Rheological Properties of Weak Gel System Cross-Linked from Chromium Acetate and Polyacrylamide and Its Application in Enhanced Oil Recovery After Polymer Flooding for Heterogeneous Reservoir
Abstract
1. Introduction
2. Results and Discussion
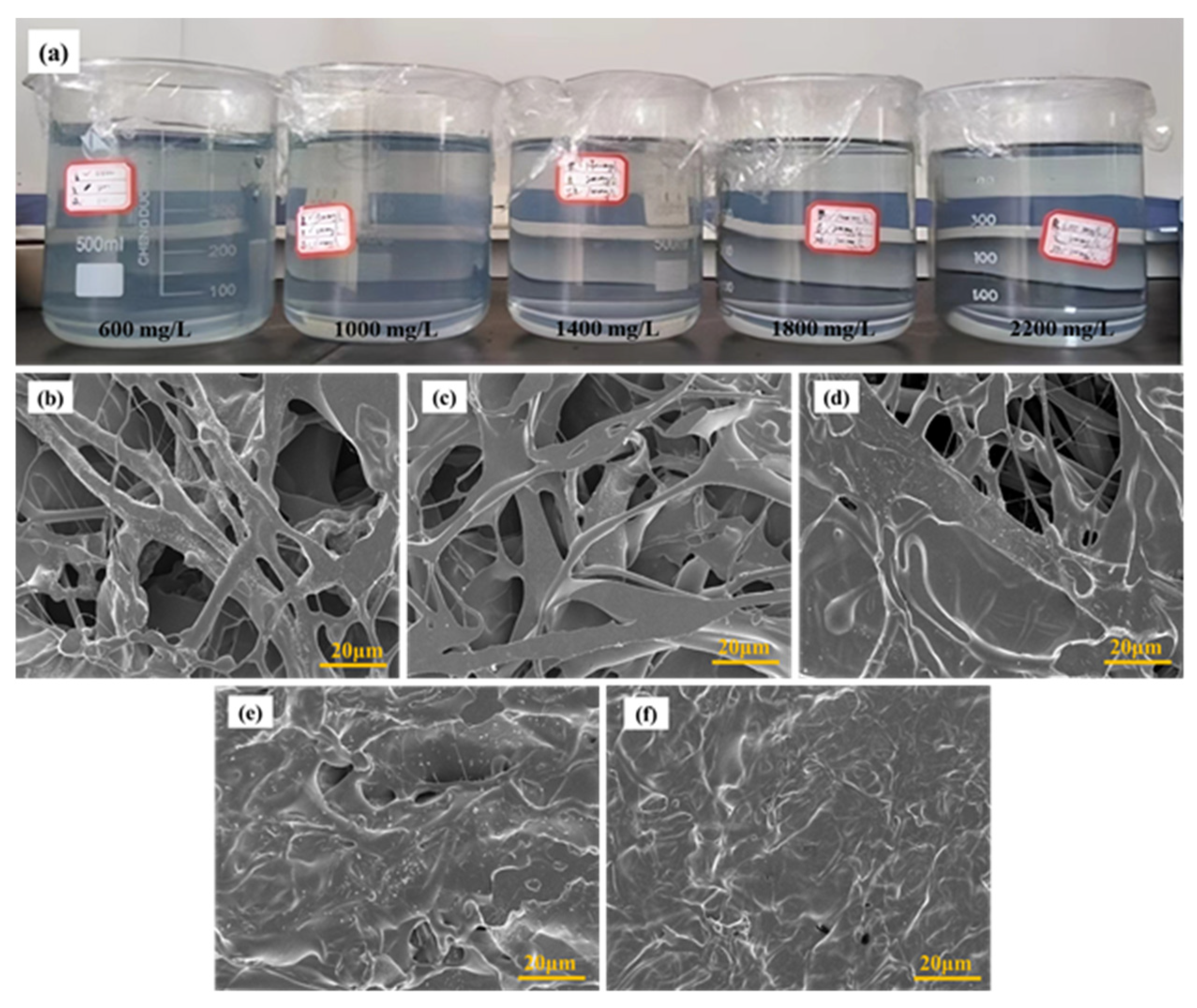
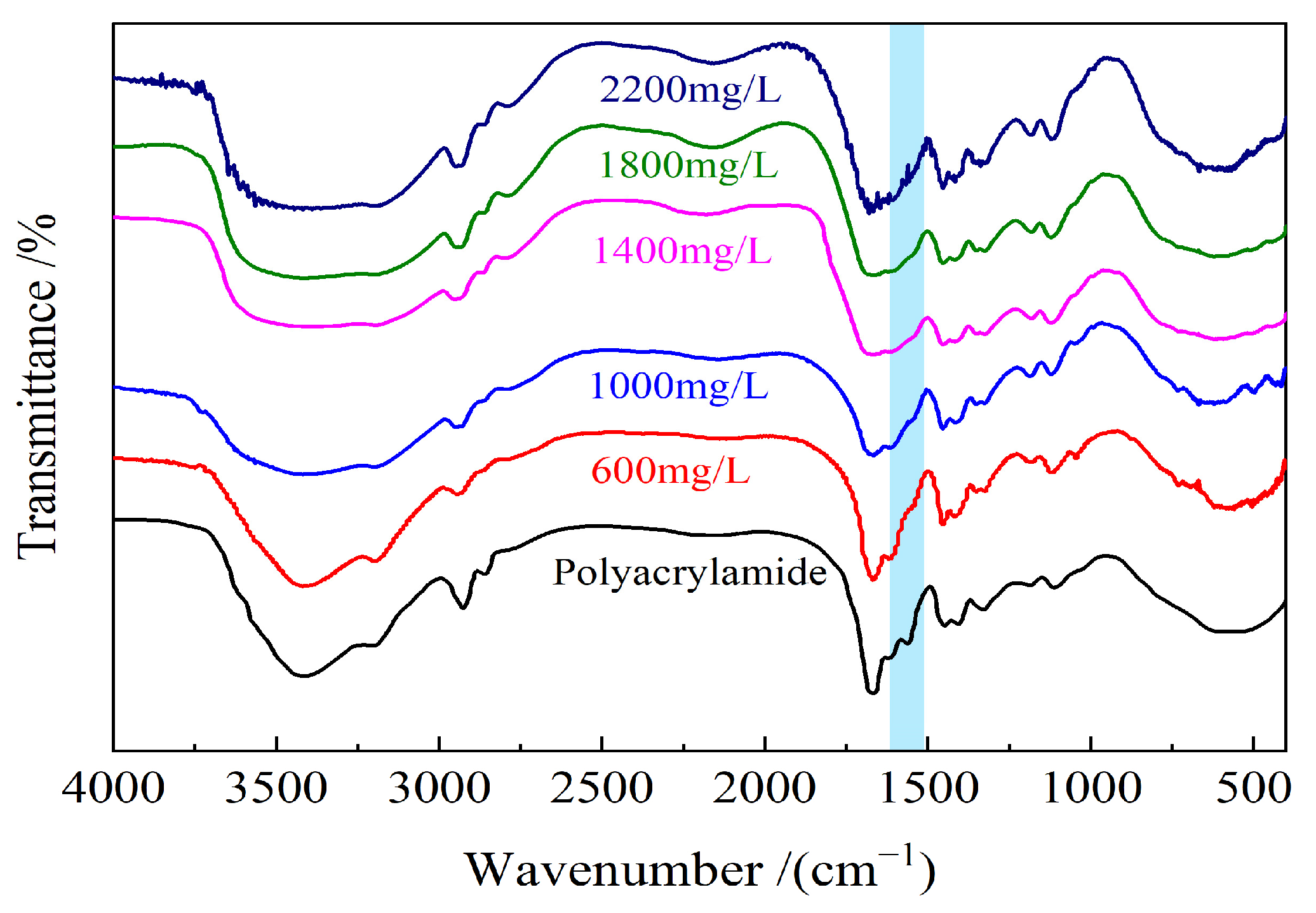
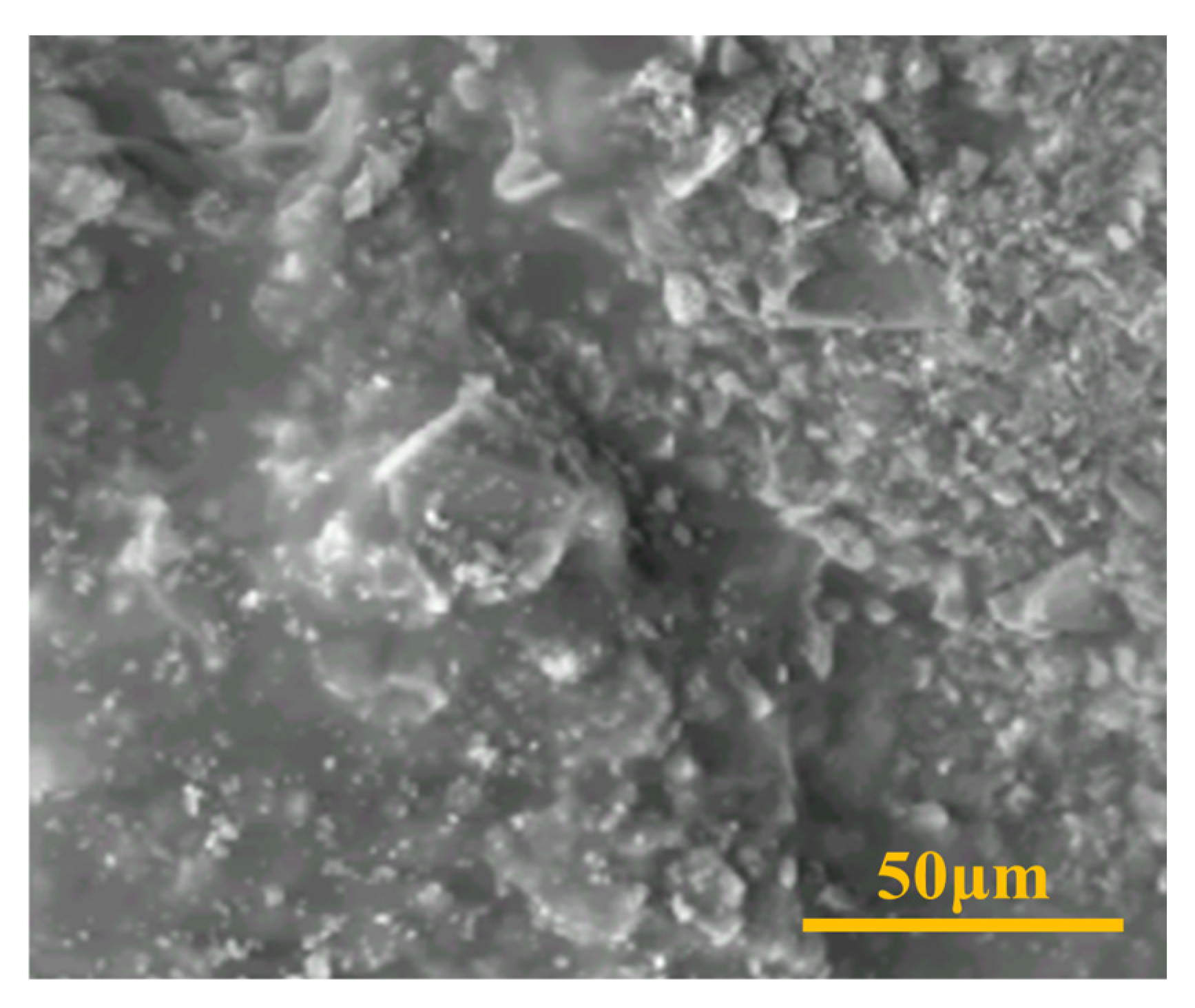
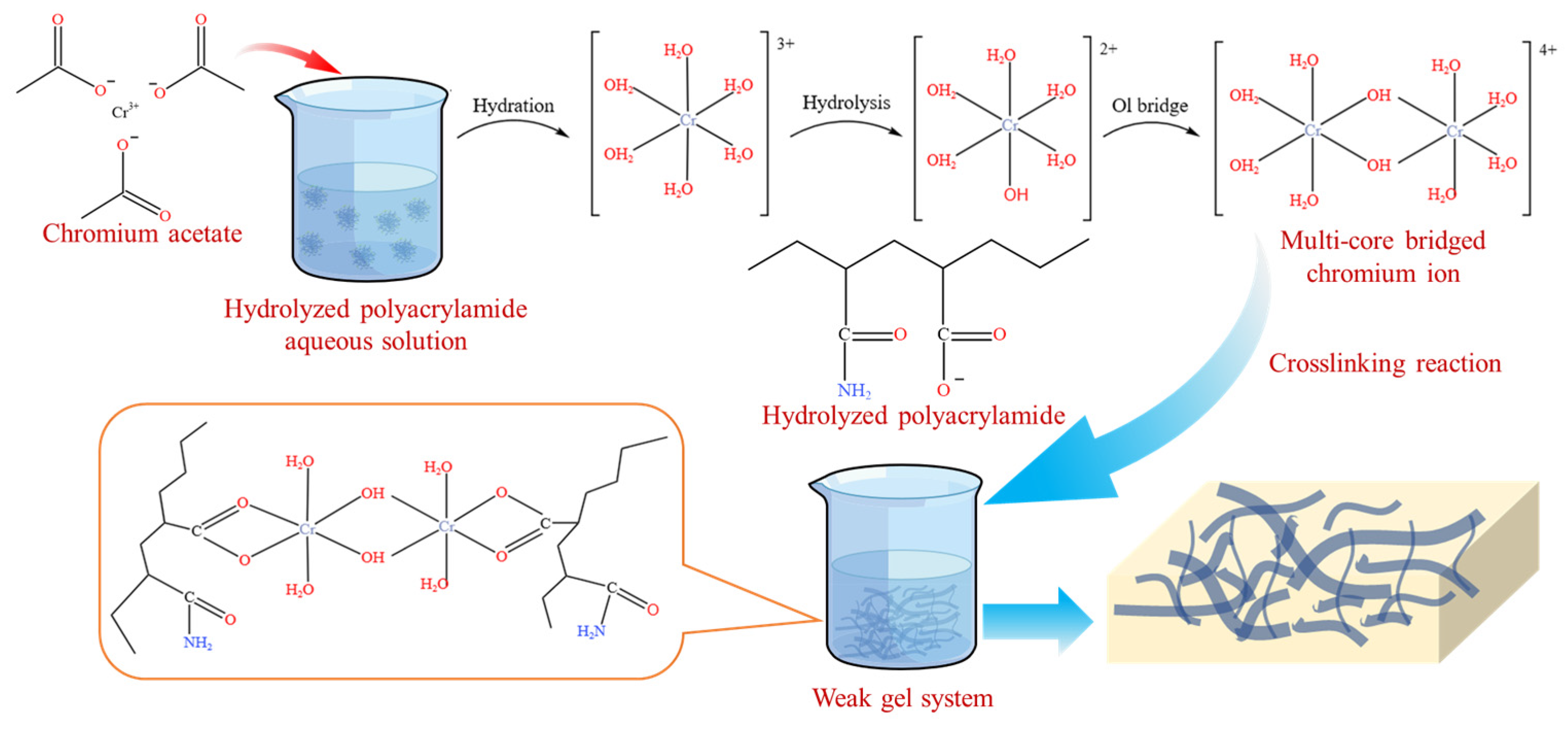
2.1. Morphology and Microstructure Characterization
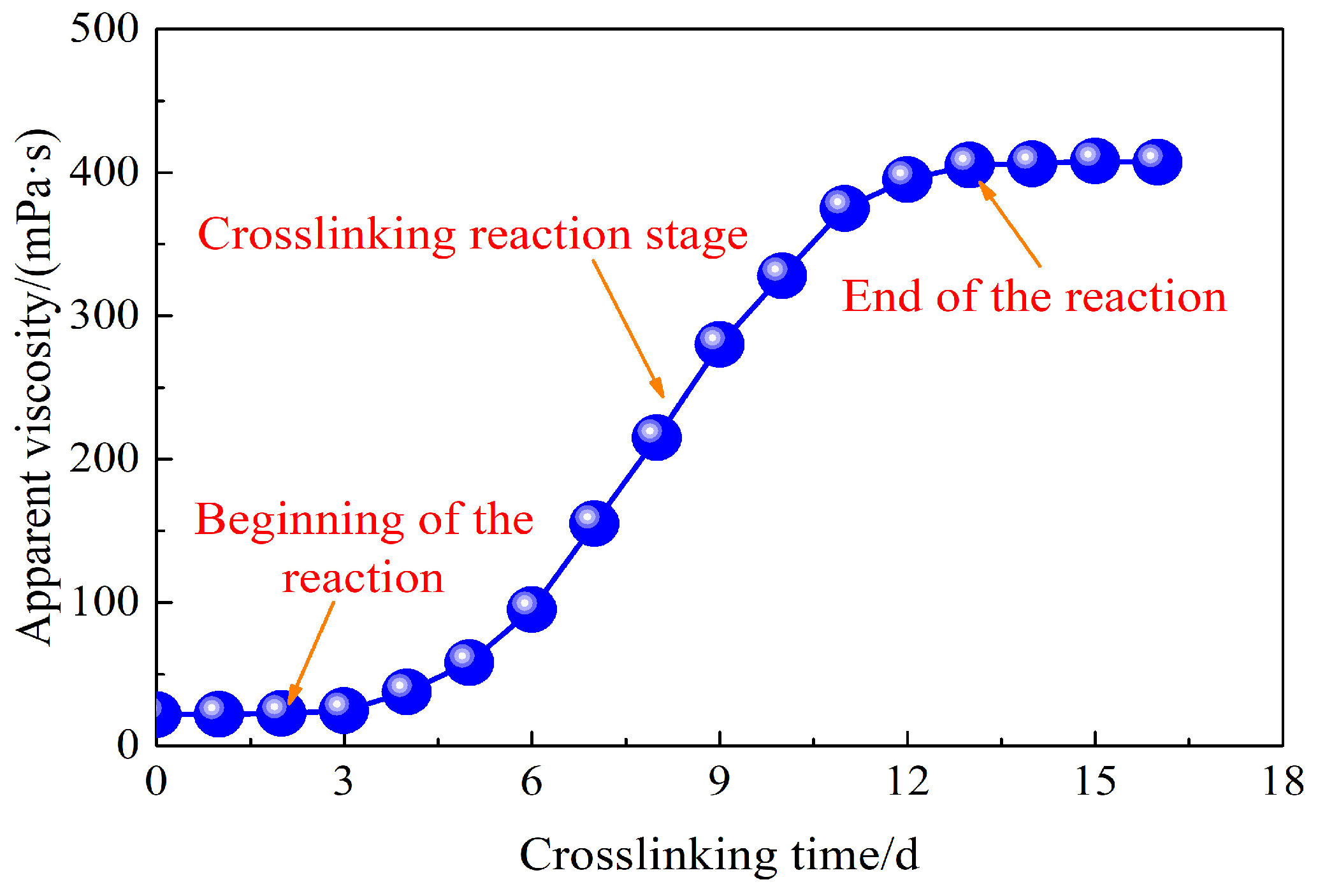
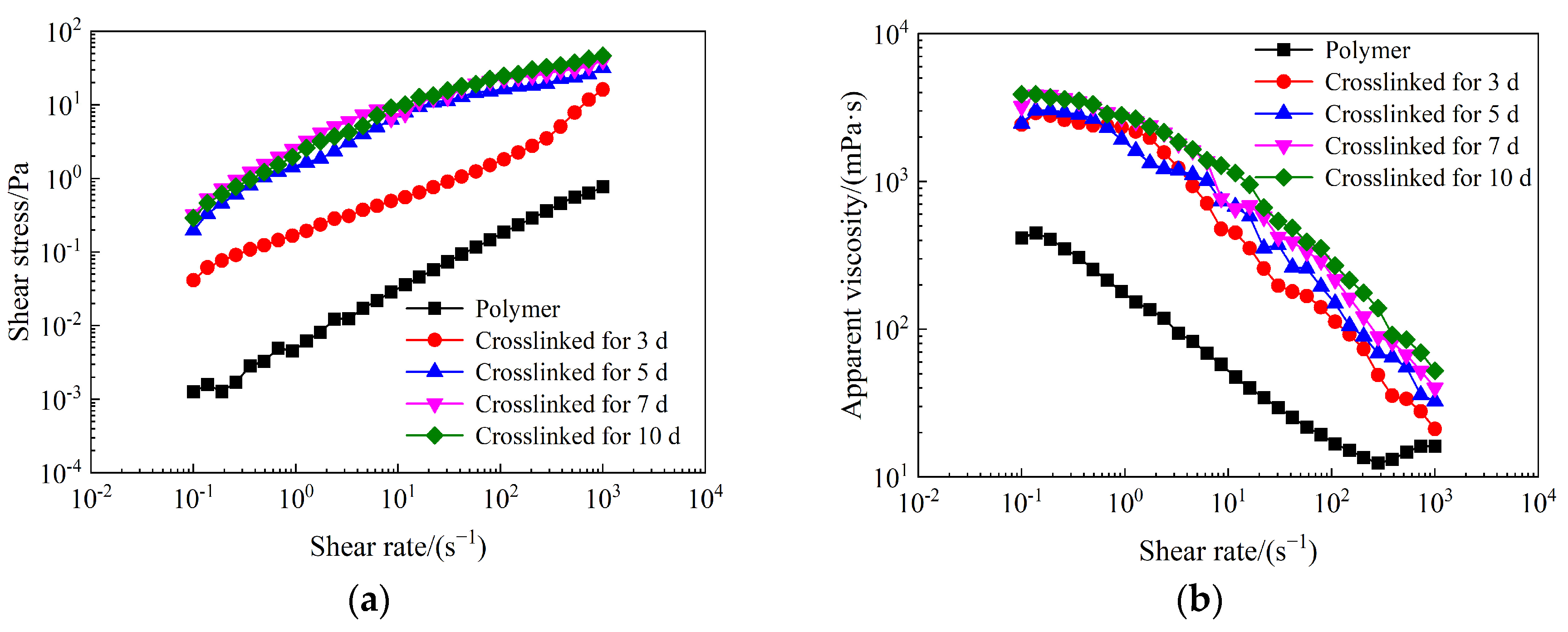
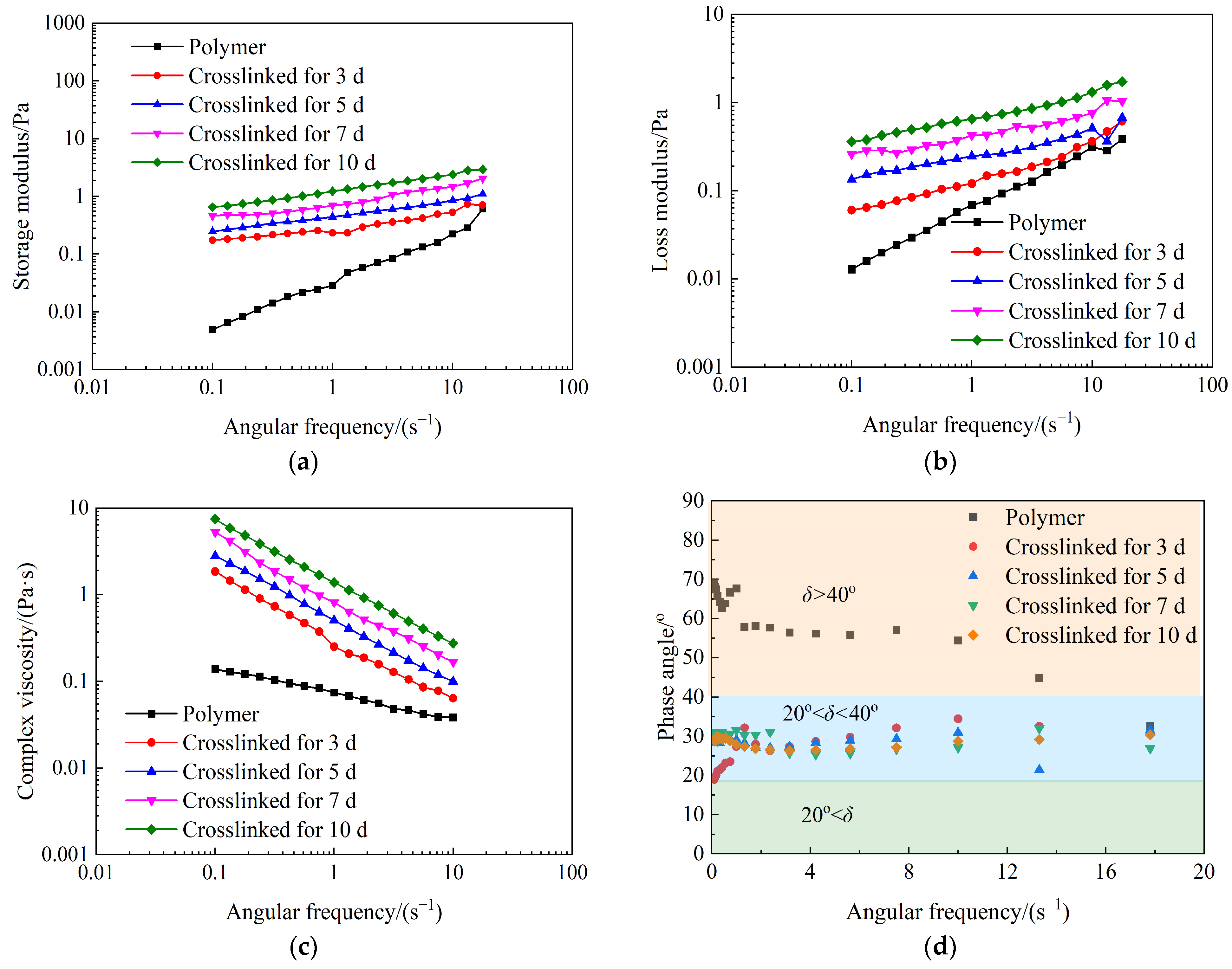
2.2. Rheological Properties of Weak Gel System
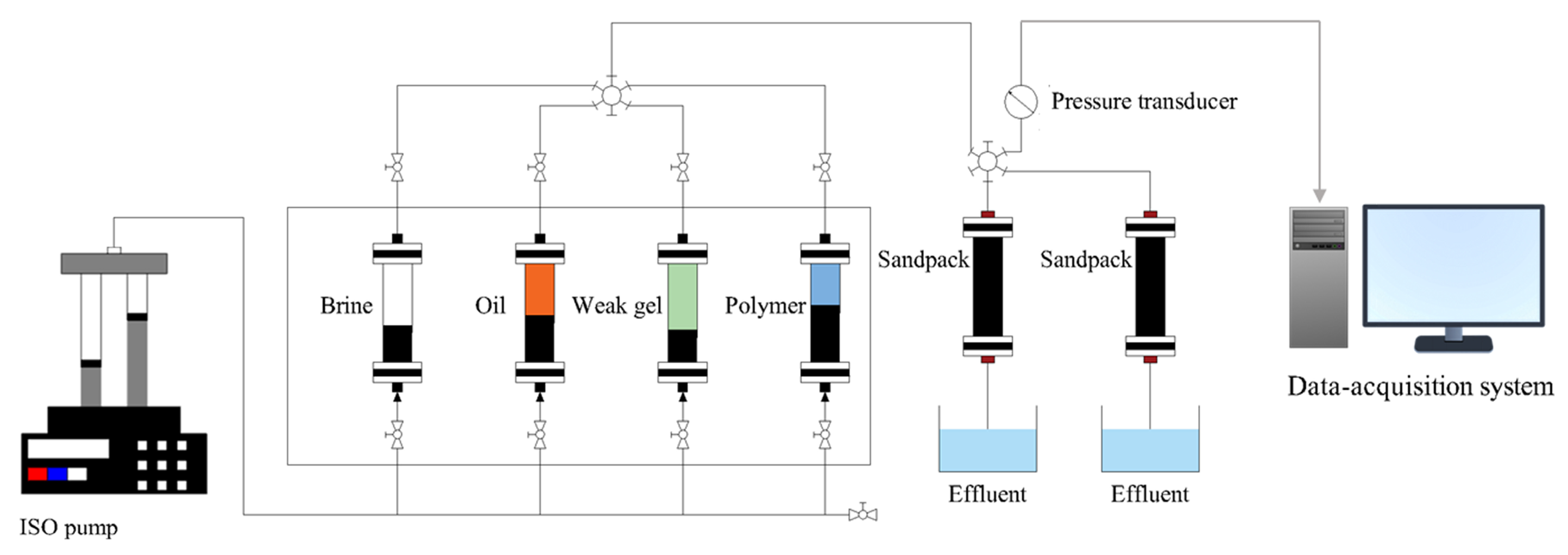
2.3. Enhanced Oil Recovery in Parallel Sandpack Models
2.3.1. Pressure Drop
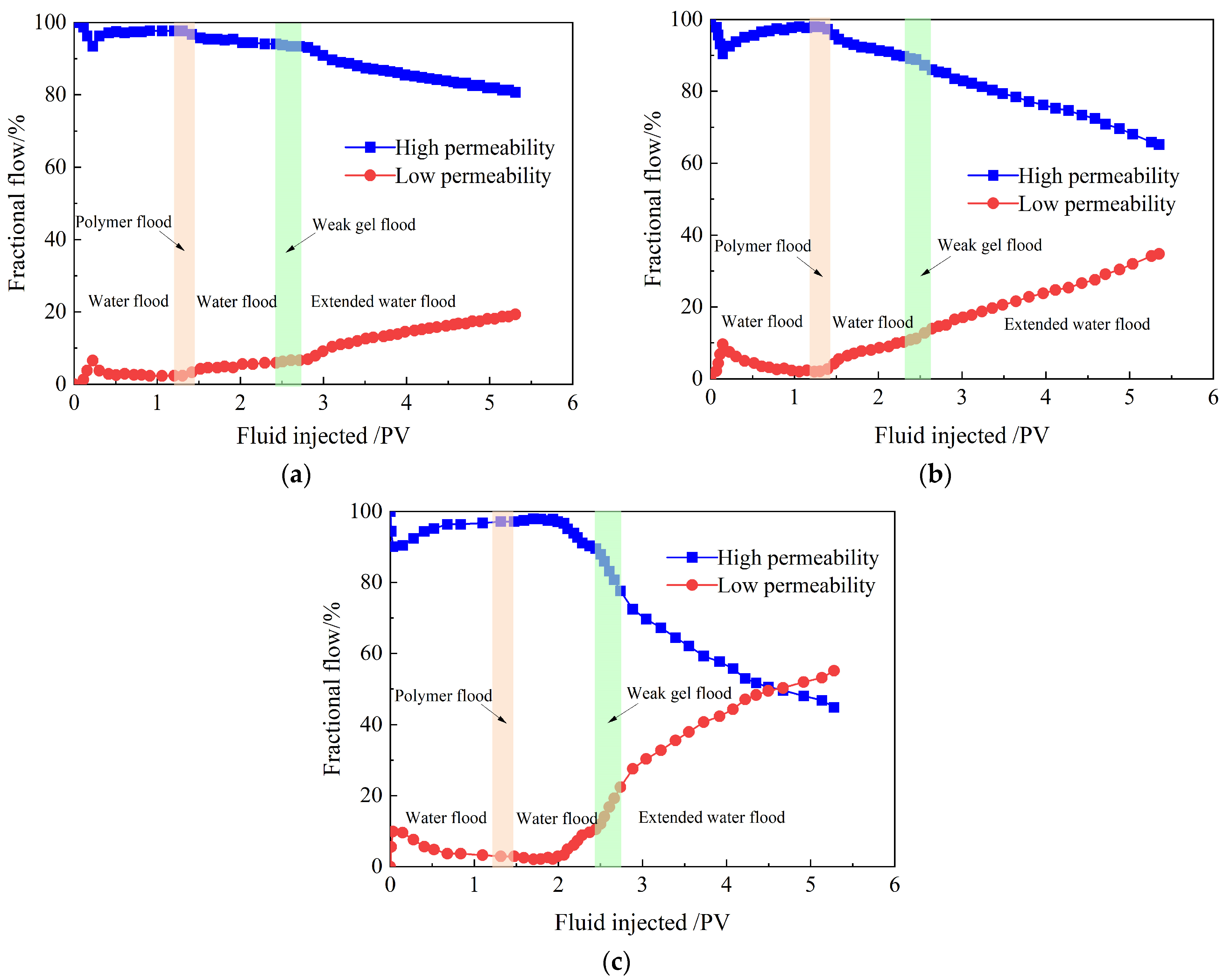
2.3.2. Fractional Flows
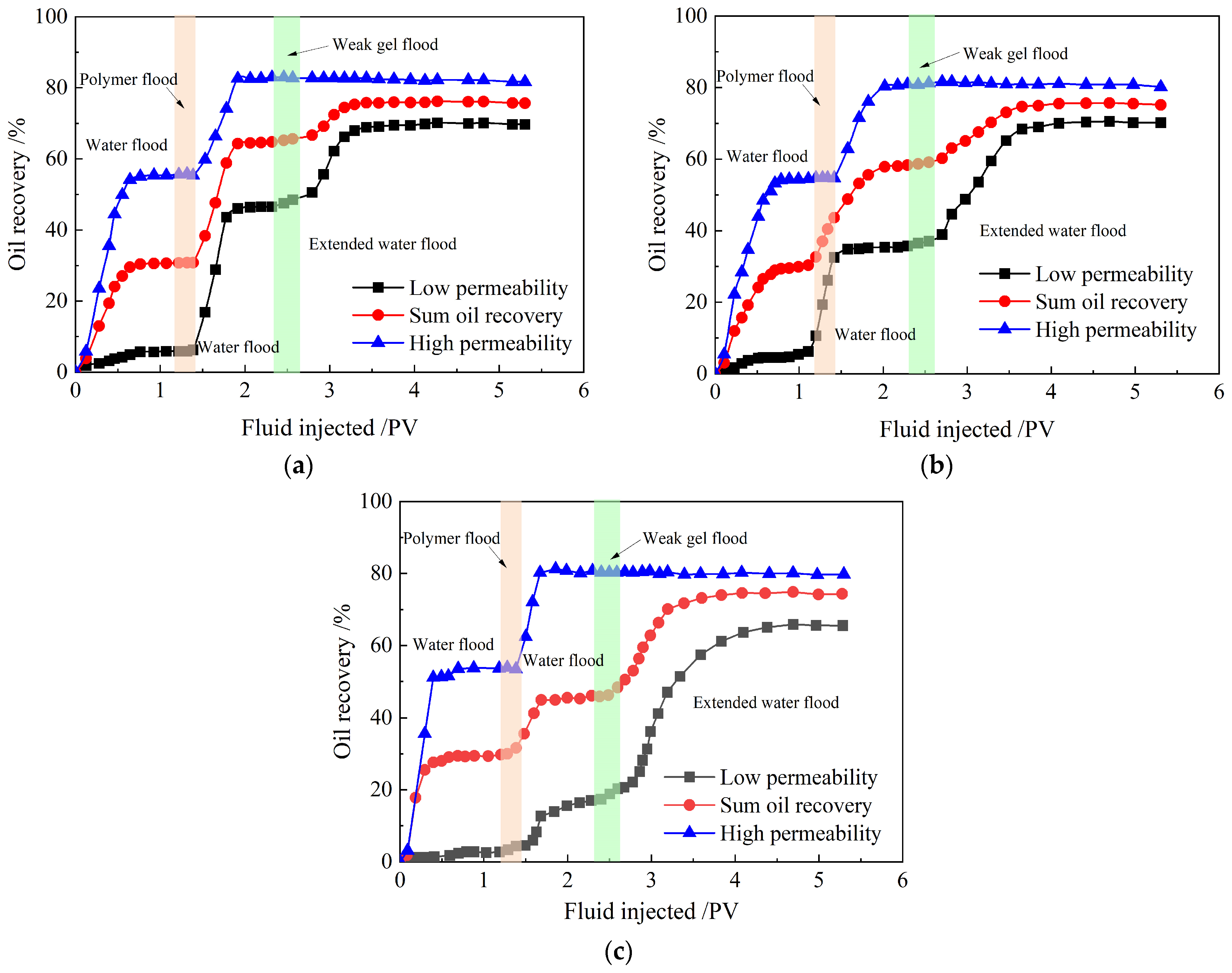
2.3.3. Enhanced Oil Recovery
3. Conclusions
- (1)
- The synthesized weak gel system flaunts a distinct network structure, endowing it with excellent shear resistance. Its apparent viscosity is notably higher than that of the partially hydrolyzed polyacrylamide solution and rises with prolonged crosslinking time. Moreover, it exhibits typical pseudo-plastic behavior.
