Dual Functionalization of Hyaluronan Dermal Fillers with Vitamin B3: Efficient Combination of Bio-Stimulation Properties with Hydrogel System Resilience Enhancement
Abstract
1. Introduction
2. Results and Discussion
2.1. HAR Hydrogel Systems: Technical Benchmarking Study and Product Formulation Analyses
2.2. HAR Hydrogel Rheological Characterization
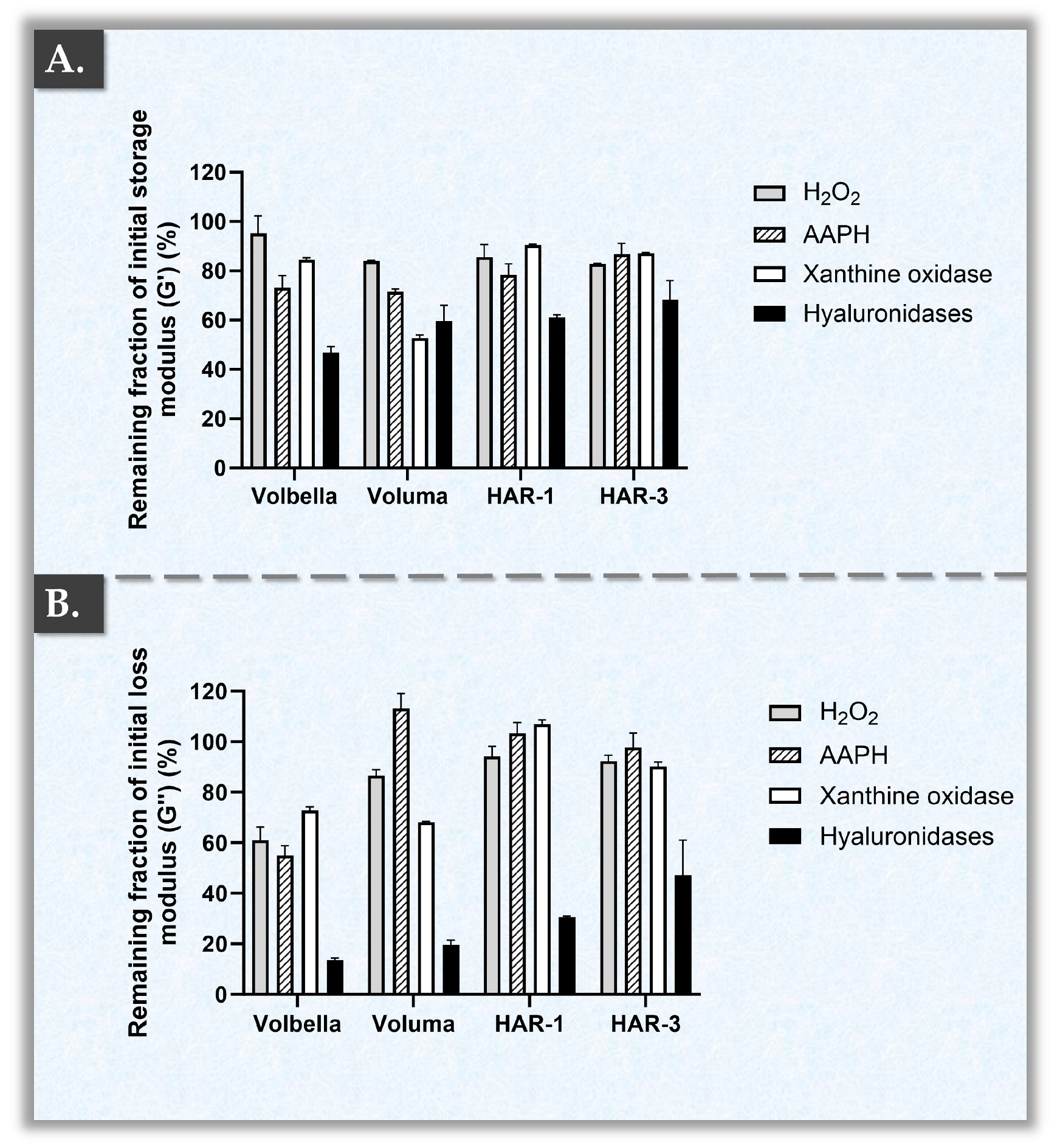
2.3. HAR Hydrogel Multi-Parametric Accelerated Degradation Assays
2.4. HAR Hydrogel System Cohesivity and Injectability Assays
2.5. HAR Hydrogel Bio-Stimulatory Attribute Assessment in a Cutaneous Cell Model
2.6. Study Limitations and Future Perspectives
3. Conclusions
4. Materials and Methods
4.1. Reagents and Consumables Used for the Study
4.2. Hydrogel Rheological Characterization Method
4.3. Hydrogel Accelerated Degradation Assays with Rheological Readouts
4.4. Hydrogel Cohesivity Analysis Method
4.5. Hydrogel Injectability Analysis Method
4.6. Product Biological Effect Evaluations in an In Vitro Dermal Fibroblast Model
4.6.1. In Vitro Assessment of Cellular Viability/Metabolic Activity
4.6.2. In Vitro Assessment of Collagen Synthesis Stimulation Activity
4.6.3. In Vitro Assessment of Total Protein Synthesis Stimulation Activity
4.7. Statistical Analyses and Data Presentation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride |
| BCA | Bicinchoninic acid |
| BDDE | 1,4-butanediol diglycidylether |
| BSA | Bovine serum albumin |
| CaHA | Calcium hydroxylapatite |
| CHUV | Lausanne University Hospital |
| CMC | Carboxymethylcellulose |
| DMEM | Dulbecco’s modified Eagle medium |
| ECM | Extracellular matrix |
| FBS | Fetal bovine serum |
| FDA | US Food and Drug Administration |
| G′ | Storage modulus |
| G″ | Loss modulus |
