A Comparative Study of Soy Protein Isolate-κ-Carrageenan Emulsion Gels and Bigels for the Encapsulation, Protection, and Delivery of Curcumin
Abstract
1. Introduction
2. Results and Discussion
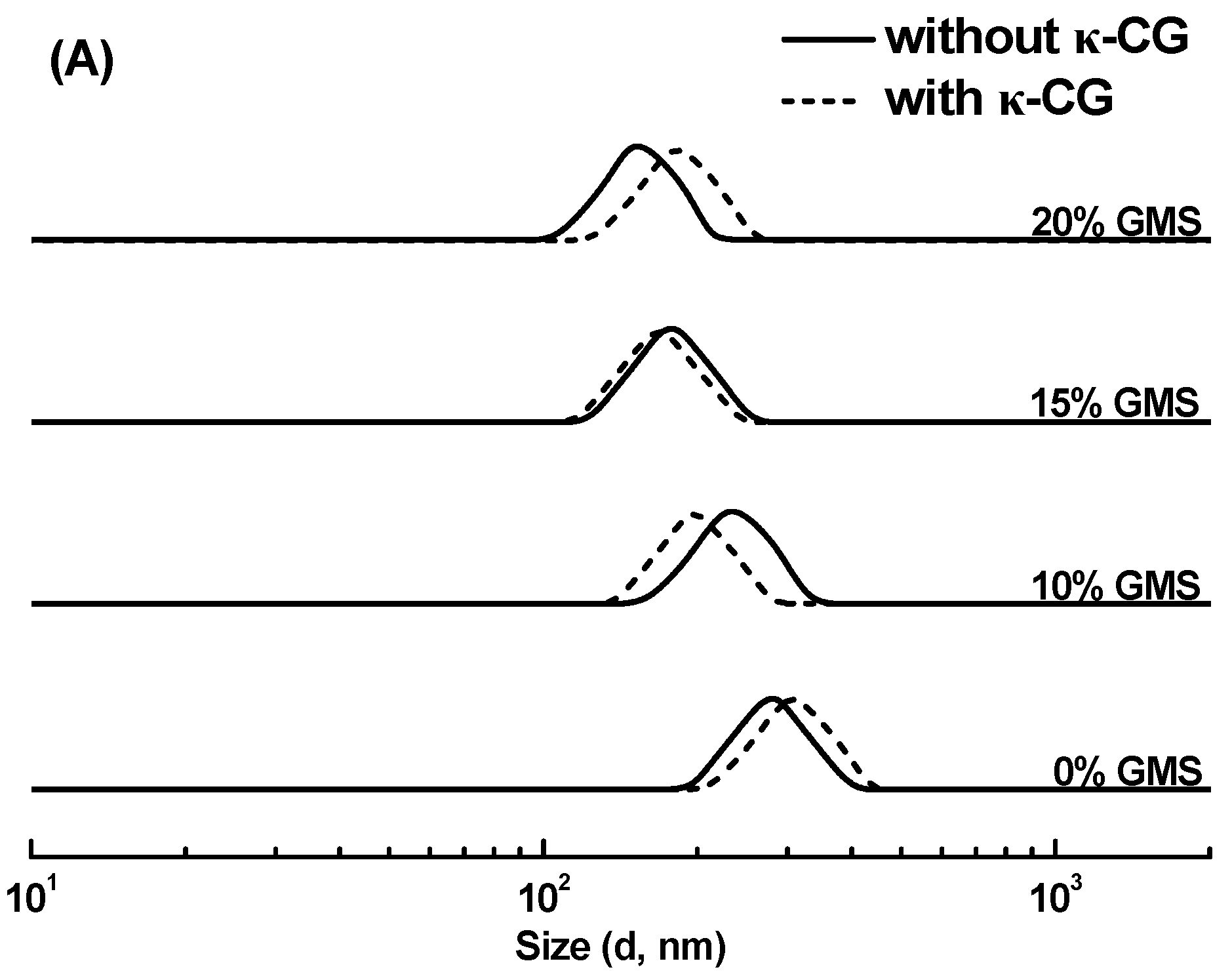
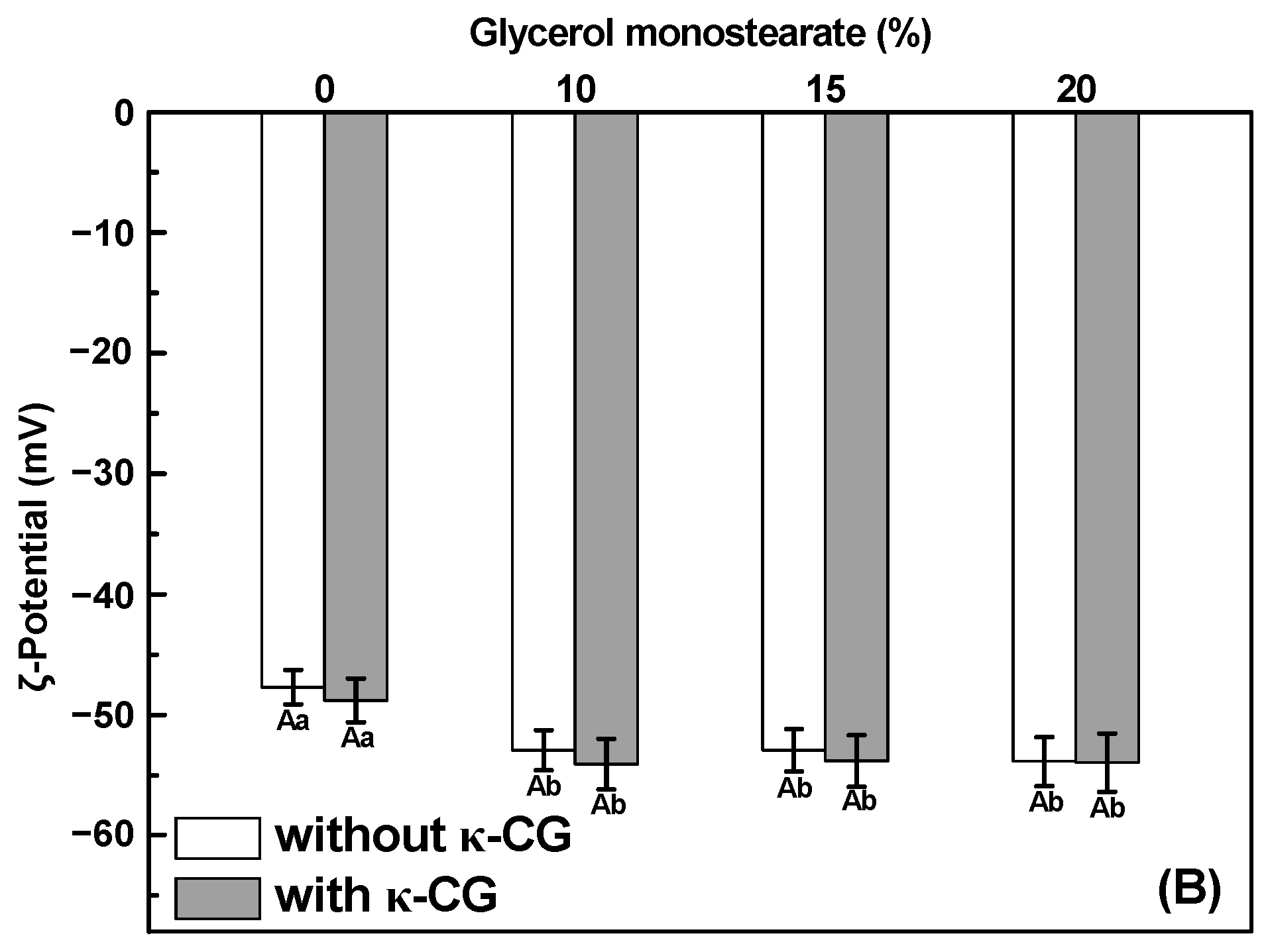
2.1. Size Distribution and ζ-Potential of Emulsions
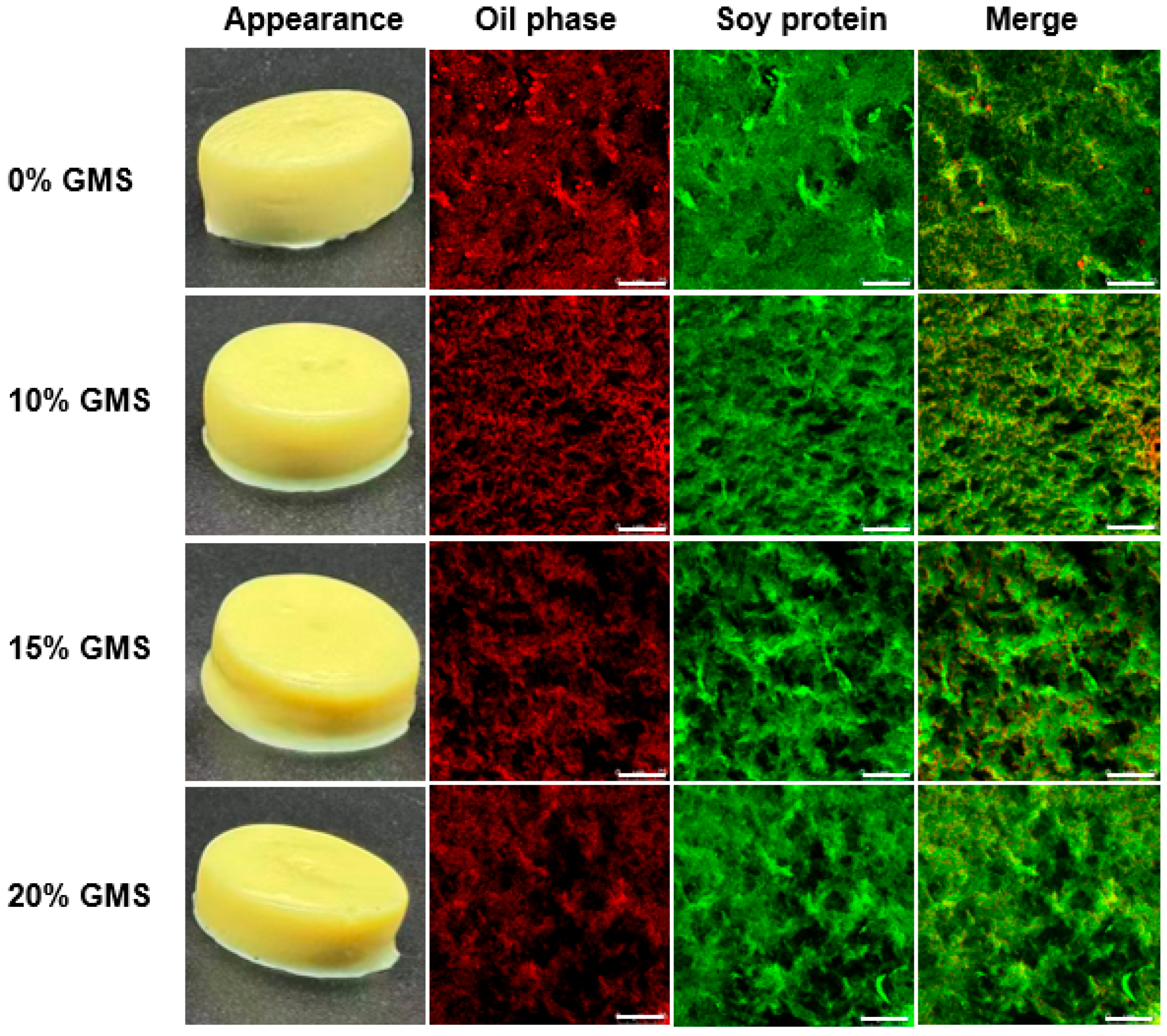
2.2. Gel Formation and Microstructure
2.3. Encapsulation Efficiency of Curcumin
2.4. Textural Properties
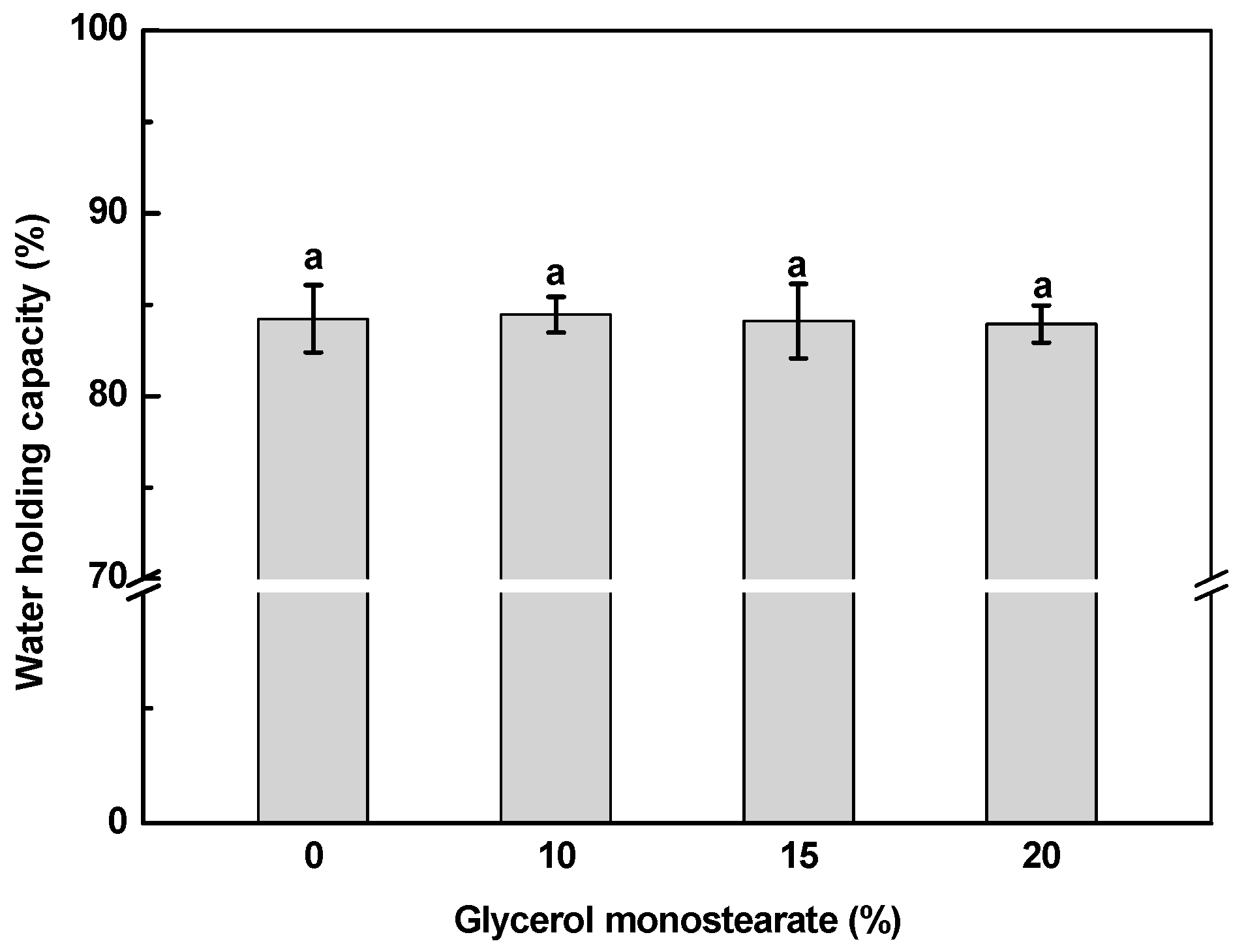
2.5. Water Holding Capacity
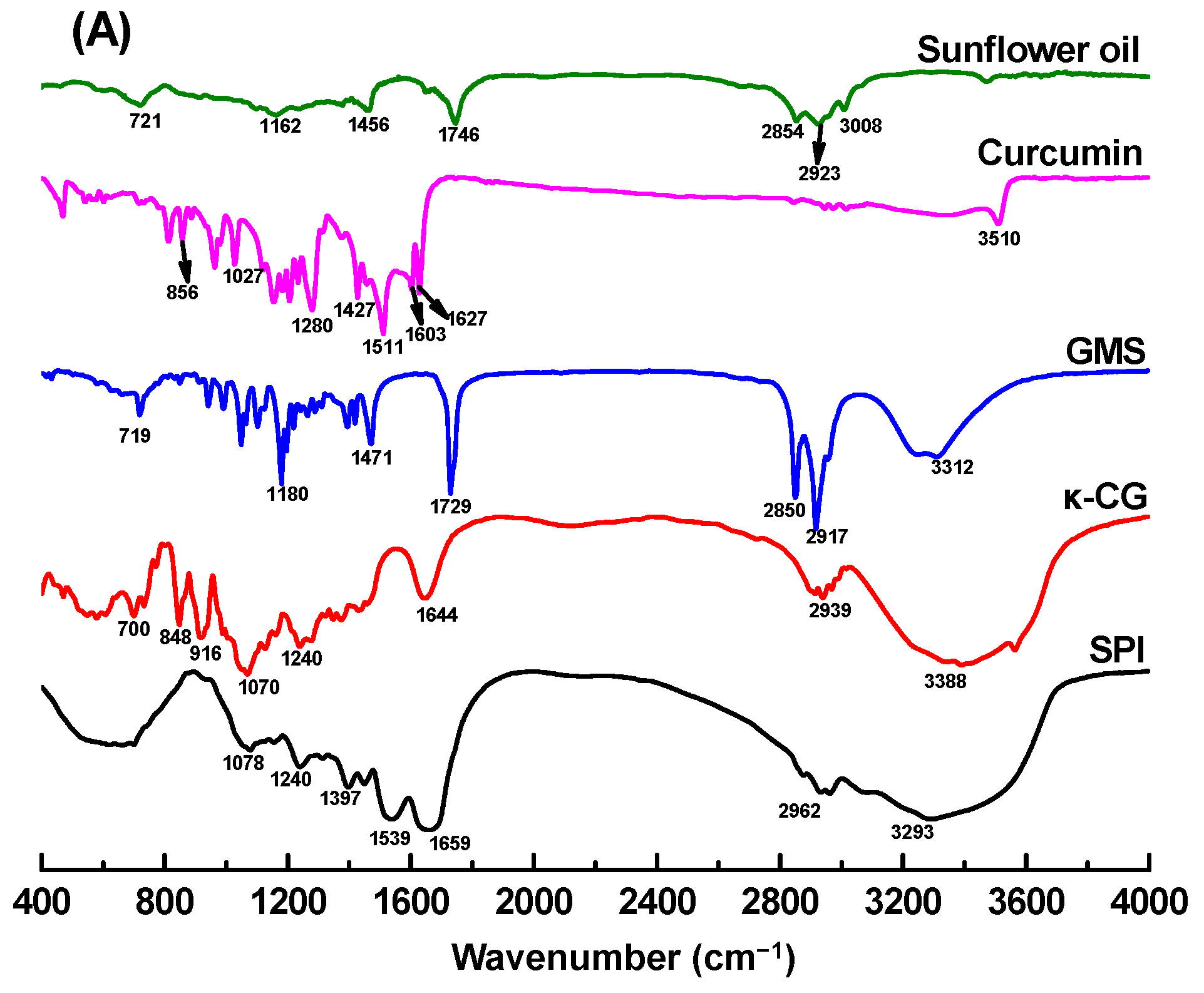
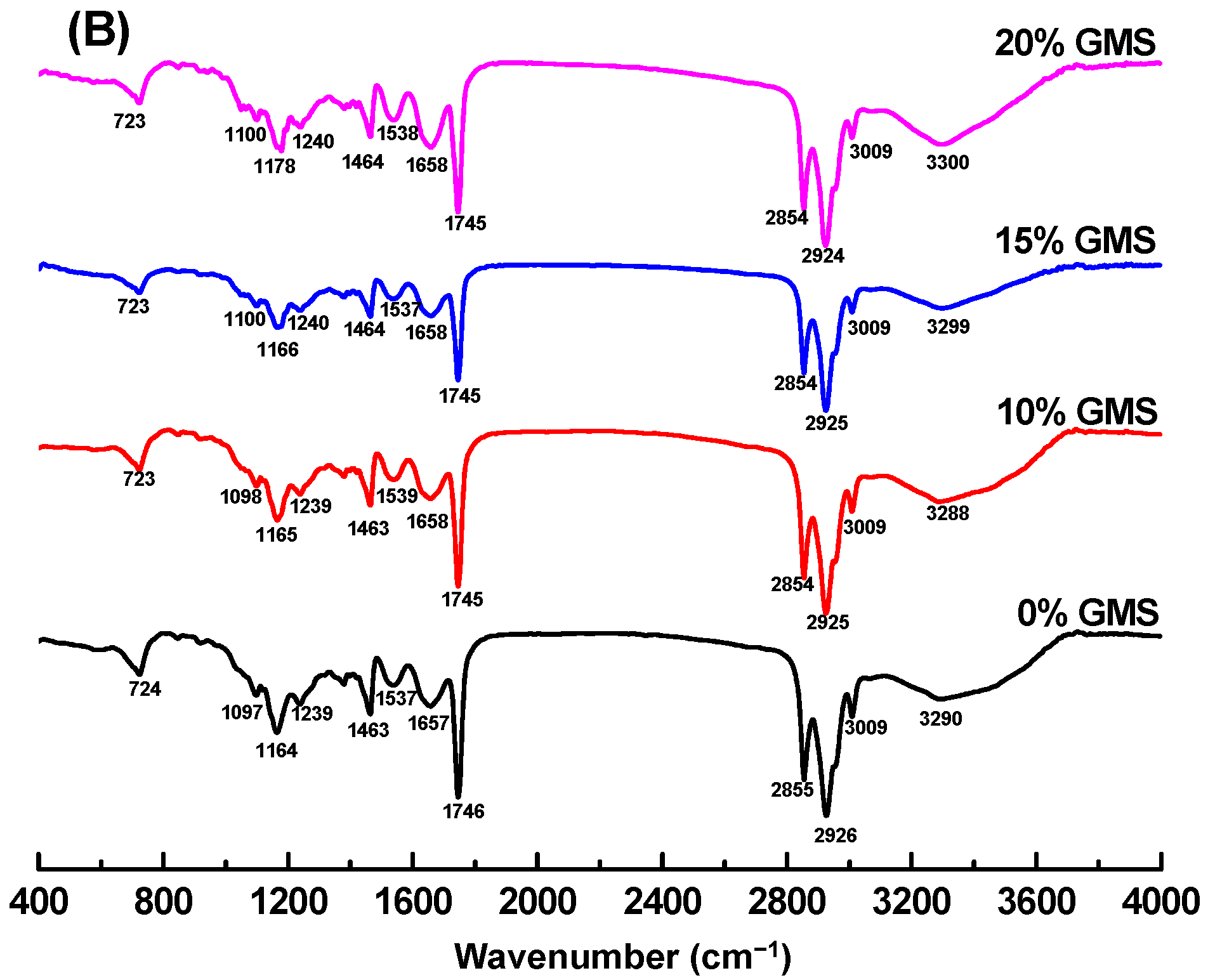
2.6. FTIR
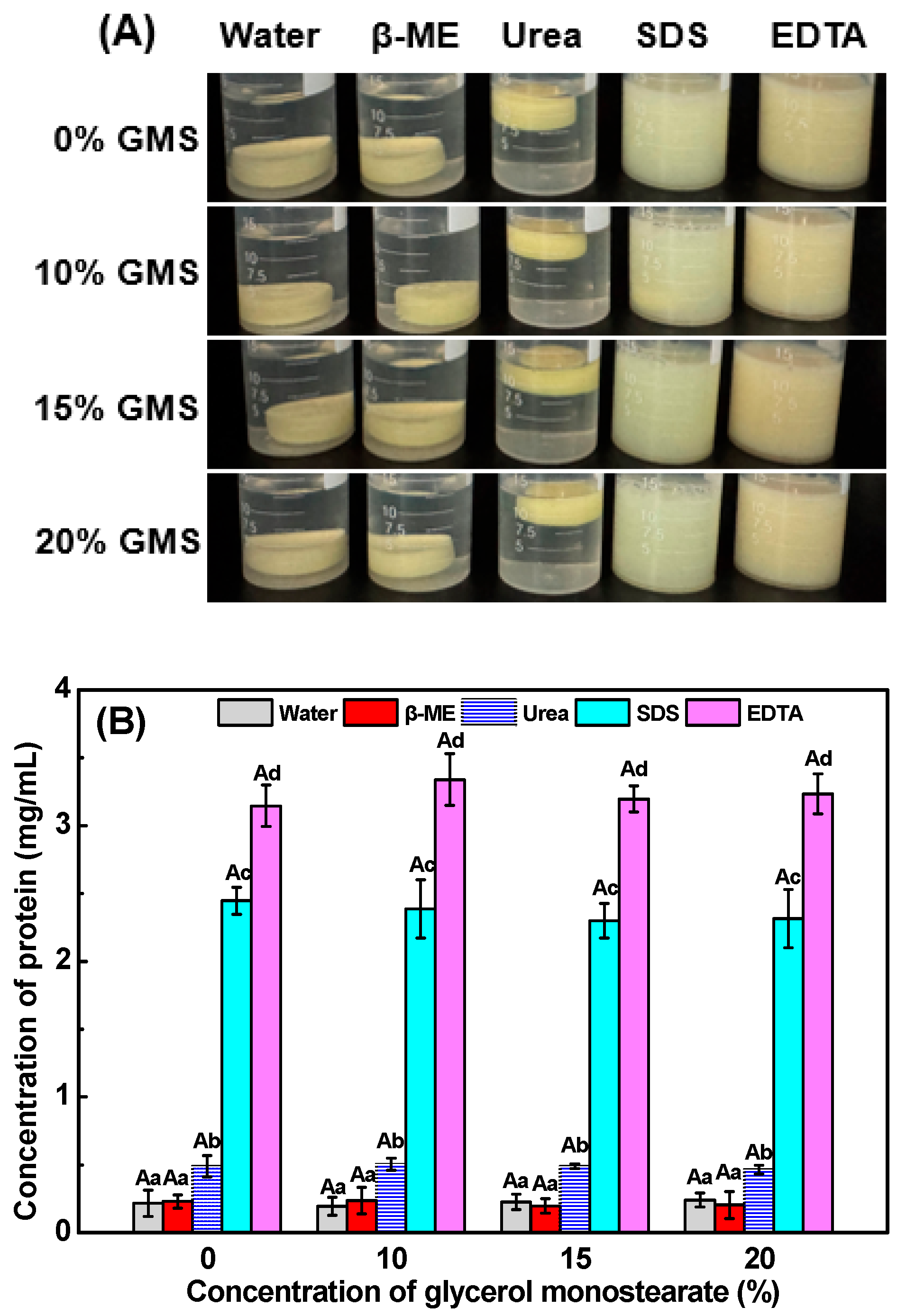
2.7. Intermolecular Force
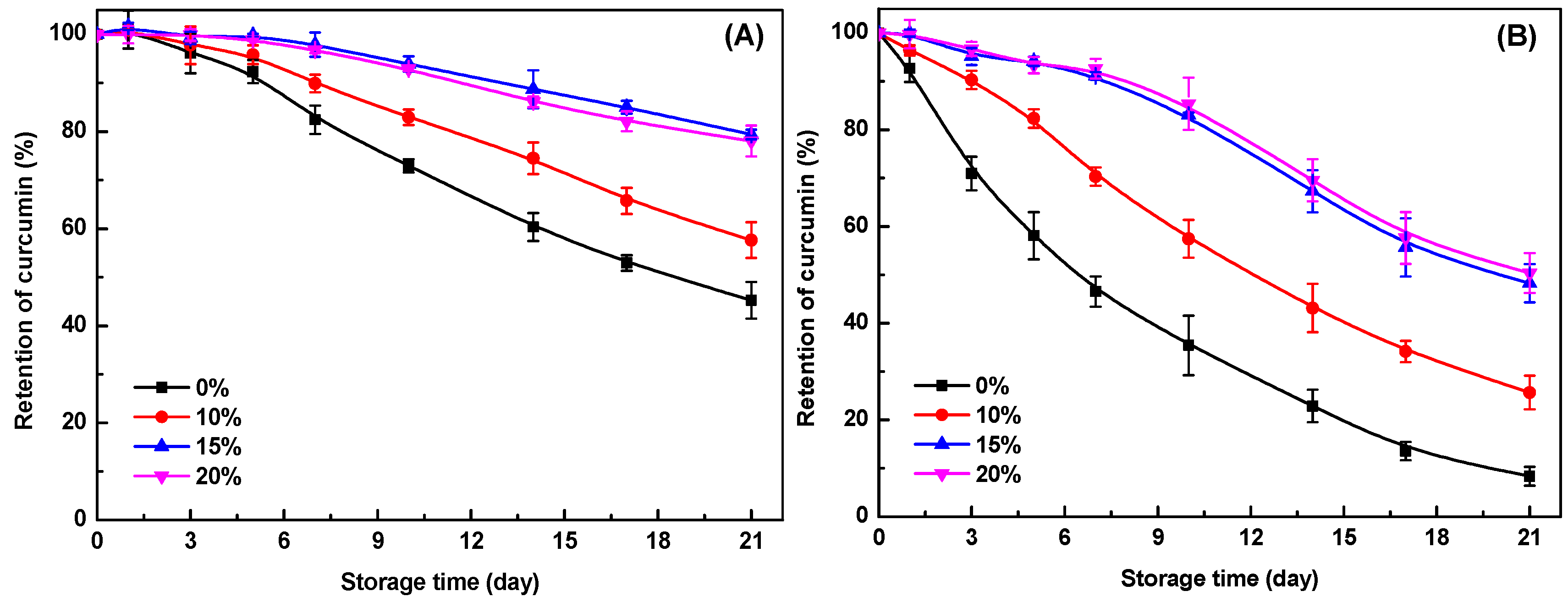
2.8. Chemical Stability of Curcumin in Emulsion Gels and Bigels
2.9. In Vitro Digestion
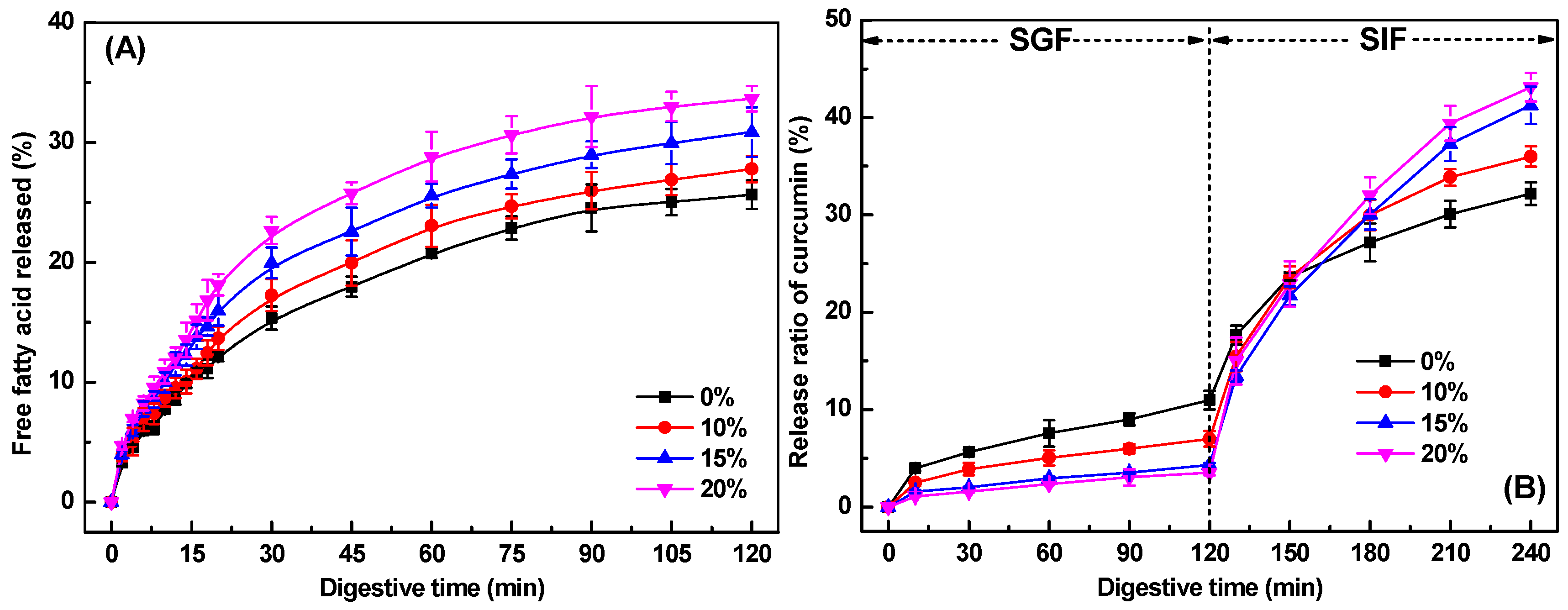
2.9.1. Free Fatty Acids Release
2.9.2. In Vitro Release Profile of Curcumin
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Emulsion Preparation
4.3. Emulsion Size Distribution and ζ-Potential Measurement
4.4. Fabrication of Emulsion Gels and Bigels
4.5. Encapsulation Efficiency of Curcumin in the Emulsions and Gels
4.6. Water Holding Capacity (WHC)
4.7. Texture Profile Analysis (TPA)
4.8. Microstructure Characterization
4.9. Fourier Infrared Spectroscopy (FTIR)
4.10. Intermolecular Force Analysis
4.11. Stability of Curcumin in Emulsion Gels and Bigels
4.12. In Vitro Digestion of Emulsion Gels and Bigels
4.12.1. Simulated Gastrointestinal Tract Digestion
4.12.2. Release Profile of Curcumin During the Digestion
4.13. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.N.; Han, Y.H.; Chen, Y.L.; Du, H.J.; Chen, B.; Gao, Z.L.; Wang, Q.; Cao, Y.; Xiao, H. Unveiling the role of gut microbiota in curcumin metabolism using antibiotic-treated mice. Food Chem. 2024, 460, 140706. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between gut microbiota and curcumin: A new key of understanding for the health effects of curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Mao, L.K.; Lu, Y.; Cui, M.N.; Miao, S.; Gao, Y.X. Design of gel structures in water and oil phases for improved delivery of bioactive food ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 1651–1666. [Google Scholar] [CrossRef]
- Zhang, B.; Meng, R.; Li, X.L.; Liu, W.J.; Cheng, J.S.; Wang, W. Preparation of Pickering emulsion gels based on κ-carrageenan and covalent crosslinking with EDC: Gelation mechanism and bioaccessibility of curcumin. Food Chem. 2021, 357, 129726. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Jin, W.; Ma, X.Y.; Wen, H.B.; Xu, G.C.; Xu, P.; Cheng, H. Impact of κ-carrageenan on the freshwater mussel (Solenaia oleivora) protein emulsion gels: Gel formation, stability, and curcumin delivery. Gels 2024, 10, 659. [Google Scholar] [CrossRef]
- Zhang, L.M.; Zheng, J.Q.; Wang, Y.; Ye, X.Q.; Chen, S.G.; Pan, H.B.; Chen, J.L. Fabrication of rhamnogalacturonan-I enriched pectin-based emulsion gels for protection and sustained release of curcumin. Food Hydrocoll. 2022, 128, 107592. [Google Scholar] [CrossRef]
- Su, J.Q.; Wang, L.L.; Dong, W.X.; Wei, J.; Liu, X.; Yan, J.X.; Ren, F.Z.; Yuan, F.; Wang, P.J. Fabrication and characterization of ultra-high-pressure (UHP)-induced whey protein isolate/κ-carrageenan composite emulsion gels for the delivery of curcumin. Front. Nutr. 2022, 9, 839761. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, W.W.; Jiang, J.; Khan, M.A.; Wusigale; Liang, L. A comprehensive review of protein-based carriers with simple structures for the co-encapsulation of bioactive agents. Compr. Rev. Food. Sci. Food Saf. 2023, 22, 2017–2042. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Guo, Q.; Bellissimo, N.; Rousseau, D. Role of gel structure in controlling in vitro intestinal lipid digestion in whey protein emulsion gels. Food Hydrocoll. 2017, 69, 264–272. [Google Scholar] [CrossRef]