- (2)
- The weak gel system belongs to the category of viscoelastic fluids, wherein its elastic properties prevail over the viscous ones. The weak gel system exhibits notably enhanced viscoelastic properties. As the crosslinking process extends over a longer duration, the viscoelasticity of the weak gel system experiences a progressive augmentation, manifesting a more pronounced and resilient rheological behavior.
- (3)
- Compared with the partially hydrolyzed polyacrylamide solution, the weak gel system is better suited for highly heterogeneous, low-permeability reservoirs. The more heterogeneous the reservoir, the more effective the weak gel’s conformance control. After partially hydrolyzed polyacrylamide solution flooding, as the permeability ratio climbs from 14.39 to 35.64, enhanced oil recoveries in low-permeability sandpacks soar from 22% to 48%.
4. Materials and Methods
4.1. Materials
4.2. Preparation of Weak Gel
4.3. Characterization
4.4. Rheological Experiment
4.5. Parallel Sandpack Flooding Experiment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mogensen, K.; Masalmeh, S. A review of EOR techniques for carbonate reservoirs in challenging geological settings. J. Pet. Sci. Eng. 2020, 195, 107889. [Google Scholar] [CrossRef]
- Shakiba, M.; Ayatollahi, S.; Riazi, M. Investigation of oil recovery and CO2 storage during secondary and tertiary injection of carbonated water in an Iranian carbonate oil reservoir. J. Pet. Sci. Eng. 2016, 137, 134–143. [Google Scholar] [CrossRef]
- Afra, M.J.S.; Horeh, M.B.; Rostami, B.; Norouzi, H. Laboratory investigation of oil viscosity effect during carbonated water injection: Comparison of secondary and tertiary recovery. Can. J. Chem. Eng. 2018, 96, 1805–1813. [Google Scholar] [CrossRef]
- Gandomkar, A.; Torabi, F.; Riazi, M. CO2 mobility control by small molecule thickeners during secondary and tertiary enhanced oil recovery. Can. J. Chem. Eng. 2021, 99, 1352–1362. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Long, Y.; Liu, J.; Zheng, H.; Nie, W.; Han, H. Experimental research on seepage law and migration characteristics of core-shell polymeric nanoparticles dispersion system in porous media. Polymers 2022, 14, 1803. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Long, Y.; Xuan, G.; Wang, Y.; Huang, X.; Xu, Y.; Liu, J.; Wang, B.; Song, F. Optimized hydrophobic magnetic nanoparticles stabilized Pickering emulsion for enhanced oil recovery in complex porous media of reservoir. Front. Energy Res. 2023, 11, 1212664. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Use of microorganisms in the recovery of oil from recalcitrant oil reservoirs: Current state of knowledge, technological advances and future perspectives. Front. Microbiol. 2020, 10, 2996. [Google Scholar] [CrossRef]
- Sarvestani, A.D.; Ayatollahi, S.; Moghaddam, M.B. Smart water flooding performance in carbonate reservoirs: An experimental approach for tertiary oil recovery. J. Pet. Explor. Prod. Te. 2019, 9, 2643–2657. [Google Scholar] [CrossRef]
- Shafiabadi, A.; Parsaei, R.; Rezaeiakmal, F.; Dehdari, B. Investigating the impact of wettability heterogeneity on tertiary oil recovery by foam flooding: A macroscopic visualization study. Colloid. Surface A. 2023, 675, 132022. [Google Scholar] [CrossRef]
- Kalyanaraman, N.; Arnold, C.; Gupta, A.; Tsau, J.S.; Ghahfarokhi, R.B. Stability improvement of CO2 foam for enhanced oil-recovery applications using polyelectrolytes and polyelectrolyte complex nanoparticles. J. Appl. Polym. Sci. 2017, 134, 44491. [Google Scholar] [CrossRef]
- Pogaku, R.; Fuat, N.H.M.; Sakar, S.; Cha, Z.; Musa, N.; Tajudin, D.N.A.A.; Morris, L.O. Polymer flooding and its combinations with other chemical injection methods in enhanced oil recovery. Polym.Bull. 2018, 75, 1753–1774. [Google Scholar] [CrossRef]
- Mandal, A. Chemical flood enhanced oil recovery: A review. Int. J. Oil. Gas. Coal. T. 2015, 9, 241–264. [Google Scholar] [CrossRef]
- Du, Q.; Zhao, D.; Wu, J.; Hou, J.; Wei, Z.; Shi, L.; Zhou, K.; Zheng, H.; Zhang, J.; Liu, Y. Early warning methods of chemical agent channeling in polymer-surfactant flooding reservoirs. Energy. Sci. Eng. 2024, 12, 2180–2197. [Google Scholar] [CrossRef]
