Preparation of Gel Forming Polymer-Based Sprays for First Aid Care of Skin Injuries
Abstract
1. Introduction
2. Results and Discussion
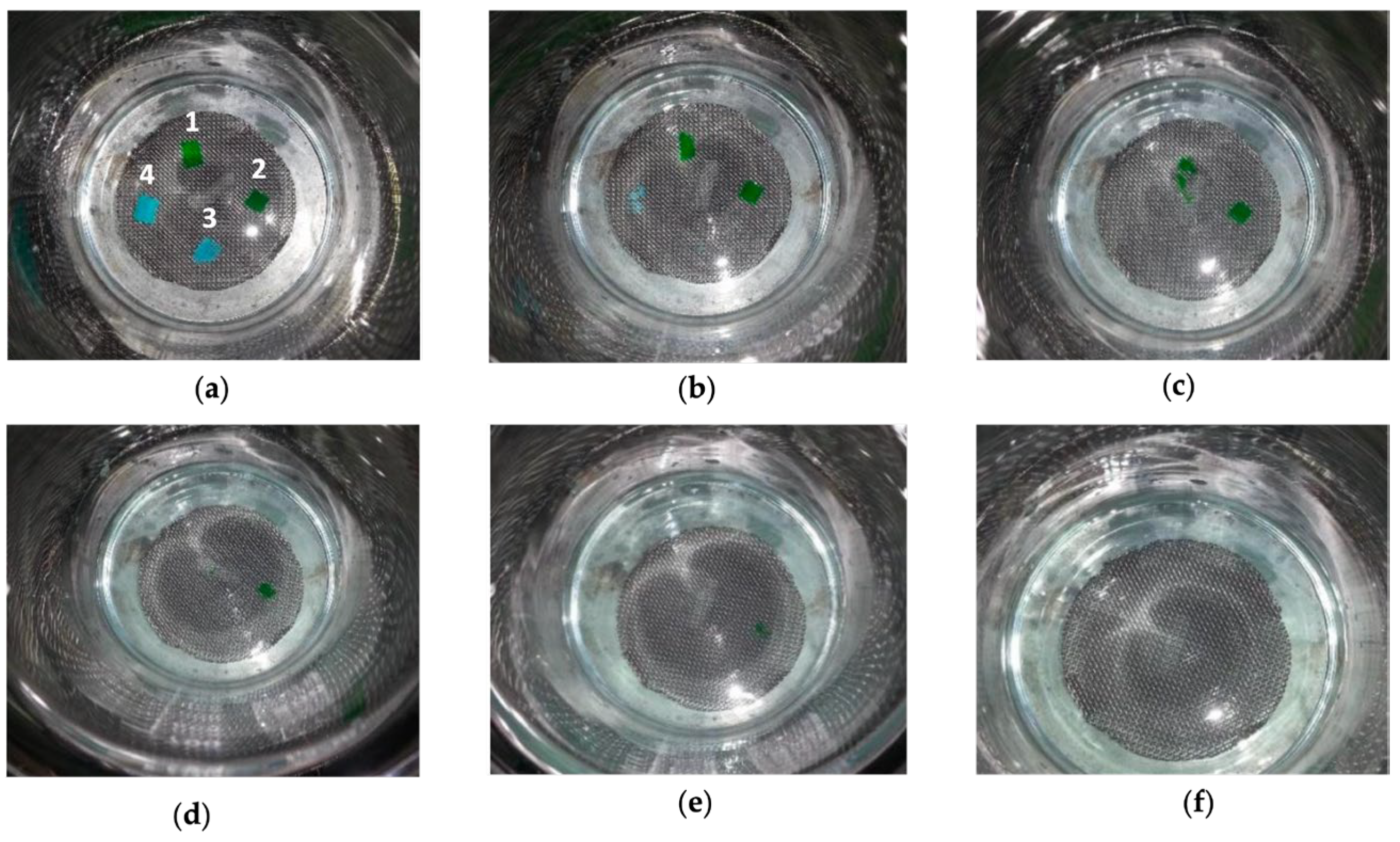
2.1. Evaluation of Spraying Capacity and Adhesion to Biological Substrates
2.2. Characterization Techniques
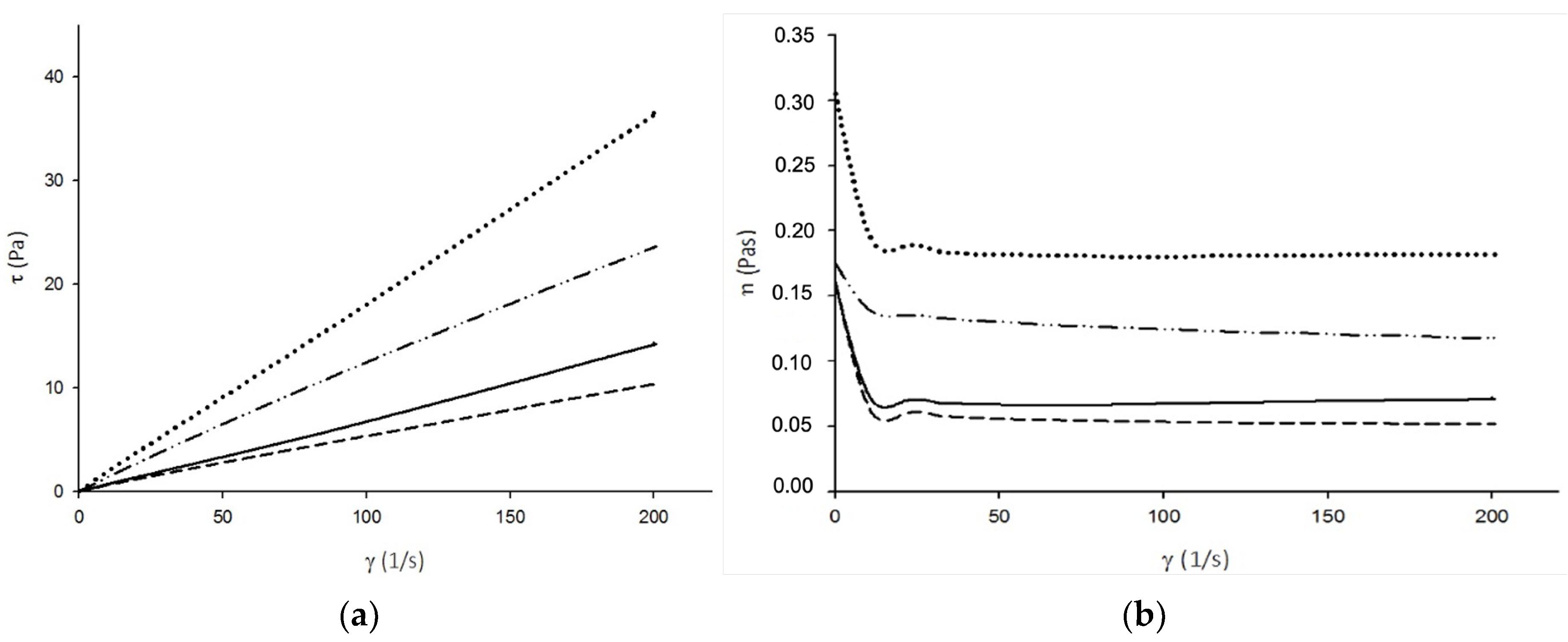
2.2.1. Rheology
2.2.2. Solubility Test in Saline Solution
2.2.3. Dynamic–Mechanical Thermal Analysis
2.2.4. Surface Free Energy Assessment
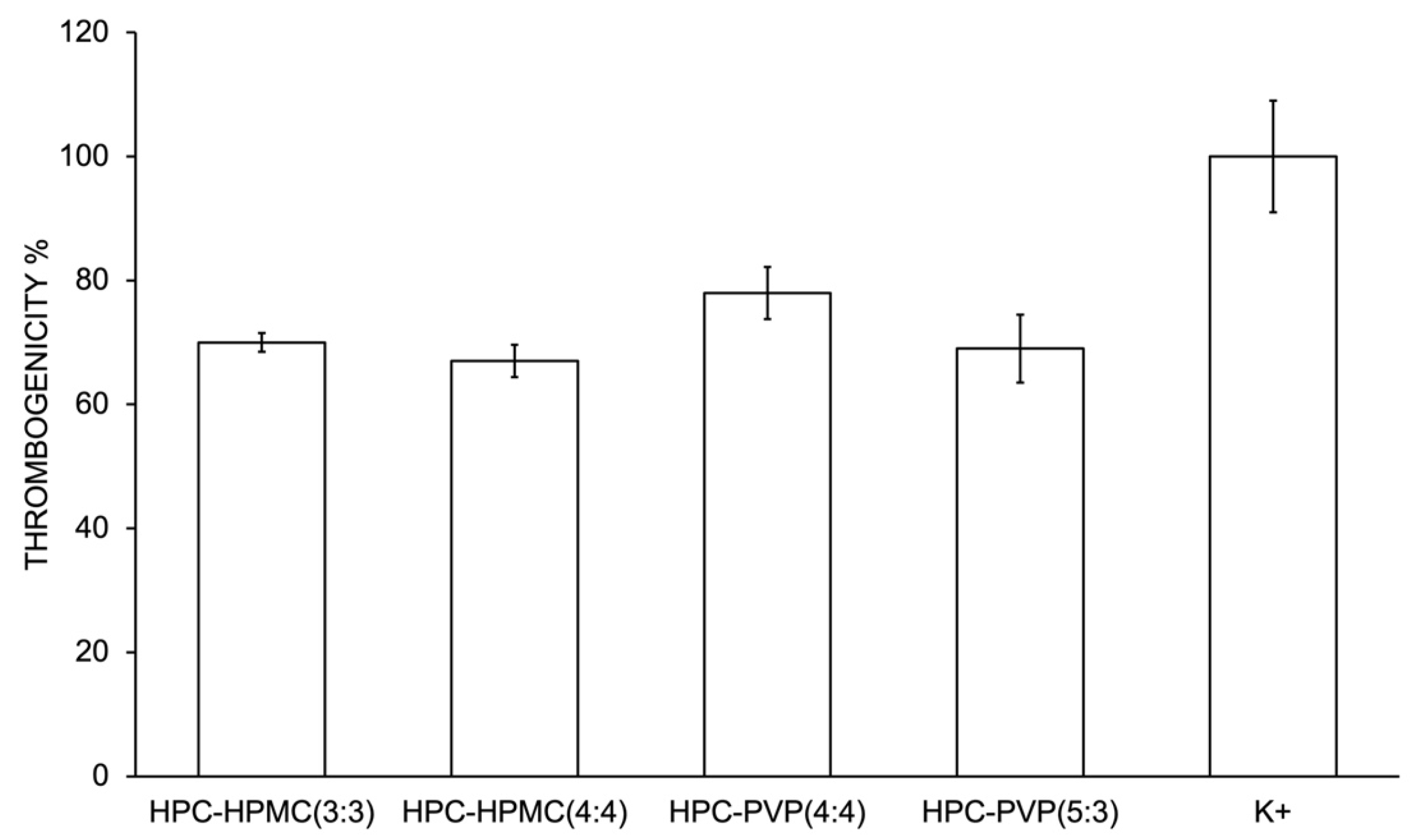
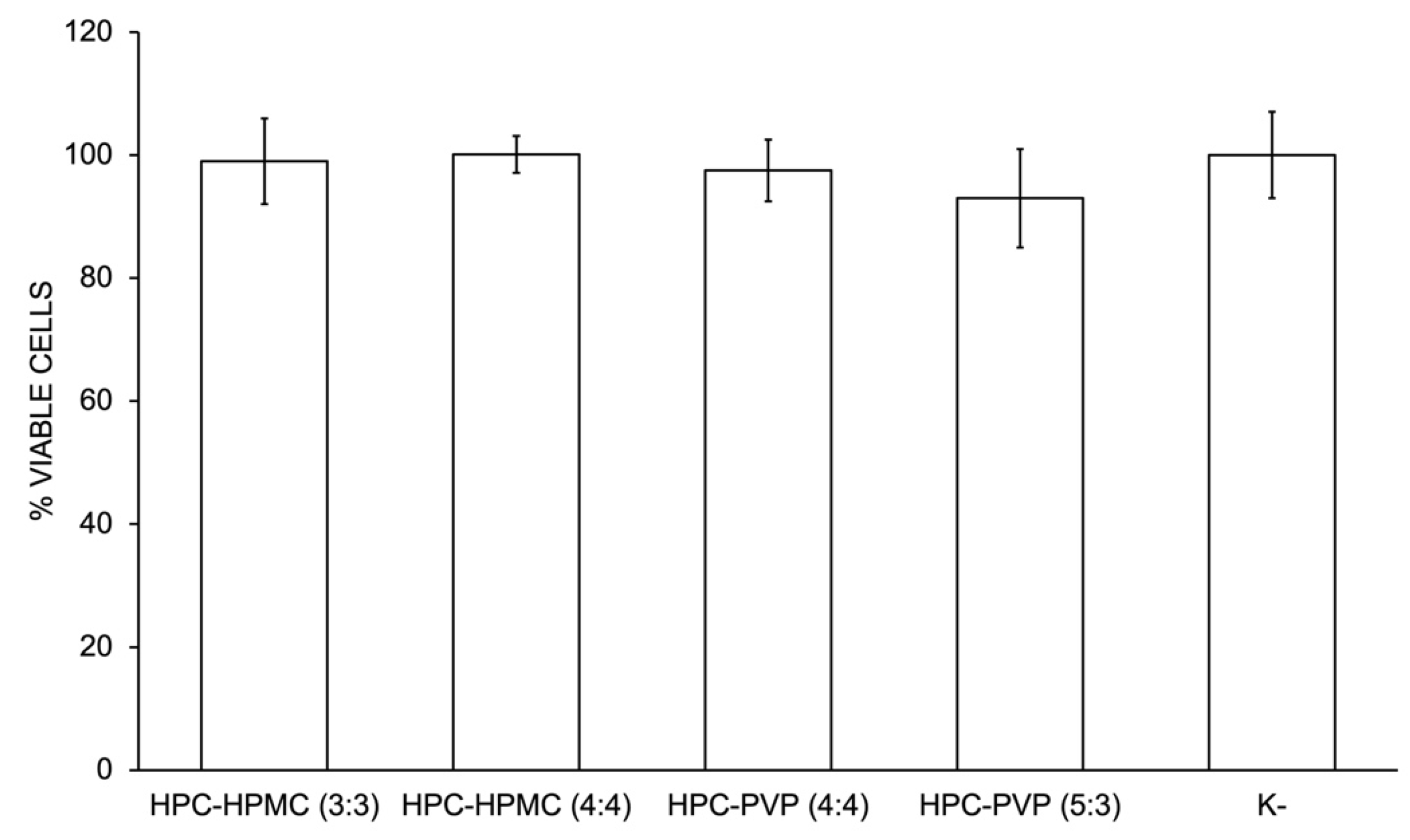
2.2.5. Characterization of the Biological Properties
Thrombogenicity Assessment
Evaluation of Materials Cytotoxicity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Polymeric Solutions and Films Preparation
4.2.2. Evaluation of Spraying Suitability and In Vitro Adhesion to Biological Substrates
4.3. Characterization Techniques
4.3.1. Rheology
4.3.2. Solubility Test in Saline Solution
4.3.3. Dynamic–Mechanical Thermal Analysis
4.3.4. Surface Tension and Surface Free Energy Assessment
4.3.5. Characterization of the Biological Properties
Thrombogenicity Assessment
Evaluation of Materials Cytotoxicity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhoyar, S.D.; Malhotra, K.; Madke, B. Dressing Materials: A Comprehensive Review. J. Cutan. Aesthet. Surg. 2023, 16, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Atkin, L.; Stephenson, J.; Bateman, S.D. Foam Dressings: A Review of the Literature and Evaluation of Fluid-Handling Capacity of Four Leading Foam Dressings. Wounds UK 2015, 11, 75–81. [Google Scholar]
