A Bioactive Emulgel Formulation of Equisetum telmateia Ehrh. Methanol Extract: Integrating Antioxidant Activity, Skin Enzyme Inhibition, and Permeation Kinetics
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Evaluation
2.2. Preparation and Characterization of Emulgel Formulation
2.3. Texture Profile and Spreadability Analysis
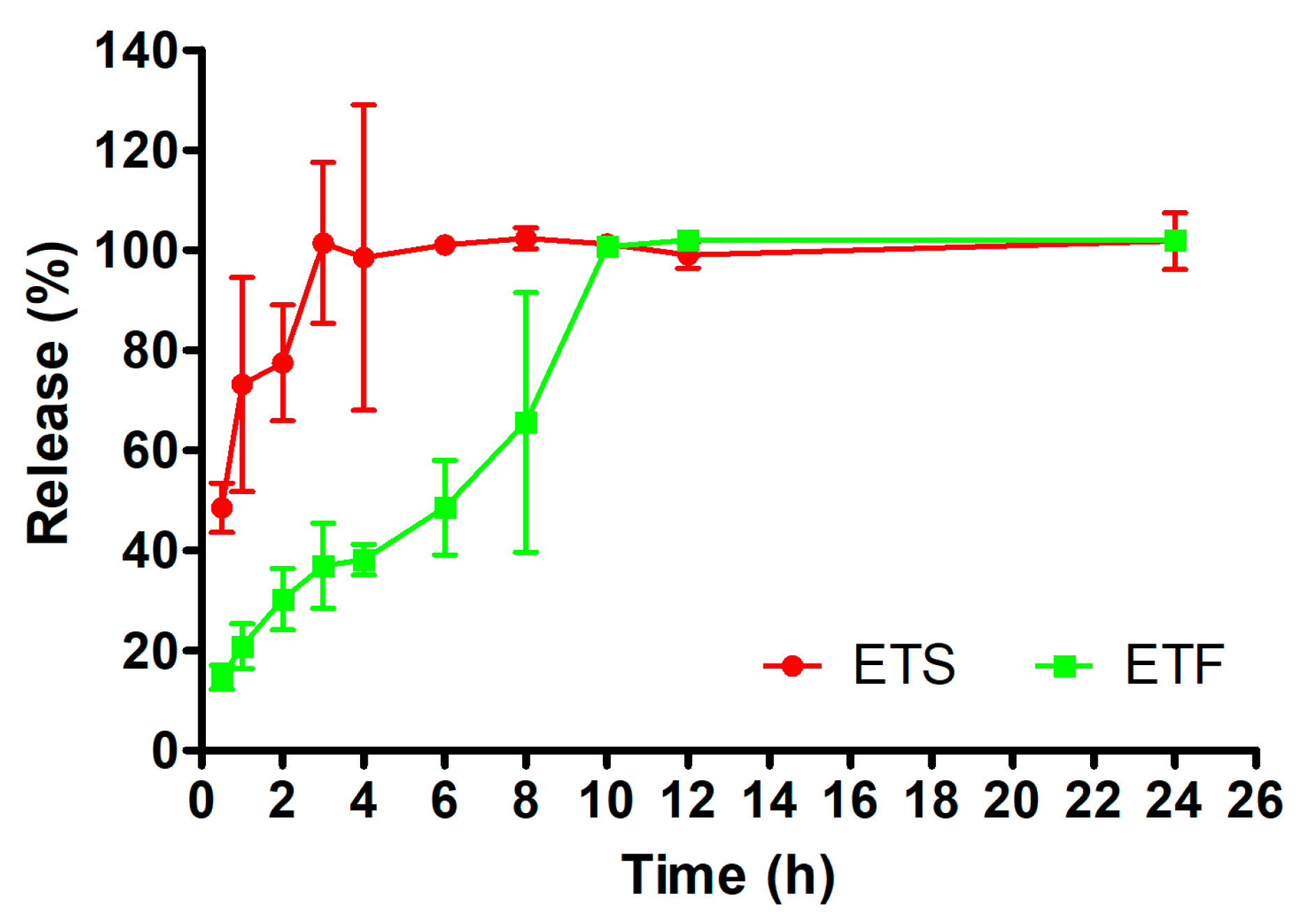
2.4. In Vitro Release Studies
2.4.1. Kinetic Release Modeling
2.4.2. Similarity Studies
2.5. Ex Vivo Permeation and Penetration Studies
2.6. In Vitro Antioxidant Tests
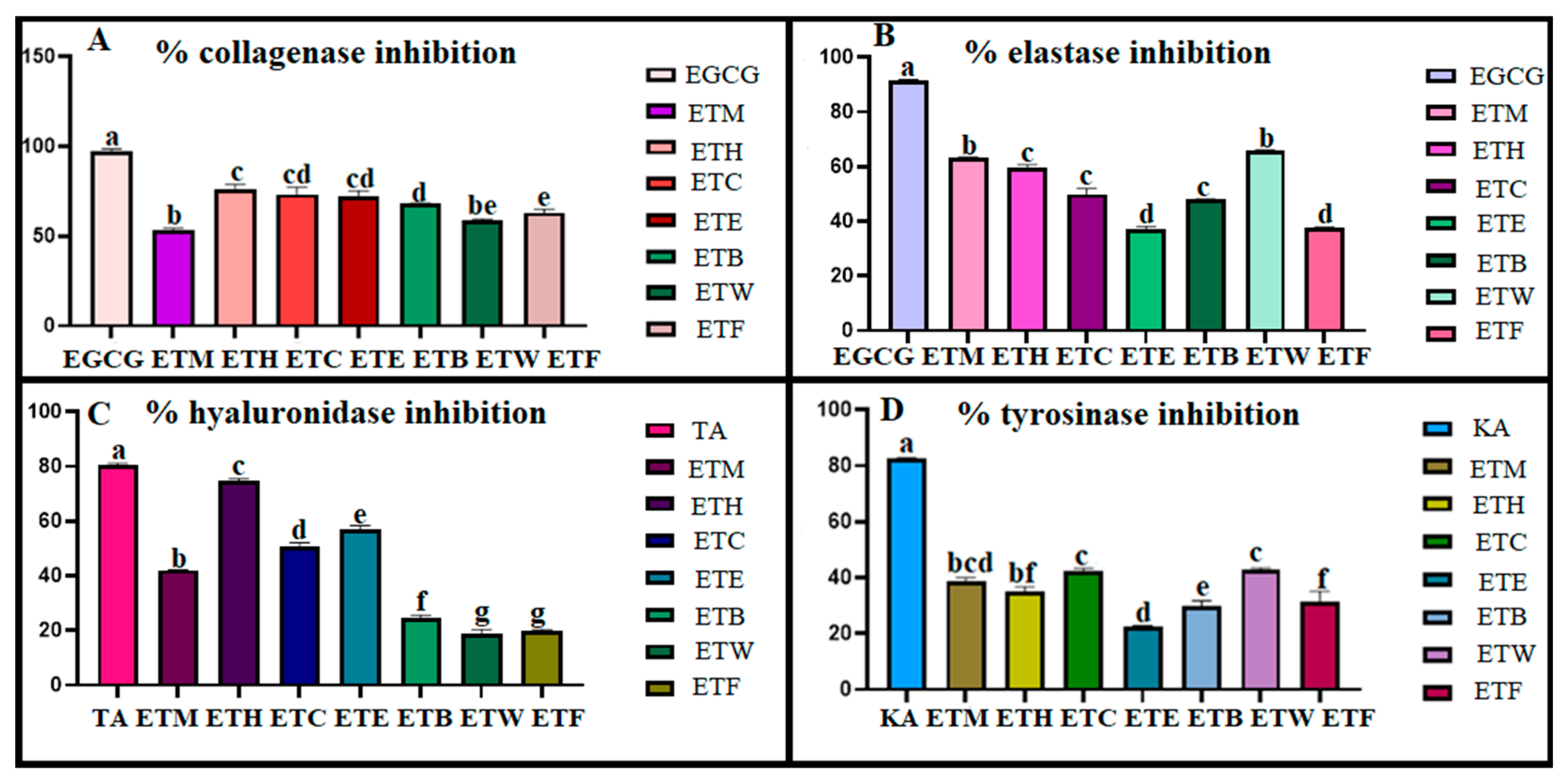
2.7. Inhibition Potential on Skin-Related Enzymes
2.8. Stability Studies
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Preparation of the Extract
4.4. Phytochemical Investigation
4.4.1. Determination of Phenolic Compounds by LC-MS/MS
4.4.2. Quantification of Marker Bioactive Compounds by HPTLC
4.5. Development of Emulgel Formulation
4.5.1. Characterization Studies of the Formulation
pH Measurement
Viscosity Measurement
Determination of Content Quantity in the Formulations
Spreadability and Texture Profile Analysis
4.5.2. In Vitro Release Studies
Kinetic Release Modeling
Similarity Studies
Ex Vivo Permeation and Penetration Studies
4.6. In Vitro Investigation of Antioxidant Potential
4.7. Skin Enzyme Inhibition Assays
4.7.1. Anticollagenase Activity
4.7.2. Antielastase Activity
4.7.3. Antihyaluronidase Activity
4.7.4. Antityrosinase Activity
4.8. Stability Studies
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Makgobole, M.U.; Mpofana, N.; Ajao, A.A. Medicinal Plants for Dermatological Diseases: Ethnopharmacological Significance of Botanicals from West Africa in Skin Care. Cosmetics 2023, 10, 167. [Google Scholar] [CrossRef]
- Genc, Y.; Dereli-Guragac, F.T.; Saracoglu, I.; Kupeli-Akkol, E. The inhibitory effects of isolated constituents from Plantago major subsp. major L. on collagenase, elastase and hyaluronidase enzymes: Potential wound healer. Saudi Pharm. J. 2020, 28, 101–106. [Google Scholar] [CrossRef]
- Rexcida Janthark Mary, S.; Josephinol, S.; Ramya, B. Anti-Ageing activity and identification of bio active compound from the seed of Linum usitatissimum by GC-MS techniques. Int. J. Biol. Pharm. Allied Sci. 2022, 11, 50–65. [Google Scholar]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.T.; Jang, Y.; Myung, S.W.; Lee, J.M.; Kim, H.S.; Ko, S.C.; Lee, S.H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Ran, L.Y.; Li, C.Y.; Chen, X.L. Diversity, structures, and collagen-degrading mechanisms of bacterial collagenolytic proteases. Appl. Environ. Microbiol. 2015, 81, 6098–6107. [Google Scholar] [CrossRef]
- Arseni, L.; Lombardi, A.; Orioli, D. From structure to phenotype: Impact of collagen alterations on human health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Weiss, A.S. The role of elastin in wound healing and dermal substitue design. In Dermal Replacements in General, Burn, and Plastic Surgery; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bahadır-Acıkara, Ö.; Ilhan, M.; Kurtul, E.; Šmejkal, K.; Küpeli -Akkol, E. Inhibitory activity of Podospermum canum and its active components on collagenase, elastase and hyaluronidase enzymes. Bioorg Chem. 2019, 93, 103330. [Google Scholar] [CrossRef]
- da Costa Gomes, A.; Figueiredo, C.C.M.; Granero, F.O.; Junior, J.L.B.; Ximenes, V.F.; Silva, L.P.; Nicolau-Junior, N.; da Silva, R.M.G. Antioxidant and antiglycation activities and inhibitory action of Passiflora cincinnata on collagenase, elastase and tyrosinase: In vitro and In Silico study. Biocatal. Agric. Biotechnol. 2022, 44, 102464. [Google Scholar] [CrossRef]
- Manjia, J.N.; Njoya, E.M.; Harishchander, A.; Munvera, A.M.; Ogundolie, F.A.; Mkounga, P.; Mcgaw, L.J.; Njayou, F.N.; Moundipa, P.F. Anti-elastase, anti-tyrosinase, and anti-inflammatory activities of three compounds isolated from Psorospermum aurantiacum: In Silico and In vitro assays. Rev. Bras. Farmacogn. 2024, 34, 1116–1128. [Google Scholar] [CrossRef]
