Formulation and Optimization of a Melissa officinalis-Loaded Nanoemulgel for Anti-Inflammatory Therapy Using Design of Experiments (DoE)
Abstract
1. Introduction
2. Results and Discussion
2.1. FT-IR Analysis of Melissa Oil
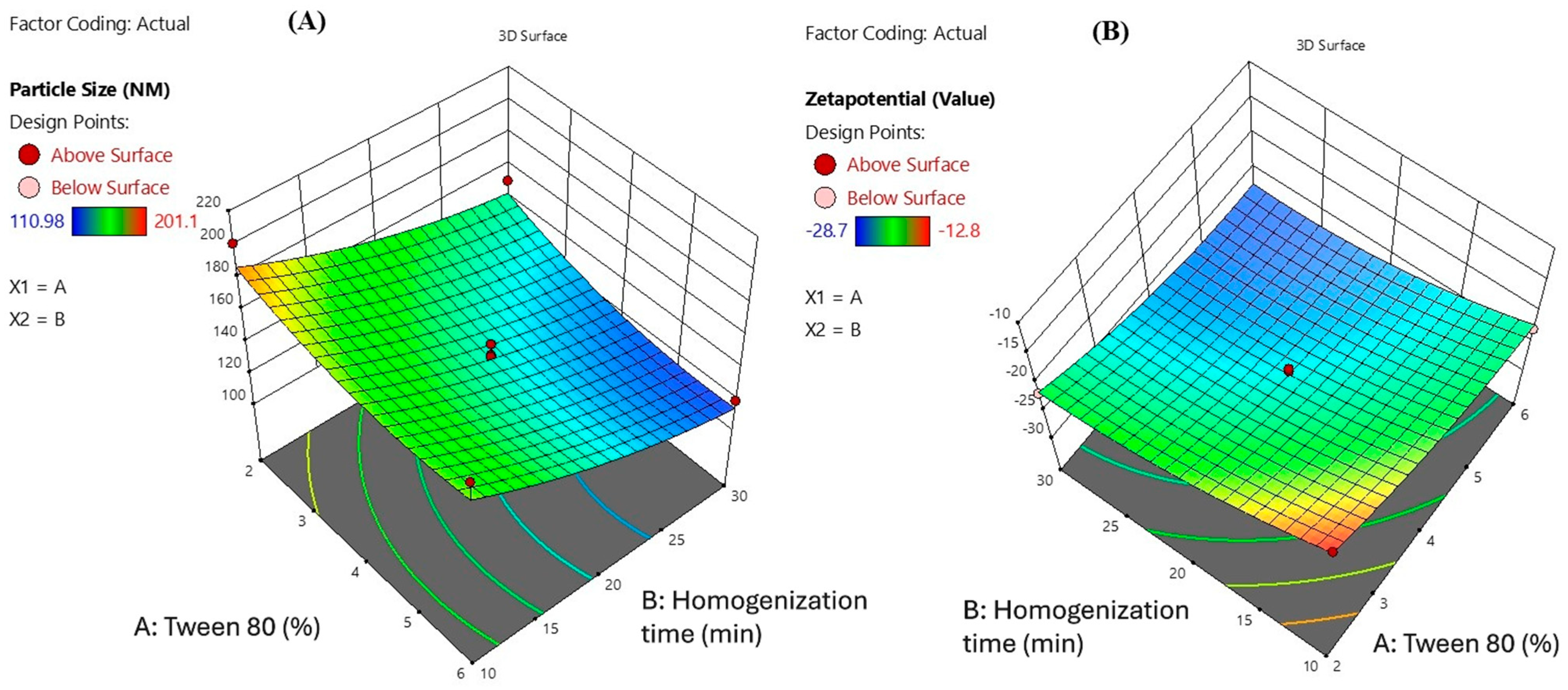
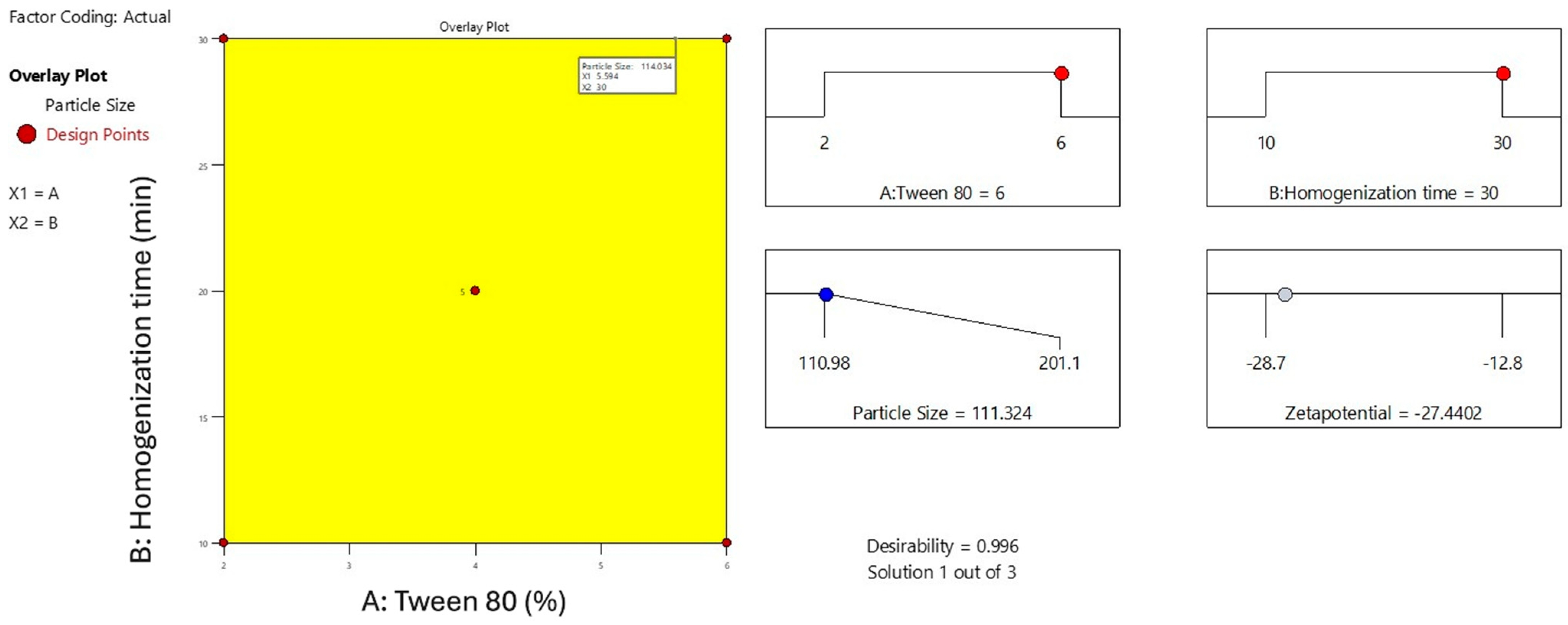
2.2. Preparation and Optimization of MelissaNanoemulsions
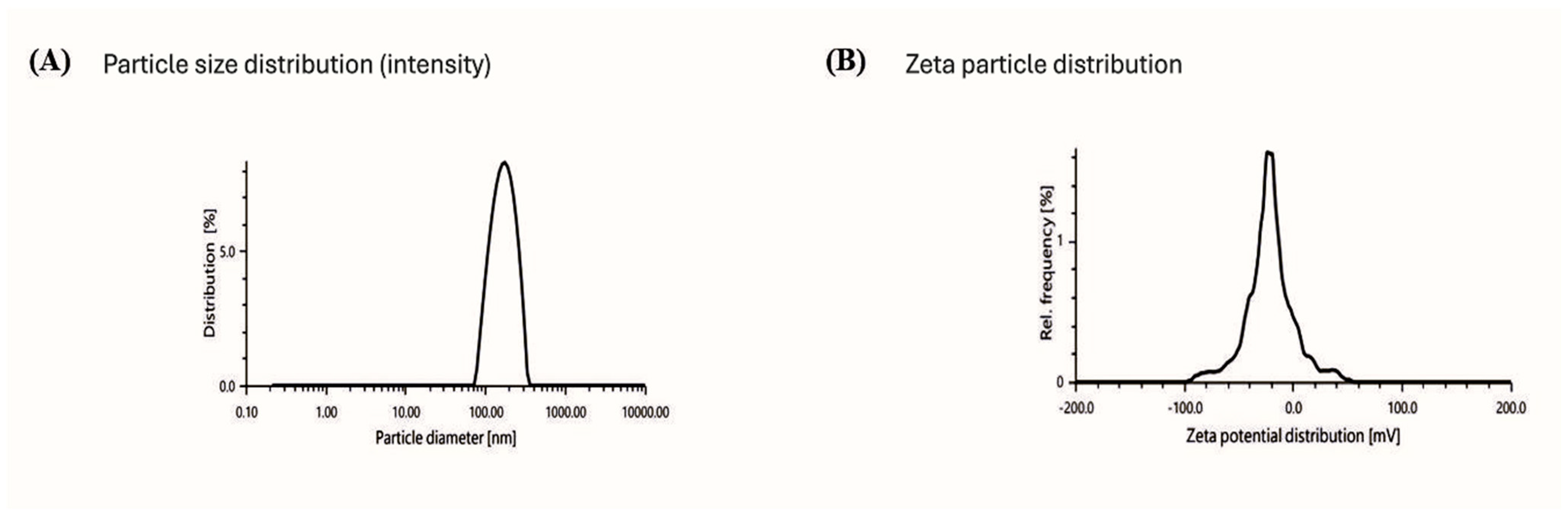
2.3. Characterization Study of Melissa-Loaded Nanoemulsions (M-NE)
2.4. Physicochemical Characteristics and Morphological Analysis
2.5. Scanning Electron Microscopy (SEM) Assessment
2.6. Entrapment Efficiency of Melissa Nanoemulsion
2.7. Design and Evaluation of a Melissa Nanoemulgel (M-NG)
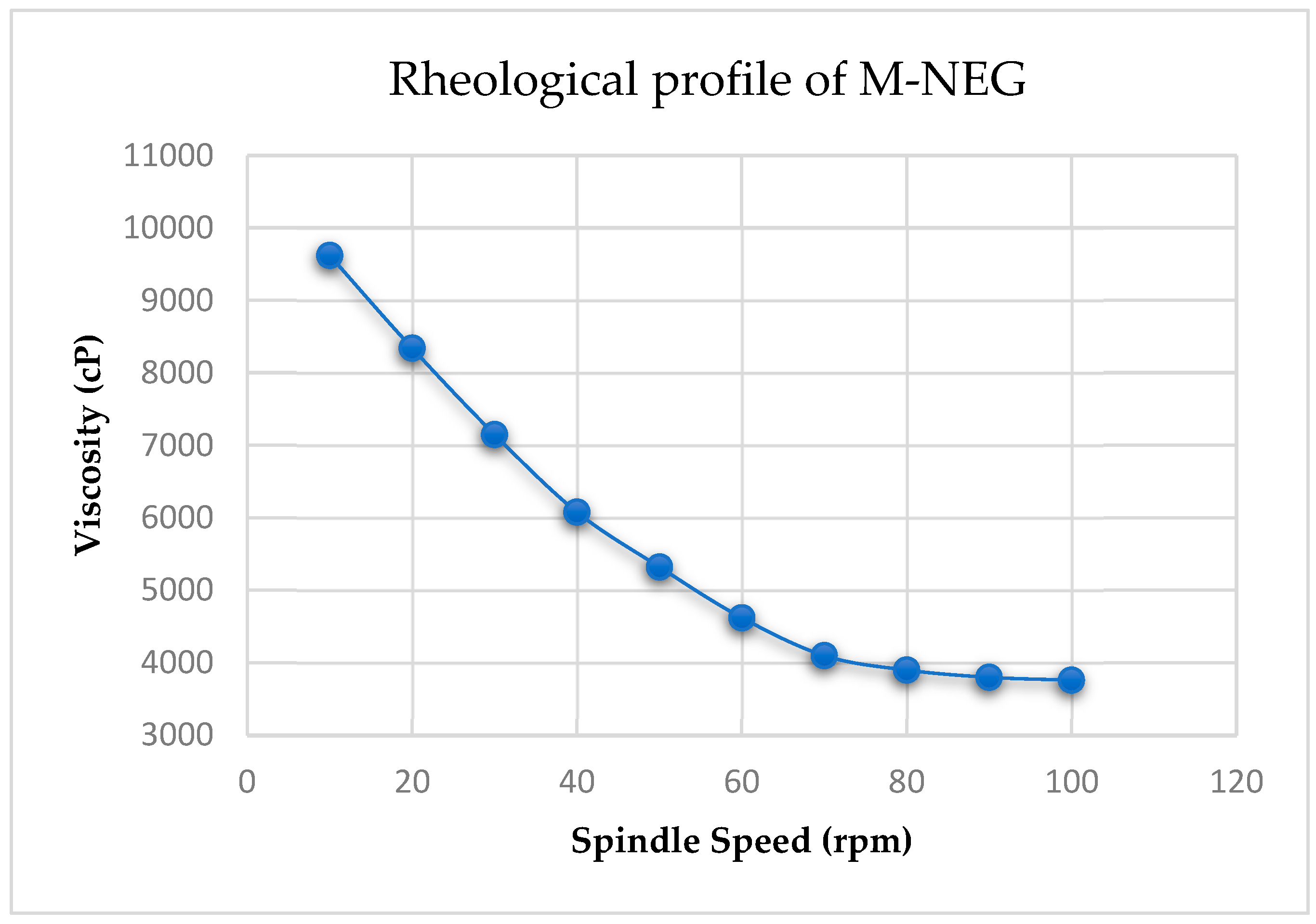
2.8. Physicochemical Characterization of Nanoemulgel
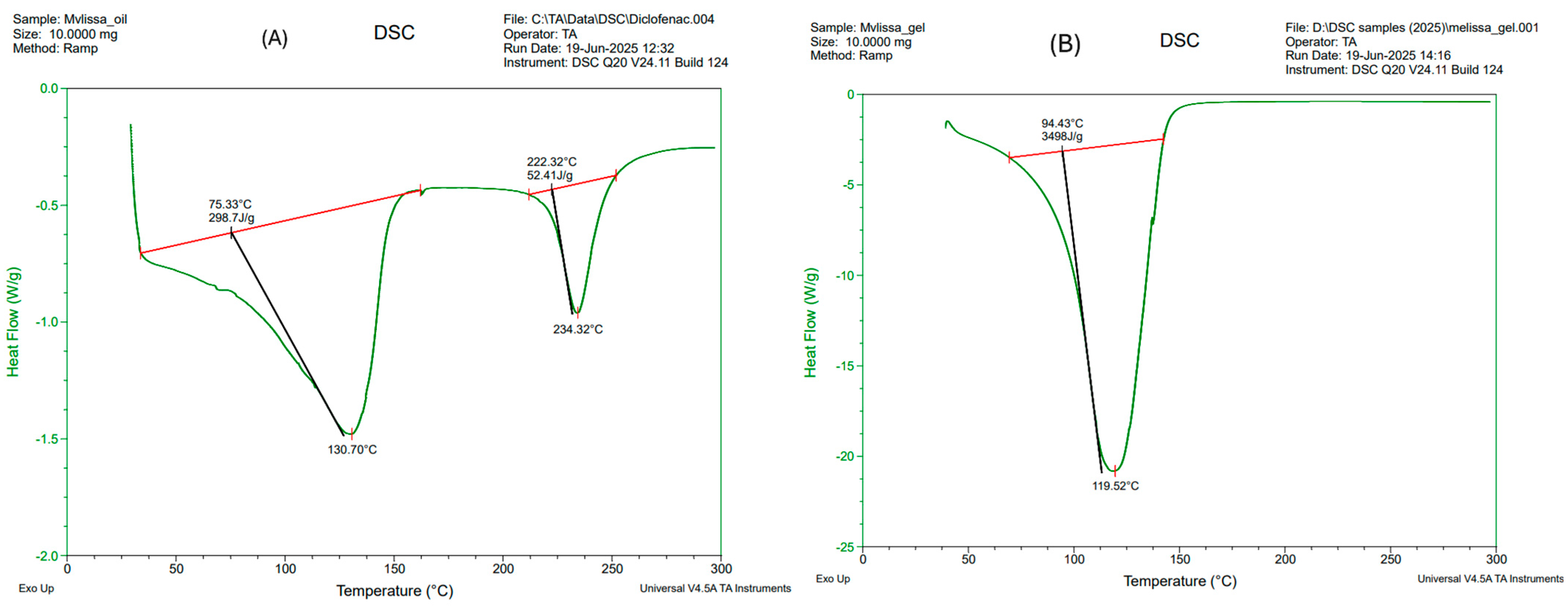
2.9. DSC Thermogram Analysis
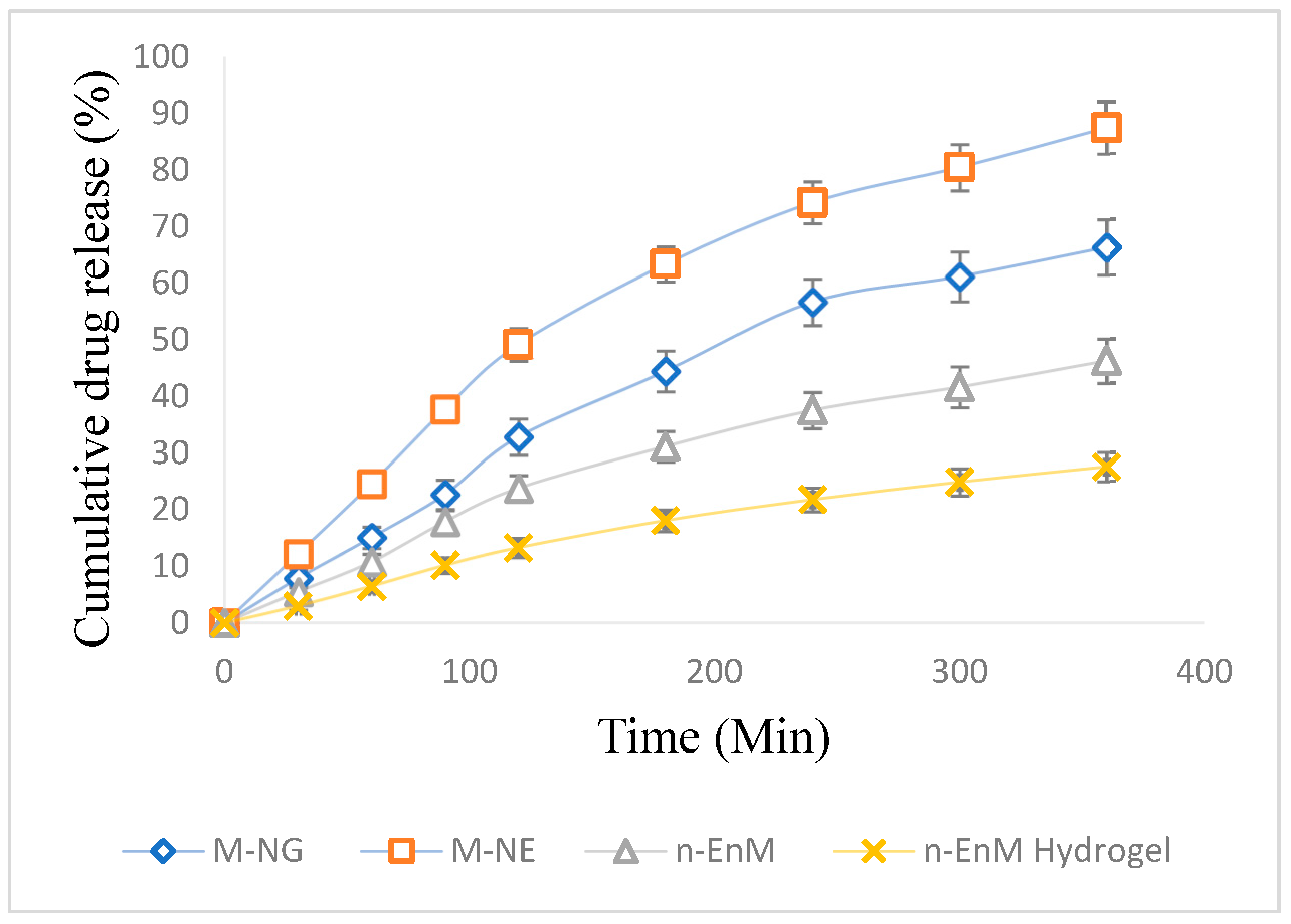
2.10. In Vitro Evaluation of Drug Release and Diffusion Profiles
2.11. Accelerated Stability Evaluation
2.12. Assessment of Antibacterial Efficacy
2.13. Examination of Dermal Effects in the Acute Irritation Study
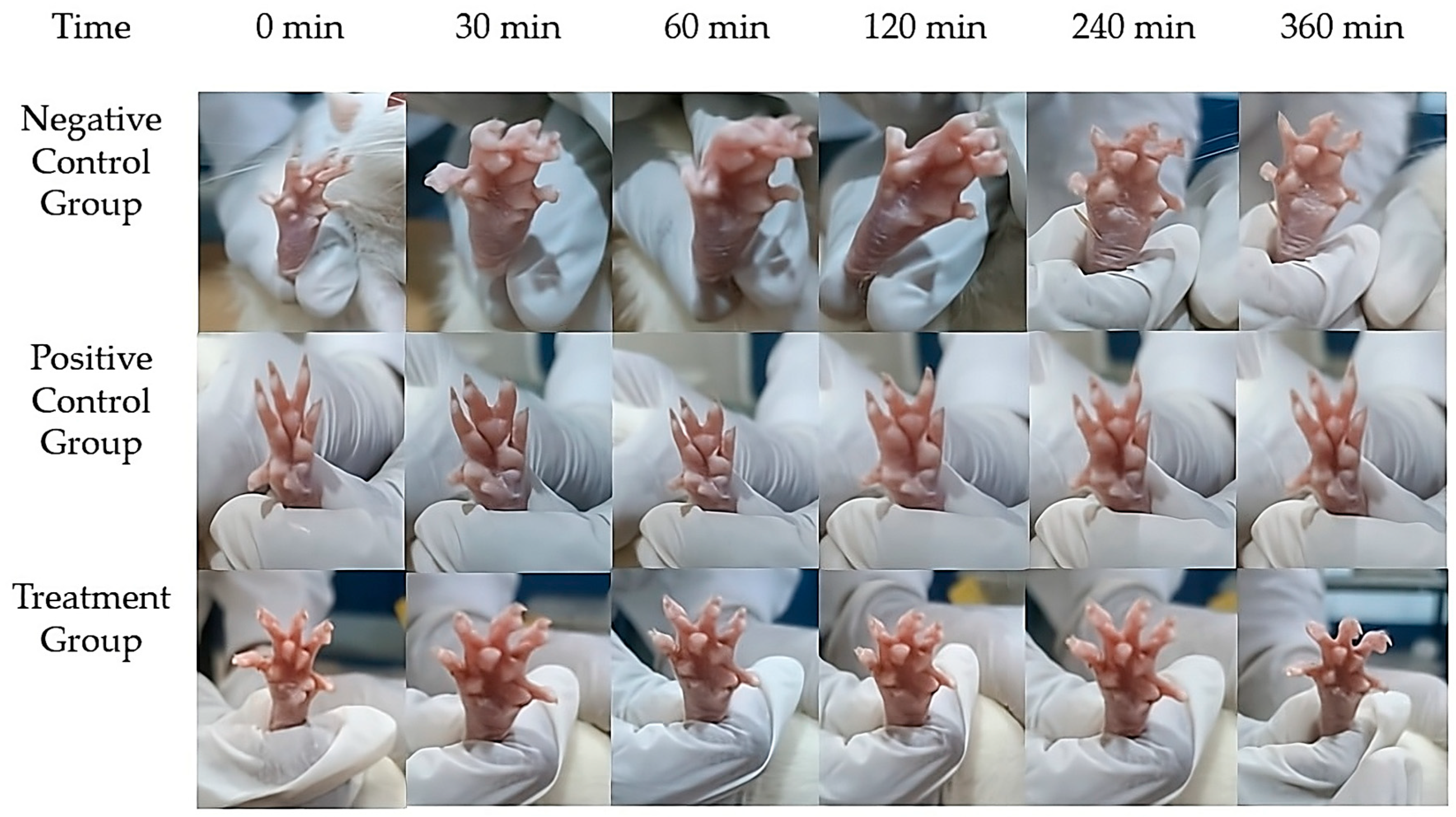
2.14. In Vivo Assessment of Anti-Inflammatory Activity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Surfactant Screening and FT-IR Analysis of Melissa Oil