- Qu, Z.; Wang, Y.; Niu, Q.; Wen, W.; Ding, G.; Liu, W. Alginate Dressings in Wound Care: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Exp. Dermatol. Res. 2023, 14, 640. [Google Scholar]
- Su, L.; Jia, Y.; Fu, L.; Guo, K.; Xie, S. The Emerging Progress on Wound Dressings and Their Application in Clinic Wound Management. Heliyon 2023, 9, e22520. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The Production and Application of Hydrogels for Wound Management: A Review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Daunton, C.; Kothari, S.; Smith, L.; Steele, D. A History of Materials and Practices for Wound Management. Wound Pract. Res. Aust. J. Wound Manag. 2012, 20, 174–186. [Google Scholar]
- Bakhrushina, E.O.; Shumkova, M.M.; Sergienko, F.S.; Novozhilova, E.V.; Demina, N.B. Spray Film-Forming Systems as Promising Topical in Situ Systems: A Review. Saudi Pharm. J. 2023, 31, 154–169. [Google Scholar] [CrossRef]
- Brodovsky, S.; Dagan, R.; Ben-Bassatt, M. Nobecutane Spray as a Temporary Dressing of Skin Graft Donor Sites. J. Dermatol. Surg. Oncol. 1986, 12, 386–387. [Google Scholar] [CrossRef]