- Li, X.J.; Chen, X.; Cheng, H. Impact of κ-carrageenan on the cold-set pea protein isolate emulsion-filled gels: Mechanical property, microstructure, and in vitro digestive behavior. Foods 2024, 13, 483. [Google Scholar] [CrossRef]
- Hashemi, B.; Assadpour, E.; Jafari, S.M. Bigels as novel carriers of bioactive compounds: Applications and research trends. Food Hydrocoll. 2024, 147, 109427. [Google Scholar] [CrossRef]
- Shakeel, A.; Farooq, U.; Iqbal, T.; Yasin, S.; Lupi, F.R.; Gabriele, D. Key characteristics and modelling of bigels systems: A review. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 97, 932–953. [Google Scholar] [CrossRef]
- Lu, Y.; Zhong, Y.; Guo, X.; Zhang, J.; Gao, Y.; Mao, L. Structural modification of O/W bigels by glycerol monostearate for improved co-delivery of curcumin and epigallocatechin gallate. ACS Food Sci. Technol. 2022, 2, 975–983. [Google Scholar] [CrossRef]
- Tian, W.; Huang, Y.; Liu, L.; Yu, Y.; Cao, Y.; Xiao, J. Tailoring the oral sensation and digestive behavior of konjac glucomannan-gelatin binary hydrogel based bigel: Effects of composition and ratio. Int. J. Biol. Macromol. 2024, 256, 127963. [Google Scholar] [CrossRef]
- Xie, D.; Hu, H.; Huang, Q.; Lu, X. Influence of oleogel/hydrogel ratios and emulsifiers on structural and digestion properties of food-grade 3D printed bigels as carriers for quercetin and catechin. Food Hydrocoll. 2023, 144, 108948. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Yang, J.; Wang, Y.; He, H.; Mao, L. Roles of different polysaccharides on the structures of alginate-based Bigel beads and co-delivery of bioactives. Food Chem.-X 2025, 27, 102359. [Google Scholar] [CrossRef]
- Chao, E.; Li, J.; Duan, Z.; Fan, L. Bigels as emerging biphasic systems: Properties, applications, and prospects in the food industry. Food Hydrocoll. 2024, 154, 110089. [Google Scholar] [CrossRef]
