Properties, Antioxidant and Antibacterial Activity of Southern Meagre Fish (Argyrosomus hololepidotus) Skin Gelatin Reinforced with Clove Bud Extract
Abstract
1. Introduction
2. Results and Discussion
2.1. Film Thickness and Water Solubility
2.2. Water Vapor Permeability
2.3. Light Transmission and Transparency
2.4. Microstructure of the Films
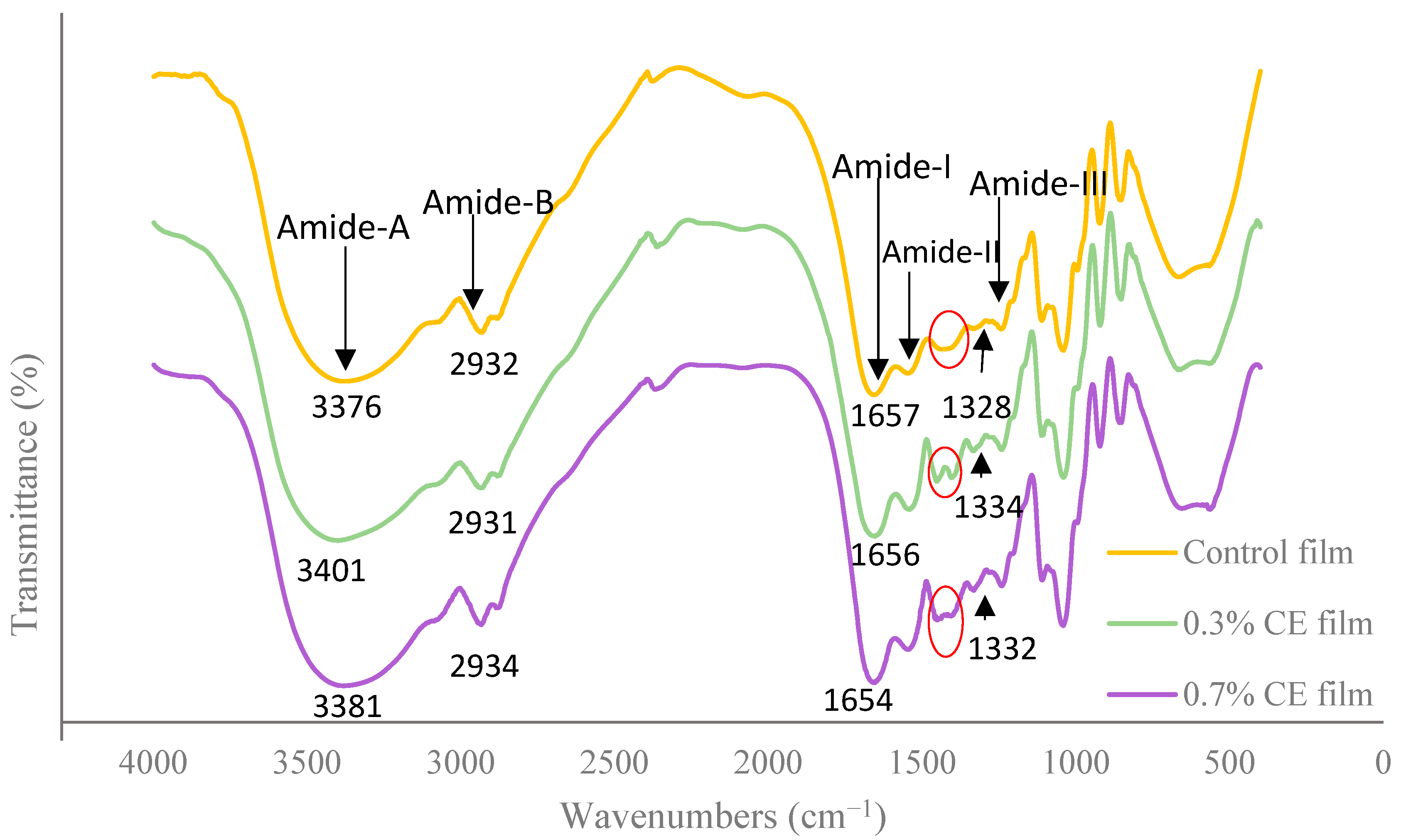
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
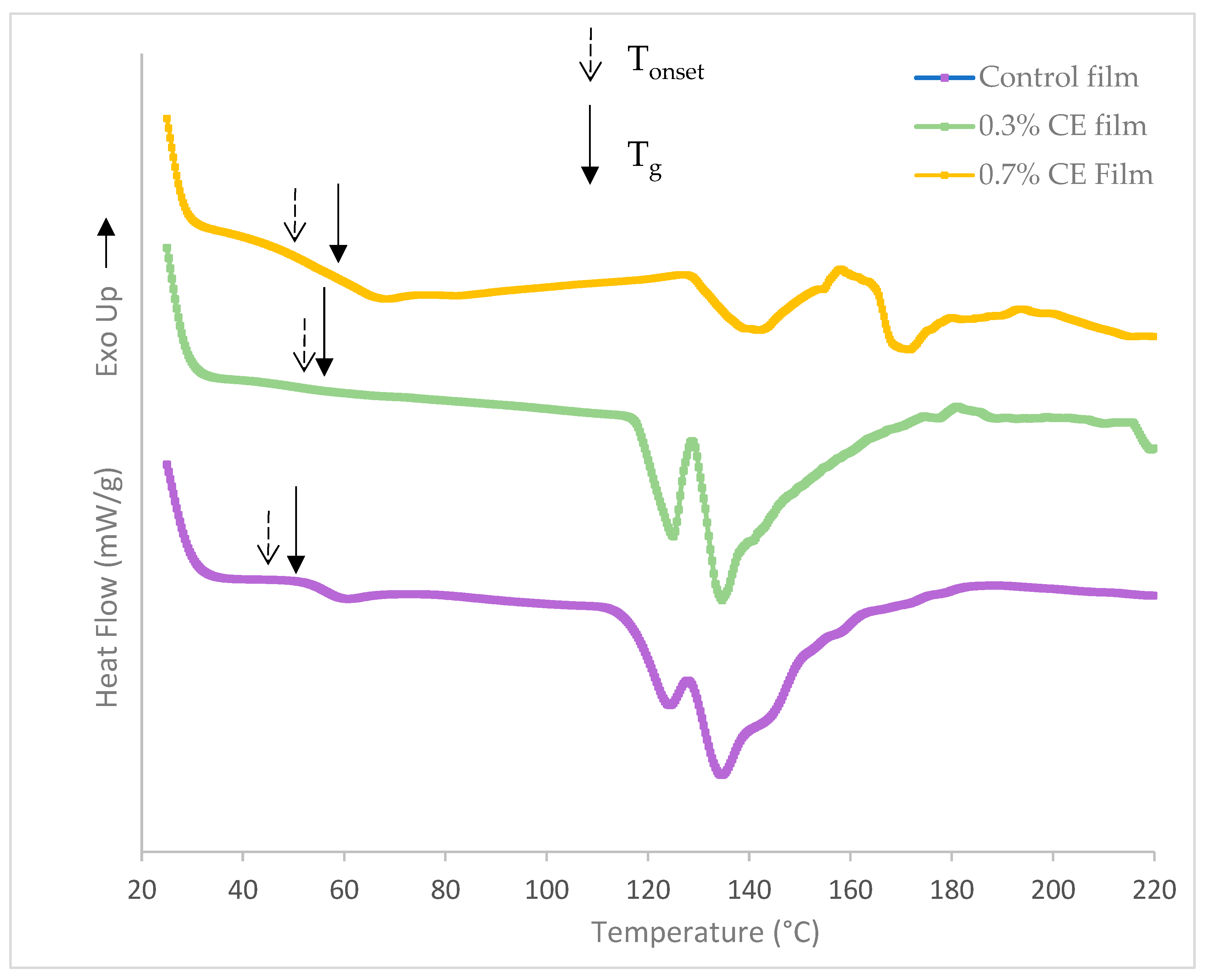
2.6. Differential Scanning Calorimetry
2.7. Bioactivity of the Films
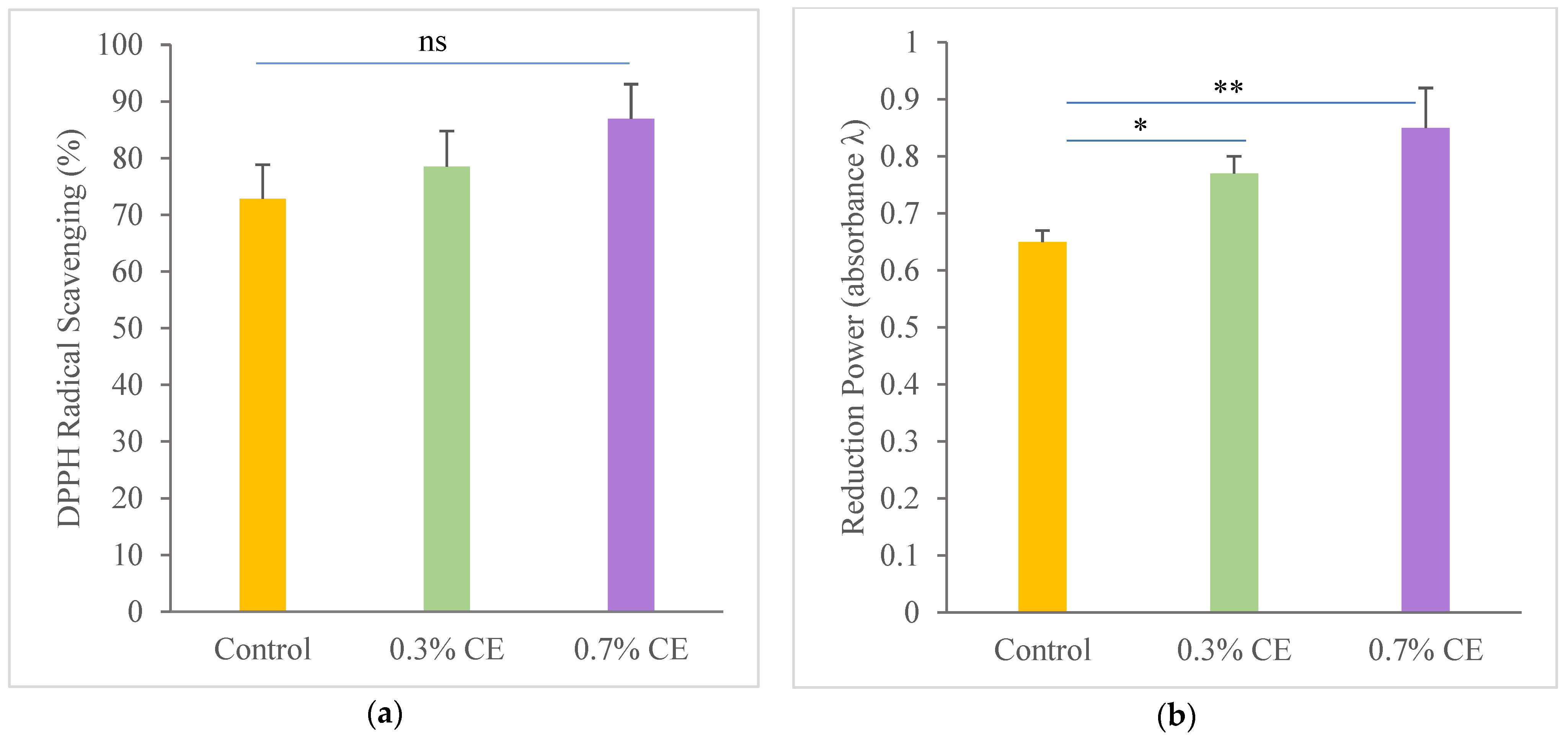
2.7.1. Antioxidant Properties of the Films
2.7.2. Antibacterial Properties of the Films
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fish Skin Preparation
4.3. Fish Skin Gelatin Preparation
4.4. Preparation of Clove Bud Extract (CE)
4.5. Film Preparation
4.6. Film Thickness
4.7. Solubility of the Film
4.8. Water Vapor Permeability (WVP)
4.9. Light Transmittance (T%) and Transparency Value
4.10. Scanning Electron Microscopy (SEM)
4.11. Fourier Transform Infrared (FTIR) Spectroscopy
4.12. Differential Scanning Calorimetry (DSC)
4.13. Bioactivity Assay
4.13.1. Antioxidative Effect of the Films
Film Preparation for Antioxidant Assay
DPPH Free Radical Scavenging Assay
Reduction Power of the Film
4.13.2. Antibacterial Effect
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babaremu, K.; Oladijo, O.P.; Akinlabi, E. Biopolymers: A suitable replacement for plastics in product packaging. Adv. Ind. Eng. Polym. Res. 2023, 6, 333–340. [Google Scholar] [CrossRef]
- Ran, C.; Li, Q.; Zhao, M.; Cui, H.; Yang, Y.; Diao, K.; Liu, Y.; Lu, S.; Dong, J.; Wang, Q. Gelatin/polyvinyl alcohol films loaded with doubly stabilized clove essential oil chitosomes: Preparation, characterization, and application in packing marinated steaks. Food Chem. 2024, 460, 140673. [Google Scholar] [CrossRef] [PubMed]
- Umaraw, P.; Munekata, P.E.; Verma, A.K.; Barba, F.J.; Singh, V.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Cruz-Galvez, A.M.; Gomez-Aldapa, C.A.; Falfan-Cortes, R.N.; Guzman-Ortiz, F.A.; Rodríguez-Marín, M.L. Biopolymer films and the effects of added lipids, nanoparticles and antimicrobials on their mechanical and barrier properties: A review. Int. J. Food Sci. Technol. 2016, 51, 1967–1978. [Google Scholar] [CrossRef]
- Diaz-Montes, E.; Castro-Munoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 10020249. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, A.Y.; Fang, D.L.; Lu, T.; Wang, J.Y.; Song, Y.X.; Li, W.L. Oregano essential oil/β-cyclodextrin inclusion compound polylactic acid/polycaprolactone electrospun nanofibers for active food packaging. Chem. Eng. J. 2022, 445, 136746. [Google Scholar] [CrossRef]