- Ellerker, A.G. Nobecutane as a wound dressing. Lancet 1955, 265, 200. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Du, S.; Lu, Y.; Li, Y.; Wang, D. The Application of Biomedical Polymer Material Hydroxy Propyl Methyl Cellulose(HPMC) in Pharmaceutical Preparations. J. Chem. Pharm. Res. 2014, 6, 155–160. [Google Scholar]
- Takeuchi, Y.; Umemura, K.; Tahara, K.; Takeuchi, H. Formulation Design of Hydroxypropyl Cellulose Films for Use as Orally Disintegrating Dosage Forms. J. Drug Deliv. Sci. Technol. 2018, 46, 93–100. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly(N-Vinyl Pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.S.; Prabhakar, M.N.; Reddy, V.N.; Sathyamaiah, G.; Maruthi, Y.; Subha, M.C.S.; Chowdoji Rao, K. Miscibility Studies of Hydroxypropyl Cellulose/Poly(Vinyl Pyrrolidone) in Dilute Solutions and Solid State. J. Appl. Polym. Sci. 2012, 125, 2289–2296. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Yeh, J.; Chen, C.; Huang, K.S.; Nien, Y.H.; Chen, J.L.; Huang, P.Z. Synthesis, Characterization, and Application of PVP/Chitosan Blended Polymers. J. Appl. Polym. Sci. 2006, 101, 885–891. [Google Scholar] [CrossRef]
- Kadajji, V.G.; Betageri, G.V. Water Soluble Polymers for Pharmaceutical Applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef]
- George, H.F.; Qureshi, F. Newton’s Law of Viscosity, Newtonian and Non-Newtonian Fluids. In Encyclopedia of Tribology; Springer: Boston, MA, USA, 2013; pp. 2416–2420. [Google Scholar]
