Development of Low-Molecular-Weight Gelator/Polymer Composite Materials Utilizing the Gelation and Swelling Process of Polymeric Materials
Abstract
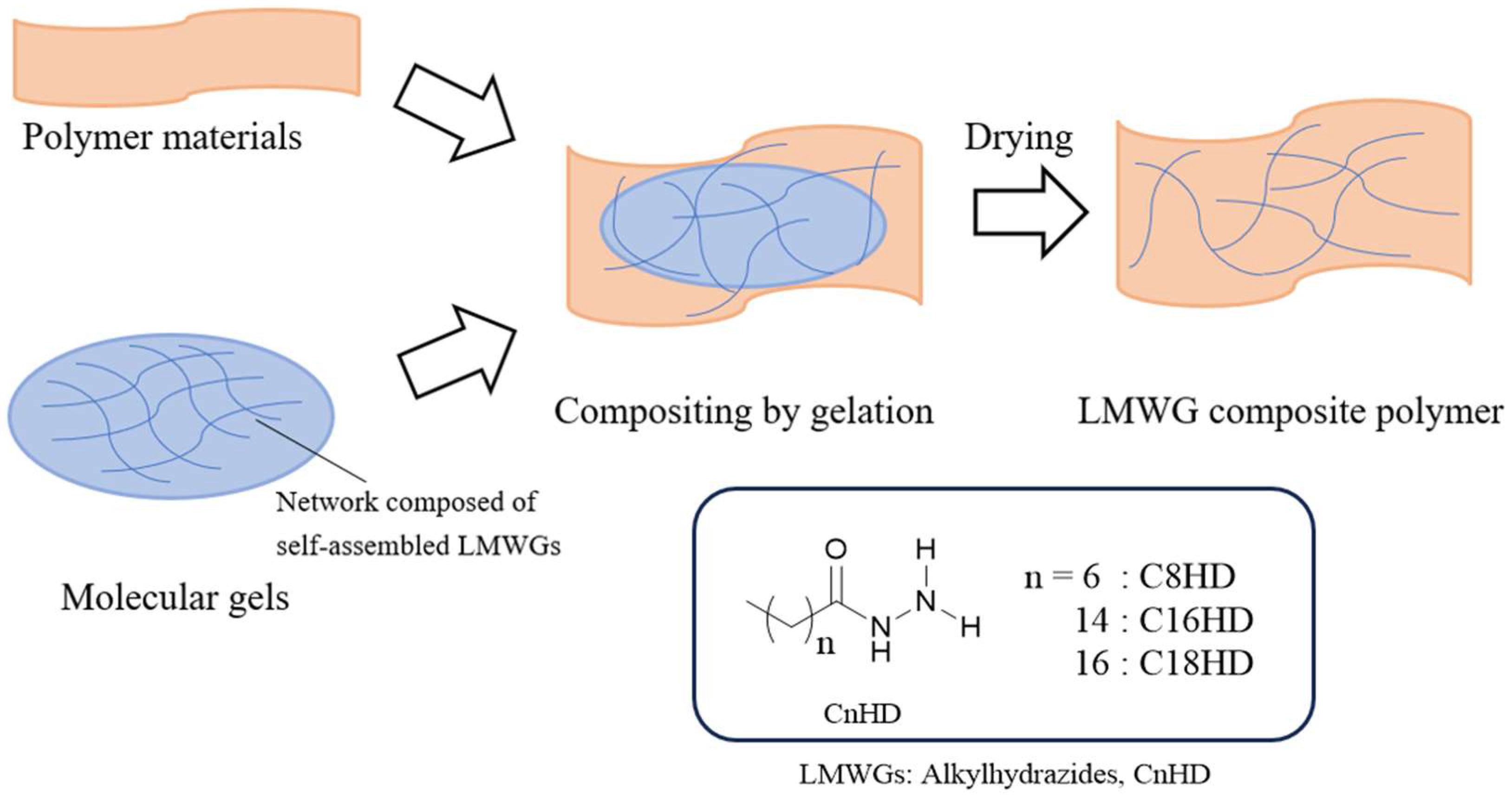
1. Introduction
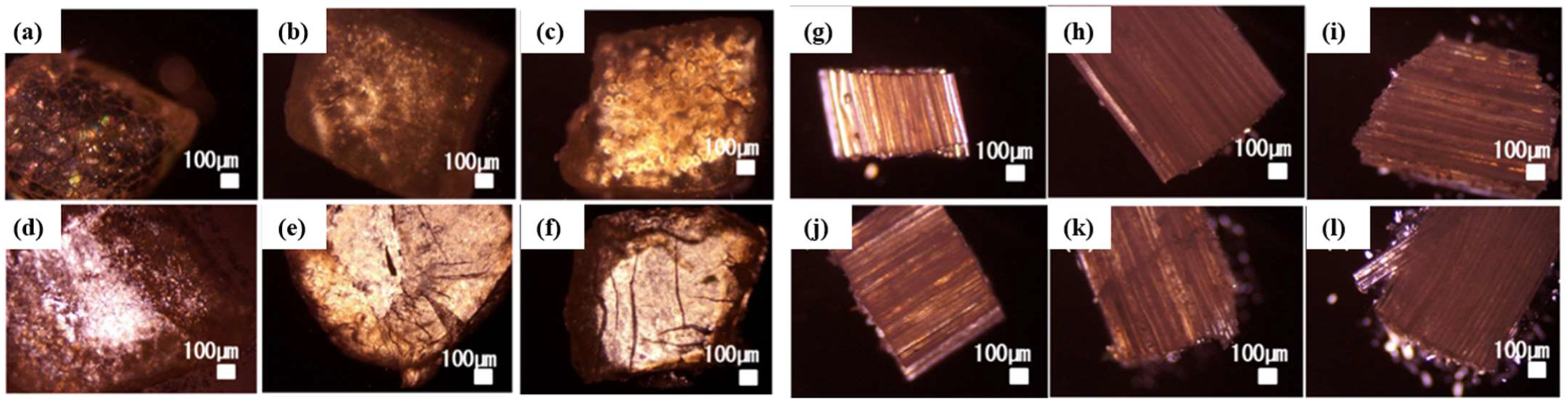
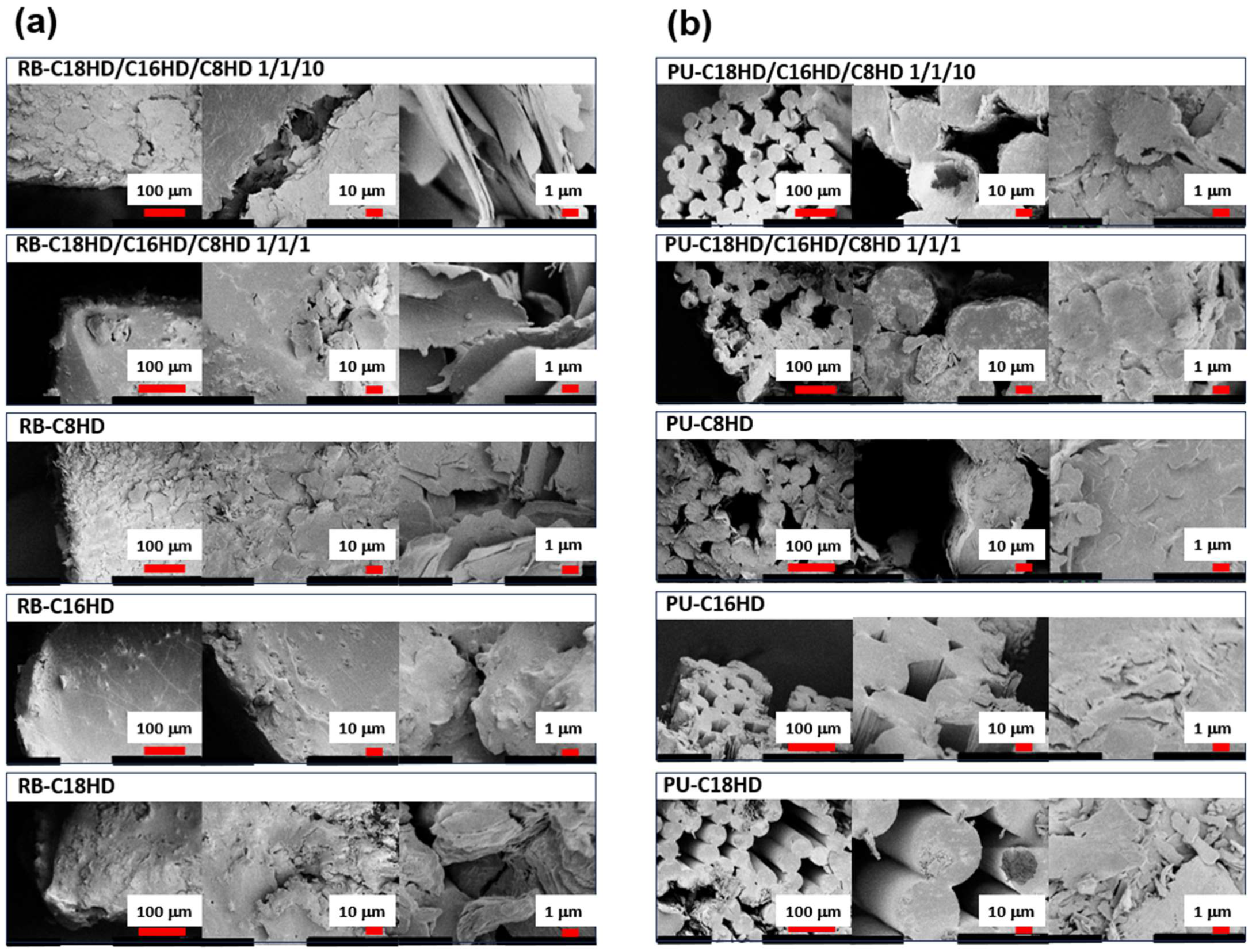
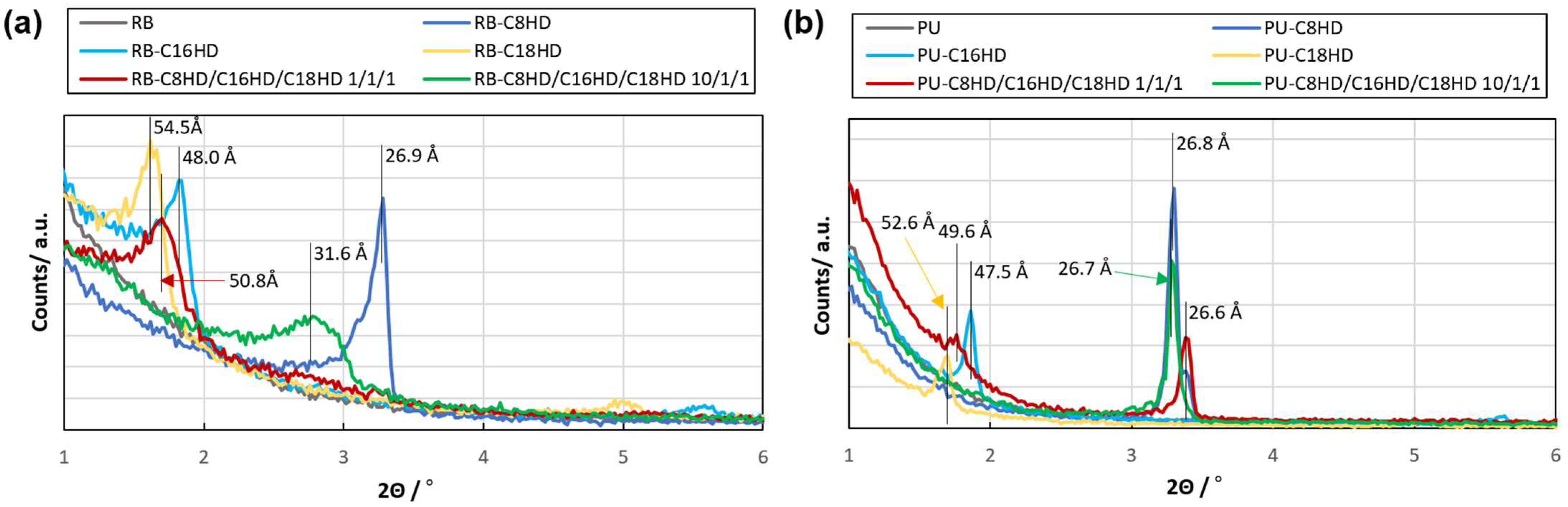
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, R.G.; Terech, P. (Eds.) Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Seiffert, S. (Ed.) Supramolecular Polymer Networks and Gels; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Guenet, J.-M. Organogels Thermodynamics, Structure, Solvent Role, and Properties; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Weiss, R.G. (Ed.) Molecular Gels, Structure and Dynamics; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Rogers, M.A.; Weiss, R.G. Systematic Modifications of Alkane-Based Molecular Gelators and the Consequences to the Structures and Properties of Their Gels. New J. Chem. 2015, 39, 785–799. [Google Scholar] [CrossRef]
- Miao, R.; Peng, J.; Fang, Y. Molecular Gels as Intermediates in the Synthesis of Porous Materials and Fluorescent Films: Concepts and Applications. Langmuir 2017, 33, 10419–10428. [Google Scholar] [CrossRef]
- Weiss, R.G. Controlling Variables in Molecular Gel Science: How Can We Improve the State of the Art? Gels 2018, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Chivers, P.R.A.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef]
- Dawn, A. Supramolecular Gel as the Template for Catalysis, Inorganic Superstructure, and Pharmaceutical Crystallization. Int. J. Mol. Sci. 2019, 20, 781. [Google Scholar] [CrossRef]