4.3. Development and Optimization of Melissa Nanoemulsion
4.4. Assessment of Entrapment Efficiency
4.5. Physicochemical Characterization and Stability Assessment
4.6. Scanning Electron Microscopy Analysis
4.7. Preparation of Melissa Oil-Loaded Nanoemulgel
4.8. Characterization of Melissa Nanoemulgel
4.9. Differential Scanning Calorimetry Analysis
4.10. Analysis of In Vitro Drug Diffusion and Kinetic Modeling
4.11. Accelerated Stability Study
4.12. Assessment of Antibacterial Potential of M-NG Formulation
4.13. Preclinical Evaluation Using Animal Models
4.14. Evaluation of Skin Irritation Following Topical Application
4.15. Investigation of Anti-Inflammatory Efficacy in Animal Models
4.16. Statistical Analysis and Interpretation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QbD | Quality by Design |
| NE | Nanoemulsion |
| NEs | Nanoemulsions |
| M-NE | Melissa Nanoemulsion |
| n-EnM | Nanoemulsion in Matrix |
| n-EnM Hydrogel | Nanoemulsion in Hydrogel |
| M-NG | Meliss Nanoemulgel |
| CCD | Central Composite Design |
| ANOVA | Analysis of Variance |
| PDI | Polydispersity Index |
| CI | Confidence Interval |
| EE | Entrapment Efficiency |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| SEM | Scanning Electron Microscopy |