| HA | Hyaluronic acid |
| HAR | Boosting dermal fillers |
| HYAL | Hyaluronidase |
| H2O2 | Hydrogen peroxide |
| LVE | Linear viscoelastic region |
| MD | Medical device |
| min | Minute |
| NA | Non-applicable |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| ns | Non-significant |
| Pa | Pascals |
| Pa·s | Pascal seconds |
| PBS | Phosphate-buffered saline |
| PLLA | Poly-l-lactic acid |
| ROS | Reactive oxygen species |
| s | Second |
| SEC-MS | Size-exclusion chromatography with mass spectrometry |
| USA | United States of America |
| UV | Ultraviolet |
References
- Olczyk, P.; Komosińska-Vassev, K.; Winsz-Szczotka, K.; Kuźnik-Trocha, K.; Olczyk, K. Hyaluronan: Structure, metabolism, functions, and role in wound healing. Adv. Hyg. Exp. Med. 2008, 62, 651–659. [Google Scholar]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Park, S.; Santosh, S.S.; Ganeshalingam, A.; Thiripuranathar, G.; Sathiyaseelan, A.; Vijayasarathy, S.; Swaminathan, A.; Priya, V.V.; Wang, M.H. Application of hyaluronic acid in tissue engineering, regenerative medicine, and nanomedicine: A review. Int. J. Biol. Macromol. 2022, 222, 2744–2760. [Google Scholar] [CrossRef] [PubMed]
- Huynh, A.; Priefer, R. Hyaluronic acid applications in ophthalmology, rheumatology, and dermatology. Carbohydr. Res. 2020, 489, 107950. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/dermal-fillers-soft-tissue-fillers (accessed on 9 April 2024).
- Allergan Aesthetics, Allergan. Instructions for Use of the Juvéderm® Gel Range (Volbella®, Volift®, Voluma®, Juvéderm® Ultra 2, 3); Allergan: Annecy, France, 2022; unpublished work; Available online: https://media.allergan.com/actavis/actavis/media/general/Juvederm-voluma-IFU.pdf (accessed on 3 April 2024).
