Fibrin-Based Hydrogels with Reactive Amphiphilic Copolymers for Mechanical Adjustments Allow for Capillary Formation in 2D and 3D Environments
Abstract
1. Introduction
Impact Statement
2. Results and Discussion
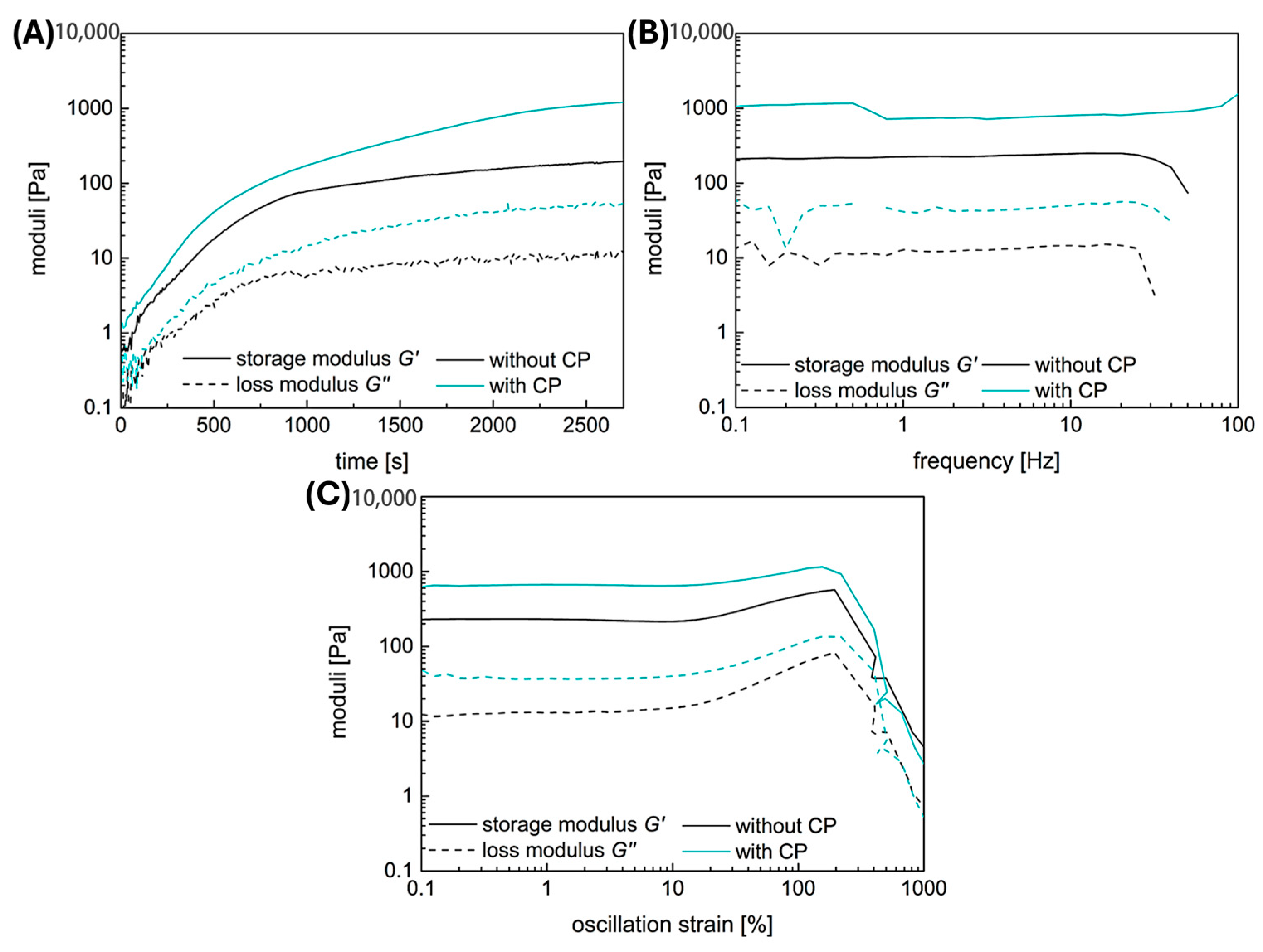
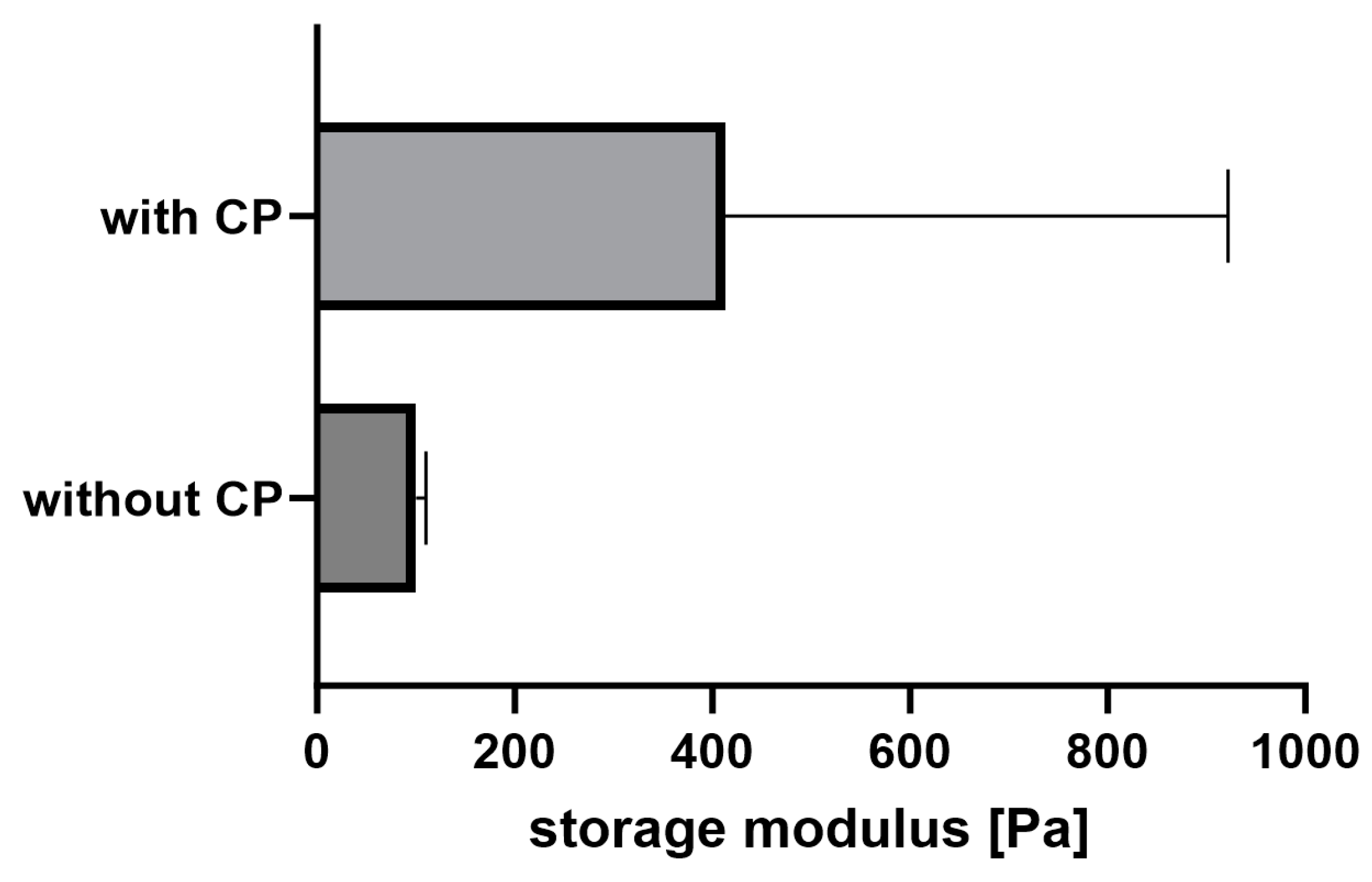
2.1. Rheology
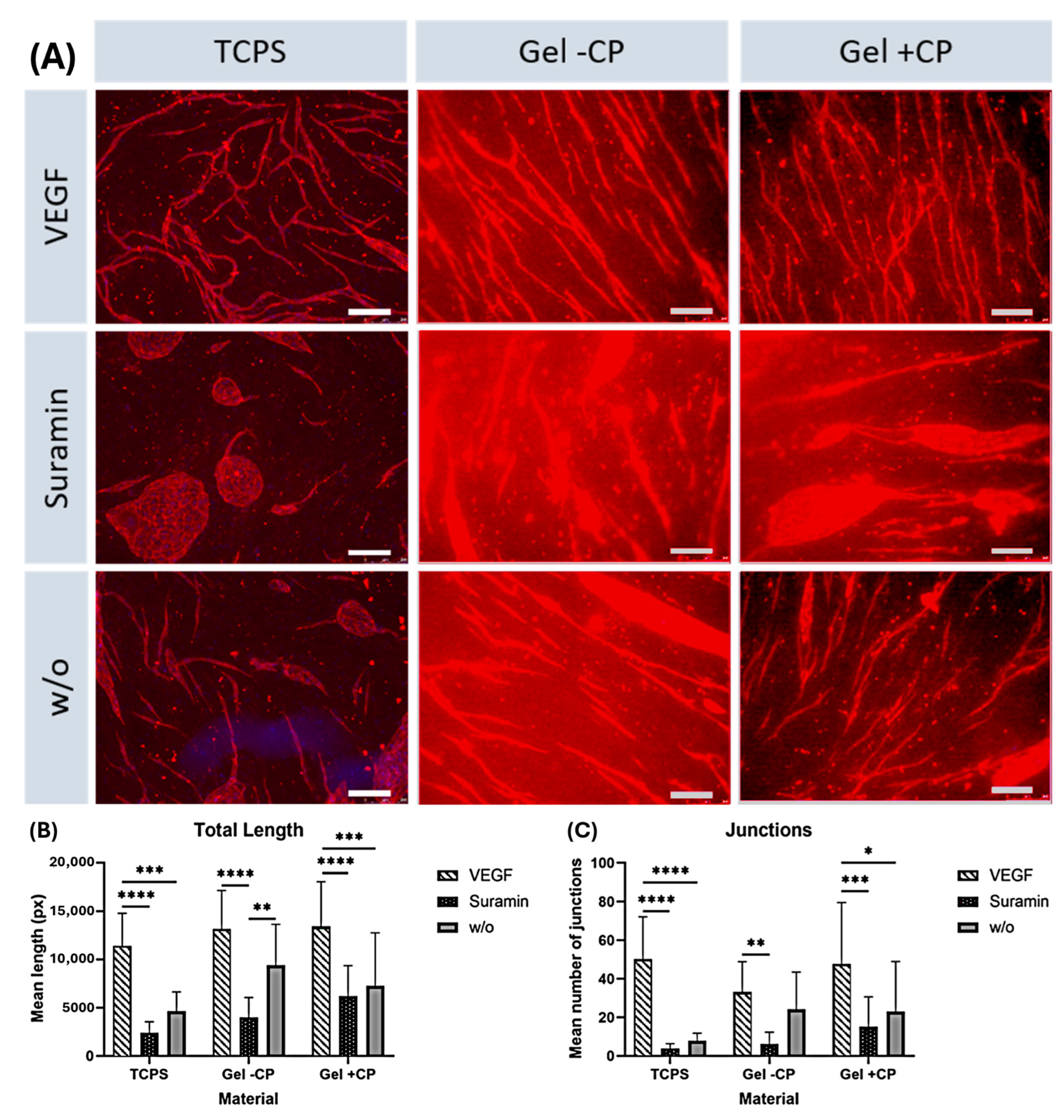
2.2. Capillary-like Structures on Hydrogels with and without Copolymer
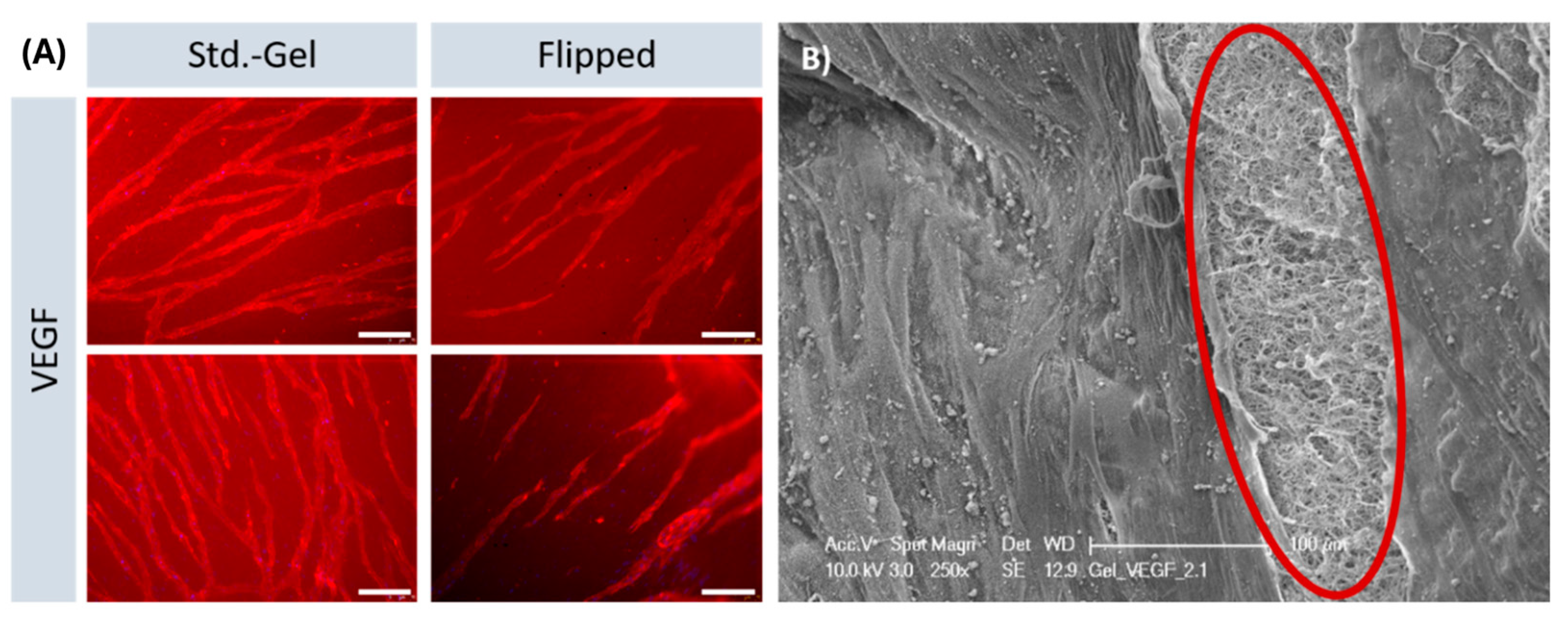
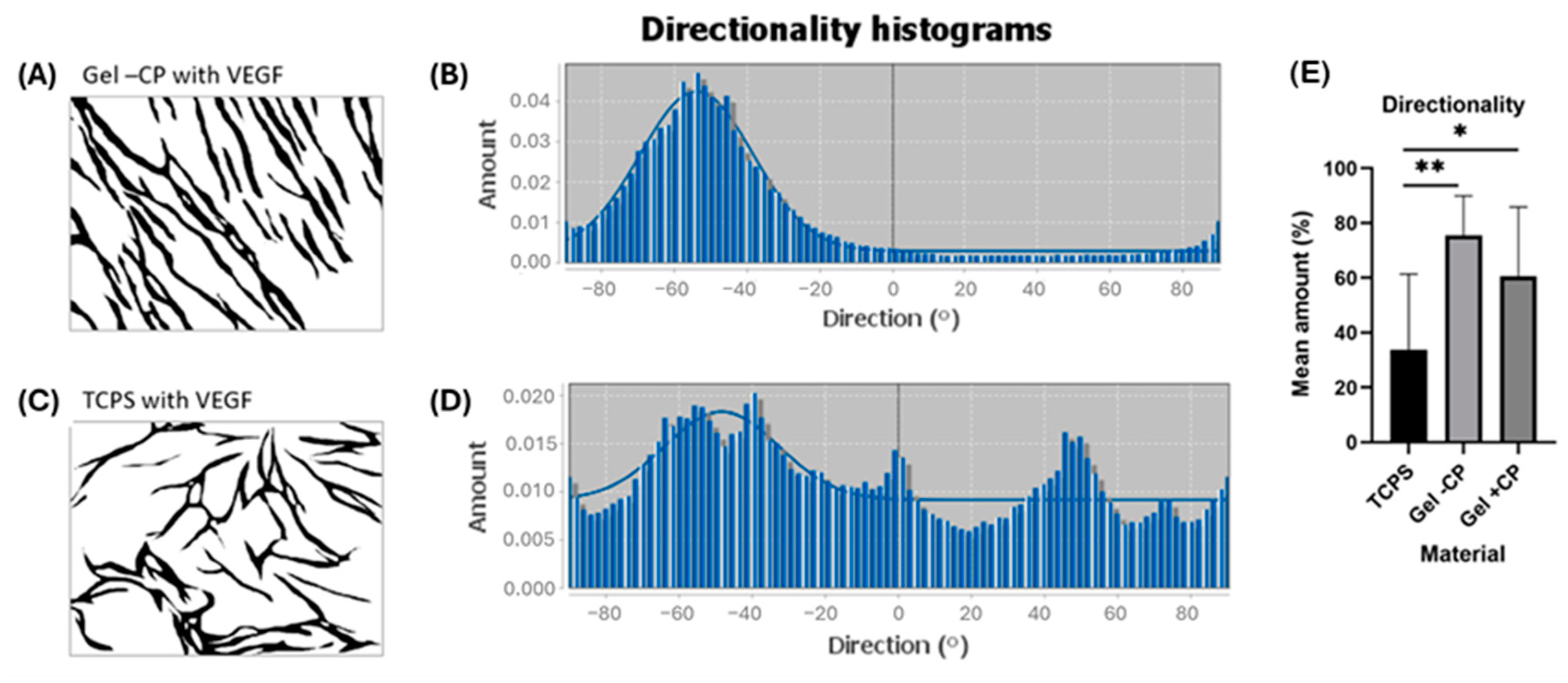
2.3. Parallel Alignment of Capillary-like Structures on Hydrogel Surfaces
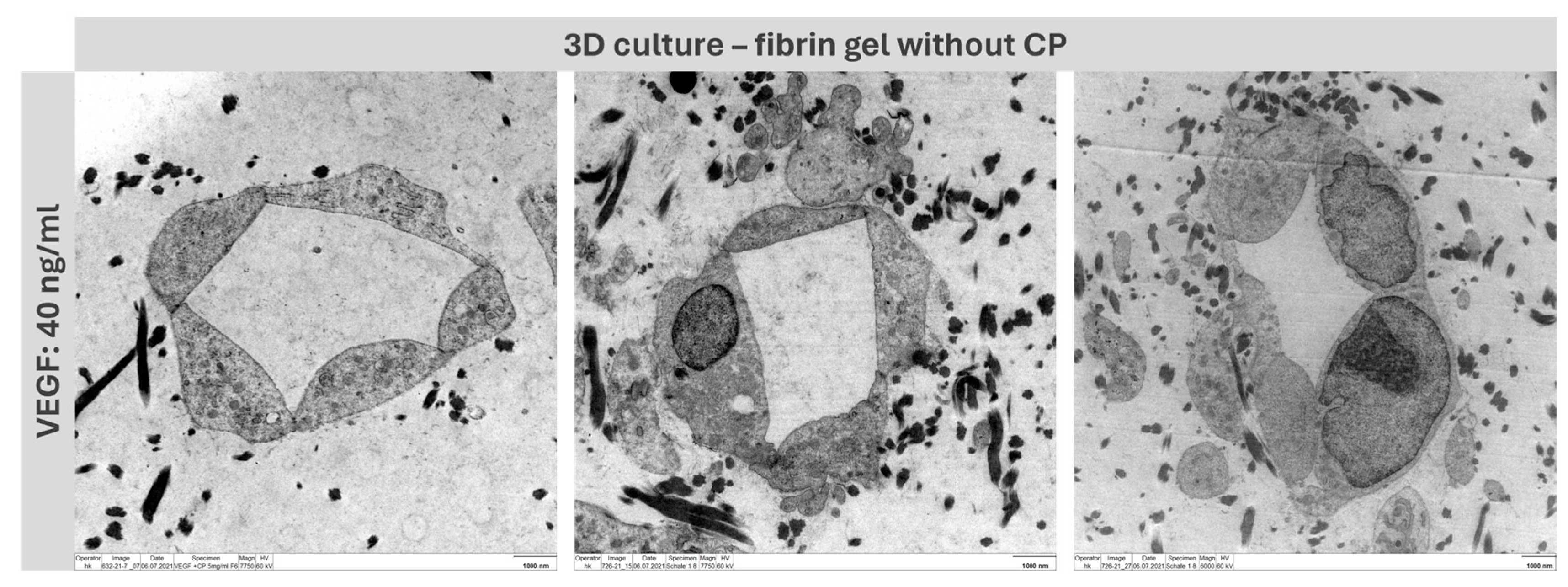
2.4. Formation of Capillary-like Structures in a 3D Environment
2.5. Lumen Detection
2.6. Mechanical Adjustability of Fibrin Gels
2.7. Capillary-like Structures on Hydrogels with and without Copolymer
2.8. Formation of Capillary-like Structures in a 3D Environment
2.9. Lumen Detection
3. Conclusions
4. Materials and Methods
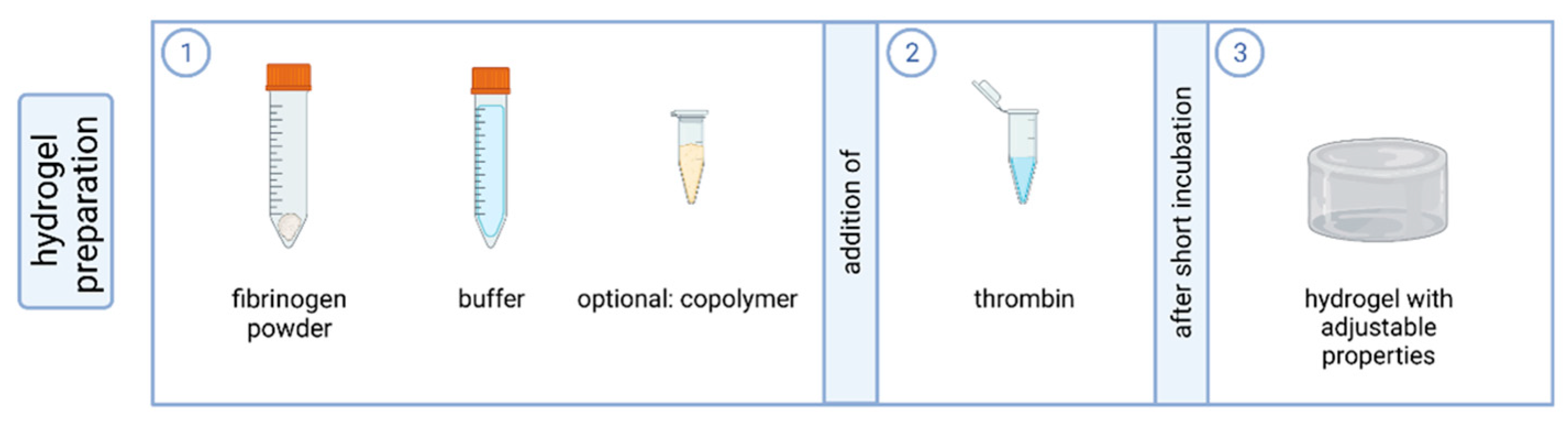
4.1. Preparation of Fibrin-Based Hydrogels
4.2. Rheology
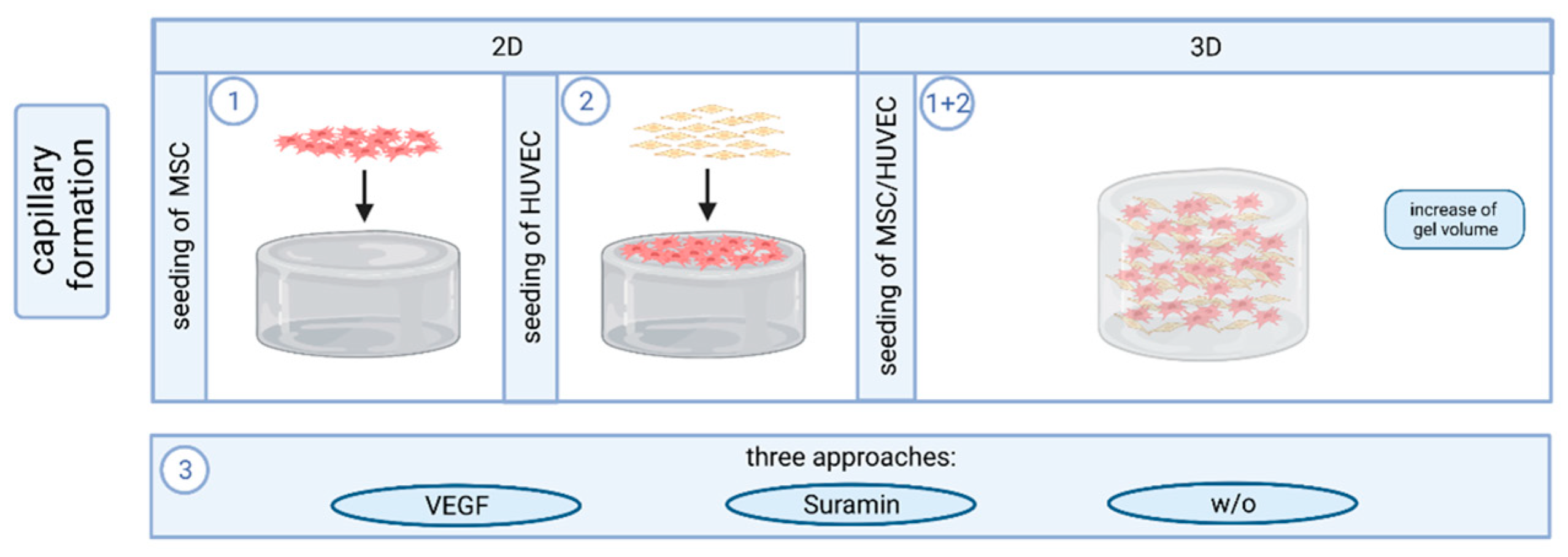
4.3. Cell Culture and Capillary Formation In Vitro
4.4. Immunofluorescence Staining and Microscopy
4.5. VEGF Detection