- Ramos, M.; Valdes, A.; Beltran, A.; Garrigos, M.C. Gelatin-based films and coatings for food packaging applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef]
- Yu, D.; Regenstein, J.M.; Xia, W. Bio-based edible coatings for the preservation of fishery products: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2481–2493. [Google Scholar] [CrossRef]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive characterization of active chitosan-gelatin blend films enriched with different essential oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Guo, B.; Liu, G.; Ye, W.; Xu, Z.; Li, W.; Zhuang, J.; Dong, H. Multifunctional carbon dots reinforced gelatin-based coating film for strawberry preservation. Food Hydrocoll. 2024, 147, 109327. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Kord, Z.; Taheri, A.; Ghaffari, M.; Sharifian, S. Incorporation of Prosopis cineraria extract improved the mechanical, barrier and antioxidant properties but not the antibacterial activity of Tigertooth croaker fish scale gelatin film. Foods 2024, 13, 538. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Fauzan, H.R.; Ningrum, A.; Supriyadi, S. Evaluation of a Fish Gelatin-Based Edible Film Incorporated with Ficus carica L. Leaf Extract as Active Packaging. Gels 2023, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, M.; Aleman, A.; Romani, V.P.; Lopez-Caballero, M.E.; Gomez-Guillen, M.C.; Montero, P.; Prenticea, C. Effects of agar films incorporated with fish protein hydrolysate or clove essential oil on flounder (Paralichthys orbignyanus) fillets shelf-life. Food Hydrocoll. 2018, 81, 351–363. [Google Scholar] [CrossRef]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Gandra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of salmon bone gelatin with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Jiang, J.; Watowita, P.S.M.S.L.; Chen, R.; Shi, Y.; Geng, J.T.; Takahashi, K.; Li, L.; Osako, K. Multilayer gelatin/myofibrillar films containing clove essential oil: Properties, protein-phenolic interactions, and migration of active compounds. Food Pack. Shelf Life 2022, 32, 100842. [Google Scholar] [CrossRef]
- Fathiraja, P.; Gopalrajan, S.; Kumar, K.; Obaiah, M.C. Augmentation of bioactivity with addition of clove essential oil into fish scale gelatin, agar and chitosan composite film and biodegradable features. Polymer Bullet. 2024, 81, 5329–5357. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT Food Sci. Technol. 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Khalil, W.A.; Atta, A.M.I.; El-Ratel, I.T.; Abdelnour, S.A.; Abdel-Khalek, E.; Fouda, S.F. Influence of nano-formulations of clove bud (Syzygium aromaticum) extract on freezing ability, antioxidant capacity, caspase-3 activity, acrosome reaction and fertility of frozen rabbit semen. Reprod. Dom. Anim. 2024, 59, e14511. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojas, D.F.; de Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Singletary, K. Clove: Overview of potential health benefits. Nutr. Today 2014, 49, 207–224. [Google Scholar] [CrossRef]