- Merz Aesthetics, Anteis SA. Instructions for Use of the Belotero® Gel Range (Belotero® Soft, Balance, Intense, Volume); Anteis: Plan-les-Ouates, Switzerland, 2015; unpublished work; Available online: https://www.merz.ch/wp-content/uploads/2016/03/BELOTERO_Volume_Lidocaine.pdf (accessed on 23 January 2024).
- Teoxane, S.A. Instructions for Use of the RHA® Gel Range (RHA® 1, 2, 3, 4); Teoxane: Geneva, Switzerland, 2015; unpublished work; Available online: https://irp-cdn.multiscreensite.com/e193bd60/files/uploaded/Teosyal-Rha-Dr.-brochure-en-1.pdf (accessed on 25 January 2024).
- Sinclair Pharma. Instructions for Use of the MaiLi® Gel Range; Sinclair Pharma Ltd.: London, UK, 2023; unpublished work; Available online: https://maili.com/physicians/maili-an-expert-s-guide/ (accessed on 7 March 2024).
- Laboratoires VIVACY. Instructions for Use of the Stylage® Gel Range (Stylage® S, M, L, XL, XXL); Vivacy: Paris, France, 2024; unpublished work; Available online: https://vivacy.com/fr/produits/medecine-esthetique/ (accessed on 23 January 2024).
- Faivre, J.; Pigweh, A.I.; Iehl, J.; Maffert, P.; Goekjian, P.; Bourdon, F. Crosslinking hyaluronic acid soft-tissue fillers: Current status and perspectives from an industrial point of view. Exp. Rev. Med. Dev. 2021, 18, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Pluda, S.; Salvagnini, C.; Fontana, A.; Marchetti, A.; Di Lucia, A.; Galesso, D.; Guarise, C. Investigation of crosslinking parameters and characterization of hyaluronic acid dermal fillers: From design to product performances. Gels 2023, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- La Gatta, A.; Salzillo, R.; Catalano, C.; D’Agostino, A.; Pirozzi, A.V.A.; De Rosa, M.; Schiraldi, C. Hyaluronan-based hydrogels as dermal fillers: The biophysical properties that translate into a “volumetric” effect. PLoS ONE 2019, 14, e0218287. [Google Scholar] [CrossRef] [PubMed]
- Bokatyi, A.N.; Dubashynskaya, N.V.; Skorik, Y.A. Chemical modification of hyaluronic acid as a strategy for the development of advanced drug delivery systems. Carbohydr. Polym. 2024, 337, 122145. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carpintero, I.; Candelas, D.; Ruiz-Rodríguez, R. Dermal fillers: Types, indications, and complications. Acta Dermo-Sifilio. 2010, 101, 381–393. [Google Scholar] [CrossRef]
- Ray, S.; Ta, H.T. Investigating the effect of biomaterials such as poly-(L-lactic acid) particles on collagen synthesis in vitro: Method is matter. J. Funct. Biomater. 2020, 11, 51. [Google Scholar] [CrossRef]
- Attenello, N.H.; Maas, C.S. Injectable fillers: Review of material and properties. Facial Plast. Surg. 2015, 31, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Jacovella, P.F. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin. Interv. Aging. 2008, 3, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, V. Sculptra: A stimulatory filler. Facial Plast. Surg. 2009, 25, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Molliard, S.G.; Bétemps, J.B.; Hadjab, B.; Topchian, D.; Micheels, P.; Salomon, D. Key rheological properties of hyaluronic acid fillers: From tissue integration to product degradation. Plast. Aesthet. Res. 2018, 5, 17. [Google Scholar] [CrossRef]
- Pierre, S.; Liew, S.; Bernardin, A. Basics of dermal filler rheology. Dermatol. Surg. 2015, 41, S120–S126. [Google Scholar] [CrossRef]
