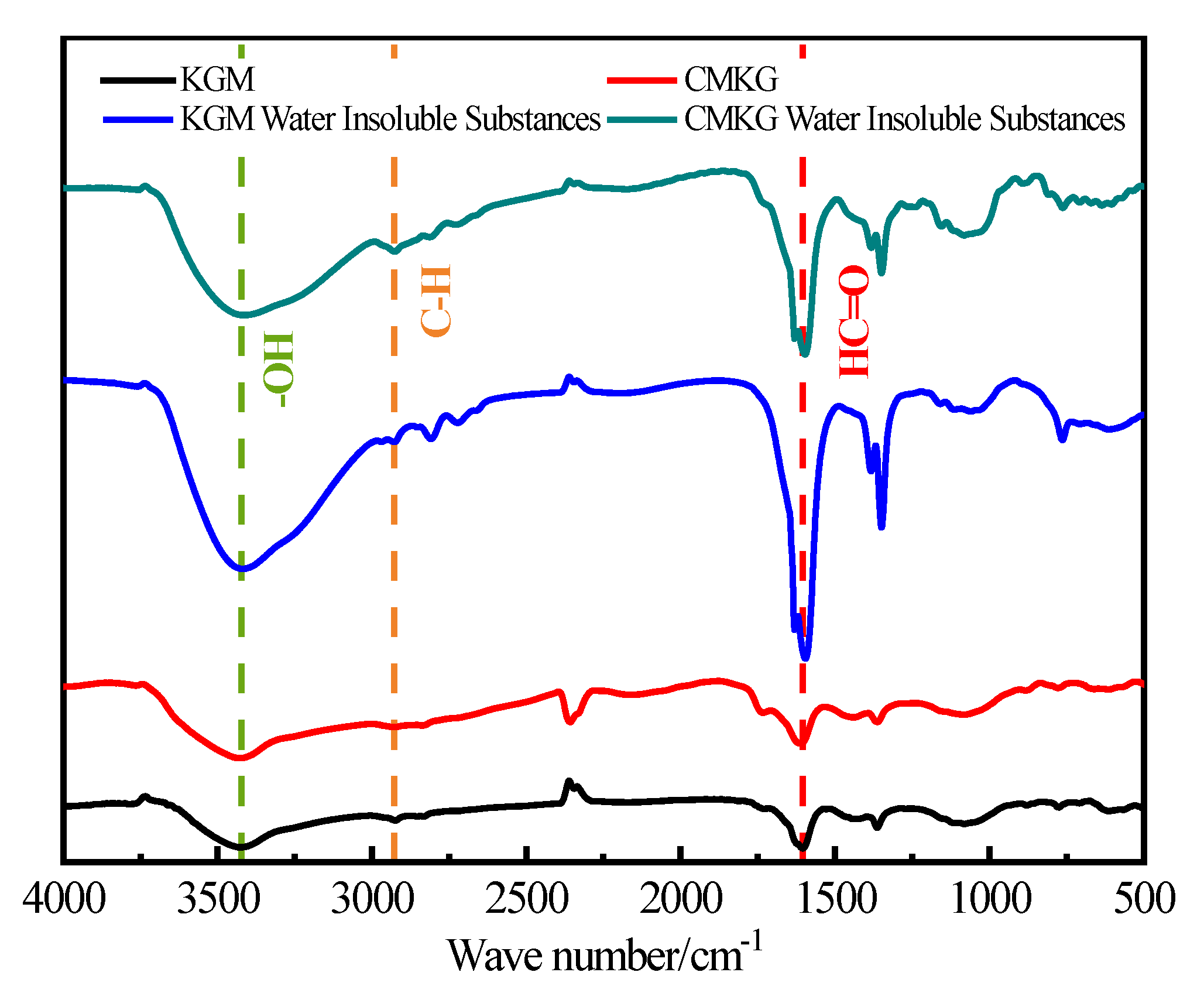
2.2.1. FTIR Analysis
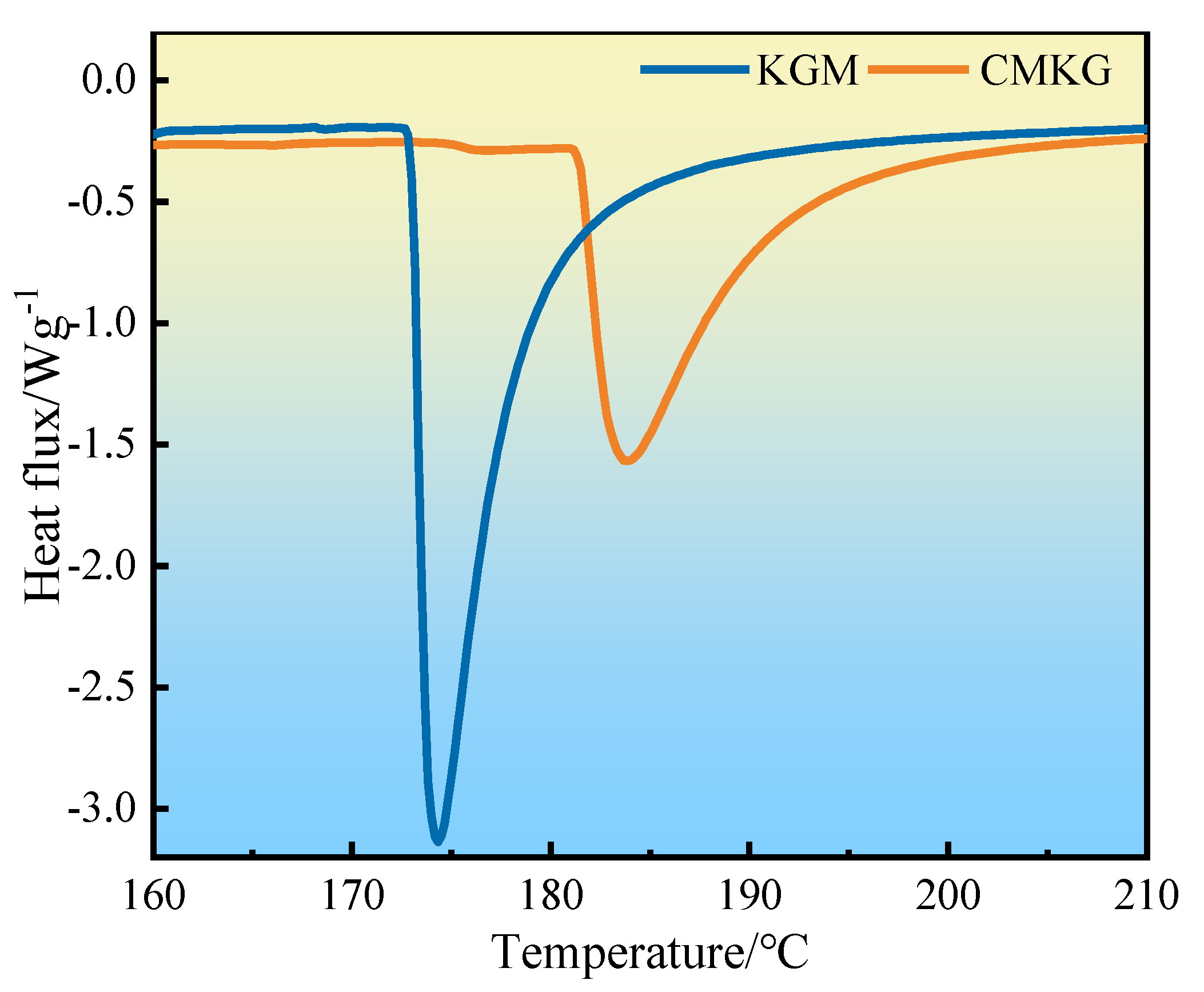
2.2.2. DSC Analysis
2.2.3. TG Analysis
2.2.4. Elemental Analysis
2.3. Application of KGM and CMKG in Drilling Fluid
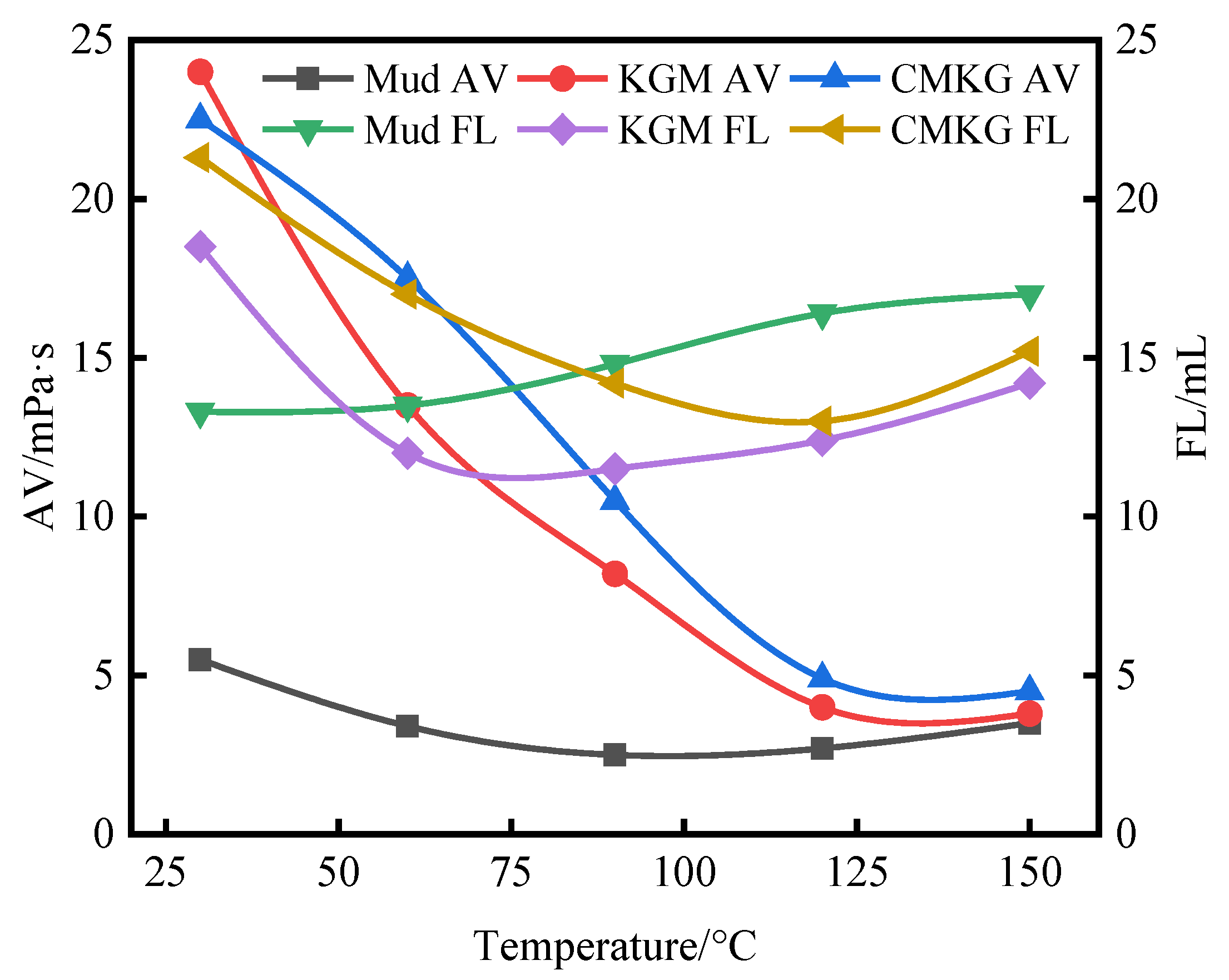
2.3.1. Drilling Fluid Performance
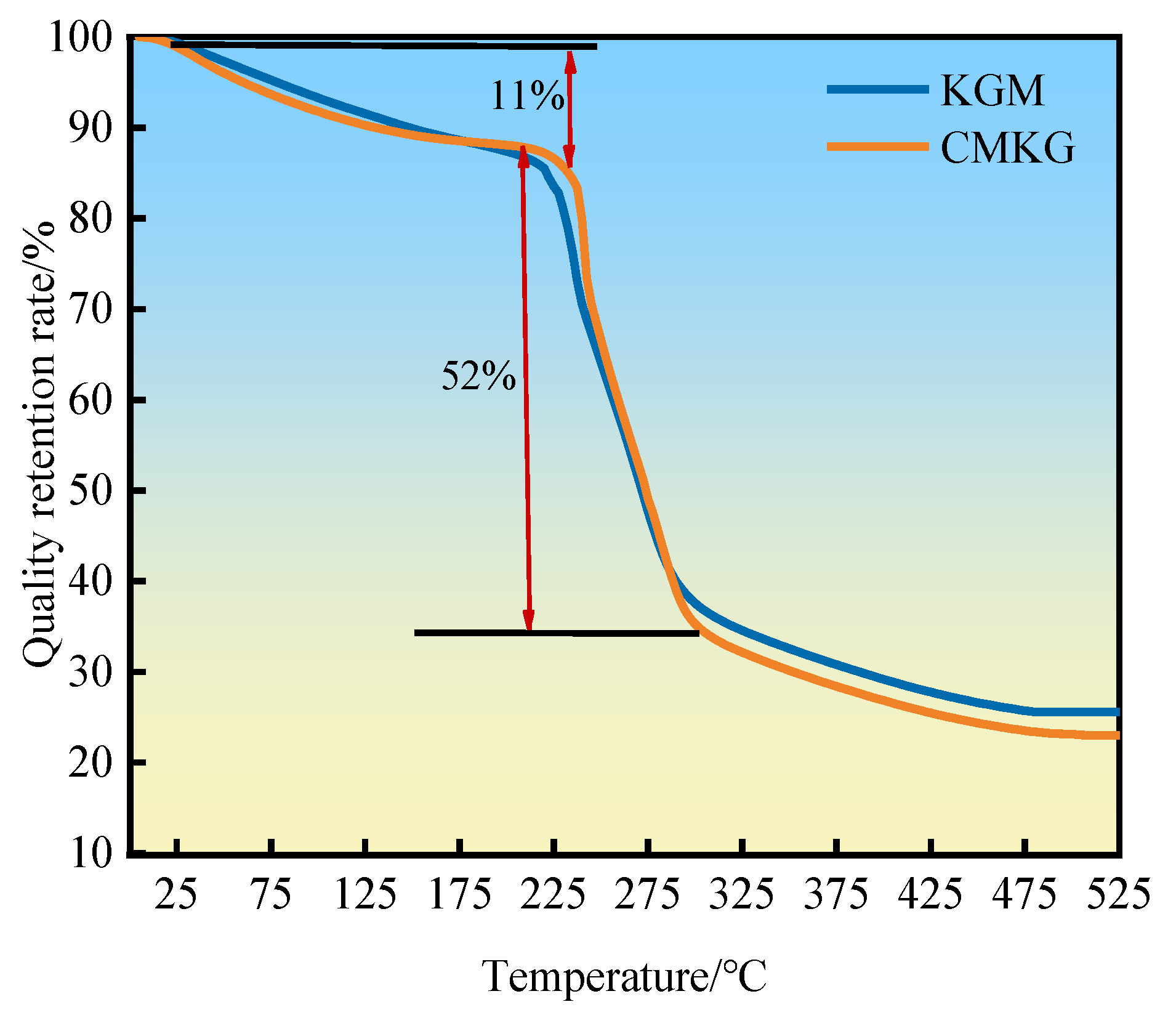
2.3.2. Evaluation of Temperature Resistance Performance
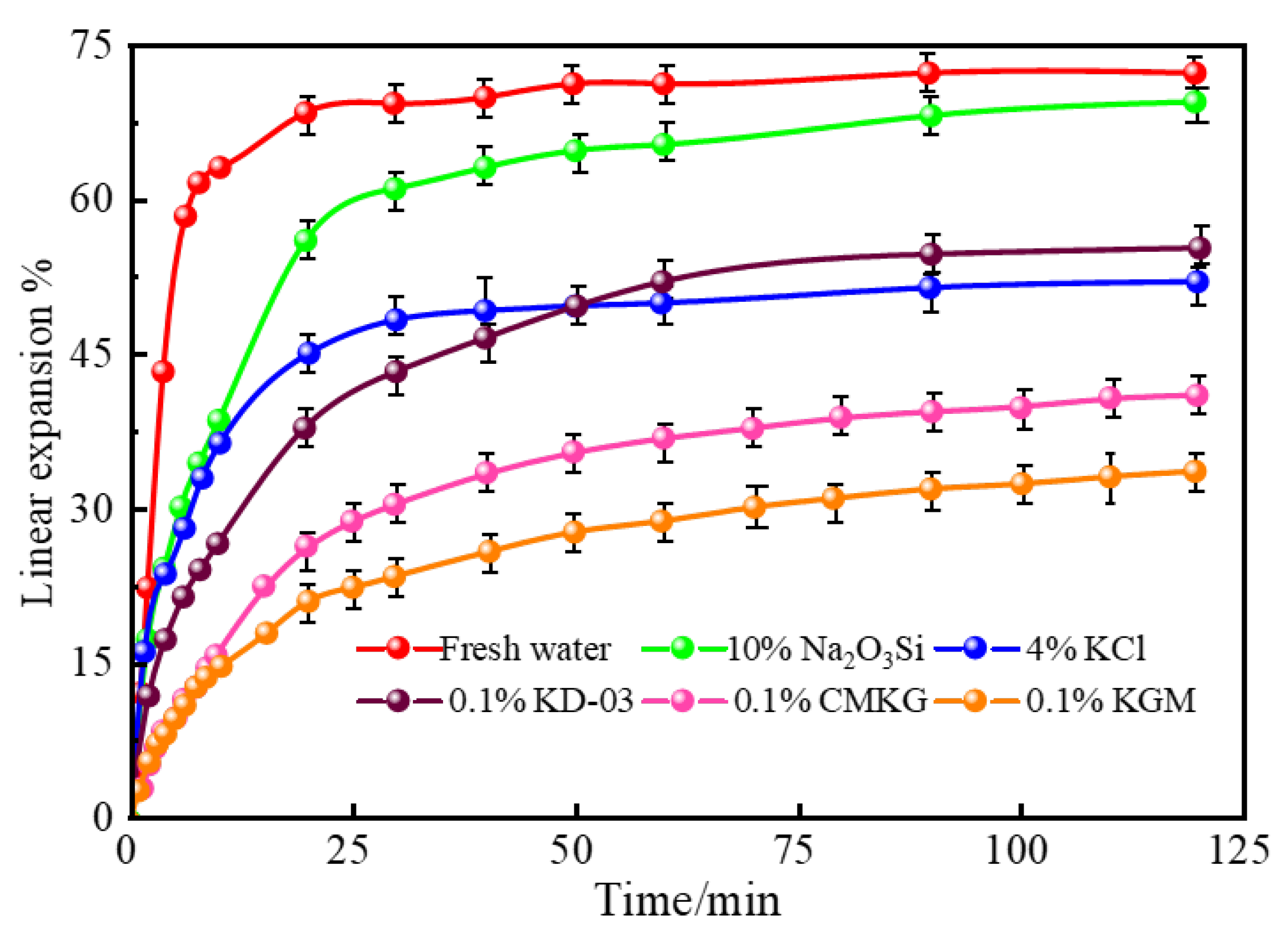
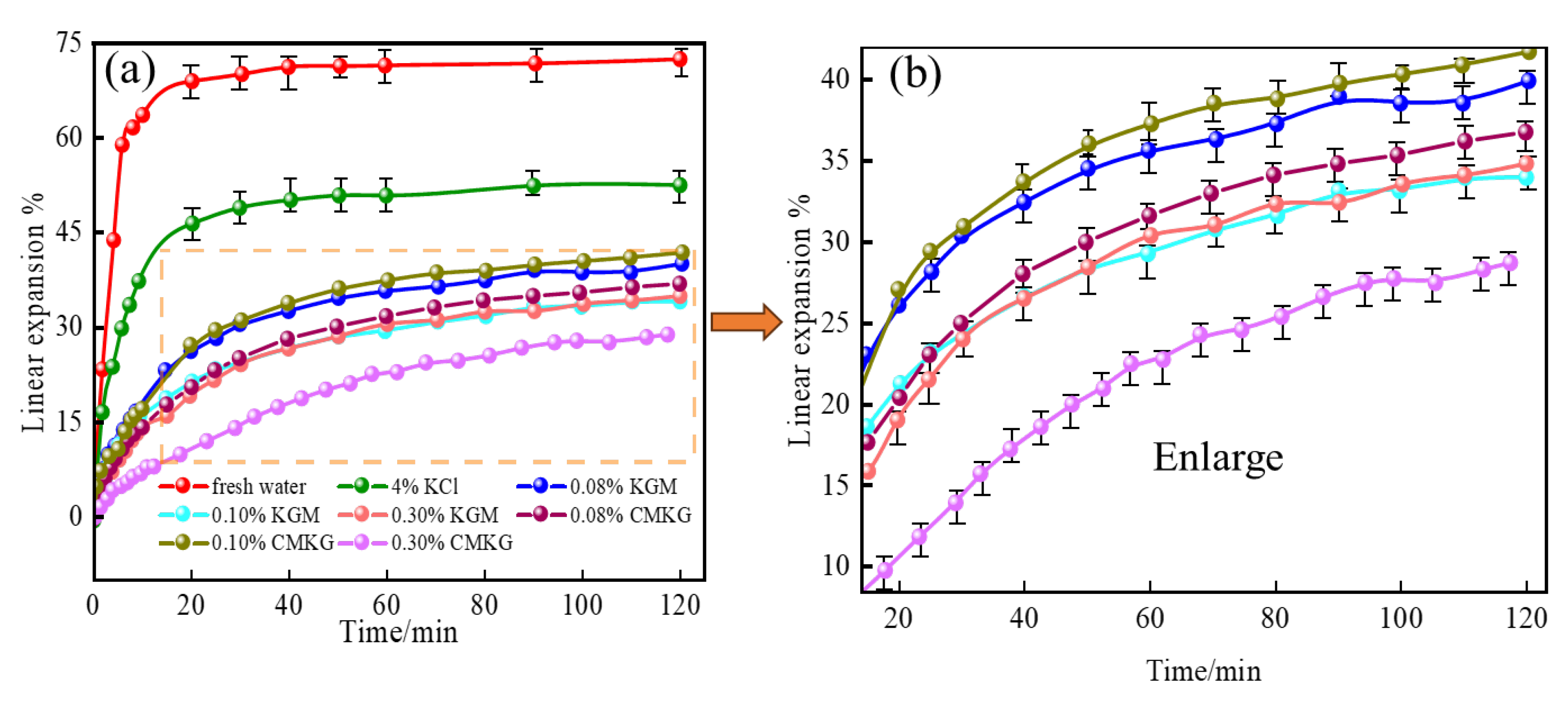
2.3.3. Linear Expansion of Bentonite
2.3.4. Mudball Experiments
2.4. Application of KGM and CMKG in Fracturing Fluid
2.4.1. Clay Compatibility
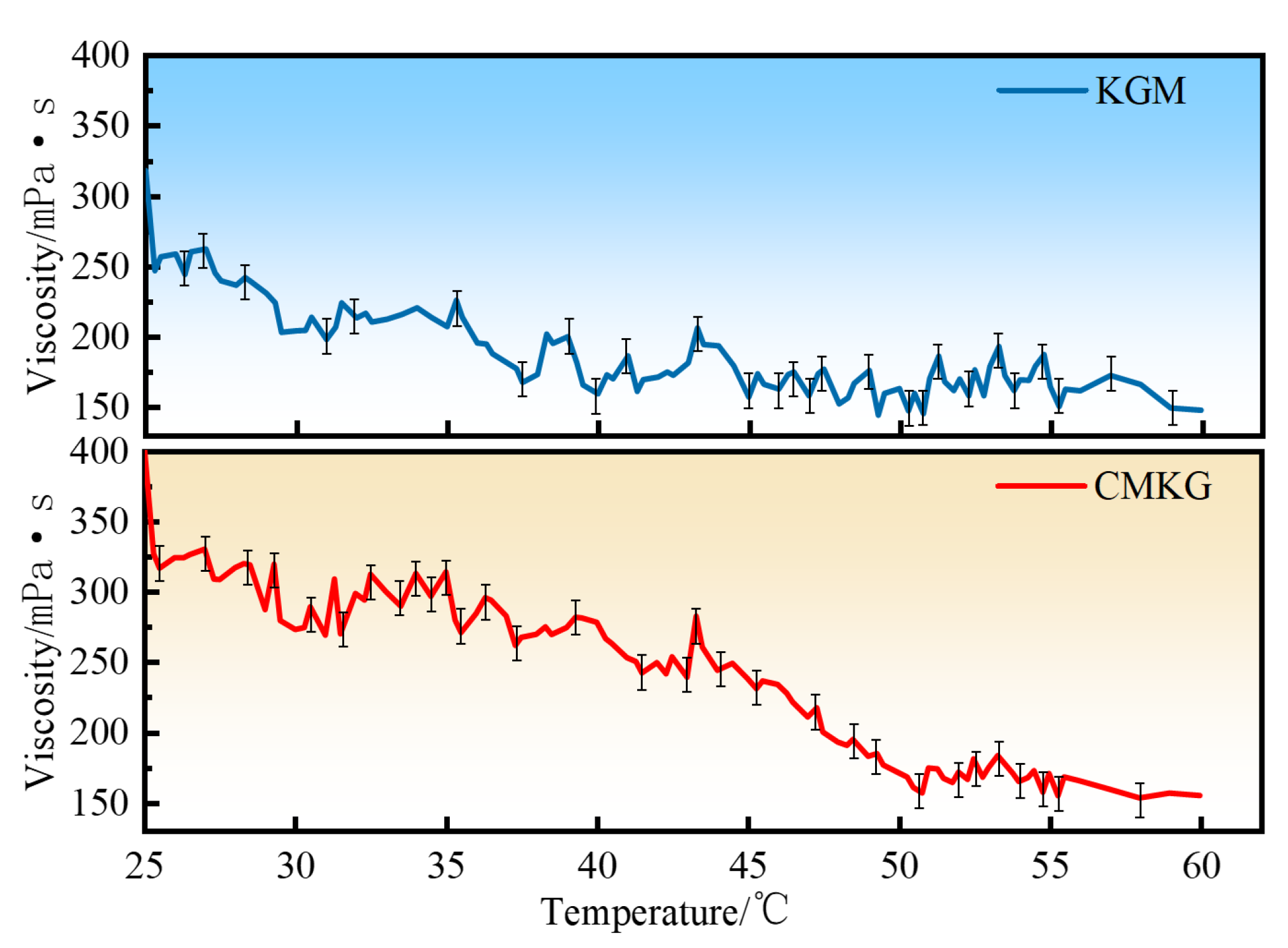
2.4.2. Temperature Resistance Performance
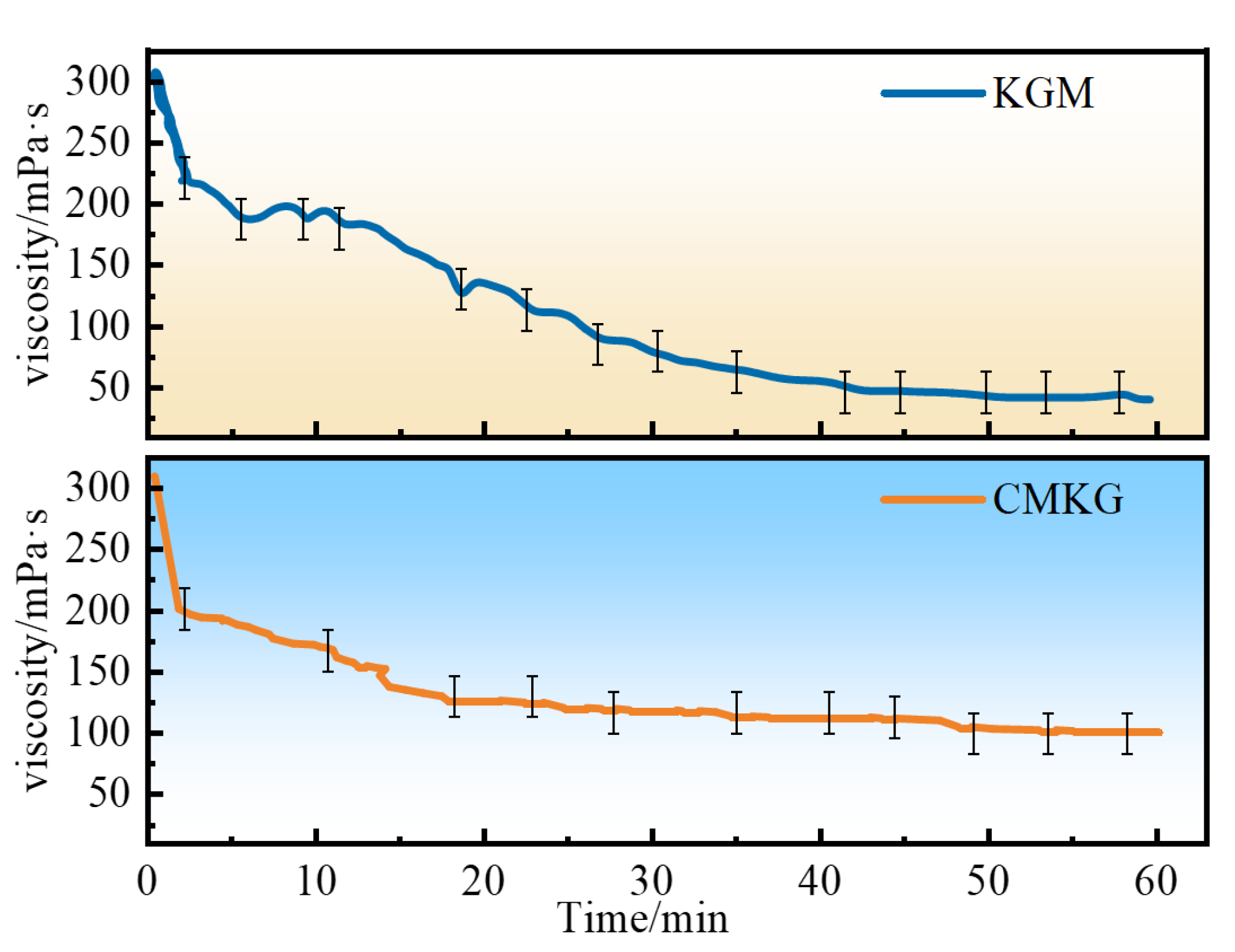
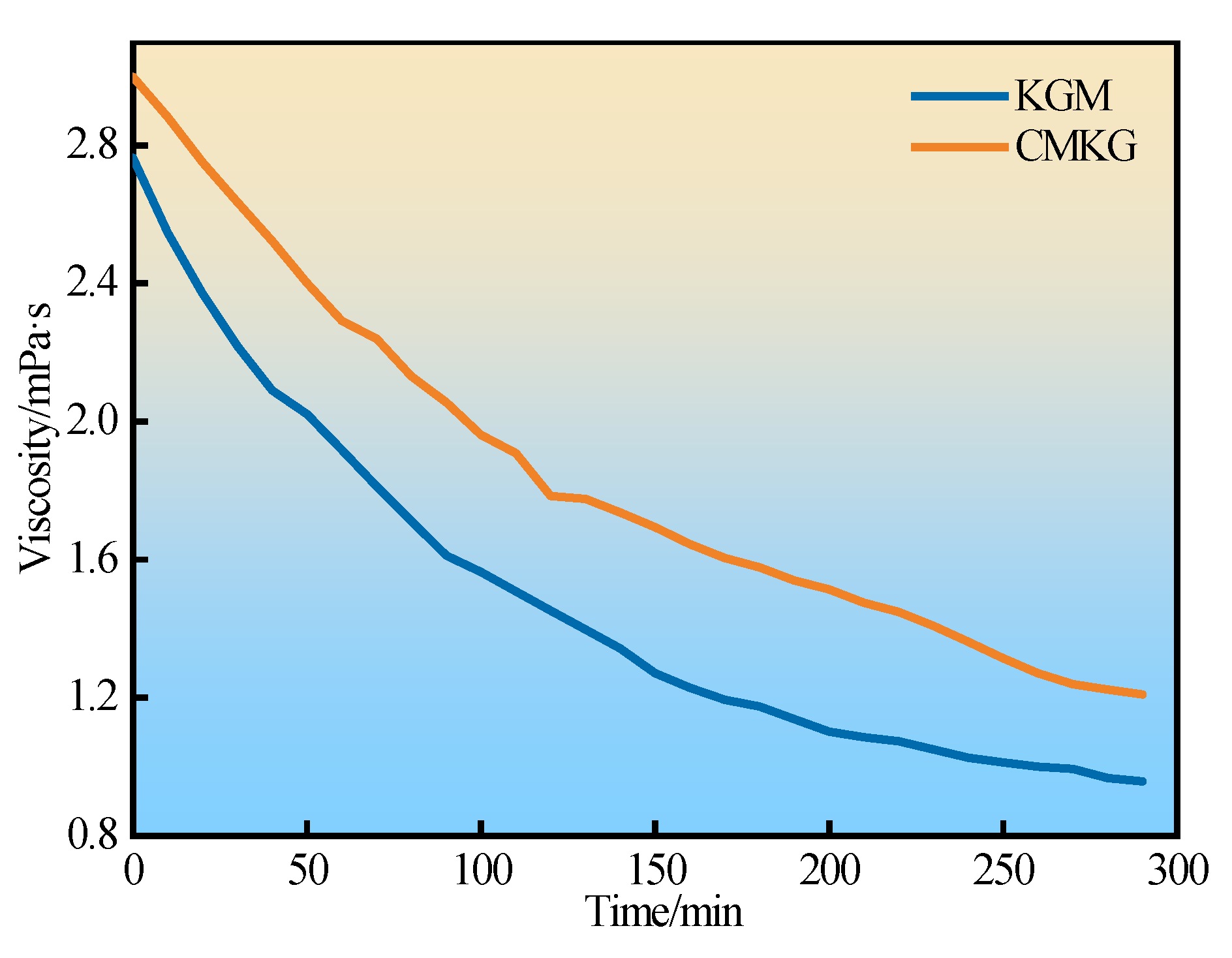
2.4.3. Shear Stability
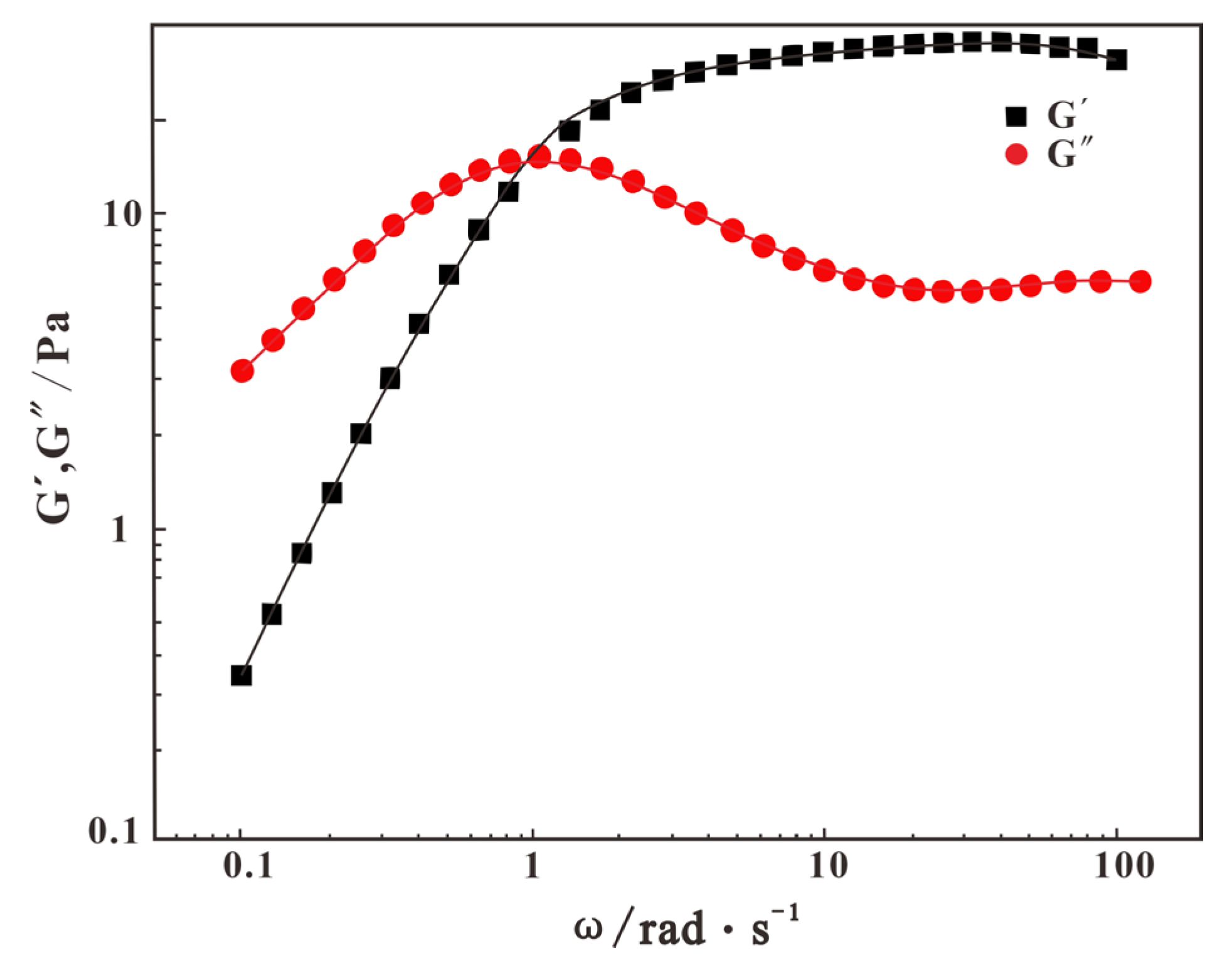
2.4.4. Viscoelastic Evaluation
2.4.5. Gel-Breaking Performance
2.5. Environment-Friendly Evaluation
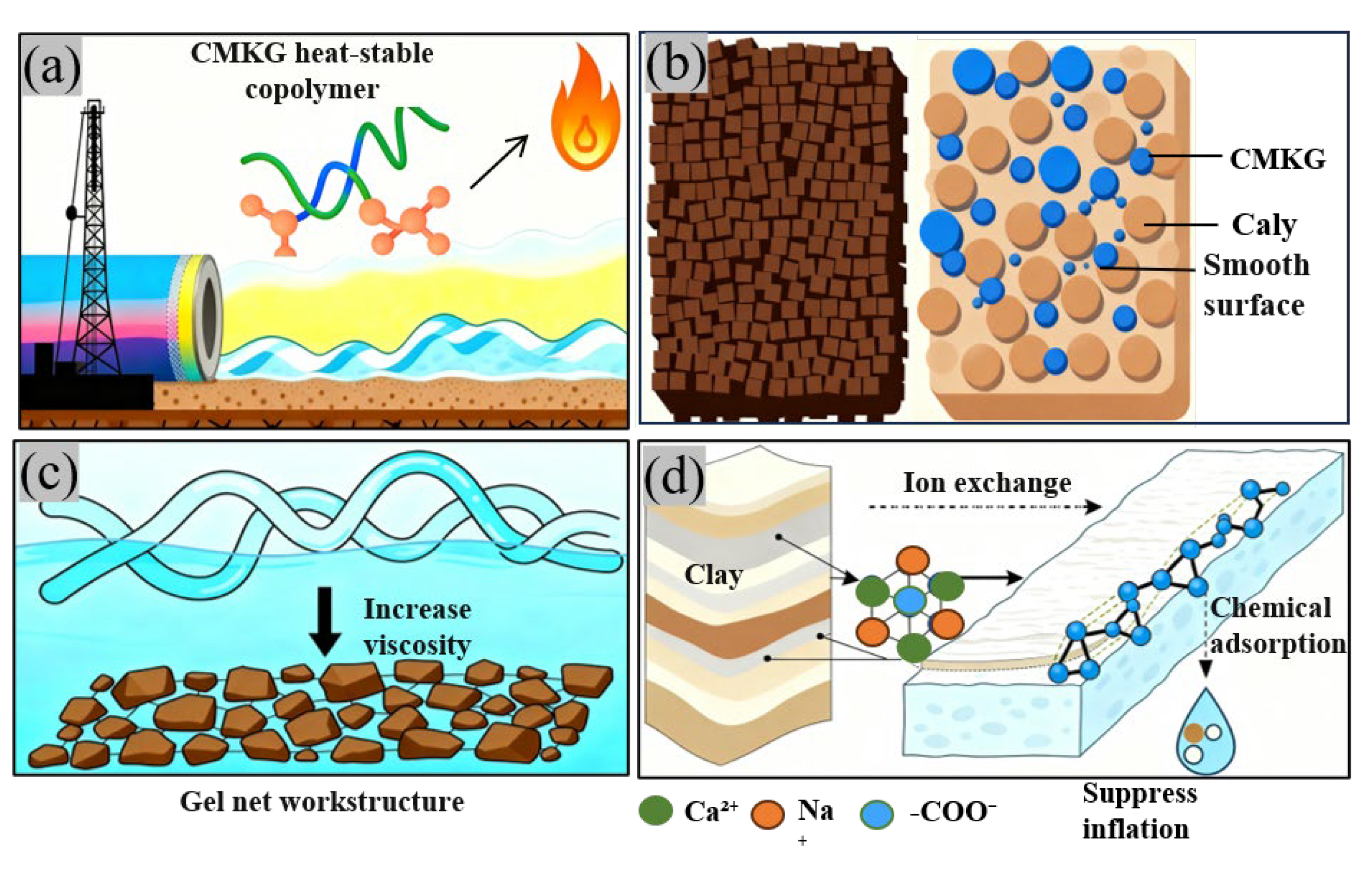
2.6. Mechanism Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Modification of KGM
4.3. Orthogonal Experiment
4.4. Characterization Methods
4.5. Drilling Fluid Performance Evaluation
4.5.1. Temperature Resistance Test
4.5.2. Linear Swelling
4.5.3. Mud Ball Test
4.6. Fracturing Fluid Performance
4.6.1. Rheological Property Test
4.6.2. Viscoelasticity Assessment
4.6.3. Determination of Gel-Breaking Performance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KGM | Konjac gum |
| CMKG | Carboxymethylated Konjac gum |
| DCA | Dichloroacetic acid |
References
- Fan, Y.; Huang, W.; Liu, Y.; Zhang, Y.; Zhao, Y. Development and Evaluation of Modified Asphalt Filtrate Loss Reducer in Oil-Based Drilling Fluids for Ultra-Deep Well. J. Phys. Conf. Ser. 2025, 2968, 012014. [Google Scholar]