- Hemalatha, R.; Nivetha, P.; Mohanapriya, C.; Sharmila, G.; Muthukumaran, C.; Gopinath, M. Phytochemical composition, GC-MS analysis, in vitro antioxidant and antibacterial potential of clove flower bud (Eugenia caryophyllus) methanolic extract. J. Food Sci. Technol. 2016, 53, 1189–1198. [Google Scholar] [CrossRef]
- Lakhan, M.N.; Chen, R.; Shar, A.H.; Chand, K.; Shah, A.H.; Ahmed, M.; Ali, I.; Ahmed, R.; Liu, J.; Takahashi, K.; et al. Eco-friendly green synthesis of clove buds extract functionalized silver nanoparticles and evaluation of antibacterial and anti-diatom activity. J. Microbiol. Met. 2020, 173, 105934. [Google Scholar] [CrossRef]
- Indiarto, R.; Herwanto, J.A.; Filianty, F.; Lembong, F.; Subroto, E.; Muhammad, D.R.A. Total phenolic and flavonoid content, antioxidant activity and characteristics of a chocolate beverage incorporated with encapsulated clove bud extract. CyTA—J. Food 2024, 22, 2329144. [Google Scholar] [CrossRef]
- Vilas Dhumal, C.; Pal, K.; Sarkar, P. Synthesis, characterization, and antimicrobial efficacy of composite films from guar gum/sago starch/whey protein isolate loaded with carvacrol, citral and carvacrol-citral mixture. J. Mater. Sci Mater. Med. 2019, 30, 117. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Krisana, N. Emulsion Film Based on Fish Skin Gelatin and Palm Oil: Physical, Structural and Thermal Properties. Food Hydrocoll. 2015, 48, 248–259. [Google Scholar] [CrossRef]
- Mutlu, M. Physicochemical and antimicrobial properties of biodegradable films based on gelatin/guar gum incorporated with grape seed oil. J. Food Measur. Character. 2023, 17, 1515–1525. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Properties of film from cuttlefish (Sepia pharaonis) skin gelatin incorporated with cinnamon, clove and star anise extracts. Food Hydrocoll. 2011, 25, 1085–1097. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Nunes, J.C.; Sousa Melo, P.T.; Lorevice, M.V.; Aouada, F.A.; de Moura, M.R. Effect of green tea extract on gelatin-based films incorporated with lemon essential oil. J. Food Sci. Technol. 2021, 58, 1–8. [Google Scholar] [CrossRef]
- Fahrullah, F.; Noersidiq, A.; Maruddin, F. Effects of Glycerol Plasticizer on Physical Characteristic of Whey-Konjac Films Enriched with Clove Essential Oil. J. Food Qual. Hazards Control 2022, 9, 226–233. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties of blend film based on cuttlefish (Sepia pharaonis) skin gelatin and mungbean protein isolate. Int. J. Biol. Macromol. 2011, 49, 663–673. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Simpson, B.K.; Dumont, M.J. Effect of beeswax and carnauba wax addition on properties of gelatin films: A comparative study. Food Biosci. 2018, 26, 88–95. [Google Scholar] [CrossRef]