- Khojah, H.; Ahmed, S.R.; Alharbi, S.Y.; AlSabeelah, K.K.; Alrayyes, H.Y.; Almusayyab, K.B.; Alrawiliy, S.R.; Alshammari, R.M.; Qasim, S. Skin anti-aging potential of Launaea procumbens extract: Antioxidant and enzyme inhibition activities supported by ADMET and molecular docking studies. Saudi Pharm. J. 2024, 32, 102107. [Google Scholar] [CrossRef]
- Apaza-Ticona, L.; Sánchez Sánchez-Corral, J.; Díaz-Guerra Martín, C.; Calderón Jiménez, S.; López-González, A.; Thiebaut -Estrada, C. Rubus urticifolius compounds with antioxidant activity, and inhibition potential against tyrosinase, melanin, hyaluronidase, elastase, and collagenase. Pharmaceuticals 2024, 17, 937. [Google Scholar] [CrossRef]
- Senol-Deniz, F.S.; Orhan, I.E.; Duman, H. Profiling cosmeceutical effects of various herbal extracts through elastase, collagenase, tyrosinase inhibitory and antioxidant assays. Phytochem. Lett. 2021, 45, 171–183. [Google Scholar] [CrossRef]
- Vigneshwaran, L.V.; Azmin Famnaz, A.H.; Anseera, F.; Nasreen, S.; Yusra Hameed, K.A.; Ajith Babu, T.K.; Sebastian, V. An overview on various formulation efforts of phytoconstituent emulgel. Int. J. Adv. Multidiscip. Res. 2024, 7, 4. [Google Scholar]
- Anasuri, S.; Sriram, P.; Singh, A.; Singh, G.; Suttee, A. A review on the development and evaluation of plant based emulgel formulations. Int. J. Res. Anal. Rev. 2019, 6, 678–684. [Google Scholar]
- Sureshkumar, J.; Amalraj, S.; Murugan, R.; Tamilselvan, A.; Krupa, J.; Sriramavaratharajan, V.; Gurav, S.S.; Ayyanar, M. Chemical profiling and antioxidant activity of Equisetum ramosissimum Desf. stem extract, a potential traditional medicinal plant for urinary tract infections. Futur. J. Pharm. Sci. 2021, 7, 192. [Google Scholar] [CrossRef]
- Boeing, T.; Tafarelo-Moreno, K.G.; Gasparotto Junior, A.; Mota Da Silva, L.; De Souza, P. Phytochemistry and pharmacology of the genus Equisetum (Equisetaceae): A narrative review of the species with therapeutic potential for kidney diseases. Evid.-Based Complement. Altern. Med. 2021, 2021, 6658434. [Google Scholar] [CrossRef]
- Jarić, S.; Kostić, O.; Mataruga, Z.; Pavlović, D.; Pavlović, M.; Mitrović, M.; Pavlović, P. Traditional wound-healing plants used in the Balkan region (Southeast Europe). J. Ethnopharmacol. 2018, 211, 311–328. [Google Scholar] [CrossRef]
- Kokten, K.; Ozel, H.B.; Yazici, H.; Iskil, R.; Kaya, Z. Fatty Acid compositions of different Equisetum species. Chem. Nat. Compd. 2020, 56, 1117–1119. [Google Scholar] [CrossRef]
- Barak, T.H.; Kurt-Celep, İ.; Celep, E. Bioaccessibility and Functional Food Potential of Equisetum telmateia Ehrh. Against Diabetes-Induced Kidney Disorders. Foods 2024, 13, 4092. [Google Scholar] [CrossRef]
- Yeganegi, M.; Tabatabaei-Yazdi, F.; Mortazavi, S.A.; Asili, J.; Alizadeh Behbahani, B.; Beigbabaei, A. Equisetum telmateia extracts: Chemical compositions, antioxidant activity and antimicrobial effect on the growth of some pathogenic strain causing poisoning and infection. Microb. Pathog. 2018, 116, 62–67. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.M.; Ćetković, G.S.; Djilas, S.M.; Tumbas, V.T.; Savatović, S.S.; Mandić, A.I.; Markov, S.L.; Cvetković, D.D. Radical scavenging and antimicrobial activity of horsetail (Equisetum arvense L.) extracts. Int. J. Food Sci. Technol. 2009, 44, 269–278. [Google Scholar] [CrossRef]
- Taşkın, T.; Yılmaz, B.N.; Doğan, A. Antioxidant, enzyme inhibitory and calcium oxalate anti-crystallization activities of Equisetum telmateia Ehrn. Int. J. Second. Metab. 2020, 7, 181–191. [Google Scholar] [CrossRef]
- Nunes, R.; Pasko, P.; Tyszka-Czochara, M.; Szewczyk, A.; Szlosarczyk, M.; Carvalho, I.S. Antibacterial, antioxidant and anti-proliferative properties and zinc content of five south Portugal herbs. Pharm. Biol. 2017, 55, 114–123. [Google Scholar] [CrossRef]