- Huang, N.; Sun, J.-S.; Liu, J.-P.; Lv, K.-H.; Deng, X.-F.; Yi, H.-J. Research on small molecule wetting agent for drilling fluids applied in Antarctic drilling engineering. Pet. Sci. 2025, 22, 2465–2477. [Google Scholar]
- Liu, K.; Qu, Y.; Zhang, D.; Liang, B.; Yan, L. The potential of the synergistic use of SiO2@Silane-PEG with molybdenum dialkyldithiocarbamate in water-based drilling fluid. Colloids Surf. A Physicochem. Eng. Asp. 2025, 709, 136101. [Google Scholar] [CrossRef]
- Saadi, R.; Hamidi, H.; Wilkinson, D. Optimizing filtration properties of water-based drilling fluids: Performance of PAC variants and synergistic effects. Colloids Surf. A Physicochem. Eng. Asp. 2025, 715, 136590. [Google Scholar] [CrossRef]
- Ching, Y.C.; Ali, M.E.; Abdullah, L.C.; Choo, K.W.; Kuan, Y.C.; Julaihi, S.J.; Chuah, C.H.; Liou, N.-S. Rheological properties of cellulose nanocrystal-embedded polymer composites: A review. Cellulose 2016, 23, 1011–1030. [Google Scholar] [CrossRef]
- Thaemlitz, C.; Patel, A.; Coffin, G.; Conn, L. New environmentally safe high-temperature water-based drilling-fluid system. SPE Drill. Complet. 1999, 14, 185–189. [Google Scholar] [CrossRef]
- You, F.; Wu, Y.; Guo, Y.; Zheng, Y. Rheological aspects of xanthan gum: Governing factors and applications in water-based drilling fluids and enhanced oil recovery. Carbohydr. Polym. 2025, 359, 123579. [Google Scholar] [CrossRef]
- Hao, S.-Q. Bentonite clay modification and the application in low solid phase drilling fluids for marine hydrate-bearing formation. Appl. Clay Sci. 2024, 250, 107285. [Google Scholar] [CrossRef]
- Nidheesh, P.; Ganiyu, S.O.; Thiam, A. Application of biochar in electro-Fenton process: Advantages and recent advancements. J. Environ. Chem. Eng. 2024, 12, 112726. [Google Scholar] [CrossRef]