- Sarode, A.; Wang, P.; Cote, C.; Worthen, D.R. Low-Viscosity Hydroxypropylcellulose (HPC) Grades SL and SSL: Versatile Pharmaceutical Polymers for Dissolution Enhancement, Controlled Release, and Pharmaceutical Processing. AAPS PharmSciTech 2013, 14, 151–159. [Google Scholar] [CrossRef]
- Swei, J.; Talbot, J.B. Viscosity Correlation for Aqueous Polyvinylpyrrolidone (PVP) Solutions. J. Appl. Polym. Sci. 2003, 90, 1153–1155. [Google Scholar] [CrossRef]
- Osborne, D.W.; Mumper, R.J. Atrix Laboratories Inc. Pharmaceutical Gel and Aerosol Formulations and Methods to Administer the Same to Skin and Mucosal Surfaces. US Patent 6432415B1, 13 August 2002. [Google Scholar]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.O.; Sykora, M.A.; Mahaguna, V. Method to Recover a Lipophilic Drug from Hydroxypropyl Methylcellulose Matrix Tablets. AAPS PharmSciTech 2001, 2, 8. [Google Scholar] [CrossRef]
- Mafi, R.; Mirabedini, S.M.; Attar, M.M.; Moradian, S. Cure Characterization of Epoxy and Polyester Clear Powder Coatings Using Differential Scanning Calorimetry (DSC) and Dynamic Mechanical Thermal Analysis (DMTA). Prog. Org. Coat. 2005, 54, 164–169. [Google Scholar] [CrossRef]
- Lim, W.-S.; Choi, J.-W.; Iwata, Y.; Koseki, H. Thermal Characteristics of Hydroxypropyl Methyl Cellulose. J. Loss Prev. Process Ind. 2009, 22, 182–186. [Google Scholar] [CrossRef]