- Abirov, R.; Ivakhnenko, A.P.; Abirov, Z.; Eremin, N.A. The associative polymer flooding: An experimental study. J. Pet. Explor. Prod. Te. 2020, 10, 447–454. [Google Scholar] [CrossRef]
- Lu, X.; Xie, K.; Cao, W.; Liu, Y.; Zhang, Y.; Wang, X.; Zhang, J. Enhanced oil recovery mechanisms of polymer flooding in a heterogeneous oil reservoir. Pet. Explor. Dev. 2021, 48, 169–178. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Cao, R.; Han, P.; Yan, W.; Sun, G.; Xia, H.; Xu, T. Microscopic plugging adjustment mechanism in a novel heterogeneous combined flooding system. Energy Rep. 2022, 8, 15350–15364. [Google Scholar] [CrossRef]
- Jacobsen, J.G.; Shiran, B.S.; Skauge, T.; Sorbie, K.S.; Skauge, A. Qualification of new methods for measuring in situ rheology of non-Newtonian fluids in porous media. Polymers 2020, 12, 452. [Google Scholar] [CrossRef]
- Navaie, F.; Esmaeilnezhad, E.; Choi, H.J. Effect of rheological properties of polymer solution on polymer flooding characteristics. Polymers 2022, 14, 5555. [Google Scholar] [CrossRef] [PubMed]