4.6. Quantification and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, B.S.; Mooney, D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998, 16, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chu, J.S.F.; Cheng, C.; Chen, F.; Chen, D.; Li, S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol. Bioeng. 2004, 88, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-A.; Lee, H.-Y.; Lee, J.H.; Kim, T.-H.; Jang, J.-H.; Kim, H.-W.; Wall, I. Collagen three-dimensional hydrogel matrix carrying basic fibroblast growth factor for the cultivation of mesenchymal stem cells and osteogenic differentiation. Tissue Eng. Part A 2012, 18, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef]
- Malda, J.; Rouwkema, J.; Martens, D.E.; Le Comte, E.P.; Kooy, F.K.; Tramper, J.; van Blitterswijk, C.A.; Riesle, J. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: Measurement and modeling. Biotechnol. Bioeng. 2004, 86, 9–18. [Google Scholar] [CrossRef]
- De la Puente, P.; Ludeña, D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp. Cell Res. 2014, 322, 1–11. [Google Scholar] [CrossRef]
- Shaikh, F.M.; Callanan, A.; Kavanagh, E.G.; Burke, P.E.; Grace, P.A.; McGloughlin, T.M. Fibrin: A natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs 2008, 188, 333–346. [Google Scholar] [CrossRef]
- Bienert, M.; Hoss, M.; Bartneck, M.; Weinandy, S.; Böbel, M.; Jockenhövel, S.; Knüchel, R.; Pottbacker, K.; Wöltje, M.; Jahnen-Dechent, W.; et al. Growth factor-functionalized silk membranes support wound healing in vitro. Biomed. Mater. 2017, 12, 45023. [Google Scholar] [CrossRef]
- Ye, Q.; Zünd, G.; Benedikt, P.; Jockenhoevel, S.; Hoerstrup, S.P.; Sakyama, S.; Hubbell, J.A.; Turina, M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur. J. Cardiothorac. Surg. 2000, 17, 587–591. [Google Scholar] [CrossRef]
- Park, C.H.; Woo, K.M. Fibrin-Based Biomaterial Applications in Tissue Engineering and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1064, 253–261. [Google Scholar] [CrossRef]
- Hokugo, A.; Takamoto, T.; Tabata, Y. Preparation of hybrid scaffold from fibrin and biodegradable polymer fiber. Biomaterials 2006, 27, 61–67. [Google Scholar] [CrossRef]
- Su, X.; Wang, T.; Guo, S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen. Ther. 2021, 16, 63–72. [Google Scholar] [CrossRef]
- Al Enezy-Ulbrich, M.A.; Malyaran, H.; Lange, R.D.; Labude, N.; Plum, R.; Rütten, S.; Terefenko, N.; Wein, S.; Neuss, S.; Pich, A. Impact of Reactive Amphiphilic Copolymers on Mechanical Properties and Cell Responses of Fibrin-Based Hydrogels. Adv. Funct. Mater. 2020, 30, 2003528. [Google Scholar] [CrossRef]