- Milovanović, V.; Radulović, N.; Todorović, Z.; Stanković, M.; Stojanović, G. Antioxidant, antimicrobial and genotoxicity screening of hydro-alcoholic extracts of five Serbian Equisetum. Plant Foods Hum. Nutr. 2007, 62, 113–119. [Google Scholar] [CrossRef]
- Francescato, L.N.; Debenedetti, S.L.; Schwanz, T.G.; Bassani, V.L.; Henriques, A.T. Identification of phenolic compounds in Equisetum giganteum by LC-ESI-MS/MS and a new approach to total flavonoid quantification. Talanta 2013, 105, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Mimica-Dukic, N.; Simin, N.; Cvejic, J.; Jovin, E.; Orcic, D.; Bozin, B. Phenolic compounds in field horsetail (Equisetum arvense L.) as natural antioxidants. Molecules 2008, 13, 1455–1464. [Google Scholar] [CrossRef]
- Marin, D.B.; Cioanca, O.; Apostu, M.; Tuchilus, G.; Mircea, C.; Robu, S.; Tutunaru, D.; Corciova, A.; Hancıanu, M. The Comparative Study of Equisetum pratense, E. sylvaticum, E. telmateia: Accumulation of silicon, antioxidant and antimicrobial screening. Rev. Chim. 2019, 70, 2519–2523. [Google Scholar] [CrossRef]
- Roumil, I.; Cheniti, W.; Baghiani, A.; Charef, N.; Arrar, L. HPLC analysis, acute toxicity and assessment of antioxidant and anti-inflammatory capacity of different extracts of Equisetum arvense. South Asian J. Exp. Biol. 2022, 12, 318–326. [Google Scholar] [CrossRef]
- Barak, T.H.; Celep, E.; İnan, Y.; Yeşilada, E. In vitro human digestion simulation of the bioavailability and antioxidant activity of phenolics from Sambucus ebulus L. fruit extracts. Food Biosci. 2020, 37, 100711. [Google Scholar] [CrossRef]
- Cho, J.; Jung, H.; Kang, D.Y.; Sp, N.; Shin, W.; Lee, J.; Bae, S.W. The skin-whitening and antioxidant effects of Protocatechuic Acid (PCA) derivatives in melanoma and fibroblast cell lines. Curr. Issues Mol. Biol. 2023, 45, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin aging properties of protocatechuic acid In vitro and In Vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Han, W.; Mai, W.; Wang, L.; Li, Q.; Lin, M.; Bai, M.; Zhang, L.; Chen, D. Antioxidant ability and mechanism of rhizoma atractylodes macrocephala. Molecules 2012, 17, 13457–13472. [Google Scholar] [CrossRef] [PubMed]
- Thi Nguyen, B.-L.; Palmieri, C.; Thi Nguyen, K.-T.; LE, D.H.; Ngo, T.T.; Tran, C.K.; Nguyen, H.Q.; Pham, H.T.; Muller, M.; Duez, P.; et al. HPTLC Fingerprinting and Cytotoxicity of Secondary Metabolites of Equisetum diffusum D. Don Extracts. Int. J. Plant Anim. Environ. Sci. 2021, 11, 596–613. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS profiling, anti-collagenase, anti-elastase, anti-tyrosinase and anti-hyaluronidase activities of a Stenocarpus sinuatus Leaves extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef]
- Talat, M.; Zaman, M.; Khan, R.; Jamshaid, M.; Akhtar, M.; Mirza, A.Z. Emulgel: An effective drug delivery system. Drug Dev. Ind. Pharm. 2021, 47, 1193–1199. [Google Scholar] [CrossRef]
- Zaka, H.S.; Khan, H.M.S.; Akhtar, N.; Khurshid, U.; Al Hagbani, T.; Saleem, H. Biotechnological exploration of Carthamus tinctorius as promising approach for cosmeceuticals: Phytochemical, biological, and micro-emulgel formulation development studies. S. Afr. J. Bot. 2022, 151, 735–742. [Google Scholar] [CrossRef]
- Kola-Mustapha, A.T.; Taiwo, S.O.; Isiaka, A.R.; Amao, S.O.; Ishola, I.O.; Ghazali, Y.O.; Usman, S.O. Evaluation of Terminalia macroptera (Combretaceae) Guill. & Perr stem bark extract incorporated into an emulgel for the potential management of rheumatoid arthritis. Sci. Afr. 2023, 19, e01557. [Google Scholar] [CrossRef]
- Kola-Mustapha, A.T.; Abdulrahman, M.O.; Ishola, I.O. Arctium lappa root extract based emulgels attenuate inducible cytokines and prostaglandins formation: Potential in the management of chronic inflammatory disorders. Sci. Afr. 2023, 22, e01942. [Google Scholar] [CrossRef]