- Kaimal, A.; Singhal, R. Bigels for controlled gastric release of ascorbic acid: Impact on rheology, texture, thermal stability and antioxidant activity. Food Hydrocoll. Health 2023, 4, 100171. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.Q.; Li, D.; Guo, Y.R.; Sun, L.J. Applications of mixed polysaccharide-protein systems in fabricating multi-structures of binary food gels-A review. Trends Food Sci. Technol. 2021, 109, 197–210. [Google Scholar] [CrossRef]
- Cornec, M.; Wilde, P.J.; Gunning, P.A.; Mackie, A.R.; Husband, F.A.; Parker, M.L.; Clark, D.C. Emulsion stability as affected by competitive adsorption between an oil-soluble emulsifier and milk proteins at the interface. J. Food Sci. 1998, 63, 39–43. [Google Scholar] [CrossRef]
- Sakuno, M.M.; Matsumoto, S.; Kawai, S.; Taihei, K.; Matsumura, Y. Adsorption and structural change of β-lactoglobulin at the diacylglycerol-water interface. Langmuir 2008, 24, 11483–11488. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, Q.; Liu, T.C.; Wusigale; Liang, L. Co-encapsulation of alpha-tocopherol and resveratrol in oil-in-water emulsion stabilized by sodium caseinate: Impact of polysaccharide on the stability and bioaccessibility. J. Food Eng. 2020, 264, 109685. [Google Scholar] [CrossRef]
- Tang, C.H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, K.; Huang, H.; Zhao, P.; Su, S.; Mcclements, D.; Chen, S.; Ma, C.; Liu, X.; Liu, F. Regulation of goat whey protein complex interfacial structures by gum Arabic to improve emulsion performance for curcumin delivery and application. Carbohydr. Polym. 2025, 366, 123782. [Google Scholar] [CrossRef]
- Li, T.; Wang, L. Improved curcumin bioaccessibility in Pickering emulsion fabricated by rice glutelin fibrils. Food Biosci. 2023, 55, 102988. [Google Scholar] [CrossRef]
- Mao, L.K.; Roos, Y.H.; Miao, S. Volatile release from self-assembly structured emulsions: Effect of monoglyceride content, oil content, and oil type. J. Agric. Food Chem. 2013, 61, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.Y.; Ni, Y.Z.; Wusigale; Dong, H.H.; Liang, L. alpha-Tocopherol and resveratrol in emulsion-filled whey protein gels: Co-encapsulation and in vitro digestion. Int. Dairy J. 2020, 104, 104649. [Google Scholar] [CrossRef]