| DSC | Differential Scanning Calorimetry |
| UV | Ultraviolet |
| O/W | Oil in Water |
| MHB | Mueller–Hinton Broth |
| MIC | Minimum Inhibitory Concentration |
| CLSI | Clinical and Laboratory Standards Institute |
| CFU | Colony-Forming Unit |
| IAEC | Institutional Animal Ethics Committee |
References
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Taherkhani, M.; Goudarzi, M.; Bakhshi, A. Antibacterial activity of Melissa officinalis essential oil against clinical isolates of methicillin-resistant Staphylococcus aureus. Iran. J. Microbiol. 2013, 5, 263–267. [Google Scholar]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Shafiq, S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J. Nanobiotechnology 2008, 6, 8. [Google Scholar] [CrossRef]
- Aqil, M.; Kamran, M.; Ahad, A.; Imam, S.S. Development of clove oil-based nanoemulsion of olmesartan for transdermal delivery: Box–Behnken design optimization and pharmacokinetic evaluation. J. Mol. Liq. 2016, 214, 23–32. [Google Scholar] [CrossRef]
- Sharma, D.; Gupta, A.; Rawat, R.; Sharma, S.; Yadav, J.S.; Saxena, A. Exploring nanoformulation drug delivery of herbal actives for enhanced therapeutic efficacy: A comprehensive review. Intell. Pharm. 