- Yan, H.; Lin, X.; Duan, N.; Khan, I.M.; Wang, Z.; Wu, S. Gelatin–carboxymethyl cellulose film incorporated porphyrin metal–organic frameworks with photodynamic/photothermal synergistic antibacterial activities for pork packaging application. J. Food Eng. 2023, 353, 111556. [Google Scholar] [CrossRef]
- Kim, H.; Beak, S.E.; Song, K.B. Development of a hagfish skin gelatin film containing cinnamon bark essential oil. LWT Food Sci. Technol. 2018, 96, 583–588. [Google Scholar] [CrossRef]
- Yavari Maroufi, L.; Ghorbani, M.; Tabibiazar, M. A gelatin-based film reinforced by covalent interaction with oxidized guar gum containing green tea extract as an active food packaging system. Food Bioprocess Technol. 2020, 13, 1633–1644. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Ihl, M.; Bifani, V.; Silva, A.; Montero, P. Edible films made from tuna-fish gelatin with antioxidant extracts of two different murta ecotypes leaves (Ugnimolinae Turcz). Food Hydrocoll. 2007, 21, 1133–1143. [Google Scholar] [CrossRef]
- Rattaya, S.; Benjakul, S.; Prodpran, T. Properties of fish skin gelatin film incorporated with seaweed extract. J. Food Eng. 2009, 95, 151–157. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties and characteristics of nanocomposite films from tilapia skin gelatin incorporated with ethanolic extract from coconut husk. J. Food Sci. Technol. 2015, 52, 7669–7682. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, C.M.; Favaro-Trindade, C.S.; Sobral, P.J.A.; Carvalho, R.A. Gelatin-based films additivated with curcuma ethanol extract: Antioxidant activity and physical properties of films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Li, J.; Miao, J.; Wu, J.; Chen, S.; Zhang, Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Montero, P.; Fernández-Martín, F.; Alemán, A.; Gómez-Guillén, M.C. Physical and chemical properties of tuna-skin and bovine-hide gelatin films with added aqueous oregano and rosemary extracts. Food Hydrocoll. 2009, 23, 1334–1341. [Google Scholar] [CrossRef]
- Hamaguchi, P.Y.; WuYin, W.; Tanaka, M. Effect of pH on the formation of edible films made from the muscle proteins of blue marlin (Makaira mazara). Food Chem. 2007, 100, 914–920. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Properties and antioxidative activity of fish gelatin-based film incorporated with epigallocatechin gallate. Food Hydrocoll. 2018, 80, 212–221. [Google Scholar] [CrossRef]