- Yokoya, M.; Kimura, S.; Yamanaka, M. Urea Derivatives as Functional Molecules: Supramolecular Capsules, Supramolecular Polymers, Supramolecular Gels, Artificial Hosts, and Catalysts. Chem. A Eur. J. 2021, 27, 5601–5614. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Adams, D.J. Stimuli Responsive Dynamic Transformations in Supramolecular Gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef]
- Sengupta, A.; Roy, G.; Likhar, A.R.; Asthana, D. A Supramolecular Assembly-Based Strategy towards the Generation and Amplification of Photon up-Conversion and Circularly Polarized Luminescence. Nanoscale 2023, 15, 18999–19015. [Google Scholar] [CrossRef]
- Mayr, J.; Saldías, C.; Díaz, D.D. Release of Small Bioactive Molecules from Physical Gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Tanaka, W.; Hamachi, I. Microscopic Imaging Techniques for Molecular Assemblies: Electron, Atomic Force, and Confocal Microscopies. Chem. Rev. 2021, 121, 14281–14347. [Google Scholar] [CrossRef] [PubMed]
- Dayan, N. Delivery System Design in Topically Applied Formulations: An Overview. In Delivery System Handbook for Personal Care and Cosmetic Products, Technology, Applications, and Formulations; Rosen, M.R., Ed.; William Andrew, Inc.: New York, NY, USA, 2005; pp. 101–118. [Google Scholar]
- Sugibayashi, K.; Morimoto, Y. Transdermal Patches. In Gels Handbook, The Fundamentals; Osada, Y., Kajiwara, K., Fushimi, T., Irasa, O., Hirokawa, Y., Matsunaga, T., Shimomura, T., Wang, L., Ishida, H., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2001; Volume 3, pp. 201–210. [Google Scholar]
- Slavkova, M.; Tzankov, B.; Popova, T.; Voycheva, C. Gel Formulations for Topical Treatment of Skin Cancer: A Review. Gels 2023, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Boekhoven, J.; Stupp, S.I. 25th Anniversary Article: Supramolecular Materials for Regenerative Medicine. Adv. Mater. 2014, 26, 1642–1659. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, J.; Du, X.; Xu, B. Supramolecular Biofunctional Materials. Biomaterials 2017, 129, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Bietsch, J.; Bashaw, K.; Wang, G. Recently Developed Carbohydrate Based Gelators and Their Applications. Gels 2021, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Ohsedo, Y.; Watanabe, H.; Oono, M.; Tanaka, A. Mixing Enhancement Effect of Low-Molecular-Weight Organogelators for Thixotropic Organogel Creation. Chem. Lett. 2013, 42, 363–365. [Google Scholar] [CrossRef]
- Hirst, A.R.; Smith, D.K. Two-Component Gel-Phase Materials—Highly Tunable Self-Assembling Systems. Chem. A Eur. J. 2005, 11, 5496–5508. [Google Scholar] [CrossRef]
- Buerkle, L.E.; Rowan, S.J. Supramolecular Gels Formed from Multi-Component Low Molecular Weight Species. Chem. Soc. Rev. 2012, 41, 6089–6102. [Google Scholar]
- Draper, E.R.; Wallace, M.; Schweins, R.; Poole, R.J.; Adams, D.J. Nonlinear Effects in Multicomponent Supramolecular Hydrogels. Langmuir 2017, 33, 2387–2395. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. How Should Multicomponent Supramolecular Gels Be Characterised? Chem. Soc. Rev. 2018, 47, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Cross, E.R.; Sproules, S.; Schweins, R.; Draper, E.R.; Adams, D.J. Controlled Tuning of the Properties in Optoelectronic Self-Sorted Gels. J. Am. Chem. Soc. 2018, 140, 8667–8670. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, W.; Shigemitsu, H.; Fujisaku, T.; Kubota, R.; Minami, S.; Urayama, K.; Hamachi, I. Post-Assembly Fabrication of a Functional Multicomponent Supramolecular Hydrogel Based on a Self-Sorting Double Network. J. Am. Chem. Soc. 2019, 141, 4997–5004. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, D.J.; Smith, D.K. Photo-Patterned Multi-Domain Multi-Component Hybrid Hydrogels. Chem. Commun. 2020, 56, 7029–7032. [Google Scholar] [CrossRef]