- Buera, M.D.P.; Levi, G.; Karel, M. Glass Transition in Poly(Vinylpyrrolidone): Effect of Molecular Weight and Diluents. Biotechnol. Prog. 1992, 8, 144–148. [Google Scholar] [CrossRef]
- Turner, D.T.; Schwartz, A. The Glass Transition Temperature of Poly(N-Vinyl Pyrrolidone) by Differential Scanning Calorimetry. Polymer 1985, 26, 757–762. [Google Scholar] [CrossRef]
- Mu, Y.; Sun, Q.; Wan, X. Impact of Polymer Chemistry on the Application of Polyurethane/Ureas in Organic Thin Film Transistors. RSC Appl. Polym. 2023, 1, 190–203. [Google Scholar] [CrossRef]
- Venkatraman, S.; Gale, R. Skin Adhesives and Skin Adhesion. 1. Transdermal Drug Delivery Systems. Biomaterials 1998, 19, 1119–1136. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Nikolopoulos, P. Wettability and Interfacial Interactions in Bioceramic-body-liquid Systems. J. Biomed. Mater. Res. 1995, 29, 421–429. [Google Scholar] [CrossRef]
- Eudier, F.; Savary, G.; Grisel, M.; Picard, C. Skin Surface Physico-Chemistry: Characteristics, Methods of Measurement, Influencing Factors and Future Developments. Adv. Colloid Interface Sci. 2019, 264, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Braune, S.; Latour, R.A.; Reinthaler, M.; Landmesser, U.; Lendlein, A.; Jung, F. In Vitro Thrombogenicity Testing of Biomaterials. Adv. Healthc. Mater. 