Antibacterial Silver Nanoparticle Containing Polydopamine Hydrogels That Enhance Re-Epithelization
Abstract
1. Introduction
2. Results and Discussion
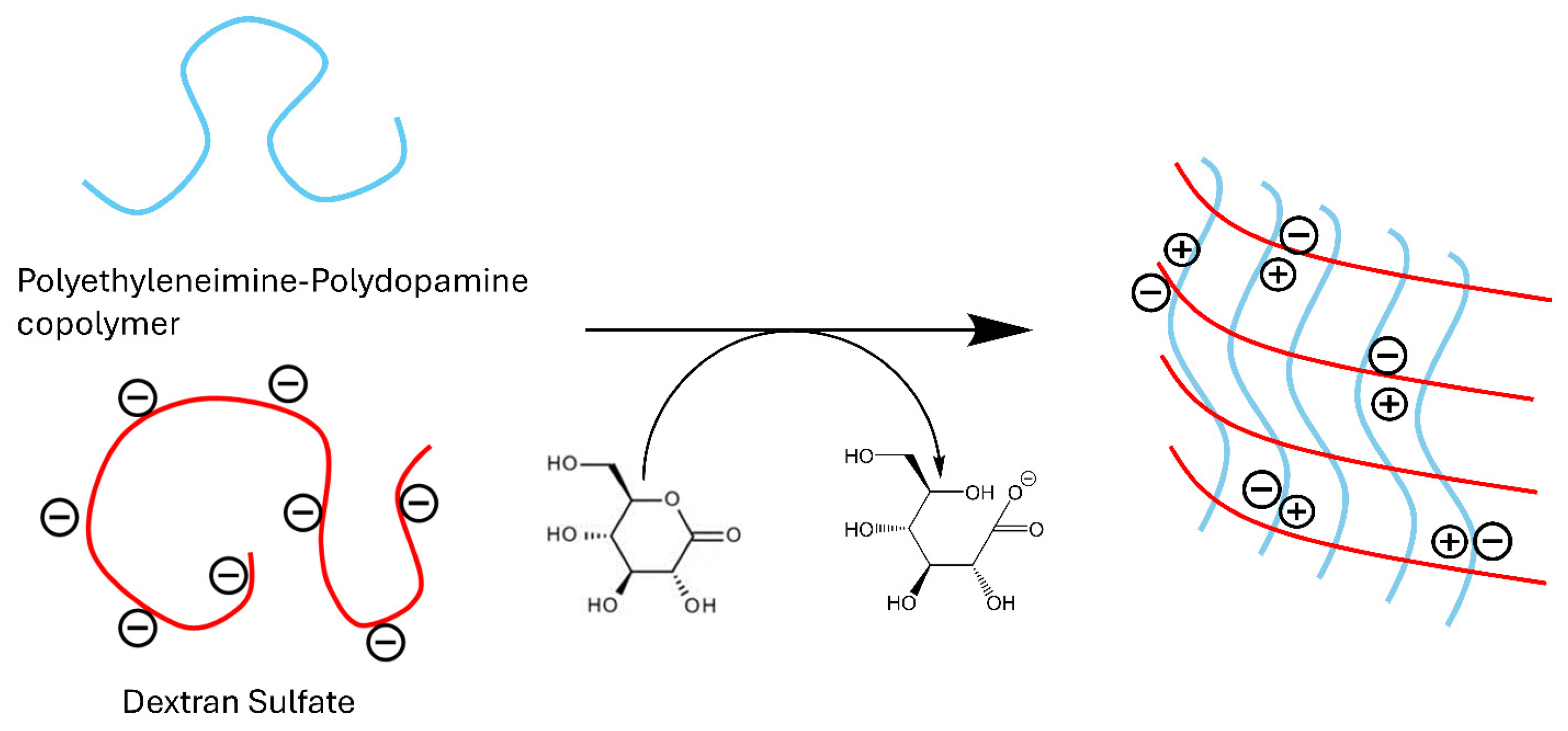
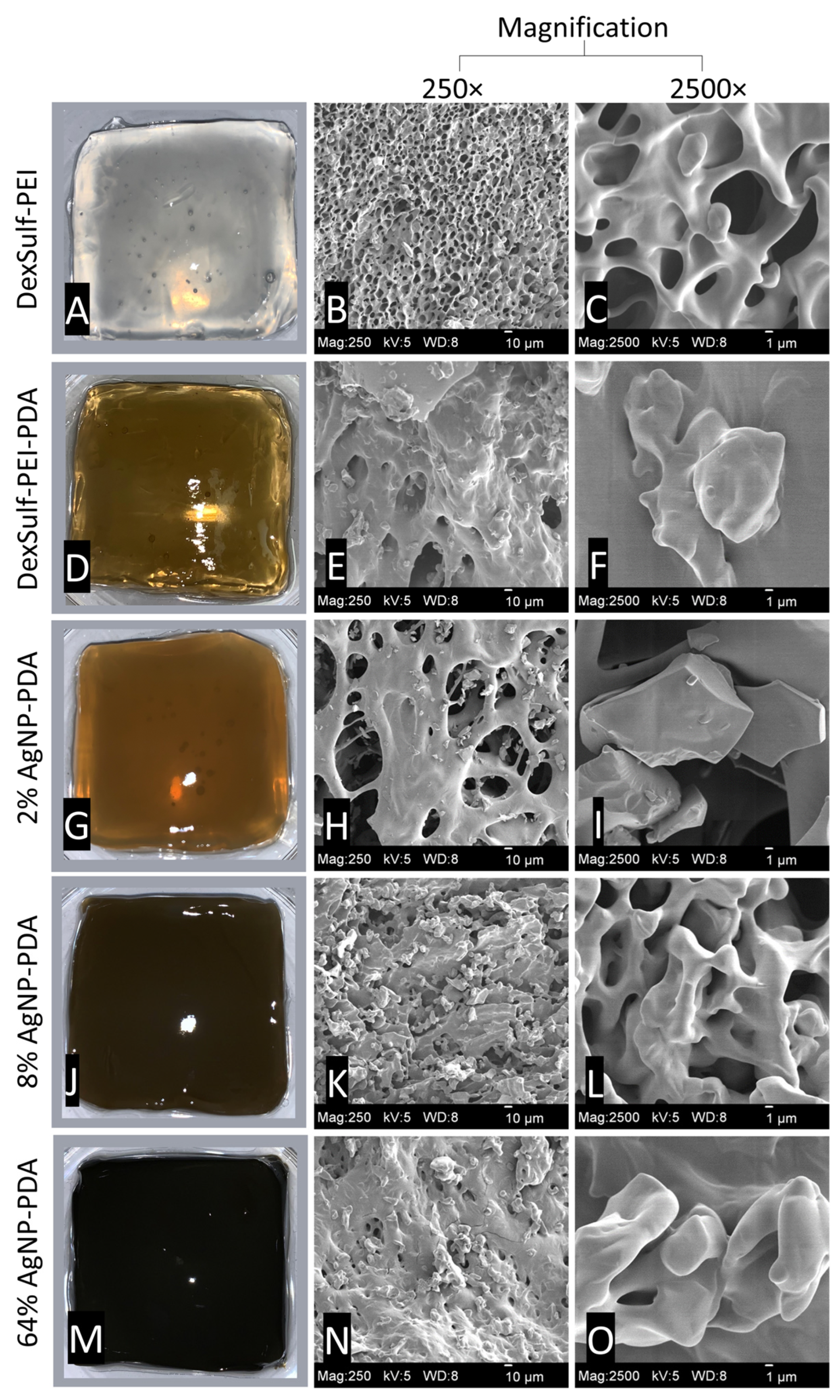
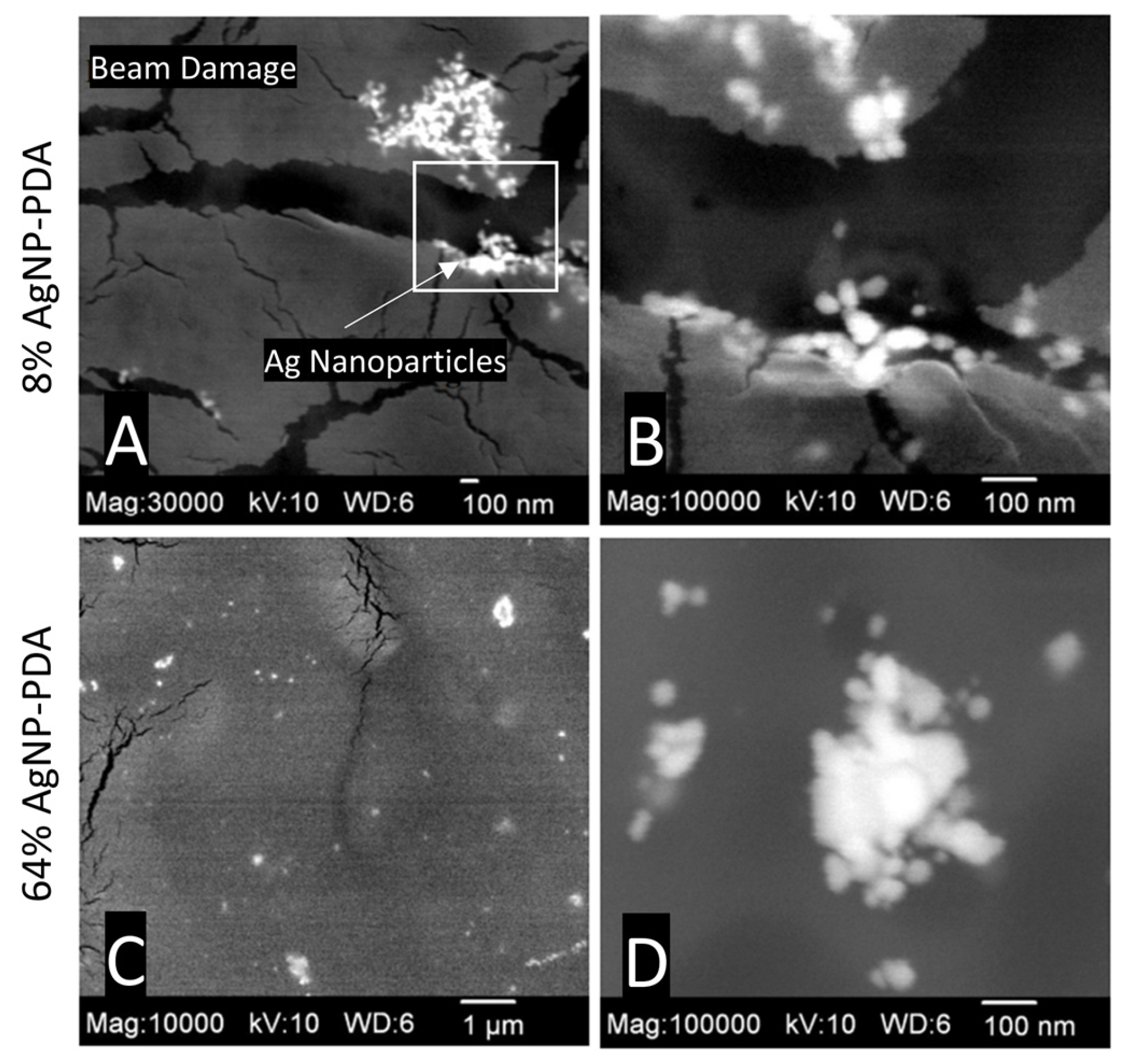
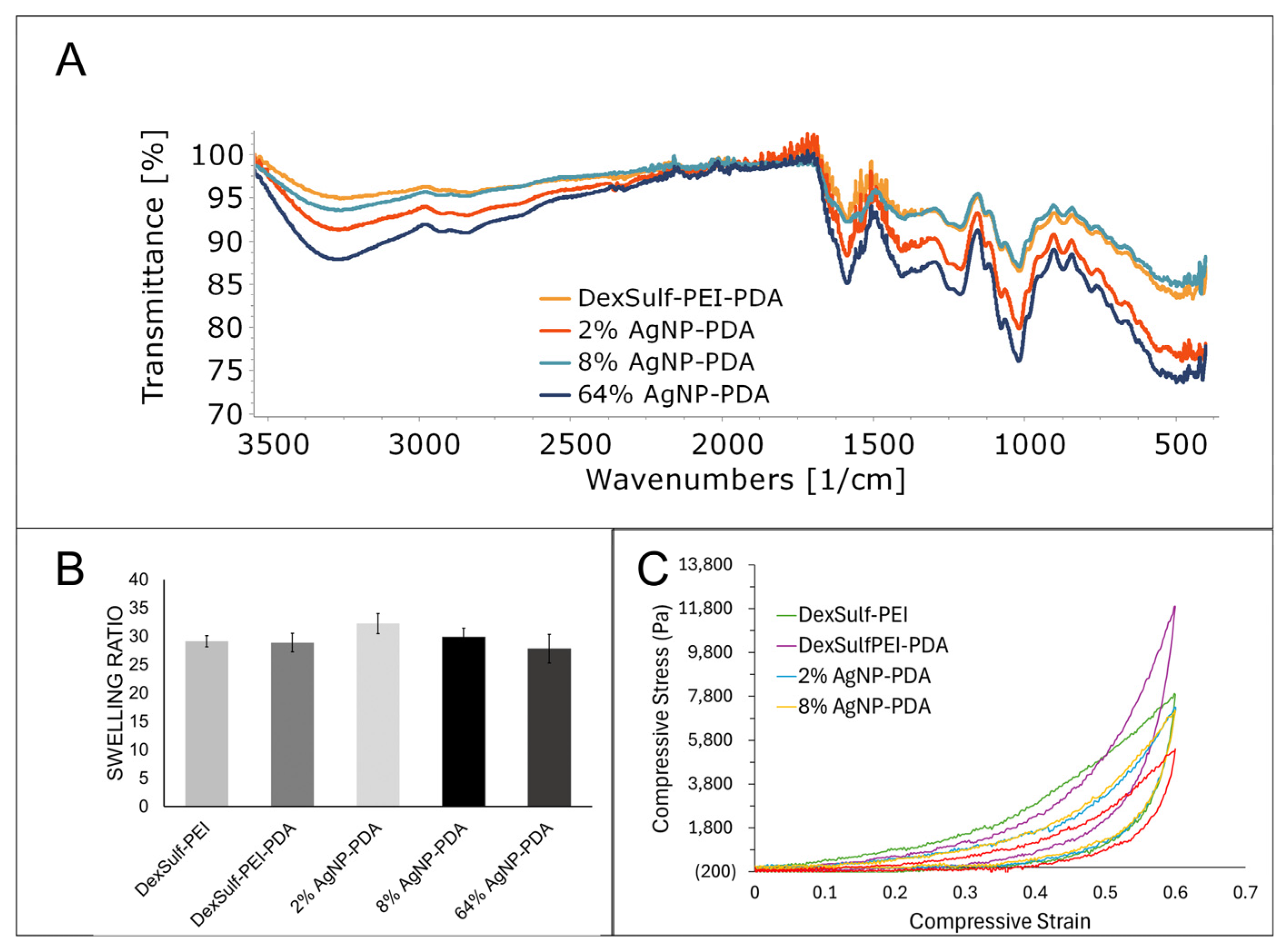
2.1. Synthesis and Structure
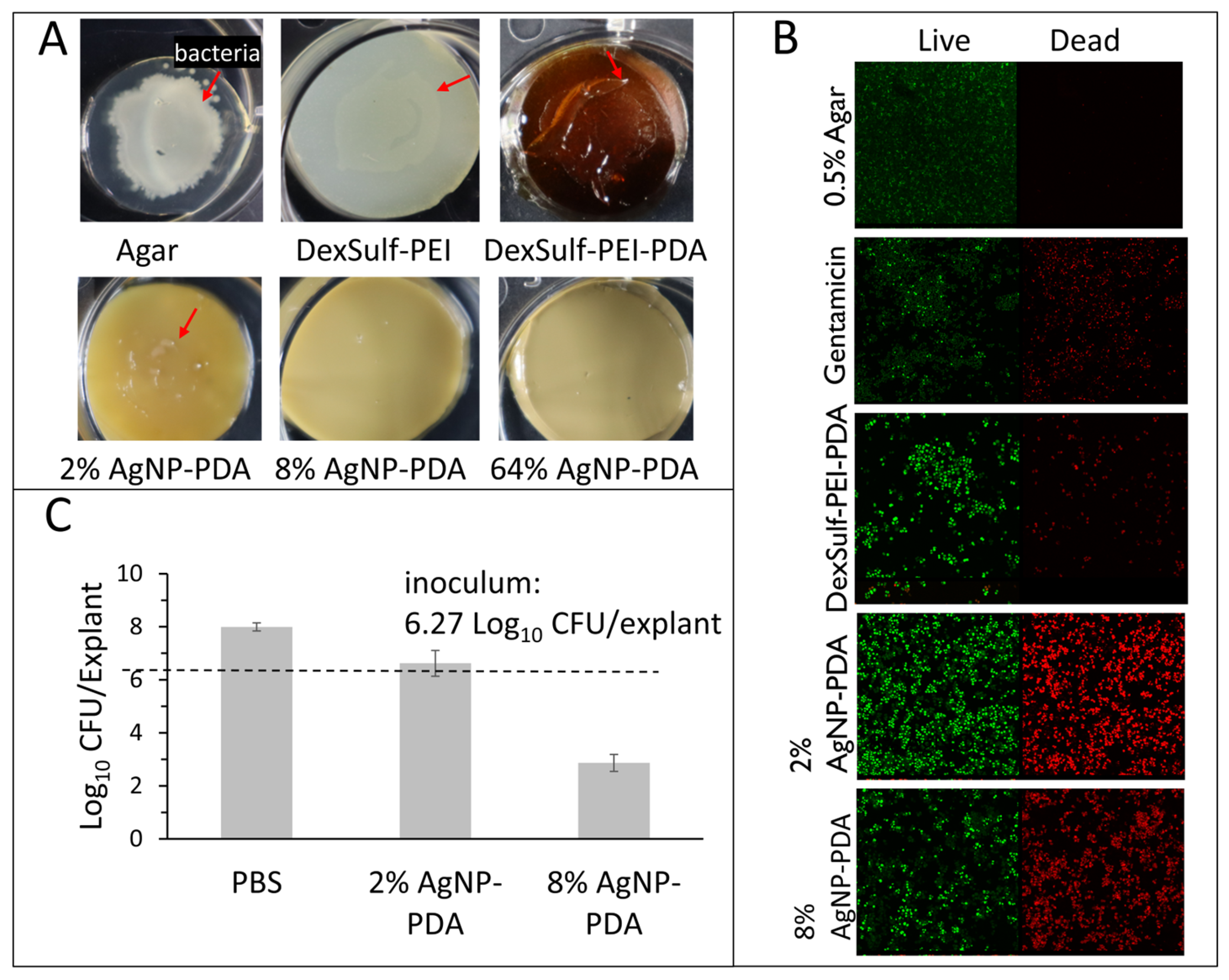
2.2. Antibacterial Activity
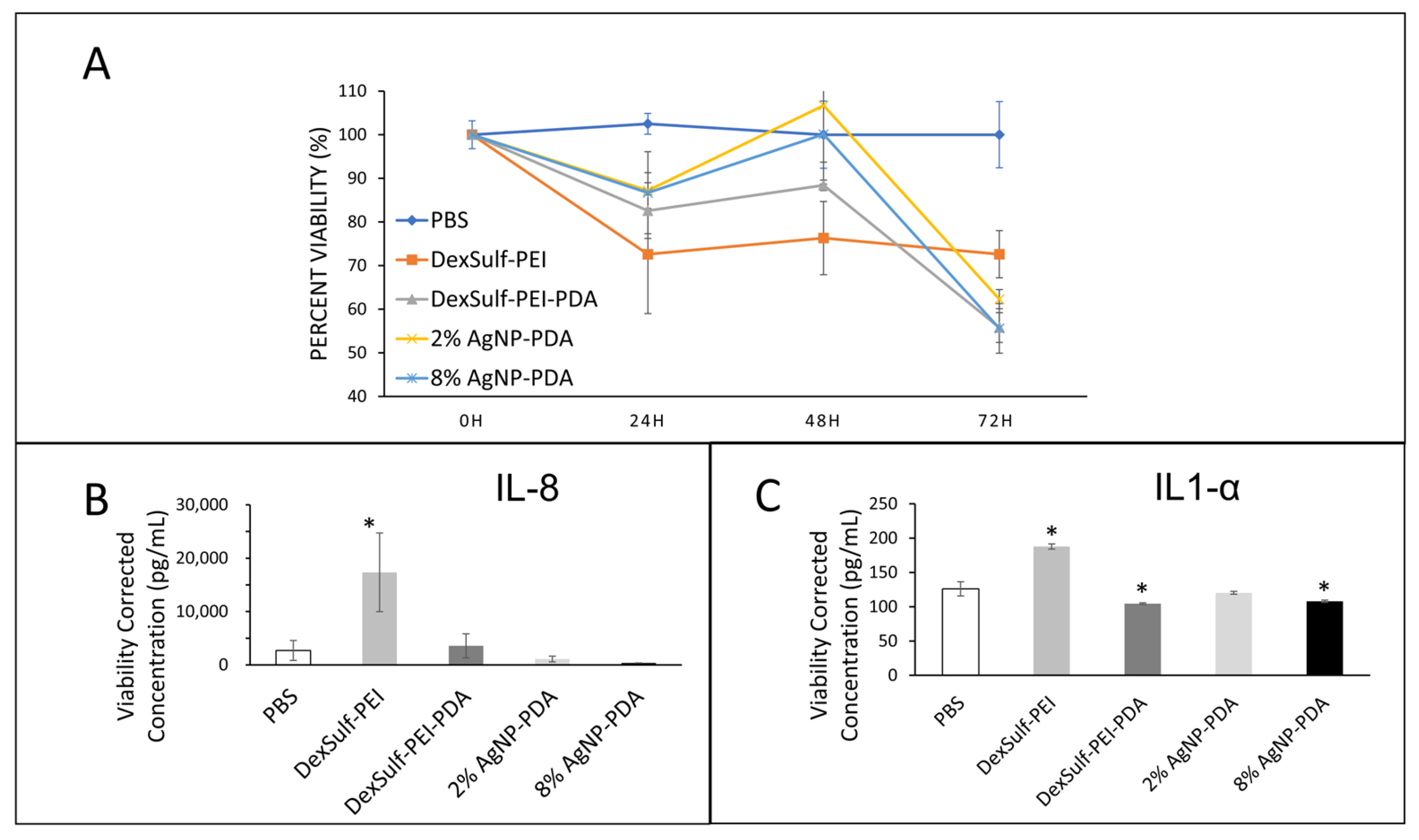
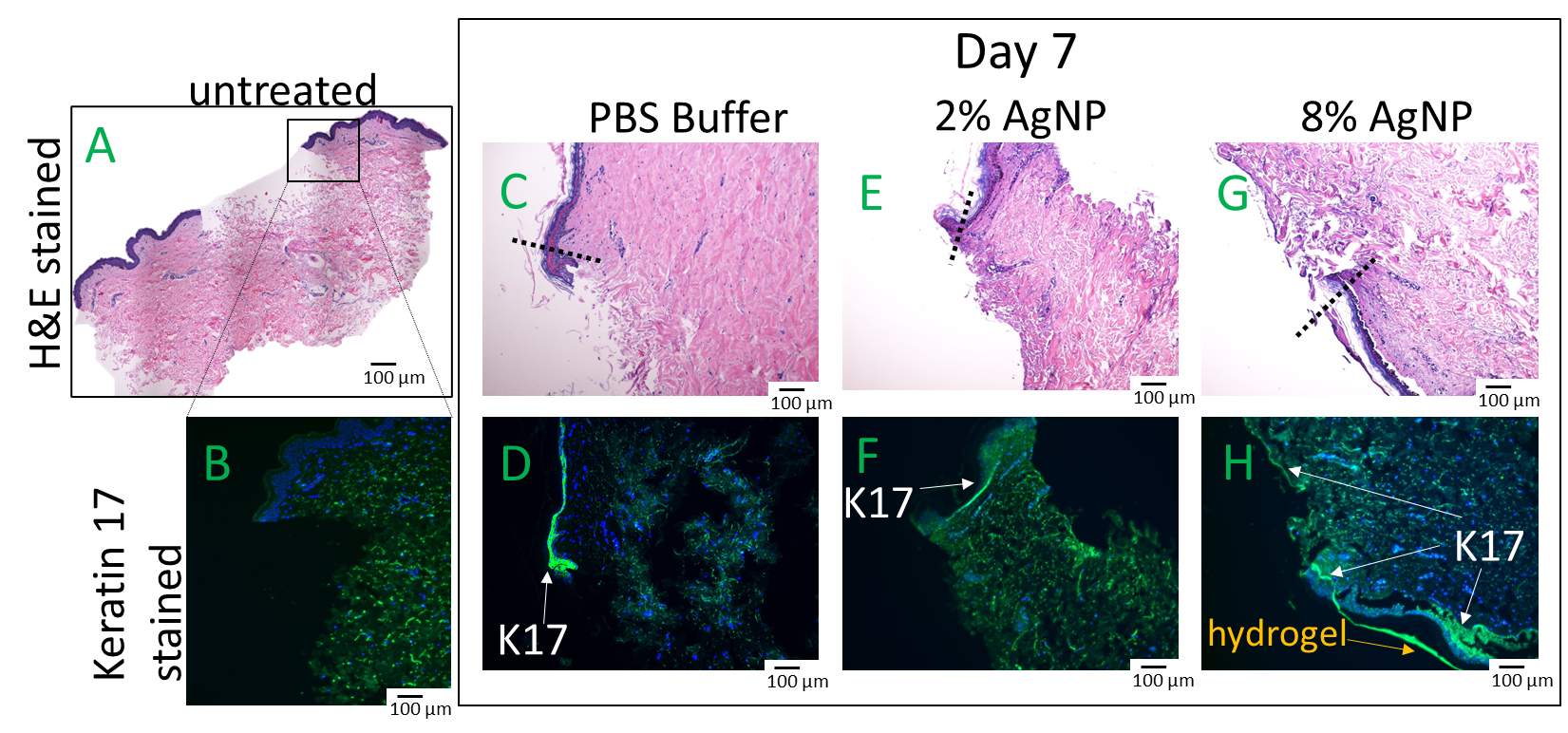
2.3. Biocompatibility and Wound Healing
3. Conclusions
4. Materials and Methods
4.1. Materials
4.1.1. Materials
4.1.2. Characterization
4.2. Hydrogel Synthesis
4.2.1. DexSulf-PEI and DexSulf-PEI-PDA Hydrogel Synthesis
4.2.2. AgNP-PDA Hydrogel Synthesis
4.3. Swelling Ratio
4.4. Compression Studies
4.5. In Vitro Antibacterial Studies
4.5.1. Hydrogel Surface Bacterial Inoculation
4.5.2. Bacterial Live/Dead Assay