- Shan, H.; Sun, Y.; Li, B.; Shen, Y.; Qi, Y.; Zhang, G. Low-temperature gel breaking fracturing fluid for marine hydrate reservoir fracturing and its effect on phase equilibrium of methane hydrates. Geoenergy Sci. Eng. 2024, 233, 212583. [Google Scholar] [CrossRef]
- Zhange, J.; Zhang, Y.; Hu, L.; Zhang, J.; Chen, G. Modification and Application of a Plant Gum as Eco-Friendly Drilling Fluid Additive. Iran. J. Chem. Chem. Eng. 2015, 34, 103–108. [Google Scholar]
- Xue, L.; Wang, Q.; Liu, L.; Zhang, L.; Wang, K.; Chen, G.; Zhang, J. Co-Gelatinization Modification of Iodine–Starch and Its Performance in Drilling Fluid. Processes 2024, 12, 2386. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Liu, B.; Zheng, M.; Chen, H.; Pang, J. Rheological behavior and molecular dynamics simulation of κ-carrageenan/casein under simulated gastrointestinal electrolyte conditions. Food Hydrocoll. 2023, 136, 108240. [Google Scholar] [CrossRef]
- Lalji, S.M.; Ali, S.I.; Khan, M.A. Study of rheological characteristics of a water-based drilling fluid in presence of biopolymers, synthetic polymer, and modified natural polymer. Pet. Chem. 2023, 63, 906–916. [Google Scholar] [CrossRef]
- Shan, H. Modification and performance evaluation of Konjac gum in drilling fluid. J. Phys. Conf. Ser. 2023, 2430, 012014. [Google Scholar] [CrossRef]
- Yi, Y.; Wu, L.; Zhang, J.; Wang, H.; Xie, X.; Gang, C. Sulfonation modification of guar gum and its performance as a fracturing fluids thickener. Pol. J. Chem. Technol. 2024, 26, 29–38. [Google Scholar] [CrossRef]