- Şentürk, T.B.; Barak, T.H.; Çağlar, E.Ş.; Nath, E.Ö.; Özdemir, Z.Ö. In vitro evaluation of skin related enzyme inhibitory effect and emulgel formulation development studies of Onobrychis argyrea subsp. argyrea with phytochemical analysis. Chem. Biodivers. 2024, 21, e202400139. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Deleebeeck, L.; Snedden, A.; Nagy, D.; Szilágyi Nagyné, Z.; Roziková, M.; Vičarová, M.; Heering, A.; Bastkowski, F.; Leito, I.; Quendera, R.; et al. Unified pH measurements of ethanol, methanol, and acetonitrile, and their mixtures with water. Sensors 2021, 21, 3935. [Google Scholar] [CrossRef]
- Fresno, M.J.C.; Ramírez, A.D.; Jiménez, M.M. Systematic study of the flow behaviour and mechanical properties of Carbopol ® Ultrez ™ 10 hydroalcoholic gels. Eur. J. Pharm. Biopharm. 2002, 54, 329–335. [Google Scholar] [CrossRef]
- Milanowski, B.; Wosicka-Frąckowiak, H.; Główka, E.; Sosnowska, M.; Woźny, S.; Stachowiak, F.; Wilkowski, D. Optimization and evaluation of the In vitro permeation parameters of topical products with non-steroidal anti-inflammatory drugs through Strat-M® membrane. Pharmaceutics 2021, 13, 1305. [Google Scholar] [CrossRef]
- Çağlar, E.Ş.; Güven, G.K.; Okur, N.Ü. Preparation and characterization of carbopol based hydrogels containing dexpanthenol. Ank. Univ. Eczaci. Fak. Derg. 2023, 47, 770–783. [Google Scholar] [CrossRef]
- Daood, N.M.; Jassim, Z.E.; Gareeb, M.M.; Zeki, H.I.B.A. Studying the effect of different gelling agent on the preparation and characterization of metronidazole as topical emulgel. Asian J. Pharm. Clin. Res. 2019, 12, 571–577. [Google Scholar] [CrossRef]
- Jones, D.S.; Bruschi, M.L.; de Freitas, O.; Gremião, M.P.D.; Lara, E.H.G.; Andrews, G.P. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int. J. Pharm. 2009, 372, 49–58. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Lochte, F.; Echeverria, C.; Pereira, L.C.J.; Coutinho, J.T.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P.M.R. Thermal and magnetic properties of iron oxide colloids: Influence of surfactants. Nanotechnology 2015, 26, 425704. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; Ferreira, S.B.D.S.; Reis, A.V.; Cook, M.T.; Bruschi, M.L. Assessing mucoadhesion in polymer gels: The effect of method type and instrument variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef]
- Santos, L.S.; Alves Filho, E.G.; Ribeiro, P.R.V. Chemotaxonomic evaluation of different species from the Myrtaceae family by UPLC-qToF/MS-MS coupled to supervised classification based on genus. Biochem. Syst. Ecol. 2020, 90, 104028. [Google Scholar] [CrossRef]
- Pérez-Zamora, C.M.; Michaluk, A.G.; Torres, C.A.; Mouriño, V.; Chiappetta, D.A.; Nuñez, M.B. Influence of herbal extracts in physicochemical properties and stability of antibacterial gels. J. Adv. Pharm. Educ. Res. 2023, 13, 16–24. [Google Scholar] [CrossRef]
- Tomić, I.; Miočić, S.; Pepić, I.; Šimić, D.; Filipović-Grčić, J. Efficacy and safety of azelaic acid nanocrystal-loaded in situ hydrogel in the treatment of acne vulgaris. Pharmaceutics 2021, 13, 567. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Çağlar, E.Ş.; Sipahi, H.; Charehsaz, M.; Aydın, A.; Üstündağ-Okur, N. Ocular microemulsion of brinzolamide: Formulation, physicochemical characterization, and In vitro irritation studies based on EpiOcularTM eye irritation assay. Pharm. Dev. Technol. 2021, 26, 765–778. [Google Scholar] [CrossRef]
- Pukale, S.; Pandya, A.; Patravale, V. Synthesis, characterization and topical application of novel bifunctional peptide metallodendrimer. J. Drug Deliv. Sci. Technol. 2021, 66, 102925. [Google Scholar] [CrossRef]
- Paolino, D.; Tudose, A.; Celia, C.; Di Marzio, L.; Cilurzo, F.; Mircioiu, C. Mathematical models as tools to predict the release kinetic of fluorescein from lyotropic colloidal liquid crystals. Materials 2019, 12, 693. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. [Google Scholar]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Madhu, A.; Venkatraman, P.D. Advances in encapsulation of organic compounds for biological protective textiles. In Advances in Healthcare and Protective Textiles; Elsevier: Amsterdam, The Netherlands, 2023; pp. 509–534. [Google Scholar]