2019, 8, 1900527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, X.; Nomizu, M.; Nishi, N. Blood Compatible Aspects of DNA-Modified Polysulfone Membrane—Protein Adsorption and Platelet Adhesion. Biomaterials 2003, 24, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Cernadas, T.; Morgado, S.; Alves, P.; Gonçalves, F.A.M.M.; Correia, T.R.; Correia, I.J.; Ferreira, P. Preparation of Functionalized Poly(Caprolactone Diol)/Castor Oils Blends to Be Applied as Photocrosslinkable Tissue Adhesives. J. Appl. Polym. Sci. 2020, 137, 49092. [Google Scholar] [CrossRef]
- Poussard, L.; Burel, F.; Couvercelle, J.P.; Lesouhaitier, O.; Merhi, Y.; Tabrizian, M.; Bunel, C. In Vitro Thrombogenicity Investigation of New Water-Dispersible Polyurethane Anionomers Bearing Carboxylate Groups. J. Biomater. Sci. Polym. Ed. 2005, 16, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.C.; Marques, D.S.; Alves, P.; Correia, T.R.; Correia, I.J.; Baptista, C.M.S.G.; Ferreira, P. Synthesis, Functionalization and Characterization of UV-Curable Lactic Acid Based Oligomers to Be Used as Surgical Adhesives. React. Funct. Polym. 2015, 94, 43–54. [Google Scholar] [CrossRef]
- Imai, Y.; Nose, Y. A New Method for Evalution of Antithrombogenicity of Materials. J. Biomed. Mater. Res. 1972, 6, 165–172. [Google Scholar] [CrossRef]
- ISO 10993-4; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. International Standard Organization (ISO): Geneva, Switzerland, 2017.