- Thibodeaux, G.M.; Baudoin, N.A.; Chirdon, W.M. Investigation of proteinaceous algal biomass as a drilling fluid component. Results Eng. 2023, 19, 101364. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, F.; Li, Y.; Liu, C.; Zhu, L.; Yao, E. Performance evaluation of oil-displacement viscoelastic zwitterionic surfactant fracturing fluid. J. Mol. Liq. 2023, 377, 121545. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, A.; Mao, J.-C.; Qin, S.-H.; Li, J.; Yang, X.-J.; Lin, C.; Huang, Z.-Y.; Liu, Y.-F. Preparation of a functional fracturing fluid with temperature- and salt-resistance, and low damage using a double crosslinking network. Pet. Sci. 2023, 20, 3223–3230. [Google Scholar] [CrossRef]
- Fu, L.; Wei, M.; Liao, K.; Qianli, M.; Shao, M.; Gu, F.; Fan, Y.; Longjie, L.; Yanfeng, H. Application of environmentally stimuli-responsive materials in the development of oil and gas field. J. Pet. Sci. Eng. 2022, 219, 111088. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Liu, W.; Liu, H.; Zhang, M.; Xu, T.; Si, C. Cellulose nanomaterials for oil exploration applications. Polym. Rev. 2022, 62, 585–625. [Google Scholar] [CrossRef]
- Ma, X.; Huang, Q.; Zhou, Z.; Mu, Y. Synthesis and evaluation of water-soluble fracturing fluid thickener based on hydrophobic association. Mater. Lett. 2022, 325, 132857. [Google Scholar] [CrossRef]
- Ruan, L.; Su, M.; Qin, X.; Ruan, Q.; Lang, W.; Wu, M.; Chen, Y.; Lv, Q. Progress in the application of sustained-release drug microspheres in tissue engineering. Mater. Today Bio 2022, 16, 100394. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, G.; Bai, Y.; Wang, P.; Wang, X.; Liu, X.; Li, D. Construction of sulfur-free gel breaker agent system and investigation on gel-breaking mechanism for association fracturing fluid. Energy Sources A Recovery Util. Environ. Eff. 2022, 44, 5665–5681. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, Y.; Zhou, M.; Chen, W.; Zeng, L.; Li, Y.; Li, B.; Fan, Y.; Bao, D. Research on the viscosity-increasing mechanism and performance analysis of modified polysaccharide natural polymer fracturing fluid gel. Can. J. Chem. Eng. 2022, 101, 4244–4254. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Y.; Zhang, X. Environmentally Friendly MEG Drilling Fluid System for Horizontal Well of Daqing Oil Field. In ICPTT 2011: Sustainable Solutions for Water, Sewer, Gas, And Oil Pipelines; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 2109–2118. [Google Scholar]
- Abidi, S.M.; Dar, A.I.; Acharya, A. Plant-Based Polymeric Nanomaterials for Biomedical Applications. In Nanomaterial-Based Biomedical Applications in Molecular Imaging, Diagnostics and Therapy; Springer: Singapore, 2020; pp. 129–158. [Google Scholar]
- Zhu, W.; Zheng, X. Effective modified xanthan gum fluid loss agent for high-temperature water-based drilling fluid and the filtration control mechanism. ACS Omega 2021, 6, 23788–23801. [Google Scholar] [CrossRef]
- Ferrer, I.; Thurman, E.M. Analysis of hydraulic fracturing additives by LC/Q-TOF-MS. Anal. Bioanal. Chem. 2015, 407, 6417–6428. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.M.; Abdel-Raouf, M.E. Applications of guar gum and its derivatives in petroleum industry: A review. Egypt. J. Pet. 2018, 27, 1043–1050. [Google Scholar] [CrossRef]
- Cui, X.; Wang, C.; Huang, W.; Zhang, S.; Chen, H.; Wu, B. Phase-Conversion Stiffened Dual-Network Hydrogel for Fracture Plugging in Oil-Based Drilling Fluid. Gels 2025, 11, 635. [Google Scholar] [CrossRef]