4.6. Ex Vivo Human Skin Studies
4.6.1. Antibacterial Assay on Human Skin
4.6.2. Human Skin Ex Vivo Biocompatibility Assays
4.6.3. Wound Healing Assay on Human Skin
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- El Yakhlifi, S.; Alfieri, M.-L.; Arntz, Y.; Eredia, M.; Ciesielski, A.; Samorì, P.; d’Ischia, M.; Ball, V. Oxidant-dependent antioxidant activity of polydopamine films: The chemistry-morphology interplay. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126134. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, R.; Lu, M.; You, G.; Wang, Y.; Chen, G.; Zhao, C.; Wang, Z.; Song, X.; Wu, Y.; et al. Bioinspired Polydopamine-Coated Hemoglobin as Potential Oxygen Carrier with Antioxidant Properties. Biomacromolecules 2017, 18, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.; Zhu, X.; Huang, H.; Xu, C. Performance of Polydopamine Complex and Mechanisms in Wound Healing. Int. J. Mol. Sci. 2021, 22, 10563. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, L.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.; Li, Y.; Gu, Z. Polydopamine antibacterial materials. Mater. Horiz. 2021, 8, 1618–1633. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yang, L.; Shi, S.; Wang, T.; Duan, G.; Liu, X.; Li, Y. Flexible Polydopamine Bioelectronics. Adv. Funct. Mater. 2021, 31, 2103391. [Google Scholar] [CrossRef]
- Siciliano, G.; Monteduro, A.G.; Turco, A.; Primiceri, E.; Rizzato, S.; Depalo, N.; Curri, M.L.; Maruccio, G. Polydopamine-Coated Magnetic Iron Oxide Nanoparticles: From Design to Applications. Nanomaterials 2022, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; van der Westen, R.; Postma, A.; Städler, B. Polydopamine—A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, N.A.; Syed, A.; Wong, M.; Hicks, J.; Nunez, G.; Jitianu, A.; Siler, Z.; Peterson, M. Polydopamine