2025, 3, 26–34. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Basak, S.; Khare, H.A.; Roursgaard, M.; Kempen, P.J.; Lee, J.H.; Bazban-Shotorbani, S.; Kræmer, M.; Chernyy, S.; Andresen, T.L.; Almdal, K.; et al. Biomimetic core–shell nanogels for spatiotemporally controlled combination delivery of chemotherapeutics and immune stimulants. Biomacromolecules 2021, 22, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Singh, P.; Weller, A.; Kadumudi, F.B.; Kempen, P.J.; Mijakovic, I.; Dolatshahi-Pirouz, A.; Almdal, K. Cost-effective and eco-friendly sprayable nanogels (ZC-CSNG) for multifunctional wound dressing applications. Chem. Eng. J. 2025, 503, 158312. [Google Scholar] [CrossRef]
- Esmaeili, F.; Zahmatkeshan, M.; Yousefpoor, Y.; Alipanah, H.; Safari, E.; Osanloo, M. Anti-inflammatory and anti-nociceptive effects of Cinnamon and Clove essential oils nanogels: An in vivo study. BMC Complement. Med. Ther. 2022, 22, 143. [Google Scholar] [CrossRef] [PubMed]
- Mimica-Dukić, N.; Božin, B.; Soković, M.; Mihajlović, B.; Matavulj, M. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004, 52, 2485–2489. [Google Scholar] [CrossRef]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Ribeiro, L.N.; Franz-Montan, M.; Breitkreitz, M.C.; de