- Smith, D.K. Supramolecular Gels—A Panorama of Low-Molecular-Weight Gelators from Ancient Origins to next-Generation Technologies. Soft Matter 2023, 20, 10–70. [Google Scholar] [CrossRef]
- Aboudzadeh, M.A.; Shaplov, A.S.; Hernandez, G.; Vlasov, P.S.; Lozinskaya, E.I.; Pozo-Gonzalo, C.; Forsyth, M.; Vygodskii, Y.S.; Mecerreyes, D. Supramolecular Ionic Networks with Superior Thermal and Transport Properties Based on Novel Delocalized Di-Anionic Compounds. J. Mater. Chem. A 2015, 3, 2338–2343. [Google Scholar] [CrossRef]
- Marrion, A.R. (Ed.) The Chemistry and Physics of Coatings, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2004. [Google Scholar]
- Iwahune, S.; Maeda, H.; Toratani, H.; Kishima, K. Viscosity Adjusting Method for Natural Rubber. Japan Patent JP2002226630A, 14 August 2002. [Google Scholar]
- Balley, J.; Seidei, A.; Arndt, E.; Thomas, S.; Parrish, K.; Gonzalez, D. (Eds.) Processing and Finishing of Polymeric Materials; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Carré, A.; Le Grel, P.; Baudy-Floc’h, M. Hydrazinoazadipeptides as Aromatic Solvent Gelators. Tetrahedron Lett. 2001, 42, 1887–1889. [Google Scholar] [CrossRef]
- Sreenivasachary, N.; Lehn, J.-M. Gelation-Driven Component Selection in the Generation of Constitutional Dynamic Hydrogels Based on Guanine-Quartet Formation. Proc. Natl. Acad. Sci. USA 2005, 102, 5938–5943. [Google Scholar] [CrossRef]
- Tan, C.; Su, L.; Lu, R.; Xue, P.; Bao, C.; Liu, X.; Zhao, Y. A Family of Low-Molecular-Weight Organogelators Based on Long Chain Substituted Benzoic Acid Hydrazides. J. Mol. Liq. 2006, 124, 32–36. [Google Scholar] [CrossRef]
- Bai, B.; Wang, H.; Xin, H.; Zhang, F.; Long, B.; Zhang, X.; Qu, S.; Li, M. Hydrazide-Based Organogels and Liquid Crystals with Columnar Order. New J. Chem. 2007, 31, 401–408. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Xiang, J.-F.; Yan, H.-J.; Chen, C.-F.; Wan, L.