- Farahnaky, A.; Mohammad, S.; Dadfar, M.; Shahbazi, M. Physical and mechanical properties of gelatin–clay nanocomposite. J. Food Eng. 2014, 122, 78–83. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Characteristics of bio-nanocomposite films from tilapia skin gelatin incorporated with hydrophilic and hydrophobic nanoclays. J. Food Eng. 2014, 143, 195–204. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties of bio-nanocomposite films from tilapia skin gelatin as affected by different nanoclays and homogenising conditions. Food Bioprocess Technol. 2014, 7, 3269–3281. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Structure and properties of gelatin/montomorillonite nanocomposite films. In Recent Advances in Research on Biodegradable Polymers and Sustainable Polymers; Jimenez, A., Zaikov, G.E., Eds.; Nova Publishers: Huntington, NY, USA, 2008; pp. 27–36. [Google Scholar]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Physical/thermal properties and heat seal ability of bilayer films based on fish gelatin and poly (lactic acid). Food Hydrocoll. 2018, 77, 248–256. [Google Scholar] [CrossRef]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Ji, M.; Wu, J.; Sun, X.; Guo, X.; Zhu, W.; Li, Q.; Shi, X.; Tian, Y.; Wang, S. Physical properties and bioactivities of fish gelatin films incorporated with cinnamaldehyde-loaded nanoemulsions and vitamin C. LWT Food Sci. Technol. 2021, 135, 110103. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, H.; Yan, T.; Li, J.; Xu, W.; Deng, Y.; Zhou, J.; Ye, X.; Liu, D.; Wang, W. Fabrication of multifunctional materials based on chitosan/gelatin incorporating curcumin-clove oil emulsion for meat freshness monitoring and shelf-life extension. Int. J. Biol. Macromol. 2022, 31, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Sobral, P.J.A. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterization of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Raucci, M.G.; Demitri, C.; Soriente, A.; Fasolino, I.; Sannino, A.; Ambrosio, L. Gelatin/Nano-Hydroxyapatite Hydrogel Scaffold Prepared by Sol-Gel Technology as Filler to Repair Bone Defects. J. Biomed. Mat. Res. A 2018, 106, 2007–2019. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrohydrodynamic encapsulation of eugenol-cyclodextrin complexes in pullulan nanofibers. Food Hydrocoll. 2021, 111, 106264. [Google Scholar] [CrossRef]
- Gao, H.; Yang, H.; Wang, C. Controllable preparation and mechanism of nanosilver mediated by the microemulsion system of the clove oil. Results Physics. 2017, 7, 3130–3136. [Google Scholar] [CrossRef]
- Murugan, G.; Benjakul, S.; Prodpran, T.; Rajasekaran, B.; Baboonsundaram, A.; Nagarajan, M. Enhancement of barrier properties of fish skin gelatin based film layered with PLA and PBAT. J. Polym. Environ. 2023, 31, 5416–5431. [Google Scholar] [CrossRef]