- Artykova, Z.; Beisenbayev, O.; Issa, A.; Kydyraliyeva, A. Modification of polymers to synthesize thermo-salt-resistant stabilizers of drilling fluids. Open Eng. 2025, 15, 20240097. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Li, Q.; Wang, F.; Cheng, Y. Sediment Instability Caused by Gas Production from Hydrate-bearing Sediment in Northern South China Sea by Horizontal Wellbore: Sensitivity Analysis. Nat. Resour. Res. 2025, 34, 1667–1699. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Wang, F.; Ning, X.; Wang, Y.; Bai, B. Settling behavior and mechanism analysis of kaolinite as a fracture proppant of hydrocarbon reservoirs in CO2 fracturing fluid. Colloids Surf. A Physicochem. Eng. Asp. 2025, 724, 137463. [Google Scholar] [CrossRef]
- Li, Q. Reservoir Science: A Multi-Coupling Communication Platform to Promote Energy Transformation, Climate Change and Environmental Protection. Reserv. Sci. 2025, 1, 1–2. [Google Scholar]
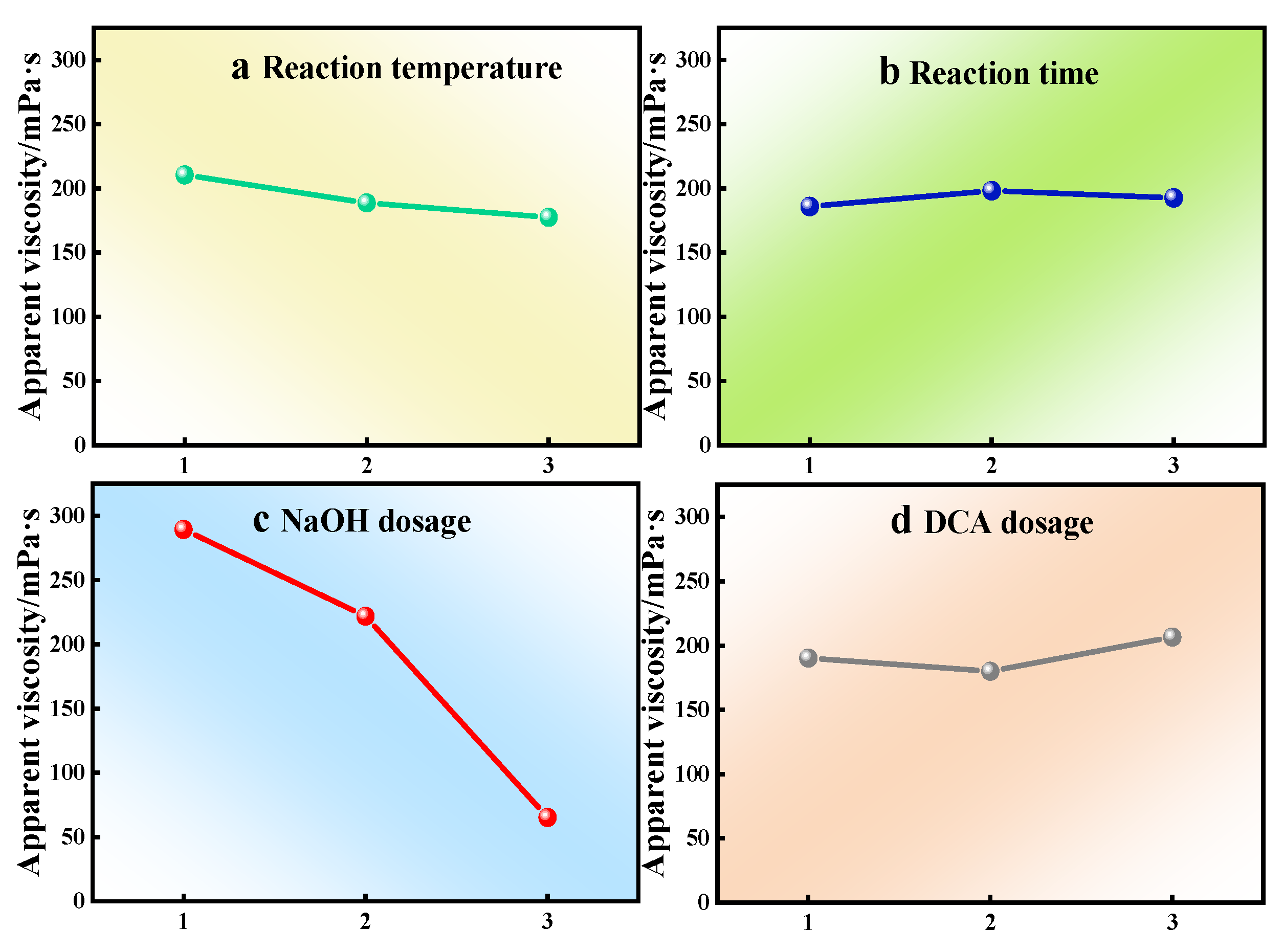
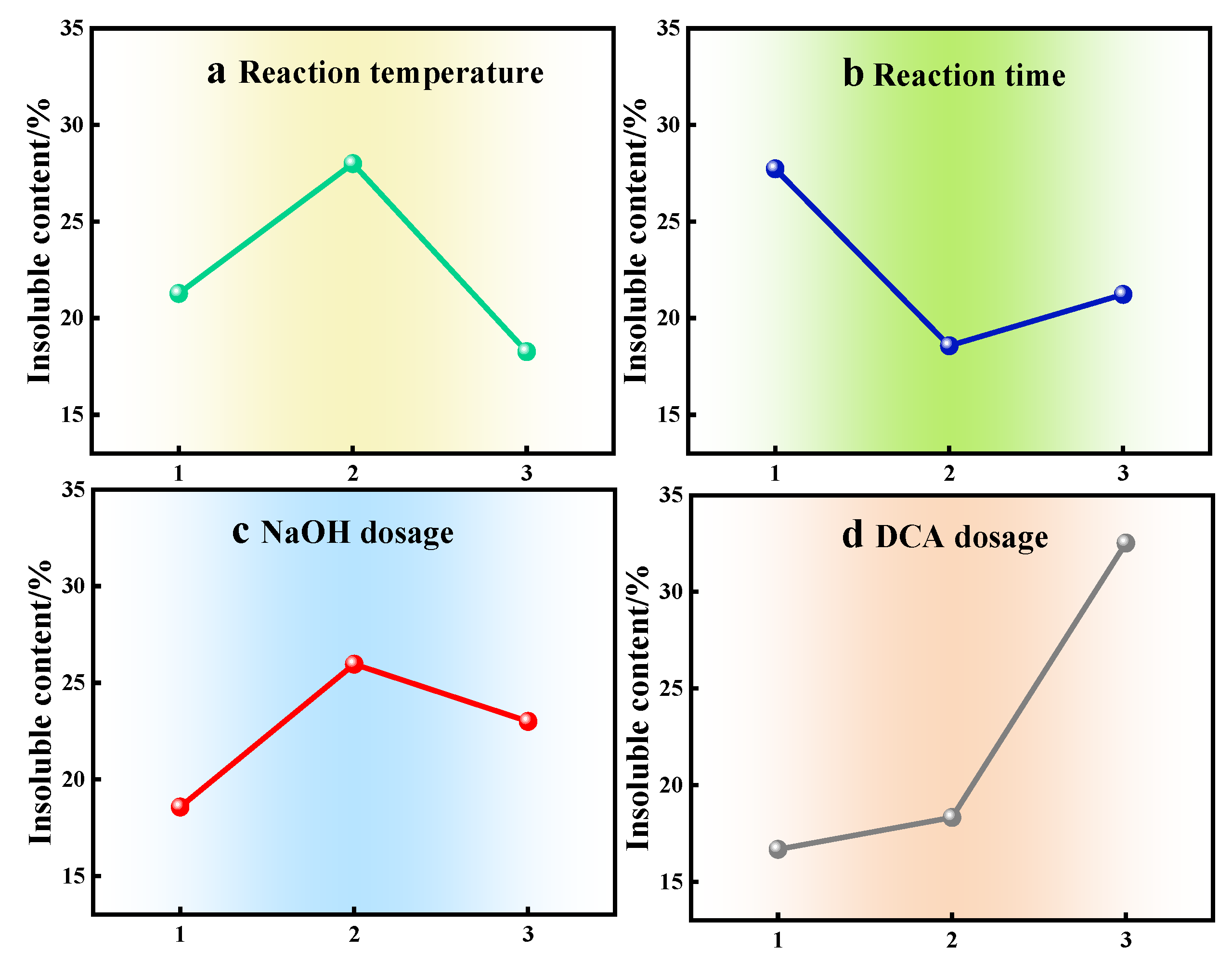

| Subjects | a | b | c | d |
|---|---|---|---|---|
| K1 | 21.27 | 27.73 | 18.57 | 16.67 |
| K2 | 28.00 | 18.57 | 25.97 | 18.33 |
| K3 | 18.27 | 21.23 | 23.00 | 32.53 |
| Range | 9.73 | 9.17 | 7.40 | 15.87 |
| Arrange in order | 2 | 3 | 4 | 1 |
| Optimization condition | a2 | b1 | c2 | d3 |
| Experiment No. | 0.6% Apparent Viscosity of Solution mPa·s | Water Insoluble Matter Content % | Crosslinkability |
|---|---|---|---|
| Bare | 199.62 | 19.31 | pickable |
| 1 | 299.42 | 16.70 | pickable |
| 2 | 233.88 | 16.60 | pickable |
| 3 | 98.32 | 30.50 | pickable |
| 4 | 226.44 | 46.70 | pickable |
| 5 | 65.73 | 18.70 | pickable |
| 6 | 274.11 | 18.60 | pickable |
| 7 | 32.17 | 19.80 | pickable |
| 8 | 294.97 | 20.40 | pickable |
| 9 | 205.58 | 14.60 | pickable |
| Subjects | a | b | c | d |
|---|---|---|---|---|
| K1 | 210.54 | 186.01 | 289.50 | 190.24 |
| K2 | 188.76 | 198.19 | 221.97 | 180.05 |