-J. Mutual Responsive Hydrazide-Based Low-Molecular-Mass Organic Gelators: Probing Gelation on the Molecular Level. Chem. Eur. J. 2008, 14, 5742–5746. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Ma, J.; Wei, J.; Song, J.; Wang, H.; Li, M. A Simple Structural Hydrazide-Based Gelator as a Fluoride Ion Colorimetric Sensor. Org. Biomol. Chem. 2014, 12, 3478–3483. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, M.; Weiss, R.G. (R)-12-Hydroxystearic Acid Hydrazides as Very Efficient Gelators: Diffusion, Partial Thixotropy, and Self-Healing in Self-Standing Gels. Chem. Asian J. 2016, 11, 3414–3422. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Liu, R.; Hu, S.; Jiang, W.; Chen, Y.; Zhong, J.; Jia, X.; Liu, H.; Luo, X. Self-Assembled Sonogels Formed from 1,4-Naphthalenedicarbonyldinicotinic Acid Hydrazide. RSC Adv. 2022, 12, 20218–20226. [Google Scholar] [CrossRef] [PubMed]
- Noteborn, W.E.M.; Vittala, S.K.; Torredemer, M.B.; Maity, C.; Versluis, F.; Eelkema, R.; Kieltyka, R.E. Switching the Mode of Drug Release from a Reaction-Coupled Low-Molecular-Weight Gelator System by Altering Its Reaction Pathway. Biomacromolecules 2023, 24, 377–386. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Miyamoto, M.; Watanabe, H.; Oono, M.; Tanaka, A. Alkylhydrazide derivatives as new organogelators and their potential ability to gel electrolytes. Bull. Chem. Soc. Jpn 2013, 86, 671–673. [Google Scholar] [CrossRef]
- Harper, C.A. Handbook of Plastics, Elastomers, and Composite, 4th ed.; McGraw Hill Companies, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Xanthos, M. (Ed.) Functional Fillers for Plastics, 2nd ed.; WILEY-VCH Verlag GmbH & CO: Weinheim, Germany, 2010. [Google Scholar]