- Qi, X.; Huang, Y.; You, S.; Xiang, Y.; Cai, E.; Mao, R.; Pan, W.; Tong, X.; Dong, W.; Ye, F.; et al. Engineering Robust Ag-Decorated Polydopamine Nano-Photothermal Platforms to Combat Bacterial Infection and Prompt Wound Healing. Adv. Sci. 2022, 9, e2106015. [Google Scholar] [CrossRef]
- Su, T.; Zhang, M.; Zeng, Q.; Pan, W.; Huang, Y.; Qian, Y.; Dong, W.; Qi, X.; Shen, J. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater. 2021, 6, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Ordeghan, A.N.; Khayatan, D.; Saki, M.R.; Alam, M.; Abbasi, K.; Shirvani, H.; Yazdanian, M.; Soufdoost, R.S.; Raad, H.T.; Karami, A.; et al. The Wound Healing Effect of Nanoclay, Collagen, and Tadalafil in Diabetic Rats: An In Vivo Study. Adv. Mater. Sci. Eng. 2022, 2022, 9222003. [Google Scholar] [CrossRef]
- Peng, H.; Rübsam, K.; Hu, C.; Jakob, F.; Schwaneberg, U.; Pich, A. Stimuli-Responsive Poly(N-Vinyllactams) with Glycidyl Side Groups: Synthesis, Characterization, and Conjugation with Enzymes. Biomacromolecules 2019, 20, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Sukmana, I.; Vermette, P. The effects of co-culture with fibroblasts and angiogenic growth factors on microvascular maturation and multi-cellular lumen formation in HUVEC-oriented polymer fibre constructs. Biomaterials 2010, 31, 5091–5099. [Google Scholar] [CrossRef] [PubMed]