- Lee, J.; Lee, Y.; Kim, J.; Yoon, M.; Choi, Y.W. Formulation of microemulsion systems for transdermal delivery of aceclofenac. Arch. Pharm. Res. 2005, 28, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, H.; Wu, S.; Ju, B.; Zhu, D.; Yan, Y.; Wang, M.; Hu, J. Preparation and evaluation of microemulsion-based transdermal delivery of Cistanche tubulosa phenylethanoid glycosides. Mol. Med. Rep. 2017, 15, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Fiqri, M.; Athiyyah, U.; Layadi, P.; Fadjar, T.G.A.; Permana, A.D. Enhanced localization of cefazoline sodium in the ocular tissue using thermosensitive-mucoadhesive hydrogels: Formulation development, hemocompatibility and in vivo irritation studies. J. Drug Deliv. Sci. Technol. 2022, 76, 103763. [Google Scholar] [CrossRef]
- Çağlar, E.Ş.; Yoltaş, A.; Özhan, Y.; Sipahi, H.; Aydın, A.; Üstündağ Okur, N.; Siafaka, P. Mucoadhesive electrospun nanofibrous poly (ε-caprolactone)/poly (lactic acid) matrices for the ocular delivery of moxifloxacin: A novel application of hyaluronic acid and xanthan gum blend as mucoadhesive coating agent. Int. J. Polym. Mater. 2025, 74, 361–376. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Ramanauskiene, K.; Kurapkiene, V.; Janulis, V. Dermal penetration studies of potential phenolic compounds ex vivo and their antioxidant activity In vitro. Plants 2022, 11, 1901. [Google Scholar] [CrossRef]
- Jaffri, J.M. Reactive oxygen species and antioxidant system in selected skin disorders. Malays. J. Med. Sci. 2023, 30, 7–20. [Google Scholar] [CrossRef]
- Baran, Ş.; Alazzawi, S.; Semerci, A.B. The effects of Equisetum arvense L. extracts prepared using different solvents and extraction methods for antioxidant and antimicrobial activity. Food Health 2024, 10, 1–11. [Google Scholar] [CrossRef]
- Radojević, I.D.; Stanković, M.S.; Stefanović, O.D.; Topuzović, M.D.; Čomić, L.R.; Ostojić, A.M. Great horsetail (Equisetum telmateia Ehrh.): Active substances content and biological effects. EXCLI J. 2012, 11, 59–67. [Google Scholar] [PubMed]
- Necip, A.; Işık, M. Bioactivities of Hypericum perforatum L. and Equisetum arvense L. fractions obtained with different solvents. Int. J. Life Sci. Biotechnol. 2019, 2, 221–230. [Google Scholar] [CrossRef]
- Dormousoglou, M.; Efthimiou, I.; Antonopoulou, M.; Fetzer, D.L.; Hamerski, F.; Corazza, M.L.; Papadaki, M.; Santzouk, S.; Dailianis, S.; Vlastos, D. Investigation of the genotoxic, antigenotoxic and antioxidant profile of different extracts from Equisetum arvense L. Antioxidants 2022, 11, 1393. [Google Scholar] [CrossRef] [PubMed]
- Hacibekiroǧlu, I.; Kolak, U. Antioxidant and anticholinesterase constituents from the petroleum ether and chloroform extracts of Iris suaveolens. Phyther Res. 2011, 25, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Nosrati Gazafroudi, K.; Mailänder, L.K.; Daniels, R.; Kammerer, D.R.; Stintzing, F.C. From stem to spectrum: Phytochemical characterization of five Equisetum species and evaluation of their antioxidant potential. Molecules 2024, 29, 2821. [Google Scholar] [CrossRef]
- Chompoo, J.; Upadhyay, A.; Fukuta, M.; Tawata, S. Effect of Alpinia zerumbet components on antioxidant and skin diseases-related enzymes. BMC Complement. Altern. Med. 2012, 12, 106. [Google Scholar] [CrossRef]
- Bose, B.; Choudhury, H.; Tandon, P.; Kumaria, S. Studies on secondary metabolite profiling, anti-inflammatory potential, In vitro photoprotective and skin-aging related enzyme inhibitory activities of Malaxis acuminata, a threatened orchid of nutraceutical importance. J. Photochem. Photobiol. B Biol. 2017, 173, 686–695. [Google Scholar] [CrossRef]
- Pintus, F.; Floris, S.; Fais, A.; Era, B.; Kumar, A.; Gatto, G.; Uriarte, E.; Matos, M.J. Hydroxy-3-phenylcoumarins as multitarget compounds for skin aging diseases: Synthesis, molecular docking and tyrosinase, elastase, collagenase and hyaluronidase inhibition, and sun protection factor. Molecules 2022, 27, 6914. [Google Scholar] [CrossRef]