- Miyamoto, N.; Shintate, M.; Ikeda, S.; Hoshida, Y.; Yamauchi, Y.; Motokawa, R.; Annaka, M. Liquid Crystalline Inorganic Nanosheets for Facile Synthesis of Polymer Hydrogels with Anisotropies in Structure, Optical Property, Swelling/Deswelling, and Ion Transport/Fixation. Chem. Commun. 2013, 49, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Saito, Y.; Park, C.; Fukushima, T.; Aida, T. Ultrahigh-Throughput Exfoliation of Graphite into Pristine ‘Single-Layer’ Graphene Using Microwaves and Molecularly Engineered Ionic Liquids. Nat. Chem. 2015, 7, 730–736. [Google Scholar] [CrossRef]
- Lekawa-Raus, A.; Patmore, J.; Kurzepa, L.; Bulmer, J.; Koziol, K. Electrical Properties of Carbon Nanotube Based Fibers and Their Future Use in Electrical Wiring. Adv. Funct. Mater. 2014, 24, 3661–3682. [Google Scholar] [CrossRef]
- Prosheva, M.; Aboudzadeh, M.A.; Leal, G.P.; Gilev, J.B.; Tomovska, R. High-Performance UV Protective Waterborne Polymer Coatings Based on Hybrid Graphene/Carbon Nanotube Radicals Scavenging Filler. Part. Part. Syst. Charact. 2019, 36, 1800555. [Google Scholar] [CrossRef]
- Cornwell, D.J.; Smith, D.K. Expanding the Scope of Gels—Combining Polymers with Low-Molecular-Weight Gelators to Yield Modified Self-Assembling Smart Materials with High-Tech Applications. Mater. Horiz. 2015, 2, 279–293. [Google Scholar] [CrossRef]
- Nakamura, K.; Kubota, R.; Aoyama, T.; Urayama, K.; Hamachi, I. Four Distinct Network Patterns of Supramolecular/Polymer Composite Hydrogels Controlled by Formation Kinetics and Interfiber Interactions. Nat. Commun. 2023, 14, 1696. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Nishitsuji, S.; Amino, N.; Ishikawa, Y.; Yamaguchi, D.; Koizumi, S. Structure Analyses of Swollen Rubber-Filler Systems by Using Contrast Variation SANS. Macromolecules 2009, 42, 308–311. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Shinoda, T. Creation of Molecular Gel Materials Using Polyrotaxane-Derived Polymeric Organogelator. Gels 2023, 9, 730. [Google Scholar] [CrossRef]