- Fei-Ping, C.; Bian-Sheng, L.; Chuan-He, T. Nanocomplexation between curcumin and soy protein isolate: Influence on curcumin stability/bioaccessibility and in vitro protein digestibility. J. Agric. Food Chem. 2015, 63, 3559–3569. [Google Scholar] [CrossRef]
- Lin, D.Q.; Kelly, A.L.; Miao, S. Preparation, structure-property relationships and applications of different emulsion gels: Bulk emulsion gels, emulsion gel particles, and fluid emulsion gels. Trends Food Sci. Technol. 2020, 102, 123–137. [Google Scholar] [CrossRef]
- Shen, X.X.; Zheng, H.; Han, M.H.; Xu, X.Y.; Li, B.Y.; Guo, Q. Intermolecular forces regulate in-vitro digestion of whey protein emulsion gels: Towards controlled lipid release. J. Colloid Interface Sci. 2023, 649, 245–254. [Google Scholar] [CrossRef]
- Choi, M.; Choi, H.W.; Kim, H.; Hahn, J.; Choi, Y.J. Mimicking animal adipose tissue using a hybrid network-based solid-emulsion gel with soy protein isolate, agar, and alginate. Food Hydrocoll. 2023, 145, 109043. [Google Scholar] [CrossRef]
- Qin, X.S.; Bo, Q.L.; Qin, P.Z.; Wang, S.F.; Liu, K.Y. Fabrication of WPI-EGCG covalent conjugates/gellan gum double network emulsion gels by duo-induction of GDL and CaCl2 for colon-controlled Lactobacillus Plantarum delivery. Food Chem. 2023, 404, 134513. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Z.; Guo, P.P.; Guo, Q.N.; Zhang, H.J.; Jiang, L.W.; Xia, N.; Xiao, B.W. Tuning egg yolk granules/sodium alginate emulsion gel structure to enhance β-carotene stability and in vitro digestion property. Int. J. Biol. Macromol. 2023, 232, 123444. [Google Scholar] [CrossRef]
- Jo, Y.J.; Chen, L.Y. Gelation behavior of lentil protein aggregates induced by sequential combination of glucono-δ-lactone and transglutaminase. Food Struct. 2023, 36, 100312. [Google Scholar] [CrossRef]