- Sparavigna, A.; La Gatta, A.; Bellia, G.; La Penna, L.; Giori, A.M.; Vecchi, G.; Tenconi, B.; Schiraldi, C. Evaluation of the volumizing performance of a new volumizer filler in volunteers with age-related midfacial volume defects. Clin. Cosmet. Investig. Dermatol. 2020, 16, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S. Basic rheology of dermal filler. Arch. Plast. Surg. 2020, 47, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Santoro, S.; Russo, L.; Argenzio, V.; Borzacchiello, A. Rheological properties of cross-linked hyaluronic acid dermal fillers. J. Appl. Biomater. Biomech. 2011, 9, 127–136. [Google Scholar] [CrossRef]
- Gou, S.; Porcello, A.; Allémann, E.; Salomon, D.; Micheels, P.; Jordan, O.; Kalia, Y.N. Injectable hyaluronan-based thermoresponsive hydrogels for dermatological applications. Pharmaceutics 2023, 15, 1708. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, H.; Rohrich, R.J.; Liew, S.; Sattler, G.; Talarico, S.; Trévidic, P.; Gavard Molliard, S. Cohesivity of hyaluronic acid fillers: Development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S. Food and Drug Administration-approved fillers. Plast. Reconstr. Surg. 2015, 136, 678–686. [Google Scholar] [CrossRef]
- Edsman, K.L.M.; Öhrlund, Å. Cohesion of hyaluronic acid fillers: Correlation between cohesion and other physicochemical properties. Dermatol. Surg. 2018, 44, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Börger, L.; Meyer, R.; Kalies, A.; Kerscher, M. Approach to differentiate between hyaluronic acid skin quality boosters and fillers based on their physicochemical properties. J. Cosmet. Dermatol. 2022, 21, 149–157. [Google Scholar] [CrossRef]
- Wongprasert, P.; Dreiss, C.A.; Murray, G. Evaluating hyaluronic acid dermal fillers: A critique of current characterization methods. Dermatol. Ther. 2022, 35, e15453. [Google Scholar] [CrossRef]
- Guo, J.; Fang, W.; Wang, F. Injectable fillers: Current status, physicochemical properties, function mechanism, and perspectives. RSC Adv. 2023, 13, 23841. [Google Scholar] [CrossRef]
- Buhren, B.A.; Schrumpf, H.; Bölke, E.; Kammers, K.; Gerber, P.A. Standardized in vitro analysis of the degradability of hyaluronic acid fillers by hyaluronidase. Eur. J. Med. Res. 2018, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Porcello, A.; Laurent, A.; Hirt-Burri, N.; Abdel-Sayed, P.; de Buys Roessingh, A.; Raffoul, W.; Jordan, O.; Allémann, E.; Applegate, L.A. Hyaluronan-based hydrogels as functional vectors for standardized therapeutics in tissue engineering and regenerative medicine. In Nanopharmaceuticals in Regenerative Medicine; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Stern, R.; Kogan, G.; Jedrzejas, M.J.; Soltés, L. The many ways to cleave hyaluronan. Biotechnol. Adv. 2007, 25, 537–557. [Google Scholar] [CrossRef]
- Žádníková, P.; Šínová, R.; Pavlík, V.; Šimek, M.; Šafránková, B.; Hermannová, M.; Nešporová, K.; Velebný, V. The degradation of hyaluronan in the skin. Biomolecules 2022, 12, 251. [Google Scholar] [CrossRef]
- Ferraz, R.M.; Sandkvist, U.; Lundgren, B. Degradation of hylauronic acid fillers using hyaluronidase in an in vivo model. J. Drugs Dermatol. 2018, 17, 548–553. [Google Scholar] [PubMed]