- Dijkstra, M.; Hansen, J.P.; Madden, P.A. Gelation of a Clay Colloid Suspension. Phys. Rev. Lett. 1995, 75, 2236–2239. [Google Scholar] [CrossRef]
- Nakato, T.; Kawamata, J.; Takagi, S. (Eds.) Inorganic Nanosheets and Nanosheet-Based Materials—Fundamentals and Applications of Two-Dimensional Systems; Springer: Tokyo, Japan, 2017. [Google Scholar]
- Sawyer, L.C.; Grubb, D.T.; Meyers, G.F. Polymer Microscopy, 3rd ed.; Springer Science+Business Media, LLC: New York, NY, USA, 2008. [Google Scholar]
- Fleming, I.; Williams, D. Spectroscopic Methods in Organic Chemistry, 7th ed.; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Bienz, S.; Bigler, L.; Fox, T.; Meier, H. Spectroscopic Methods in Organic Chemistry, 3rd ed.; Thieme Chemistry, Georg Thieme Verlag KG: Stuttgart, Germany, 2021. [Google Scholar]



| LMWG Samples | Increase in Weight after Drying for Ring Band (RB)/% | Increase in Weight after Drying for Operon Rubber (PU)/% |
|---|---|---|
| C8HD/C16HD/C18HD:10/1/1 | 19.0 | 16.2 |
| C8HD/C16HD/C18HD:1/1/1 | 14.6 | 15.7 |
| C8HD | 12.4 | 16.6 |
| C16HD | 18.4 | 14.4 |
| C18HD | 10.6 | 17.9 |
| Samples 1 | Tmelt on Heating/°C (ΔH/mJ mg−1) | Tcrystallization on Cooling/°C (ΔH/mJ mg−1) |
|---|---|---|
| RB-C8HD/C16HD/C18HD 10/1/1 | 71.4 (18.3) | 72.2 (18.0) |
| RB-C8HD/C16HD/C18HD 1/1/1 | 74.3 (13.4) | 87.4 (13.3) |
| RB-C8HD | 78.4 (16.2) | 74.2 (16.3) |
| RB-C16HD | 111.2 (35.1) | 106.2 (34.2) |
| RB-C18HD | 10.9 (28.5) | 105.2 (28.3) |
| PU-C8HD/C16HD/C18HD 10/1/1 | 39.5 (10.9) | 43.3 (10.9) |
| PU-C8HD/C16HD/C18HD 1/1/1 | 54.8 (5.5) | 50.4 (5.8) |
| PU-C8HD | 42.2 (6.1) | 32.2 (6.4) |
| PU-C16HD | 94.4 (17.0) | 84.3 (15.9) |
| PU-C18HD | 81.3 (12.3) | 84.5 (12.4) |
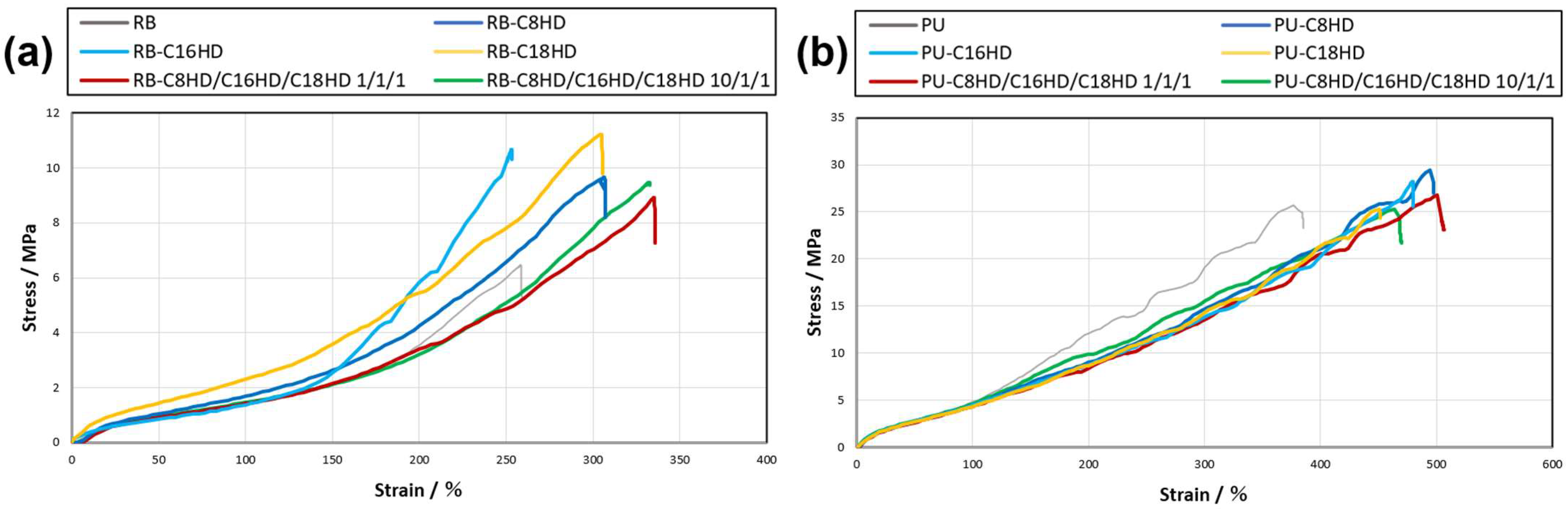
| Samples | Young’s Modulus/MPa | Tensile Strength/MPa (Elongation at Yield/%) |
|---|---|---|
| RB | 9.8 × 10−1 | 6.4 (258) |