- Tang, C.H.; Xiao, M.L.; Chen, Z.; Yang, X.Q.; Yin, S.W. Properties of cast films of vicilin-rich protein isolates from Phaseolus legumes: Influence of heat curing. LWT Food Sci. Technol. 2009, 42, 1659–1666. [Google Scholar] [CrossRef]
- Orford, P.; Parker, R.; Ring, S.; Smith, A.C. Effect of water as a diluent on the glass transition behavior of malto-oligosaccharides, amylase and amylopectin. Int. J. Biol. Macromol. 1989, 11, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Nuthong, P.; Benjakul, S.; Prodpran, T. Characterization of porcine plasma protein-based films as affected by pretreatment and cross-linking agents. Int. J. Biol. Macromol. 2009, 44, 143–148. [Google Scholar] [CrossRef]
- Vanin, F.M.; Sobral, P.J.A.; Menegalli, F.C.; Carvalho, R.A.; Habitante, A.M.Q.B. Effects of plasticizers and their concentrations on thermal and functional properties of gelatin-based films. Food Hydrocoll. 2005, 19, 899–907. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Psomiadou, E.; Nakayama, A.; Aiba, S.; Yamamoto, N. Edible films made from gelatin, soluble starch and polyols, part 3. Food Chem. 1997, 60, 593–604. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef]
- Jin, S.K.; Choi, J.S.; Jeong, J.Y.; Kim, G.D. The effect of clove bud powder at a spice level on antioxidant and quality properties of emulsified pork sausage during cold storage. J. Sci. Food Agri. 2016, 96, 4089–4097. [Google Scholar] [CrossRef] [PubMed]
- Tagrida, M.; Nilsuwan, K.; Gulzar, S.; Prodpran, T.; Benjakul, S. Fish gelatin/chitosan blend films incorporated with betel (Piper betle L.) leaf ethanolic extracts: Characteristics, antioxidant and antimicrobial properties. Food Hydrocoll. 2023, 137, 108316. [Google Scholar] [CrossRef]
- Biswas, P.; Anand, U.; Saha, S.C.; Kant, N.; Mishra, T.; Masih, H.; Bar, A.; Pandey, D.K.; Jha, N.K.; Majumder, M.; et al. Betelvine (Piper betle L.): A comprehensive insight into its ethnopharmacology, phytochemistry, and pharmacological, biomedical and therapeutic attributes. J. Cell. Mol. Med. 2022, 26, 3038–3119. [Google Scholar] [CrossRef] [PubMed]
- Azizah, F.; Nursakti, H.; Ningrum, A.; Supriyadi, S. Development of Edible Composite Film from Fish Gelatin–Pectin Incorporated with Lemongrass Essential Oil and Its Application in Chicken Meat. Polymers 2023, 15, 2075. [Google Scholar] [CrossRef]
- Chen, H.; Wu, D.; Ma, W.; Wu, C.; Liu, J.; Du, M. Strong Fish Gelatin Hydrogels Double Cross-linked by Transglutaminase and Carrageenan. Food Chem. 2022, 376, 131873. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Tajik, H.; Rohani, S.M.R.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Chin, S.S.; Lyn, F.H.; Hanani, Z.A.N. Effect of Aloe vera (Aloe barbadensis Miller) gel on the physical and functional properties of fish gelatin films as active packaging. Food Pack. Shelf Life 2017, 12, 128–134. [Google Scholar] [CrossRef]