- Schanté, C.; Zuber, G.; Herlin, C.; Vandamme, T.F. Synthesis of N-alanyl-hyaluronamide with high degree of substitution for enhanced resistance to hyaluronidase-mediated digestion. Carbohydr. Polym. 2011, 86, 747–752. [Google Scholar] [CrossRef]
- Flégeau, K.; Jing, J.; Brusini, R.; Gallet, M.; Moreno, C.; Walker, L.; Bourdon, F.; Faivre, J. Multidose hyaluronidase administration as an optimal procedure to degrade resilient hyaluronic acid soft tissue fillers. Molecules 2023, 28, 1003. [Google Scholar] [CrossRef]
- Paap, M.K.; Silkiss, R.Z. The interaction between hyaluronidase and hyaluronic acid gel fillers—A review of the literature and comparative analysis. Plast. Aesthet. Res. 2020, 7, 36. [Google Scholar] [CrossRef]
- Šoltés, L.; Mendichi, R.; Kogan, G.; Schiller, J.; Stankovska, M.; Arnhold, J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 2006, 7, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Hrabárová, E.; Valachová, K.; Juránek, I.; Soltés, L. Free-radical degradation of high-molar-mass hyaluronan induced by ascorbate plus cupric ions: Evaluation of antioxidative effect of cysteine-derived compounds. Chem. Biodivers. 2012, 9, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Darsy, G.; Patarin, J.; Conrozier, T. Large variations in resistance to degradation between hyaluronic acid viscosupplements: A comparative rheological study. Cartilage 2023, 19476035231205696. [Google Scholar] [CrossRef] [PubMed]
- Barygina, V.; Becatti, M.; Lotti, T.; Moretti, S.; Taddei, N.; Fiorillo, C. ROS-challenged keratinocytes as a new model for oxidative stress-mediated skin diseases. J. Cell. Biochem. 2019, 120, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Alsharabasy, A.M.; Glynn, S.; Farràs, P.; Pandit, A. Interactions between nitric oxide and hyaluronan implicate the migration of breast cancer cells. Biomacromolecules 2022, 23, 3621–3647. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef] [PubMed]
- Kusumawati, I.; Indrayanto, G. Chapter 15—Natural antioxidants in cosmetics. In Atta-ur-Rahman; 2013; pp. 485–505. Available online: https://repository.unair.ac.id/117006/3/C-25%20Similarity.pdf (accessed on 2 May 2024).
- Ngoc, L.T.N.; Moon, J.-Y.; Lee, Y.-C. Antioxidants for improved skin appearance: Intracellular mechanism, challenges and future strategies. Int. J. Cosmet. Sci. 2023, 45, 299–314. [Google Scholar] [CrossRef]
- Fitzgerald, R.; Bass, L.M.; Goldberg, D.J.; Graivier, M.H.; Lorenc, Z.P. Physiochemical characteristics of poly-L-lactic acid (PLLA). Aesthet. Surg. J. 2018, 38, S13–S17. [Google Scholar] [CrossRef]
- Vleggaar, D. Facial volumetric correction with injectable poly-L-lactic acid. Dermatol. Surg. 2005, 31, 1511–1518. [Google Scholar] [CrossRef]
- Lorenc, Z.P.; Bass, L.M.; Fitzgerald, R.; Goldberg, D.J.; Graivier, M.H. Physiochemical characteristics of calcium hydroxylapatite (CaHA). Aesthet. Surg. J. 2018, 38, S8–S12. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.L.; Hussain, M.; Goldberg, D.J. Calcium hydroxylapatite filler for facial rejuvenation: A histologic and immunohistochemical analysis. Dermatol. Surg. 2008, 34, S64–S67. [Google Scholar] [CrossRef] [PubMed]