- Morin, K.T.; Dries-Devlin, J.L.; Tranquillo, R.T. Engineered microvessels with strong alignment and high lumen density via cell-induced fibrin gel compaction and interstitial flow. Tissue Eng. Part A 2014, 20, 553–565. [Google Scholar] [CrossRef]
- McCoy, M.G.; Wei, J.M.; Choi, S.; Goerger, J.P.; Zipfel, W.; Fischbach, C. Collagen Fiber Orientation Regulates 3D Vascular Network Formation and Alignment. ACS Biomater. Sci. Eng. 2018, 4, 2967–2976. [Google Scholar] [CrossRef]
- Hadjizadeh, A.; Doillon, C.J. Directional migration of endothelial cells towards angiogenesis using polymer fibres in a 3D co-culture system. J. Tissue Eng. Regen. Med. 2010, 4, 524–531. [Google Scholar] [CrossRef]
- Ceccarelli, J.; Cheng, A.; Putnam, A.J. Mechanical strain controls endothelial patterning during angiogenic sprouting. Cell. Mol. Bioeng. 2012, 5, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Kniebs, C.; Kreimendahl, F.; Köpf, M.; Fischer, H.; Jockenhoevel, S.; Thiebes, A.L. Influence of Different Cell Types and Sources on Pre-Vascularisation in Fibrin and Agarose-Collagen Gels. Organogenesis 2020, 16, 14–26. [Google Scholar] [CrossRef]