Antioxidant Hydrogels for Wound Healing Applications. Gels 2020, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, N.A.; Abugharbieh, A.; Yasmeen, F.; Buabeng, E.; Mathew, S.; Samaroo, D.; Cheng, H.P. The crosslinking of polysaccharides with polyamines and dextran-polyallylamine antibacterial hydrogels. Int. J. Biol. Macromol. 2015, 72, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Wang, H.; Deng, H.; Gao, M.; Zhang, W. Self-Assembled Nanogels Based on Ionic Gelation of Natural Polysaccharides for Drug Delivery. Front. Bioeng. Biotechnol. 2021, 9, 703559. [Google Scholar] [CrossRef] [PubMed]
- Komoto, D.; Furuike, T.; Tamura, H. Preparation of polyelectrolyte complex gel of sodium alginate with chitosan using basic solution of chitosan. Int. J. Biol. Macromol. 2019, 126, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Piras, C.C.; Smith, D.K. Multicomponent polysaccharide alginate-based bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Liu, X. Stretchable Ionic Conductors for Soft Electronics. Macromol. Rapid Commun. 2022, 43, e2200512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zuo, F.; Liao, Z.; Qin, Z.; Du, S.; Zhao, Z. Mussel-Inspired One-Pot Synthesis of a Fluorescent and Water-Soluble Polydopamine-Polyethyleneimine Copolymer. Macromol. Rapid Comm. 2015, 36, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, Y.; Wang, J.; Cao, B.; Tang, C.Y. In Situ Reduction of Silver by Polydopamine: A Novel Antimicrobial Modification of a Thin-Film Composite Polyamide Membrane. Environ. Sci. Technol. 2016, 50, 9543–9550. [Google Scholar] [CrossRef] [PubMed]
- Pallavali, R.R.; Degati, V.L.; Lomada, D.; Reddy, M.C.; Durbaka, V.R.P. Isolation and in vitro evaluation of bacteriophages against MDR-bacterial isolates from septic wound infections. PLoS ONE 2017, 12, e0179245. [Google Scholar] [CrossRef]