- Porcello, A.; Hadjab, F.; Ajouaou, M.; Philippe, V.; Martin, R.; Abdel-Sayed, P.; Hirt-Burri, N.; Scaletta, C.; Raffoul, W.; Applegate, L.A.; et al. Ex vivo functional benchmarking of hyaluronan-based osteoarthritis viscosupplement products: Comprehensive assessment of rheological, lubricative, adhesive, and stability attributes. Gels 2023, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Zhen, A.X.; Piao, M.J.; Kang, K.A.; Madushan Fernando, P.D.S.; Kang, H.K.; Koh, Y.S.; Yi, J.M.; Hyun, J.W. Niacinamide protects skin cells from oxidative stress induced by particulate matter. Biomol. Ther. 2019, 27, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Mechanistic basis and clinical evidence for the applications of nicotinamide (niacinamide) to control skin aging and pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.T.; Kam, J.; Bloom, J.D. Hyaluronic acid basics and rheology. Facial Plast. Surg. Clin. N. Am. 2022, 30, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Fundarò, S.P.; Salti, G.; Malgapo, D.M.H.; Innocenti, S. The rheology and physicochemical characteristics of hyaluronic acid fillers: Their clinical implications. Int. J. Mol. Sci. 2022, 23, 10518. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhang, J.; Jiang, X. Comparative properties of hyaluronic acid hydrogel cross-linked with 1,4-butanediol diglycidyl ether assayed using a marine hyaluronidase. Mat. Sci. Eng. 2019, 493, 012007. [Google Scholar] [CrossRef]
- Hino, T.; Ford, J.L. Effect of nicotinamide on the properties of aqueous HPMC solutions. Int. J. Pharm. 2001, 226, 53–60. [Google Scholar] [CrossRef]
- Cysewski, P.; Przybyłek, M.; Kowalska, A.; Tymorek, N. Thermodynamics and intermolecular interactions of nicotinamide in neat and binary solutions: Experimental measurements and COSMO-RS concentration dependent reactions investigations. Int. J. Mol. Sci. 2021, 22, 7365. [Google Scholar] [CrossRef]
- Zhai, C.; Hou, B.; Peng, P.; Zhang, P.; Li, L.; Chen, X. Hydrogen bonding interaction of ascorbic acid with nicotinamide: Experimental and theoretical study. J. Mol. Liq. 2018, 249, 9–15. [Google Scholar] [CrossRef]
- De la Guardia, C.; Virno, A.; Musumeci, M.; Bernardin, A.; Silberberg, M.B. Rheologic and physicochemical characteristics of hyaluronic acid fillers: Overview and relationship to product performance. Facial Plast. Surg. 2022, 38, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.postersessiononline.eu/173580348_eu/congresos/WBC2020/aula/-WBC2020-LATE_4410_WBC2020.pdf (accessed on 10 February 2024).
- Battelli, M.G.; Bortolotti, M.; Bolognesi, A.; Polito, L. Pro-aging effects of xanthine oxidoreductase products. Antioxidants 2020, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Meneshian, A.; Bulkley, G.B. The physiology of endothelial xanthine oxidase: From urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation 2002, 9, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Vergara, C.; López-Alarcón, C.; Alvarez-Figueroa, M.J. Transdermal penetration of diclofenac in the presence of AAPH-derived peroxyl radicals. Drug Dev. Industr. Pharm. 2009, 35, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, V.F.; Pessoa, A.S.; Padovan, C.Z.; Abrantes, D.C.; Gomes, F.H.; Maticoli, M.A.; de Menezes, M.L. Oxidation of melatonin by AAPH-derived peroxyl radicals: Evidence of a pro-oxidant effect of melatonin. Biochim. Biophys. Acta 2009, 1790, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Ouyang, S.H.; Tu, L.F.; Wang, X.; Yuan, W.L.; Wang, G.E.; Wu, Y.P.; Duan, W.J.; Yu, H.M.; Fang, Z.Z.; et al. Caffeine protects skin from oxidative stress-induced senescence through the activation of autophagy. Theranostics 2018, 8, 5713–5730. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Hadjab, F.; Porcello, A.; Lourenço, K.; Scaletta, C.; Abdel-Sayed, P.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Mechanistic insights into the multiple functions of niacinamide: Therapeutic implications and cosmeceutical applications in functional skincare products. Antioxidants 2024, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.M.; Lengfelder, E. Hyaluronic acid degradation by ascorbic acid and influence of iron. Free Radic. Res. Commun. 1987, 3, 85–92. [Google Scholar] [CrossRef]
- Micheels, P.; Porcello, A.; Bezzola, T.; Perrenoud, D.; Quinodoz, P.; Kalia, Y.; Allémann, E.; Laurent, A.; Jordan, O. Clinical perspectives on the injectability of cross-linked hyaluronic acid dermal fillers: A standardized methodology for commercial product benchmarking with inter-injector assessments. Gels 2024, 10, 101. [Google Scholar] [CrossRef]
- Chang, T.-M.; Yang, T.-Y.; Huang, H.-C. Nicotinamide mononucleotide and coenzyme Q10 protects fibroblast senescence induced by particulate matter preconditioned mast cells. Int. J. Mol. Sci. 2022, 23, 7539. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.R.; Tan, C.L.; Chin, T.; Morenc, M.; Ho, C.Y.; Rovito, H.A.; Quek, L.S.; Soon, A.L.; Lim, J.S.Y.; Dreesen, O.; et al. Nicotinamide prevents UVB- and oxidative stress–induced photoaging in human primary keratinocytes. J. Investig. Dermatol. 2022, 142, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Bierman, J.C.; Laughlin, T.; Tamura, M.; Hulette, B.C.; Mack, C.E.; Sherrill, J.D.; Tan, C.Y.R.; Morenc, M.; Bellanger, S.; Oblong, J.E. Niacinamide mitigates SASP-related inflammation induced by environmental stressors in human epidermal keratinocytes and skin. Int. J. Cosmet. Sci. 2020, 42, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Alyoussef, A.; Nasr, M.; Ahmed, R.F.; Ahmed Farid, O.A.H.; Bakeer, R.; Karandikar, H.; Paradkar, A. Nicotinamide extrudates as novel anti-aging and collagen promoting platform: A comparative cosmeceutical study versus the gel form. Pharm. Dev. Technol. 2020, 25, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.J. Optimizing poly-L-lactic acid use. J. Cosmet. Laser Ther. 2008, 10, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.; Vitavska, O.; Kind, P.; Hoppe, W.; Wieczorek, H.; Schürer, N.Y. The biological basis for poly-L-lactic acid-induced augmentation. J. Dermatol. Sci. 2015, 78, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Nowag, B.; Casabona, G.; Kippenberger, S.; Zöller, N.; Hengl, T. Calcium hydroxylapatite microspheres activate fibroblasts through direct contact to stimulate neocollagenesis. J. Cosmet. Dermatol. 2023, 22, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Meçani, R.; Niehot, C.D.; Phillips, T.; Kolb, J.; Daughtry, H.; Muka, T. Skin regeneration-related mechanisms of Calcium Hydroxylapatite (CaHA): A systematic review. Front. Med. 2023, 10, 1195934. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]



| Product Commercial Name | Intended Product Uses 1 | Needle Gauge 2 (G) | HA Concentration 3 | Cross-Linker | Manufacturing Technology |
|---|---|---|---|---|---|