- Farrukh, A.; Paez, J.I.; del Campo, A. 4D Biomaterials for Light-Guided Angiogenesis. Adv. Funct. Mater. 2019, 29, 1807734. [Google Scholar] [CrossRef]
- Oh, H.H.; Lu, H.; Kawazoe, N.; Chen, G. Spatially guided angiogenesis by three-dimensional collagen scaffolds micropatterned with vascular endothelial growth factor. J. Biomater. Sci. Polym. Ed. 2012, 23, 2185–2195. [Google Scholar] [CrossRef]
- Andrée, B.; Ichanti, H.; Kalies, S.; Heisterkamp, A.; Strauß, S.; Vogt, P.-M.; Haverich, A.; Hilfiker, A. Formation of three-dimensional tubular endothelial cell networks under defined serum-free cell culture conditions in human collagen hydrogels. Sci. Rep. 2019, 9, 5437. [Google Scholar] [CrossRef] [PubMed]
- Grainger, S.J.; Putnam, A.J. Assessing the permeability of engineered capillary networks in a 3D culture. PLoS ONE 2011, 6, e22086. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Azari, Z.; Nazarnezhad, S.; Webster, T.J.; Hoseini, S.J.; Brouki Milan, P.; Baino, F.; Kargozar, S. Stem cell-mediated angiogenesis in skin tissue engineering and wound healing. Wound Repair Regen. 