- Perkins, M.A.; Osterhues, M.A.; Farage, M.A.; Robinson, M.K. A noninvasive method to assess skin irritation and compromised skin conditions using simple tape adsorption of molecular markers of inflammation. Skin Res. Technol. 2001, 7, 227–237. [Google Scholar] [CrossRef]
- Cakic, M.; Nikolić, G.M.; Ilić, L.; Stanković, S.M. Synthesis and FTIR characterization of some dextran sulphates. Chem. Ind. Chem. Eng. Q. 2005, 11, 74–78. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Saravanakumar, G.; Sobha, S.; Oh, T.H. Dextran Sulfate Nanocarriers: Design, Strategies and Biomedical Applications. Int. J. Mol. Sci. 2022, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Ammassam Veettil, R.; Marcano, D.C.; Yuan, X.; Zaheer, M.; Adumbumkulath, A.; Lee, R.; Isenhart, L.C.; Soriano, N.; Mhatre, K.; Joseph, R.; et al. Dextran Sulfate Polymer Wafer Promotes Corneal Wound Healing. Pharmaceutics 2021, 13, 1628. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Sanderson, I.R. Dextran sulfate sodium-induced inflammation is enhanced by intestinal epithelial cell chemokine expression in mice. Pediatr. Res. 2003, 53, 143–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Freedberg, I.M.; Tomic-Canic, M.; Komine, M.; Blumenberg, M. Keratins and the keratinocyte activation cycle. J. Investig. Dermatol. 2001, 116, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Lee, K.J.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef] [PubMed]
| Hydrogels | Mass Ratio | |||
|---|---|---|---|---|
| Polyethylene Amine | Dextran Sulfate | Dopamine | AgNO3 | |
| DexSulf-PEI | 2 | 1 | — | — |
| DexSulf-PEI-PDA | 2 | 1 | 0.17 | — |
| 2% AgNP-PDA | 2 | 1 | 0.17 | 0.0034 |
| 8% AgNP-PDA | 2 | 1 | 0.17 | 0.014 |
| 64% AgNP-PDA | 2 | 1 | 0.17 | 0.108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, N.A.; Syed, A.; Kastrat, E.; Cheng, H.-P. Antibacterial Silver Nanoparticle Containing Polydopamine Hydrogels That Enhance Re-Epithelization. Gels 2024, 10, 363. https://doi.org/10.3390/gels10060363
O’Connor NA, Syed A, Kastrat E, Cheng H-P. Antibacterial Silver Nanoparticle Containing Polydopamine Hydrogels That Enhance Re-Epithelization. Gels. 2024; 10(6):363. https://doi.org/10.3390/gels10060363
Chicago/Turabian StyleO’Connor, Naphtali A., Abdulhaq Syed, Ertan Kastrat, and Hai-Ping Cheng. 2024. "Antibacterial Silver Nanoparticle Containing Polydopamine Hydrogels That Enhance Re-Epithelization" Gels 10, no. 6: 363. https://doi.org/10.3390/gels10060363
APA StyleO’Connor, N. A., Syed, A., Kastrat, E., & Cheng, H.-P. (2024). Antibacterial Silver Nanoparticle Containing Polydopamine Hydrogels That Enhance Re-Epithelization. Gels, 10(6), 363. https://doi.org/10.3390/gels10060363