| HAR-1 “Instant Refine” | Fine lines; Peri-oral lines; Tear through | 30 G | 15 mg/mL | BDDE | Boost and Fusion |
| HAR-3 “Maxi Lift” | Volumizer | 27 G | 20 mg/mL | BDDE | Boost and Fusion |
| JUVÉDERM® VOLBELLA® | Fine lines; Tear through | 30 G | 15 mg/mL | BDDE | VYCROSS® |
| JUVÉDERM® VOLUMA® | Volumizer | 27 G | 20 mg/mL | BDDE | VYCROSS® |
| Product Parameters | Hydrogel Product | |
|---|---|---|
| HAR-1 | HAR-3 | |
| HA Concentration (mg/mL) | 15.0 | 20.0 |
| Vitamin B3 Concentration 1 (mg/mL) | 7.50 | 7.50 |
| Lidocaine Concentration (mg/mL) | 3.00 | 3.00 |
| Degree of HA Modification (%) | ~3.8 | ~4.2 |
| Product Name | Plateau Injection Force (N) | Maximum Injection Force (N) |
|---|---|---|
| HAR-1 “Instant Refine” | 43.78 ± 0.51 | 44.62 ± 0.58 |
| HAR-3 “Maxi Lift” | 23.33 ± 0.65 | 23.49 ± 0.65 |
| Empty Syringe Control 1 | 2.39 ± 0.13 | 2.48 ± 0.16 |
| Product Name | Intended Clinical Uses (Experimental Uses) | Product Conditioning 1 (Sample Preparation Method) | Main Product Composition | Ratio Prepared Product: Culture Medium 2 |
|---|---|---|---|---|
| HAR-1 “Instant Refine” | Fine lines; Peri-oral lines; Tear through | 1 mL syringe | HA; Vitamin B3 | 1:1 |
| HAR-3 “Maxi Lift” | Volumizer | 1 mL syringe | HA; Vitamin B3 | 1:1 |
| Radiesse® | Moderate to severe facial wrinkles and folds; Lipoatrophy (Bio-stimulant control) | 1.5 mL syringe (mixed with 6 mL water) | CaHA; CMC; Glycerin | 1:1 |
| Sculptra™ | Volumizer; Lipoatrophy (Bio-stimulant control) | 1 vial (lyophilizate resuspended in 5 mL water) | PLLA; CMC; Mannitol | 1:1 |
| JUVÉDERM® VOLUMA® | Volumizer (Volumizer control) | 1 mL syringe | HA | 1:1 |
| PBS | (Sham control) | 500 mL bottle | Monopotassium phosphate; Disodium phosphate; Sodium chloride | 1:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcello, A.; Chemali, M.; Marques, C.; Scaletta, C.; Lourenço, K.; Abdel-Sayed, P.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Dual Functionalization of Hyaluronan Dermal Fillers with Vitamin B3: Efficient Combination of Bio-Stimulation Properties with Hydrogel System Resilience Enhancement. Gels 2024, 10, 361. https://doi.org/10.3390/gels10060361
Porcello A, Chemali M, Marques C, Scaletta C, Lourenço K, Abdel-Sayed P, Raffoul W, Hirt-Burri N, Applegate LA, Laurent A. Dual Functionalization of Hyaluronan Dermal Fillers with Vitamin B3: Efficient Combination of Bio-Stimulation Properties with Hydrogel System Resilience Enhancement. Gels. 2024; 10(6):361. https://doi.org/10.3390/gels10060361
Chicago/Turabian StylePorcello, Alexandre, Michèle Chemali, Cíntia Marques, Corinne Scaletta, Kelly Lourenço, Philippe Abdel-Sayed, Wassim Raffoul, Nathalie Hirt-Burri, Lee Ann Applegate, and Alexis Laurent. 2024. "Dual Functionalization of Hyaluronan Dermal Fillers with Vitamin B3: Efficient Combination of Bio-Stimulation Properties with Hydrogel System Resilience Enhancement" Gels 10, no. 6: 361. https://doi.org/10.3390/gels10060361
APA StylePorcello, A., Chemali, M., Marques, C., Scaletta, C., Lourenço, K., Abdel-Sayed, P., Raffoul, W., Hirt-Burri, N., Applegate, L. A., & Laurent, A. (2024). Dual Functionalization of Hyaluronan Dermal Fillers with Vitamin B3: Efficient Combination of Bio-Stimulation Properties with Hydrogel System Resilience Enhancement. Gels, 10(6), 361. https://doi.org/10.3390/gels10060361