2022, 30, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Werlein, C.; Ackermann, M.; Stark, H.; Shah, H.R.; Tzankov, A.; Haslbauer, J.D.; von Stillfried, S.; Bülow, R.D.; El-Armouche, A.; Kuenzel, S.; et al. Inflammation and vascular remodeling in COVID-19 hearts. Angiogenesis 2023, 26, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Neuss, S.; Becher, E.; Wöltje, M.; Tietze, L.; Jahnen-Dechent, W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 2004, 22, 405–414. [Google Scholar] [CrossRef]
- Anderson, S.M.; Siegman, S.N.; Segura, T. The effect of vascular endothelial growth factor (VEGF) presentation within fibrin matrices on endothelial cell branching. Biomaterials 2011, 32, 7432–7443. [Google Scholar] [CrossRef]
- Meyers, M.O.; Gagliardi, A.R.; Flattmann, G.J.; Su, J.L.; Wang, Y.Z.; Woltering, E.A. Suramin analogs inhibit human angiogenesis in vitro. J. Surg. Res. 2000, 91, 130–134. [Google Scholar] [CrossRef] [PubMed]



| Component | µL per Well |
|---|---|
| Fibrinogen (6.2 mg/mL) | 207 |
| CaCl2 (50 mM) | 11.5 |
| GBSH5 incomplete or PVP12400-co-GMA10mol% in GBSH5 complete (30 mg/mL) | 4.6 |
| Tranexamic acid (100 mg/mL) | 4.6 |
| Thrombin (10U) | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wein, S.; Schemmer, C.; Al Enezy-Ulbrich, M.A.; Jung, S.A.; Rütten, S.; Kühnel, M.; Jonigk, D.; Jahnen-Dechent, W.; Pich, A.; Neuss, S. Fibrin-Based Hydrogels with Reactive Amphiphilic Copolymers for Mechanical Adjustments Allow for Capillary Formation in 2D and 3D Environments. Gels 2024, 10, 182. https://doi.org/10.3390/gels10030182