- Fang, H.C.; Li, J.Y.; Huo, T.Y.; Niu, Y.G.; Yu, L.L. Novel double cross-linked gels of soybean protein isolates and soluble dietary fiber from soybean coats with their functionalities. Food Hydrocoll. 2021, 113, 106474. [Google Scholar] [CrossRef]
- Zheng, H.X.; Mao, L.K.; Cui, M.N.; Liu, J.F.; Gao, Y.X. Development of food-grade bigels based on κ-carrageenan hydrogel and monoglyceride oleogels as carriers for β-carotene: Roles of oleogel fraction. Food Hydrocoll. 2020, 105, 105855. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr. Polym. 2018, 180, 264–275. [Google Scholar] [CrossRef]
- Hu, K.; Huang, X.X.; Gao, Y.Q.; Huang, X.L.; Xiao, H.; McClements, D.J. Core-shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein-pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef]
- Guo, Q.; Su, J.; Shu, X.; Yuan, F.; Mao, L.; Liu, J.; Gao, Y. Fabrication, structural characterization and functional attributes of polysaccharide-surfactant-protein ternary complexes for delivery of curcumin. Food Chem. 2021, 337, 128019. [Google Scholar] [CrossRef]
- Sen, M.; Erboz, E.N. Determination of critical gelation conditions of kappa-carrageenan by viscosimetric and FT-IR analyses. Food Res. Int. 2010, 43, 1361–1364. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.Y.; Zhang, G.D.; McClements, D.J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct. Foods 2017, 34, 139–151. [Google Scholar] [CrossRef]
- Zhao, K.; Hao, Y.L.; Gan, J.L.; Ye, H.Q.; Shen, X. Development of quinoa protein emulsion gels to deliver curcumin: Influence of oil type. J. Food Eng. 2025, 384, 112260. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, R.; Gao, Y.; He, Q.; Sun, R. Influence of monoglyceride on curcumin-loaded nanostructured lipid carriers: Stability, antioxidant activity, digestion behavior, and intestinal absorption. Food Biosci. 2025, 71, 107150. [Google Scholar] [CrossRef]
- O’Sullivan, C.; Davidovich-Pinhas, M.; Wright, A.; Barbut, S.; Marangoni, A. Ethylcellulose oleogels for lipophilic bioactive delivery—Effect of oleogelation on in vitro bioaccessibility and stability of beta-carotene. Food Funct. 2017, 8, 1438–1451. [Google Scholar] [CrossRef]