Paula, E.; Groppo, F.C.; Duarte, J.L.; Barbosa, R.M.; de Paula, E. Nanostructured lipid carriers versus nanoemulsions: Effects on lidocaine topical delivery. J. Nanoparticle Res. 2012, 14, 1–10. [Google Scholar]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimization. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef]
- Mishra, N.; Tiwari, S.; Verma, A.; Tiwari, G. UV spectrophotometric method development and validation of essential oil of Eucalyptus globulus. J. Pharm. Sci. Biosci. Res. 2013, 3, 28–32. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Yetukuri, K.; Umashankar, M.S. Development and Optimization of Kunzea ericoides Nanoemulgel Using a Quality by Design Approach for Transdermal Anti-Inflammatory Therapy. Gels 2025, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Haq, N.; Alanazi, F.K.; Alsarra, I.A.; Ramadan, W.; Faiyazuddin, M. Formulation and optimization of nanoemulsions for topical delivery of hydrophobic drugs: Evaluation of physical stability and particle size. Pharm. Dev. Technol. 2015, 20, 188–196. [Google Scholar]
- Gadkari, P.N.; Patil, P.B.; Saudagar, R.B. Formulation, development and evaluation of topical nanoemulgel of tolnaftate. J. Drug Deliv. Ther. 2019, 9, 208–213. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent update on nanoemulgel as topical drug delivery system. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef]
- Bashir, M.; Ahmad, J.; Asif, M.; Khan, S.U.; Irfan, M.; Ibrahim, A.Y. Nanoemulgel: An innovative carrier for diflunisal topical delivery with profound anti-inflammatory effect: In vitro and in vivo evaluation. Int. J. Nanomed. 2021, 16, 1457–1472. [Google Scholar] [CrossRef]
- Oktay, A.N.; Karakucuk, A.; Ilbasmis Tamer, S.; Celebi, N. Dermal flurbiprofen nanosuspensions: Optimization with design of experiment approach and in vitro evaluation. Eur. J. Pharm. Sci. 2018, 122, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.A.; Hussein, A.K.; Nasef, G.A.; Mansour, H.F. Oxiconazole nitrate solid lipid nanoparticles: Formulation, in vitro characterization and clinical assessment of an analogous loaded carbopol gel. Drug Dev. Ind. Pharm. 2020, 46, 706–716. [Google Scholar] [CrossRef]
- International Council for Harmonisation (ICH). ICH Harmonised Tripartite Guideline: Stability Testing of New Drug Substances and Products Q1A(R2); ICH Steering Committee; International Council for Harmonisation (ICH): Geneva, Switzerland, 2003; Available online: https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf (accessed on 24 March 2025).