Wein S, Schemmer C, Al Enezy-Ulbrich MA, Jung SA, Rütten S, Kühnel M, Jonigk D, Jahnen-Dechent W, Pich A, Neuss S. Fibrin-Based Hydrogels with Reactive Amphiphilic Copolymers for Mechanical Adjustments Allow for Capillary Formation in 2D and 3D Environments. Gels. 2024; 10(3):182. https://doi.org/10.3390/gels10030182
Chicago/Turabian StyleWein, Svenja, Carina Schemmer, Miriam Aischa Al Enezy-Ulbrich, Shannon Anna Jung, Stephan Rütten, Mark Kühnel, Danny Jonigk, Wilhelm Jahnen-Dechent, Andrij Pich, and Sabine Neuss. 2024. "Fibrin-Based Hydrogels with Reactive Amphiphilic Copolymers for Mechanical Adjustments Allow for Capillary Formation in 2D and 3D Environments" Gels 10, no. 3: 182. https://doi.org/10.3390/gels10030182
APA StyleWein, S., Schemmer, C., Al Enezy-Ulbrich, M. A., Jung, S. A., Rütten, S., Kühnel, M., Jonigk, D., Jahnen-Dechent, W., Pich, A., & Neuss, S. (2024). Fibrin-Based Hydrogels with Reactive Amphiphilic Copolymers for Mechanical Adjustments Allow for Capillary Formation in 2D and 3D Environments. Gels, 10(3), 182. https://doi.org/10.3390/gels10030182