| RB-C8HD/C16HD/C18HD 10/1/1 | 1.0 | 9.5 (332) |
| RB-C8HD/C16HD/C18HD 1/1/1 | 1.0 | 8.8 (335) |
| RB-C8HD | 1.3 | 9.8 (305) |
| RB-C16HD | 1.1 | 1.0 × 10 (253) |
| RB-C18HD | 1.8 | 1.1 × 10 (305) |
| PU | 5.2 | 2.5 × 10 (380) |
| PU-C8HD/C16HD/C18HD 10/1/1 | 4.6 | 2.5 × 10 (464) |
| PU-C8HD/C16HD/C18HD 1/1/1 | 3.7 | 2.7 × 10 (501) |
| PU-C8HD | 4.3 | 2.8 × 10 (497) |
| PU-C16HD | 4.0 | 2.8 × 10 (479) |
| PU-C18HD | 3.6 | 2.5 × 10 (451) |
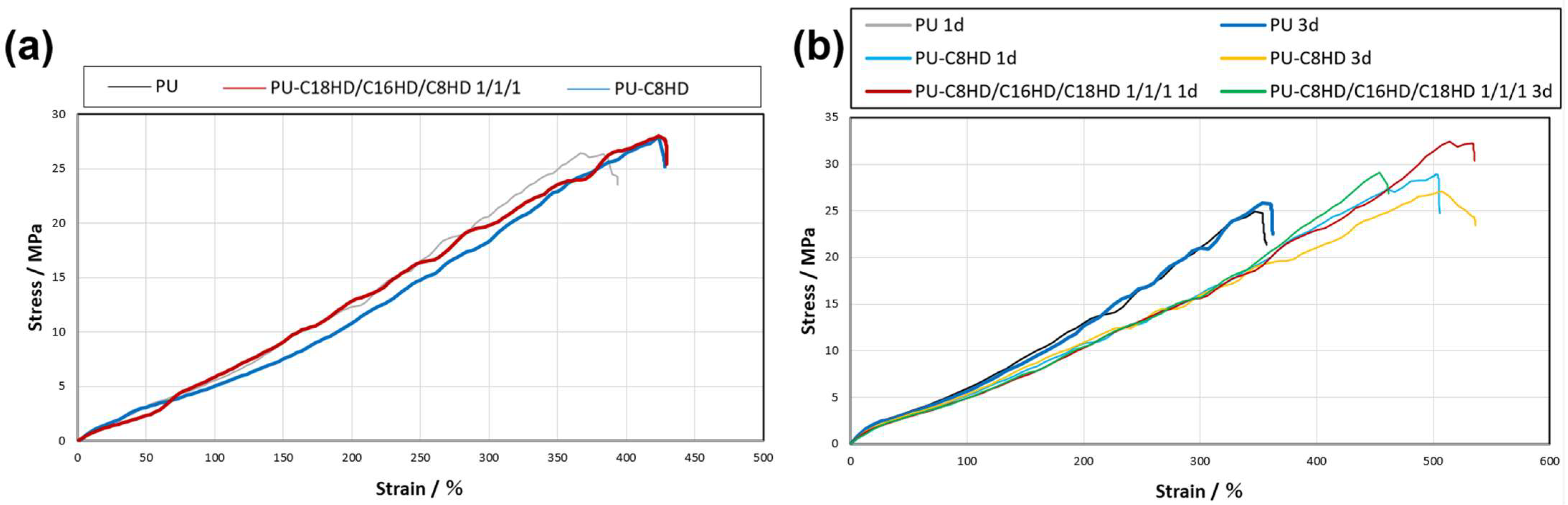
| Samples 1 | Young’s Modulus/MPa | Tensile Strength/MPa (Elongation at Yield/%) |
|---|---|---|
| PU (before heat treatment) | 5.2 | 2.5 × 10 (380) |
| PU 1d | 6.3 | 2.5 × 10 (347) |
| PU 3d | 6.2 | 2.5 × 10 (361) |
| PU-C8HD/C16HD/C18HD 1/1/1 1d | 5.0 | 2.9 × 10 (503) |
| PU-C8HD/C16HD/C18HD 1/1/1 3d | 5.2 | 2.7 × 10 (514) |
| PU-C8HD 1d | 5.0 | 3.2 × 10 (521) |
| PU-C8HD 3d | 5.0 | 2.9 × 10 (447) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohsedo, Y.; Takagi, C. Development of Low-Molecular-Weight Gelator/Polymer Composite Materials Utilizing the Gelation and Swelling Process of Polymeric Materials. Gels 2024, 10, 298. https://doi.org/10.3390/gels10050298
Ohsedo Y, Takagi C. Development of Low-Molecular-Weight Gelator/Polymer Composite Materials Utilizing the Gelation and Swelling Process of Polymeric Materials. Gels. 2024; 10(5):298. https://doi.org/10.3390/gels10050298
Chicago/Turabian StyleOhsedo, Yutaka, and Chinatsu Takagi. 2024. "Development of Low-Molecular-Weight Gelator/Polymer Composite Materials Utilizing the Gelation and Swelling Process of Polymeric Materials" Gels 10, no. 5: 298. https://doi.org/10.3390/gels10050298
APA StyleOhsedo, Y., & Takagi, C. (2024). Development of Low-Molecular-Weight Gelator/Polymer Composite Materials Utilizing the Gelation and Swelling Process of Polymeric Materials. Gels, 10(5), 298. https://doi.org/10.3390/gels10050298