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Shadab, M.D.; Nabil, A.A.; Aldawsari, H.M.; Kotta, S.; Ahmad, J.; Akhter, S. Improved analgesic and anti-inflammatory effect of diclofenac sodium by topical nanoemulgel: Formulation development, in vitro and in vivo studies. J. Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- El-Leithy, E.S.; Makky, A.M.; Khattab, A.M.; Hussein, D.G. Nanoemulsion gel of nutraceutical co-enzyme Q10 as an alternative to conventional topical delivery system to enhance skin permeability and anti-wrinkle efficiency. Int. J. Pharm. Pharm. Sci. 2017, 9, 207–217. [Google Scholar] [CrossRef]
- Burki, I.K.; Khan, M.K.; Khan, B.A.; Uzair, B.; Braga, V.A.; Jamil, Q.A. Formulation development, characterization, and evaluation of a novel dexibuprofen-capsaicin skin emulgel with improved in vivo anti-inflammatory and analgesic effects. AAPS PharmSciTech 2020, 21, 1–4. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. Transdermal delivery of ketoprofen using lecithin organogels: Mechanistic studies. AAPS PharmSciTech 2005, 6, E237–E245. [Google Scholar] [CrossRef]
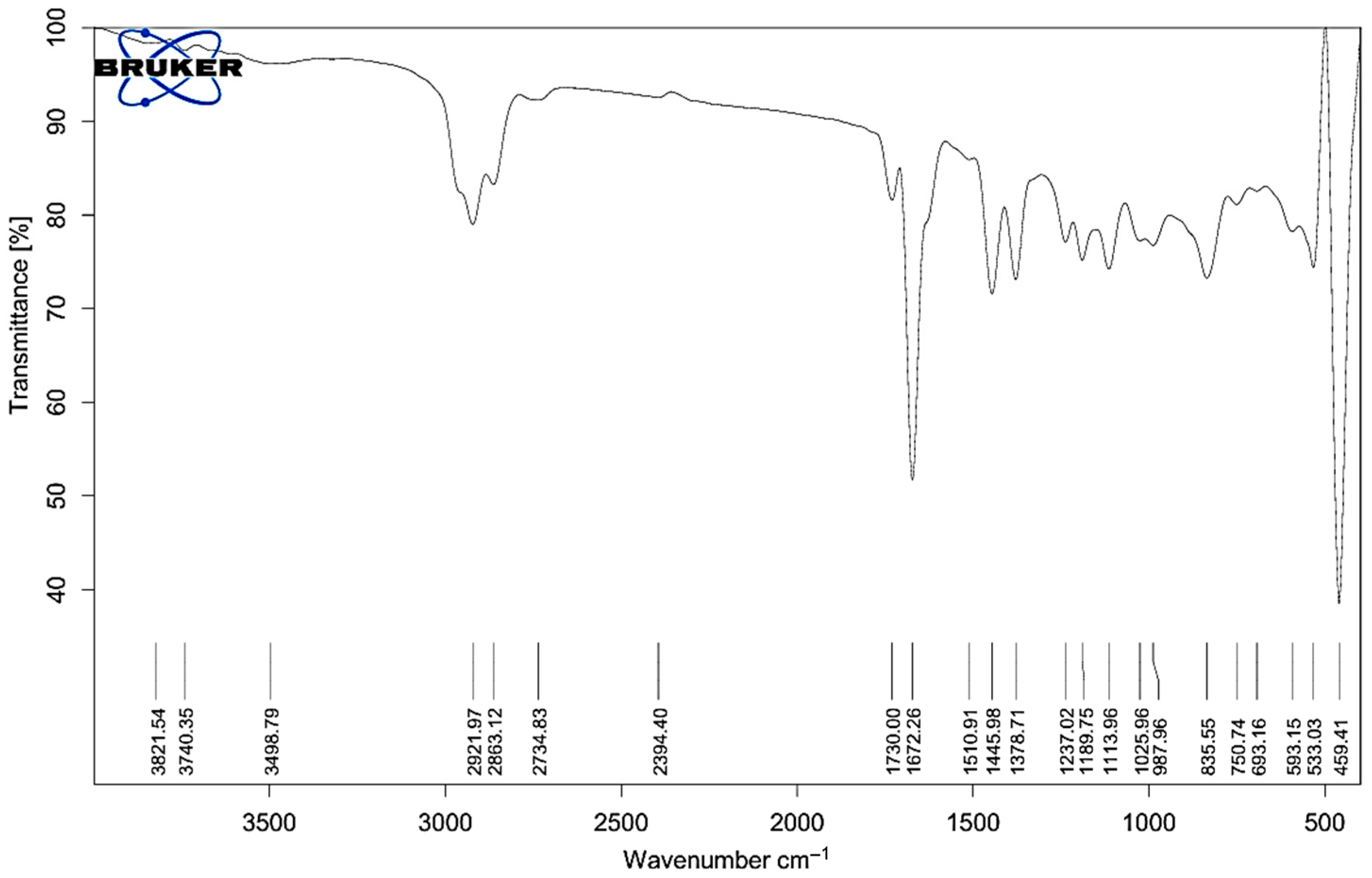


| Observed Wavenumber (cm−1) | Assigned Functional Group | Typical Wavenumber Range (cm−1) | Presumed Compound Class |
|---|---|---|---|
| 3821.54, 3740.35 | O–H stretch | 3200–3700 | Alcohols, Phenols |
| 3498.79 | H-bonded O–H stretch | 3200–3600 | Phenolics, Hydroxyl groups |
| 2921.97, 2863.12, 2734.83 | C–H stretch | 2800–3000 | Alkanes (saturated hydrocarbons) |
| 1730.00, 1672.26 | C=O stretch | 1650–1750 | Esters, Ketones |
| 1510.91, 1445.89, 1378.71 | C=C stretch, CH bend | 1350–1600 | Aromatics, Alkanes, Alkenes |
| 1237.02, 1180.75, 1113.96, 1025.96 | C–O stretch | 1000–1300 | Alcohols, Ethers |
| 987.69, 925.59 | =C–H bending | 800–1000 | Terpenes, Aromatics |
| 835.55, 750.74, 693.16 | C–H out-of-plane bend | 650–900 | Aromatic or cyclic compounds |
| 593.15, 533.13, 459.41 | Fingerprint region | <600 | Specific spectral features |
| Formulations | X1 (Coded Level) | X2 (Coded Level) | Tween 80 (%) X1 | Homogenization Time (min) X2 | Particle Size (nm) | Zetapotential |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 4 | 20 | 147.7 | −25 |
| 2 | 0 | 0 | 4 | 20 | 129.5 | −24.9 |
| 3 | − | − | 2 | 10 | 201.1 | −12.8 |
| 4 | 0 | 0 | 4 | 20 | 132.9 | −25.4 |
| 5 | 0 | A | 4 | 34.1421 | 110.98 | −25 |
| 6 | 0 | 0 | 4 | 20 | 140.8 | −24.1 |
| 7 | + | + | 6 | 30 | 119.1 | −28.7 |
| 8 | − | + | 2 | 30 | 150.6 | −21.8 |
| 9 | a | 0 | 1.17157 | 20 | 160.28 | −15.9 |
| 10 | + | − | 6 | 10 | 181.34 | −22.8 |
| 11 | 0 | 0 | 4 | 20 | 139.67 | −23.7 |
| 12 | A | 0 | 6.82843 | 20 | 132.89 | −25.1 |
| 13 | 0 | a | 4 | 5.85786 | 173.52 | −15.8 |
| Factor | df | Overall Variability | Mean Square | F-Statistic | p-Value | Statistical Significance |
|---|---|---|---|---|---|---|
| Model | 5 | 6564.29 | 1312.86 | 12.47 | 0.0021 | Significant |
| Error | 7 | 736.71 | 105.24 | – | – | – |
| Total | 12 | 7301 | – | – | – | – |
| Intercept | – | – | 143.92 | 28.72 | <0.0001 | Highly significant |
| Tween 80 | 1 | – | −23.56 | −4.11 | 0.0047 | Highly significant |
| Homogenization Time | 1 | – | −27.89 | −5.24 | 0.0022 | Highly significant |