| K3 | 177.57 | 192.67 | 65.41 | 206.58 |
| Range | 32.97 | 12.18 | 224.09 | 26.52 |
| Arrange in order | 2 | 4 | 1 | 3 |
| Optimization condition | a1 | b2 | c1 | d3 |
| Sample | Element % (Mass) | ||||
|---|---|---|---|---|---|
| C | H | N | S | O | |
| KGM | 37.80 | 6.16 | 0.62 | 0.33 | 55.09 |
| CMKG | 39.37 | 6.41 | 0.18 | 0.31 | 53.72 |
| Additive | AV/ mPa·s | PV/mPa·s | YP/ Pa | YP/PV/ Pa/mPa·s | FL/ mL | tg |
|---|---|---|---|---|---|---|
| Mud | 5.50 | 4.00 | 1.40 | 0.36 | 13.30 | 0.0963 |
| Mud + 0.05%KGM | 12.50 | 7.50 | 4.80 | 0.64 | 12.10 | 0.0437 |
| Mud + 0.10%KGM | 24.00 | 15.00 | 8.60 | 0.58 | 18.50 | 0.0699 |
| Mud + 0.30%KGM | 27.20 | 10.00 | 16.30 | 1.63 | 52.00 | - |
| Mud + 0.05%CMKG | 16.10 | 9.50 | 6.20 | 0.65 | 12.70 | 0.0875 |
| Mud + 0.10%CMKG | 25.70 | 9.50 | 10.80 | 1.14 | 18.80 | 0.0875 |
| Mud + 0.30%CMKG | 28.50 | 11.00 | 11.00 | 1.01 | 21.30 | 0.0963 |
| T/°C | Additive | AV /mPa·s | PV /mPa·s | YP /Pa | YP/PV /Pa/mPa·s | tg | FL /mL |
|---|---|---|---|---|---|---|---|
| 30 | Mud | 5.50 | 4.00 | 1.40 | 0.36 | 0.0963 | 13.30 |
| KGM | 24.00 | 15.20 | 8.64 | 0.58 | 0.0699 | 18.50 | |
| CMKG | 22.50 | 11.00 | 11.04 | 1.01 | 0.0963 | 21.30 | |
| 60 | Mud | 3.40 | 2.50 | 0.34 | 0.14 | 0.1152 | 13.50 |
| KGM | 13.50 | 7.00 | 6.24 | 0.89 | 0.0787 | 12.00 | |
| CMKG | 17.50 | 8.00 | 9.12 | 1.14 | 0.0699 | 17.00 | |
| 90 | Mud | 2.50 | 2.10 | 0.05 | 0.02 | 0.1584 | 14.80 |
| KGM | 8.20 | 5.00 | 2.88 | 0.58 | 0.0524 | 11.50 | |
| CMKG | 10.50 | 6.20 | 4.13 | 0.66 | 0.0612 | 14.20 | |
| 120 | Mud | 2.70 | 2.80 | 0.10 | 0.04 | 0.2315 | 16.40 |
| KGM | 4.00 | 3.50 | 0.48 | 0.14 | 0.0699 | 12.40 | |
| CMKG | 4.90 | 3.80 | 1.06 | 0.13 | 0.0524 | 13.00 | |
| 150 | Mud | 3.50 | 3.20 | 0.20 | 0.06 | 0.2168 | 17.00 |