- An, B.J.; Kwak, J.H.; Park, J.M.; Lee, J.; Park, T.; Son, J.; Jo, C.; Byun, M. Inhibition of enzyme activities and the antiwrinkle effect of polyphenol isolated from the persimmon leaf (Diospyros kaki folium) on human skin. Dermatol. Surg. 2005, 31, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Liceaga, A.M. Identification of chia seed (Salvia hispanica L.) peptides with enzyme inhibition activity towards skin-aging enzymes. Amino Acids 2020, 52, 1149–1159. [Google Scholar] [CrossRef]
- Abed-Savaya, N.S.; Issa, R.A.; Talib, W.H. In vitro evaluation of the antioxidant, anti-Propioni bacterium acne and antityrosinase effects of Equisetum ramosissimum (Jordanian horsetail). Trop. J. Pharm. Res. 2020, 19, 2147–2152. [Google Scholar] [CrossRef]
- Li, P.H.; Chiu, Y.P.; Shih, C.C.; Wen, Z.-H.; Ibeto, L.K.; Huang, S.-H.; Chiu, C.C.; Ma, D.-L.; Leung, C.-H.; Chang, Y.-N.; et al. Biofunctional activities of Equisetum ramosissimum extract: Protective effects against oxidation, melanoma, and melanogenesis. Oxid. Med. Cell Longev. 2016, 2016, 2853543. [Google Scholar] [CrossRef]
- Birgül, B. Determination of Antienzyme and Antioxidant Activities of Echinops Borae and Echinops Pungens Species. Master’s Thesis, Kayseri University, Kayseri, Turkey, 2023. [Google Scholar]
- Ibrahim, N.; Abbas, H.; El-Sayed, N.S.; Gad, H.A. Rosmarinus officinalis L. hexane extract: Phytochemical analysis, nanoencapsulation, and In Silico, In vitro and In Vivo anti-photoaging potential evaluation. Sci. Rep. 2022, 12, 13102. [Google Scholar] [CrossRef]
- Yilmaz, M.A. Simultaneous quantitative screening of 53 phytochemicals in 33 species of medicinal and aromatic plants: A detailed, robust and comprehensive LC–MS/MS method validation. Ind. Crops Prod. 2020, 149, 112347. [Google Scholar] [CrossRef]
- Bardakci, H.; Barak, T.H.; Özdemir, K.; Celep, E. Effect of brewing material and various additives on polyphenolic composition and antioxidant bioactivity of commercial Tilia platyphyllos Scop. infusions. J. Pharm. Res. 2020, 24, 1–9. [Google Scholar] [CrossRef]
- Hua, S. Comparison of In vitro dialysis release methods of loperamide-encapsulated liposomal gel for topical drug delivery. Int. J. Nanomed. 2014, 9, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Abdelbary, G. Ocular ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes. Pharm. Dev. Technol. 2011, 16, 44–56. [Google Scholar] [CrossRef]
- Barak, T.H.; Bardakcı, H.; Kurt-Celep, İ.; Özdemir, K.; Celep, E. Evaluation of the influence of In vitro human digestion simulation on the chemical composition and bioactivities of Ziziphus jujuba Mill. Acta Aliment. 2022, 51, 105–114. [Google Scholar] [CrossRef]
- Barak, T.H.; Kurt-Celep, İ.; Şentürk, T.B.; Bardakci, H.; Celep, E. In vitro Anti-aging potential evaluation of Maclura pomifera (Rafin.) Schneider 80% methanol extract with quantitative HPTLC analysis. Turk. J. Pharm. Sci. 2022, 19, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Barak, T.H.; Kurt-Celep, I.; Dilek-Tepe, H.; Bardakcı, H.; Akaydın, G.; Yesilada, E.; Celep, E. In vitro assessment of dermatological activity potential of Achillea clypeolata Sm. in H2O2-treated human dermal fibroblasts. S. Afr. J. Bot. 2023, 160, 1–8. [Google Scholar] [CrossRef]
- Ersoy, E.; Ozkan, E.E.; Boga, M.; Yilmaz, M.A.; Mat, A. Anti-aging potential and anti-tyrosinase activity of three Hypericum species with focus on phytochemical composition by LC–MS/MS. Ind. Crops Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Okur, N.Ü.; Apaydın, Ş.; Yavaşoğlu, N.Ü.K.; Yavaşoğlu, A.; Karasulu, H.Y. Evaluation of skin permeation and anti-inflammatory and analgesic effects of new naproxen microemulsion formulations. Int. J. Pharm. 2011, 416, 136–144. [Google Scholar] [CrossRef] [PubMed]
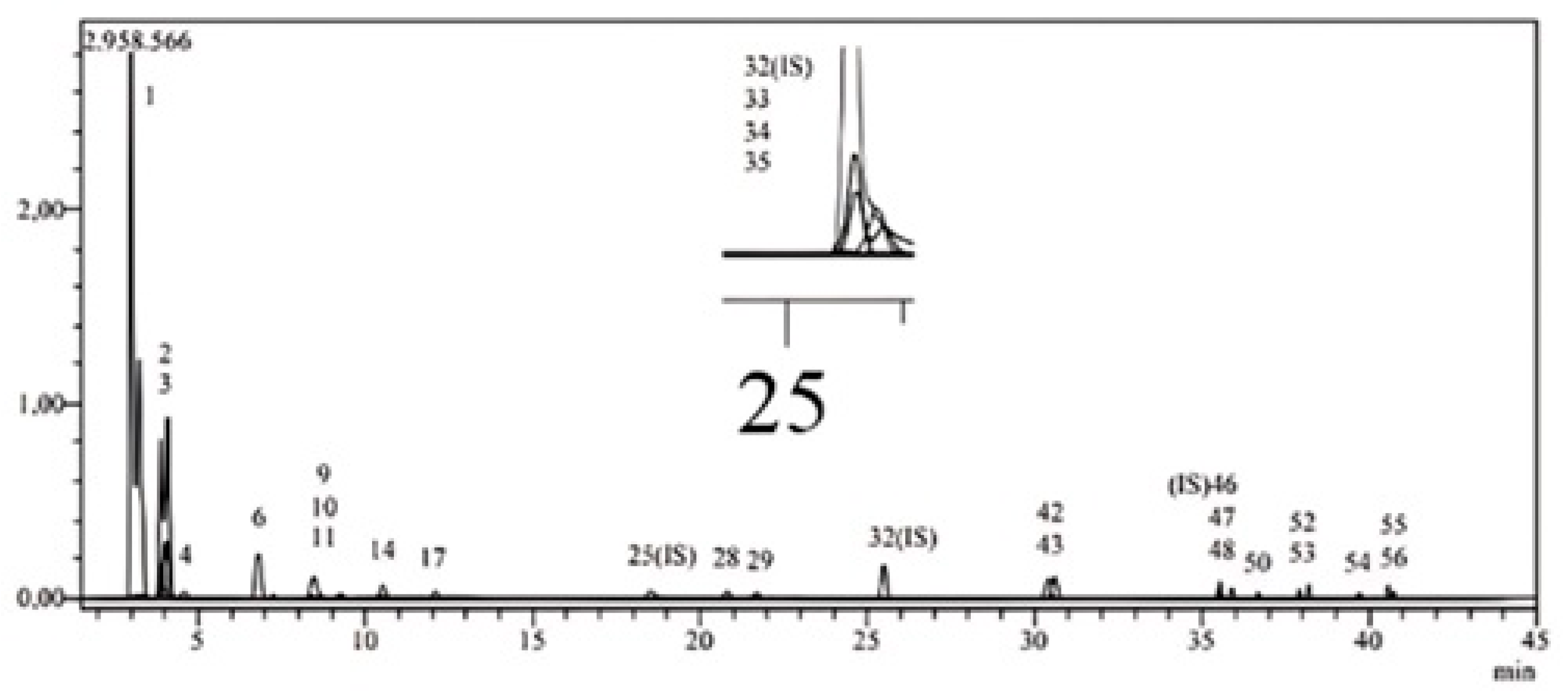


| Analytes | R.T. a | M.I.s (m/z) b | F.I.s (m/z) c | Ion. Mode | Quantification (μg/g) | |