- Kang, J.H.; Song, K.B. Characterization of Job’s tears (Coix lachrymajobi L.) starch films incorporated with clove bud essential oil and their antioxidant effects on pork belly during storage. LWT Food Sci. Technol. 2019, 111, 711–718. [Google Scholar] [CrossRef]
- Nurdiani, R.; Ma’rifah, R.D.A.; Busyro, I.K.; Jaziri, A.A.; Prihanto, A.A.; Firdaus, M.; Talib, R.A.; Huda, N. Physical and functional properties of fish gelatin-based film incorporated with mangrove extracts. PeerJ 2022, 10, e13062. [Google Scholar] [CrossRef] [PubMed]
- Tagrida, M.; Benjakul, S. Betel (Piper betle L.) leaf ethanolic extracts dechlorophyllized using different methods: Antioxidant and antibacterial activities, and application for shelf-life extension of Nile tilapia (Oreochromis niloticus) fillets. RSC Adv. 2021, 11, 17630–17641. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Deng, Y.; Wang, M.; Li, C.; Xie, P.; Sun, B.; Yang, X.; Lang, Y. Effect of chitosan-gelatin edible coating containing nano-encapsulated clove ethanol extract on cold storage of chilled pork. Meat Sci. 2023, 204, 109288. [Google Scholar] [CrossRef] [PubMed]
- Sutrisno, E.; Ningrum, A.; Supriyadi, S.; Munawaroh, H.S.H.; Siti Aisyah, S.; Susanto, E. Characterization of tuna (Thunnus albacares) skin gelatin edible film incorporated with clove and ginger essential oils and different surfactants. Food Res. 2021, 5, 440–450. [Google Scholar] [CrossRef]
- Oroh, S.B.; Kandou, F.E.F.; Pelealu, J.; Pandiangan, D. The inhibitory test of Selaginella delicatula and Diplazium methanol extracts against Staphylococcus aureus and Escherichia coli (in Bahasa Indonesia). J. Ilmiah Sains 2015, 15, 52–58. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, L.; Han, L.; Li, S.; Song, L. Characterization of Antioxidant and Antibacterial Gelatin Films Incorporated with: Ginkgo Biloba Extract. RSC Adv. 2019, 9, 27449–27454. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.L.; Yang, S.Y.; Song, K.B. Development of burdock root inulin/chitosan blend films containing oregano and thyme essential oils. Int. J. Mol. Sci. 2018, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in Foods-a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Nouri, L.; Nafchi, A.M. Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. Int. J. Biol. Macromol. 2014, 66, 254–259. [Google Scholar] [CrossRef]
- ASTM D1653-13. Standard test methods for water vapor transmission of organic coating films. ASTM International, West Conshohocken. 2021. Available online: www.astm.org (accessed on 20 April 2024).