| Tween 80 × Homogenization Time | 1 | – | 1.89 | 0.34 | 0.7442 | Not significant |
| Tween 80 × Tween 80 | 1 | – | 9.63 | 2.71 | 0.0481 | Marginally significant |
| Homogenization Time × Time | 1 | – | 5.74 | 1.28 | 0.2417 | Not significant |
| R2 | – | 0.8992 | – | – | – | Indicates strong model fit |
| Adjusted R2 | – | 0.8465 | – | – | – | Adjusted for predictors |
| Kinetic Model | Equation | R2 Value | Interpretation |
|---|---|---|---|
| Zero-order | 0.825 | Constant release rate (less suited here) | |
| First-order | 0.841 | Concentration-dependent release | |
| Higuchi | 0.900 | Best fit: diffusion-controlled release | |
| Korsmeyer– Peppas | 0.895 | Non-Fickian (anomalous) transport (n = 0.88) | |
| Hixson–Crowell (optional) | 0.810 | Suggests erosion/dissolution (less relevant here) |
| Time Point | Melissa Nanoemulgel 1 | Positive Control (Formalin) 1 | Negative Control (Blank Gel) 1 | p-Value 2 |
|---|---|---|---|---|
| 1 h | 0.5 ± 0.5 | 2.5 ± 0.5 | 0.0 ± 0.0 | 0.0274 |
| 24 h | 0.0 ± 0.0 | 2.0 ± 0.5 | 0.0 ± 0.0 | 0.0196 |
| 48 h | 0.0 ± 0.0 | 1.5 ± 0.5 | 0.0 ± 0.0 | 0.0221 |
| 72 h | 0.0 ± 0.0 | 1.0 ± 0.0 | 0.0 ± 0.0 | 0.0303 |
| Time (min) | Negative Control Group (mL) 1 | Positive Control Group (mL) 1 | Treatment Group (mL) 1 | Inhibition of Edema (%) | Group Comparisons | Mean Difference (mL) | p-Value 4 |
|---|---|---|---|---|---|---|---|
| 0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | - | - | - |
| 30 | 0.42 ± 0.007 | 0.32 ± 0.005 | 0.25 ± 0.006 | 23.8 2/ 40.5 3 | Negative Control vs. Standard | −0.17 | 0.029 |
| 60 | 0.41 ± 0.006 | 0.28 ± 0.005 | 0.17 ± 0.004 | 33.2 2/ 60.3 3 | Negative Control vs. Treatment | −0.24 | 0.008 |
| 120 | 0.39 ± 0.005 | 0.19 ± 0.004 | 0.07 ± 0.003 | 52.6 2/ 81.6 3 | Standard vs. Treatment | −0.12 | 0.076 |
| 240 | 0.36 ± 0.003 | 0.05 ± 0.001 | 0.01 ± 0.001 | 86.5 2/ 96.9 3 | - | - | - |
| 360 | 0.33 ± 0.002 | 0.00 ± 0.00 | 0.00 ± 0.00 | 100 2,3 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koushik, Y.; Rama Rao, N.; Sri Venkatesh, U.; Rami Reddy, G.V.; Surendra, A.V.; Sreenu, T. Formulation and Optimization of a Melissa officinalis-Loaded Nanoemulgel for Anti-Inflammatory Therapy Using Design of Experiments (DoE). Gels 2025, 11, 776. https://doi.org/10.3390/gels11100776
Koushik Y, Rama Rao N, Sri Venkatesh U, Rami Reddy GV, Surendra AV, Sreenu T. Formulation and Optimization of a Melissa officinalis-Loaded Nanoemulgel for Anti-Inflammatory Therapy Using Design of Experiments (DoE). Gels. 2025; 11(10):776. https://doi.org/10.3390/gels11100776
Chicago/Turabian StyleKoushik, Yetukuri, Nadendla Rama Rao, Uriti Sri Venkatesh, Gottam Venkata Rami Reddy, Amareswarapu V. Surendra, and Thalla Sreenu. 2025. "Formulation and Optimization of a Melissa officinalis-Loaded Nanoemulgel for Anti-Inflammatory Therapy Using Design of Experiments (DoE)" Gels 11, no. 10: 776. https://doi.org/10.3390/gels11100776
APA StyleKoushik, Y., Rama Rao, N., Sri Venkatesh, U., Rami Reddy, G. V., Surendra, A. V., & Sreenu, T. (2025). Formulation and Optimization of a Melissa officinalis-Loaded Nanoemulgel for Anti-Inflammatory Therapy Using Design of Experiments (DoE). Gels, 11(10), 776. https://doi.org/10.3390/gels11100776