|---|---|---|---|---|---|---|
| 1 | Quinic acid | 3.0 | 190.8 | 93.0 | Neg | 1.086 |
| 2 | Fumaric aid | 3.9 | 115.2 | 40.9 | Neg | 0.963 |
| 3 | Equisetic acid | 4.0 | 172.8 | 129.0 | Neg | 7.865 |
| 4 | Gallic acid | 4.4 | 168.8 | 79.0 | Neg | 0.091 |
| 5 | Epigallocatechin | 6.7 | 304.8 | 219.0 | Neg | ----- |
| 6 | Protocatechuic acid | 6.8 | 152.8 | 108.0 | Neg | 8.503 |
| 7 | Catechin | 7.4 | 288.8 | 203.1 | Neg | ----- |
| 8 | Gentisic acid | 8.3 | 152.8 | 109.0 | Neg | ----- |
| 9 | Chlorogenic acid | 8.4 | 353.0 | 85.0 | Neg | 0.013 |
| 10 | Protocatechuic aldehyde | 8.5 | 137.2 | 92.0 | Neg | 2.607 |
| 11 | Tannic acid | 9.2 | 182.8 | 78.0 | Neg | 0.199 |
| 12 | Epigallocatechin gallate | 9.4 | 457.0 | 305.1 | Neg | ----- |
| 13 | Cynarin | 9.8 | 515.0 | 191.0 | Neg | ----- |
| 14 | 4-OH Benzoic acid | 10.5 | 137,2 | 65.0 | Neg | 0.602 |
| 15 | Epicatechin | 11.6 | 289.0 | 203.0 | Neg | ----- |
| 16 | Vanilic acid | 11.8 | 166.8 | 108.0 | Neg | ----- |
| 17 | Caffeic acid | 12.1 | 179.0 | 134.0 | Neg | 0.149 |
| 18 | Syringic acid | 12.6 | 196.8 | 166.9 | Neg | ----- |
| 19 | Vanillin | 13.9 | 153.1 | 125.0 | Poz | ----- |
| 20 | Syringic aldehyde | 14.6 | 181.0 | 151.1 | Neg | ----- |
| 21 | Daidzin | 15.2 | 417.1 | 199.0 | Poz | ----- |
| 22 | Epicatechin gallate | 15.5 | 441.0 | 289.0 | Neg | ----- |
| 23 | Piceid | 17.2 | 391.0 | 135/106.9 | Poz | ----- |
| 24 | p-Coumaric acid | 17.8 | 163.0 | 93.0 | Neg | ----- |
| 25 | Ferulic acid-D3-IS d | 18.8 | 196.2 | 152.1 | Neg | N.A. |
| 26 | Ferulic acid | 18.8 | 192.8 | 149.0 | Neg | ----- |
| 27 | Sinapic acid | 18.9 | 222.8 | 193.0 | Neg | ----- |
| 28 | Coumarin | 20.9 | 146.9 | 103.1 | Poz | 0.034 |
| 29 | Salicylic acid | 21.8 | 137.2 | 65.0 | Neg | 0.028 |
| 30 | Luteolin 7-O-glucoside | 23.7 | 447.0 | 284.0 | Neg | ----- |
| 31 | Miquelianin | 24.1 | 477.0 | 150.9 | Neg | ----- |
| 32 | Rutin-D3-IS | 25.5 | 612.2 | 304.1 | Neg | N.A. |
| 33 | Rutin | 25.6 | 608.9 | 301.0 | Neg | 0.076 |
| 34 | Isoquercetin | 25.6 | 463.0 | 271.0 | Neg | 0.097 |
| 35 | Hesperidin | 25.8 | 611.2 | 449.0 | Poz | 0.025 |
| 36 | o-Coumaric acid | 26.1 | 162.8 | 93.0 | Neg | ----- |
| 37 | Genistin | 26.3 | 431.0 | 239.0 | Neg | ----- |
| 38 | Rosmarinic acid | 26.6 | 359.0 | 197.0 | Neg | ----- |
| 39 | Ellagic acid | 27.6 | 301.0 | 284.0 | Neg | ----- |
| 40 | Apigenin 7-glucoside | 28.2 | 431.0 | 269.0 | Neg | 0.035 |
| 41 | Quercitrin | 29.8 | 447.0 | 301.0 | Neg | ----- |
| 42 | Astragalin | 30.4 | 447.0 | 255.0 | Neg | 4.764 |
| 43 | Nicotiflorin | 30.6 | 592.9 | 255.0/284.0 | Neg | 5.334 |
| 44 | Fisetin | 30.6 | 285.0 | 163.0 | Neg | ----- |
| 45 | Daidzein | 34.0 | 253.0 | 223.0 | Neg | ----- |
| 46 | Quercetin-D3-IS | 35.6 | 304.0 | 275.9 | Neg | N.A. |
| 47 | Quercetin | 35.7 | 301.0 | 272.9 | Neg | 0.059 |
| 48 | Naringenin | 35.9 | 270.9 | 119.0 | Neg | 0.214 |
| 49 | Hesperetin | 36.7 | 301.0 | 136.0/286.0 | Neg | ----- |
| 50 | Luteolin | 36.7 | 284.8 | 151.0/175.0 | Neg | 0.012 |
| 51 | Genistein | 36.9 | 269.0 | 135.0 | Neg | ----- |
| 52 | Kaempferol | 37.9 | 285.0 | 239.0 | Neg | 0.861 |
| 53 | Apigenin | 38.2 | 268.8 | 151.0/149.0 | Neg | 0.045 |
| 54 | Amentoflavone | 39.7 | 537.0 | 417.0 | Neg | 0.005 |
| 55 | Chrysin | 40.5 | 252.8 | 145.0/119.0 | Neg | 0.177 |
| 56 | Acacetin | 40.7 | 283.0 | 239.0 | Neg | 0.038 |
| Compound | ETM | ETH | ETC | ETE | ETB | ETW |
|---|---|---|---|---|---|---|
| Protocatechuic acid | 8.49 ± 0.15 | nd | nd | 137.4 ± 1.99 | 31.95 ± 1.44 | nd |
| Formulation/Characterization | F * | ETF |
|---|---|---|
| pH | 5.4 ± 0.1 | 6.5 ± 0.1 |
| Viscosity (P) | 24.227 ± 0.228 | 6.440 ± 0.080 |
| Drug Content (%) | - | 77.19 ± 2.07 |
| Formulation | Hardness (g) ± SS | Adhesiveness (g·s) ± SS | Elasticity ± SS | Cohesiveness ± SS |
|---|---|---|---|---|
| F | −3.345 ± 0.423 | −90.678 ± 0.391 | 0.235 ± 0.053 | 0.855 ± 0.139 |
| ETF | −3.699 ± 0.273 | −58.000 ± 1.665 | 0.143 ± 0.004 | 0.903 ± 0.058 |