- Weng, W.; Zheng, H. Effect of transglutaminase on properties of tilapia scale gelatin films incorporated with soy protein isolate. Food Chem. 2015, 169, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Bouhanna, I.; Boussaa, A.; Boumaza, A.; Rigano, D.; Maisto, M.; Basile, A.; Rollini, M.; Limbo, S.; Idoui, T. Characterization and antibacterial activity of gelatin-based film incorporated with Arbutus unedo L. fruit extract on Sardina pilchardus. J. Food Process Preserv. 2021, 45, e15424. [Google Scholar] [CrossRef]
- Huang, P.H.; Chiu, C.S.; Lu, W.C.; Li, P.H. Effect of compositions on physicochemical properties and rheological behavior of gelatinized adzuki-bean cake (Yokan). LWT Food Sci. Technol. 2022, 168, 113870. [Google Scholar] [CrossRef]
- Thivya, P.; Bhosale, Y.K.; Anandakumar, S.; Hema, V.; Sinija, V.R. Study on the characteristics of gluten/alginate-cellulose/onion waste extracts composite film and its food packaging application. Food Chem. 2022, 390, 133221. [Google Scholar] [CrossRef]
- Cai, J.; Bai, J.; Luo, B.; Ni, Y.; Tian, F.; Yan, W. In vitro evaluation of probiotic properties and antioxidant activities of Bifidobacterium strains from infant feces in the Uyghur population of northwestern China. Ann. Microbiol. 2022, 72, 14. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crop Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]


| Film Type | Thickness (mm) | WS (%) | WVP (×10−13 g s−1m−1Pa−1) |
|---|---|---|---|
| Control without CE | 0.26 ± 0.01 a | 89.20 ± 2.40 a | 1.68 ± 0.13 b |
| Film incorporating 0.3% CE | 0.24 ± 0.01 a | 84.44 ± 6.28 ab | 1.31 ± 0.21 b |
| Film incorporating 0.7% CE | 0.24 ± 0.01 a | 69.04 ± 3.37 b | 0.85 ± 0.10 a |
| Film Type | Transmittance (nm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 500 | 600 | 800 | Transparency Values | |
| Control without CE | 0.06 | 0.118 | 0.306 | 0.447 | 0.668 | 0.804 | 0.825 | 0.36 ± 0.014 a |
| Film incorporating 0.3% CE | 0.01 | 0.03 | 0.115 | 0.216 | 0.21 | 0.318 | 0.456 | 2.07 ± 0.086 b |
| Film incorporating 0.7% CE | 0.002 | 0.009 | 0.100 | 0.115 | 0.104 | 0.305 | 0.455 | 2.15 ± 0.089 b |
| Film Type | Tg | Tonset | Tm1 | Tm2 |
|---|---|---|---|---|
| Control film without CE | 51.04 | 45.62 | 124.65 | 134.30 |
| Film incorporating 0.3% CE | 57.12 | 53.30 | 124.19 | 134.20 |
| Film incorporating 0.7% CE | 58.80 | 47.35 | 141.92 | 171.83 |
| Inhibition Zone (mm) | ||||
|---|---|---|---|---|
| Film Type | Staphylococcus aureus | Pseudomonas aeruginosa | Escherichia coli | Listeria monocytogenes |
| Control without CE | 0 c | 0 c | 0 c | 0 c |
| Film incorporating 0.3% CE | 3.9 ± 0.10 b | 3.7 ± 0.36 b | 2.77 ± 0.30 b | 3.13 ± 0.13 b |
| Film incorporating 0.7% CE | 6.27 ± 0.42 a | 6.03 ± 0.25 a | 4.90 ± 0.62 a | 6.23 ± 0.15 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostami, P.; Taheri, A.; Ghaffari, M. Properties, Antioxidant and Antibacterial Activity of Southern Meagre Fish (Argyrosomus hololepidotus) Skin Gelatin Reinforced with Clove Bud Extract. Gels 2025, 11, 21. https://doi.org/10.3390/gels11010021
Rostami P, Taheri A, Ghaffari M. Properties, Antioxidant and Antibacterial Activity of Southern Meagre Fish (Argyrosomus hololepidotus) Skin Gelatin Reinforced with Clove Bud Extract. Gels. 2025; 11(1):21. https://doi.org/10.3390/gels11010021
Chicago/Turabian StyleRostami, Parvin, Ali Taheri, and Mostafa Ghaffari. 2025. "Properties, Antioxidant and Antibacterial Activity of Southern Meagre Fish (Argyrosomus hololepidotus) Skin Gelatin Reinforced with Clove Bud Extract" Gels 11, no. 1: 21. https://doi.org/10.3390/gels11010021
APA StyleRostami, P., Taheri, A., & Ghaffari, M. (2025). Properties, Antioxidant and Antibacterial Activity of Southern Meagre Fish (Argyrosomus hololepidotus) Skin Gelatin Reinforced with Clove Bud Extract. Gels, 11(1), 21. https://doi.org/10.3390/gels11010021