- Witzleb, R.; Müllertz, A.; Kanikanti, V.; Hamann, H.; Kleinebudde, P. Dissolution of solid lipid extrudates in biorelevant media. Int. J. Pharm. 2012, 422, 116–124. [Google Scholar] [CrossRef]
- Lv, P.; Wang, D.; Dai, L.; Wu, X.; Gao, Y.; Yuan, F. Pickering emulsion gels stabilized by high hydrostatic pressure-induced whey protein isolate gel particles: Characterization and encapsulation of curcumin. Food Res. Int. 2020, 132, 109032. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.Q.; Liu, J.Y.; Liu, H.Z.; Wang, Z.Y. Preparation of transglutaminase-catalyzed rice bran protein emulsion gels as a curcumin vehicle. Foods 2024, 13, 2072. [Google Scholar] [CrossRef]
- Li, X.M.; Meng, R.; Xu, B.C.; Zhang, B. Investigation of the fabrication, characterization, protective effect and digestive mechanism of a novel Pickering emulsion gels. Food Hydrocoll. 2021, 117, 106708. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Qi, B.K.; Han, L.; Wang, D.Q.; Zhang, S.; Jiang, L.Z.; Xie, F.Y.; Li, Y. Study on the gel properties, interactions, and pH stability of pea protein isolate emulsion gels as influenced by inulin. LWT-Food Sci. Technol. 2021, 137, 110421. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, D.L.; Wang, X.F.; Xu, L.; Qian, J.Y.; He, X.D. Enzymatically modified quinoa starch based pickering emulsion as carrier for curcumin: Rheological properties, protection effect and in vitro digestion study. Food Biosci. 2022, 49, 101933. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Zhang, R.J.; Zhang, Z.P.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on gastrointestinal fate of oil-in-water emulsions containing anionic dietary fiber (pectin). Food Hydrocoll. 2015, 45, 175–185. [Google Scholar] [CrossRef]