| Formulation | Firmness (g) | Work of Shear (g·s) | Stickiness (g) | Work of Adhesion (g·s) |
|---|---|---|---|---|
| F | 1591.67 ± 6.01 | 1657.73 ± 32.20 | −1287.72 ± 14.37 | −214.75 ± 12.33 |
| ETF | 1205.36 ± 2.93 | 1078.68 ± 15.75 | −964.46 ± 10.19 | −257.98 ± 19.48 |
| Formulations | ETS | ETF | ||||
|---|---|---|---|---|---|---|
| Models | r2 | m | n | r2 | m | n |
| Zero-order | 0.305 | 1.4186 | 80.407 | 0.748 | 4.1964 | 26.354 |
| First-order | 0.278 | 0.0079 | 1.8905 | 0.676 | 0.0344 | 1.4209 |
| Hixson–Crowell | 0.288 | −0.0257 | −0.293 | 0.713 | −0.0969 | 0.9114 |
| Higuchi | 0.510 | 10.239 | 66.187 | 0.865 | 25.194 | −3.662 |
| Korsmeyer–Peppas | 0.840 | 2.4873 | 0.992 | 2.0815 | ||
| Method of Release | Reference Formulation | Experimental Formulation | f1 | f2 |
|---|---|---|---|---|
| Dialysis bag | ETS | ETF | 66 | 15 |
| ETF | ETS | 193 | 15 |
| ETM * | ETH * | ETC * | ETE * | ETB * | ETW * | ETF | |
|---|---|---|---|---|---|---|---|
| DPPH 1 | 2639 ± 9 a | 1812 ± 20 b | 1949 ± 42 c | 2654 ± 4 a | 2684 ± 7 a | 2570 ± 23 d | 1124 ± 27 e |
| FRAP 2 | 1.37 ± 0.06 a | 0.67 ± 0.02 b | 0.60 ± 0.29 b | 2.40 ± 0.18 c | 3.02 ± 0.40 d | 1.02 ± 0.09 ab | 1.17 ± 0.16 ab |
| CUPRAC 3 | 214.64 ± 10.73 a | 122.75 ± 9.95 b | 161.42 ± 8.66 cf | 428.88 ± 12.66 d | 321.28 ± 0.38 e | 147.03 ± 8.60 bc | 184.31 ± 2.91 f |
| TOAC 3 | 158.09 ± 9.81 a | 173.60 ± 8.77 a | 218.22 ± 3.41 b | 316.91 ± 13.89 c | 265.60 ± 8.39 d | 157.07 ± 10.84 a | 173.41 ± 2.23 a |
| Formulation Code | Jss (mg·cm−2·h−1) | Kp × 10−4 (cm/h) | Qn (mg·cm−2) | Skin Retention (mg·cm−2) |
|---|---|---|---|---|
| ETS | 1.1040 ± 0.1520 | 347.0 ± 48.0 | 20.17 ± 3.66 | 1.28 ± 0.47 |
| ETF | 0.1502 ± 0.0392 | 47.0 ± 14.0 | 5.16 ± 3.74 | 3.06 ± 0.21 |
| 4 ± 2 °C | |||
| Time (Month) | Content Quantification (%) | pH | Viscosity (P) |
| 0 | 100.00 ± 2.68 | 6.50 ± 0.10 | 6.440 ± 0.080 |
| 12 | 92.63 ± 8.44 | 6.33 ± 0.01 | 6.493 ± 23.09 |
| 25 ± 2 °C | |||
| Time (Month) | Content Quantification (%) | pH | Viscosity (P) |
| 0 | 100.00 ± 2.68 | 6.50 ± 0.10 | 6.440 ± 0.080 |
| 12 | 79.53 ± 8.73 | 6.31 ± 0.01 | 6.466 ± 0.023 |
| 40 ± 2 °C | |||
| Time (Month) | Content Quantification (%) | pH | Viscosity (P) |
| 0 | 100.00 ± 2.68 | 6.50 ± 0.10 | 6.440 ± 0.080 |
| 12 | 72.14 ± 0.66 | 6.26 ± 0.02 | 6.400 ± 0.040 |
| Ingredients | Amount (%) |
|---|---|
| Oil Phase | |
| Oleic acid | 5 |
| Span 80 | 1.37 |
| Methyl paraben | 0.1 |
| Water Phase | |
| Glycerine | 1.8 |
| Ethyl alcohol | 3 |
| Tween 60 | 3.63 |
| Propyl paraben | 0.1 |
| Distilled water | q.s. 100 |
| Carbopol 934 | 2 |
| Plant extract | 4 |
| Triethanolamine | q.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şentürk, T.B.; Barak, T.H.; Çağlar, E.Ş.; Saldamlı, E.; Özdemir Nath, E.; Özdemir, Z.Ö. A Bioactive Emulgel Formulation of Equisetum telmateia Ehrh. Methanol Extract: Integrating Antioxidant Activity, Skin Enzyme Inhibition, and Permeation Kinetics. Gels 2025, 11, 662. https://doi.org/10.3390/gels11080662
Şentürk TB, Barak TH, Çağlar EŞ, Saldamlı E, Özdemir Nath E, Özdemir ZÖ. A Bioactive Emulgel Formulation of Equisetum telmateia Ehrh. Methanol Extract: Integrating Antioxidant Activity, Skin Enzyme Inhibition, and Permeation Kinetics. Gels. 2025; 11(8):662. https://doi.org/10.3390/gels11080662
Chicago/Turabian StyleŞentürk, Tuğba Buse, Timur Hakan Barak, Emre Şefik Çağlar, Emine Saldamlı, Ebru Özdemir Nath, and Zafer Ömer Özdemir. 2025. "A Bioactive Emulgel Formulation of Equisetum telmateia Ehrh. Methanol Extract: Integrating Antioxidant Activity, Skin Enzyme Inhibition, and Permeation Kinetics" Gels 11, no. 8: 662. https://doi.org/10.3390/gels11080662
APA StyleŞentürk, T. B., Barak, T. H., Çağlar, E. Ş., Saldamlı, E., Özdemir Nath, E., & Özdemir, Z. Ö. (2025). A Bioactive Emulgel Formulation of Equisetum telmateia Ehrh. Methanol Extract: Integrating Antioxidant Activity, Skin Enzyme Inhibition, and Permeation Kinetics. Gels, 11(8), 662. https://doi.org/10.3390/gels11080662