| KGM | 3.80 | 2.80 | 0.35 | 0.12 | 0.0699 | 14.20 | |
| CMKG | 4.50 | 3.60 | 0.41 | 0.11 | 0.0524 | 15.20 |
| Environmental Performance Indicators | EC50 | BOD5 | CODCr | BOD5/CODCr |
|---|---|---|---|---|
| Index Indicator | 21,400 | 8345.5 | 13,562.7 | 0.61 |
| Level | A: Temperature/°C | B: Time/min | C: Additions (ω/%) | D:Additions(ω/%) (DCA/KGM) |
|---|---|---|---|---|
| (Sodium Hydroxide NaOH/KGM) | ||||
| 1 | 30 | 2 | 0.1% | 0.1% |
| 2 | 50 | 4 | 1.0% | 0.5% |
| 3 | 70 | 6 | 2.0% | 1.0% |
| Experiment No. | A | B | C | D |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 | 2 |
| 3 | 1 | 3 | 3 | 3 |
| 4 | 2 | 1 | 2 | 3 |
| 5 | 2 | 2 | 3 | 1 |
| 6 | 2 | 3 | 1 | 2 |
| 7 | 3 | 1 | 3 | 2 |
| 8 | 3 | 2 | 1 | 3 |
| 9 | 3 | 3 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Guo, P.; Qu, K.; Du, W.; Wang, Y.; Chen, G. Study on Carboxymethylation Modification of Konjac Gum and Its Effect in Drilling Fluid and Fracturing Fluid. Gels 2025, 11, 792. https://doi.org/10.3390/gels11100792
Li Y, Guo P, Qu K, Du W, Wang Y, Chen G. Study on Carboxymethylation Modification of Konjac Gum and Its Effect in Drilling Fluid and Fracturing Fluid. Gels. 2025; 11(10):792. https://doi.org/10.3390/gels11100792
Chicago/Turabian StyleLi, Yongfei, Pengli Guo, Kun Qu, Weichao Du, Yanling Wang, and Gang Chen. 2025. "Study on Carboxymethylation Modification of Konjac Gum and Its Effect in Drilling Fluid and Fracturing Fluid" Gels 11, no. 10: 792. https://doi.org/10.3390/gels11100792
APA StyleLi, Y., Guo, P., Qu, K., Du, W., Wang, Y., & Chen, G. (2025). Study on Carboxymethylation Modification of Konjac Gum and Its Effect in Drilling Fluid and Fracturing Fluid. Gels, 11(10), 792. https://doi.org/10.3390/gels11100792