- Liu, F.G.; Liang, X.P.; Yan, J.; Zhao, S.L.; Li, S.Q.; Liu, X.B.; Ngai, T.; McClements, D.J. Tailoring the properties of double-crosslinked emulsion gels using structural design principles: Physical characteristics, stability, and delivery of lycopene. Biomaterials 2022, 280, 121265. [Google Scholar] [CrossRef] [PubMed]
| GMS (%) | Encapsulation Efficiency (%) | |
|---|---|---|
| Oil Droplet of Emulsion | Gel Sample | |
| 0 | 93.2 ± 0.9 a | 98.2 ± 0.7 a |
| 10 | 95.5 ± 1.1 b | 99.1 ± 0.9 a |
| 15 | 96.4 ± 0.8 b | 98.8 ± 0.4 a |
| 20 | 97.1 ± 1.0 b | 99.3 ± 0.3 a |
| GMS (%) | Hardness (g) | Springiness | Cohesiveness | Chewiness (g) |
|---|---|---|---|---|
| 0 | 1304.6 ± 24.7 a | 0.594 ± 0.025 a | 0.506 ± 0.027 a | 392.9 ± 32.3 a |
| 10 | 940.5 ± 38.9 b | 0.592 ± 0.014 a | 0.465 ± 0.001 ab | 259.1 ± 21.1 b |
| 15 | 1117.8 ± 30.3 c | 0.573 ± 0.022 ab | 0.429 ± 0.021 bc | 274.7 ± 6.7 b |
| 20 | 1285.8 ± 15.8 c | 0.544 ± 0.003 b | 0.414 ± 0.037 c | 289.6 ± 30.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gray, E.T.; Huang, W.; Zhou, Z.; Cheng, H.; Liang, L. A Comparative Study of Soy Protein Isolate-κ-Carrageenan Emulsion Gels and Bigels for the Encapsulation, Protection, and Delivery of Curcumin. Gels 2025, 11, 782. https://doi.org/10.3390/gels11100782
Gray ET, Huang W, Zhou Z, Cheng H, Liang L. A Comparative Study of Soy Protein Isolate-κ-Carrageenan Emulsion Gels and Bigels for the Encapsulation, Protection, and Delivery of Curcumin. Gels. 2025; 11(10):782. https://doi.org/10.3390/gels11100782
Chicago/Turabian StyleGray, Emmanueline T, Weining Huang, Zhongkai Zhou, Hao Cheng, and Li Liang. 2025. "A Comparative Study of Soy Protein Isolate-κ-Carrageenan Emulsion Gels and Bigels for the Encapsulation, Protection, and Delivery of Curcumin" Gels 11, no. 10: 782. https://doi.org/10.3390/gels11100782
APA StyleGray, E. T., Huang, W., Zhou, Z., Cheng, H., & Liang, L. (2025). A Comparative Study of Soy Protein Isolate-κ-Carrageenan Emulsion Gels and Bigels for the Encapsulation, Protection, and Delivery of Curcumin. Gels, 11(10), 782. https://doi.org/10.3390/gels11100782