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Standard Organization (ISO): Geneva, Switzerland, 2009.

| Formulation | Properties Assessment | |||||
|---|---|---|---|---|---|---|
| Ease of Spraying | Detachability | Flexibility | Adhesion to Biological Substrate | Washability with Saline | ||
| (a) HPC-HPMC (3:3) |  | +++ | +++ | ++ | +++ | ++ |
| (b) HPC-HPMC (4:4) | ++ | +++ | ++ | +++ | + | |
| (c) HPC-PVP (4:4) | +++ | ++ | +++ | +++ | +++ | |
| (d) HPC-PVP (5:3) | ++ | ++ | +++ | +++ | +++ | |
| Composition | Solubilization Time (min) |
|---|---|
| HPC-HPMC (3:3) | 12 |
| HPC-HPMC (4:4) | 18 |
| HPC-PVP (4:4) | 4 |
| HPC-PVP (5:3) | 6 |
| Sample | Surface Tension (mN/m) | Surface Energy (mN/m) | ||
|---|---|---|---|---|
| Dry skin | - | 43.7 | 35.7 | 8.0 |
| Blood | 47.5 | - | - | - |
| HPC-HPMC (3:3) | 17.21 ± 1.9 | 23.02 ± 2.2 | 5.04 ± 0.8 | 17.98 ± 2.1 |
| HPC-HPMC (4:4) | 22.45 ± 2.4 | 30.1 ± 2.6 | 1.34 ± 0.4 | 28.8 ± 2.5 |
| HPC-PVP (4:4) | 17.01 ± 0.8 | 21.8 ± 3.0 | 6.8 ± 1.4 | 15.0 ± 2.6 |
| HPC-PVP (5:3) | 19.89 ± 2.3 | 26.6 ± 2.0 | 2.6 ± 0.5 | 24.0 ± 1.9 |
| Formulation | HPC (%w/w) | HPMC (%w/w) | PVP (%w/w) | PEG (%v/v) |
|---|---|---|---|---|
| HPC-HPMC (3:3) | 3 | 3 | - | 1 |
| HPC-HPMC (4:4) | 4 | 4 | - | 1 |
| HPC-PVP (4:4) | 4 | - | 4 | 1 |
| HPC-PVP (5:3) | 5 | - | 3 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, P.; Luzio, D.; de Sá, K.; Correia, I.; Ferreira, P. Preparation of Gel Forming Polymer-Based Sprays for First Aid Care of Skin Injuries. Gels 2024, 10, 297. https://doi.org/10.3390/gels10050297
Alves P, Luzio D, de Sá K, Correia I, Ferreira P. Preparation of Gel Forming Polymer-Based Sprays for First Aid Care of Skin Injuries. Gels. 2024; 10(5):297. https://doi.org/10.3390/gels10050297
Chicago/Turabian StyleAlves, Patrícia, Diana Luzio, Kevin de Sá, Ilídio Correia, and Paula Ferreira. 2024. "Preparation of Gel Forming Polymer-Based Sprays for First Aid Care of Skin Injuries" Gels 10, no. 5: 297. https://doi.org/10.3390/gels10050297
APA StyleAlves, P., Luzio, D., de Sá, K., Correia, I., & Ferreira, P. (2024). Preparation of Gel Forming Polymer-Based Sprays for First Aid Care of Skin Injuries. Gels, 10(5), 297. https://doi.org/10.3390/gels10050297