- Olabode, O.; Dike, H.; Olaniyan, D.; Oni, B.; Faleye, M. Experimental investigation of the effect of surfactant–polymer flooding on enhanced oil recovery for medium crude oil. Polymers 2024, 16, 1674. [Google Scholar] [CrossRef]
- Sidiq, H.; Abdulsalam, V.; Nabaz, Z. Reservoir simulation study of enhanced oil recovery by sequential polymer flooding method. Adv. Geo-Energy Res. 2019, 3, 115–121. [Google Scholar] [CrossRef]
- Azad, M.S.; Trivedi, J.J. Quantification of sor reduction during polymer flooding using extensional capillary number. SPE J. 2021, 26, 1469–1498. [Google Scholar] [CrossRef]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on polymer flooding: Rheology, adsorption, stability, and field applications of various polymer systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Seright, R.S.; Wang, D. Polymer flooding: Current status and future directions. Pet. Sci. 2023, 20, 910–921. [Google Scholar] [CrossRef]
- Ding, M.; Wang, Y.; Yuan, F.; Zhao, H.; Li, Z. A comparative study of the mechanism and performance of surfactantand alkali-polymer flooding in heavy-oil recovery. Chem. Eng. Sci. 2020, 219, 115603. [Google Scholar] [CrossRef]
- Musa, M.S.M.; Agi, A.; Nwaichi, P.I.; Ridzuan, N.; Mahat, S.Q.A.B. Simulation study of polymer flooding performance: Effect of salinity, polymer concentration in the Malay Basin. Geoenergy. Sci. Eng. 2023, 228, 211986. [Google Scholar] [CrossRef]
- Scott, A.J.; Bhicajee, P.; Kistamah, R.; Romero-Zerón, L.; Penlidis, A. Evaluating the performance of designed terpolymers for polymer flooding. Can. J. Chem. Eng. 2023, 101, 5072–5086. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, D.; Li, Y.; He, J.; Zhang, C. A review of crude oil emulsification and multiphase flows in chemical flooding. Energy. Sci. Eng. 2023, 11, 1484–1500. [Google Scholar] [CrossRef]
- Satapute, P.; Jogaiah, S. A biogenic microbial biosurfactin that degrades difenoconazole fungicide with potential antimicrobial and oil displacement properties. Chemosphere 2022, 286, 131694. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xie, K.; Lu, X.; Liu, Y.; Zhang, Y. Effect of profile-control oil-displacement agent on increasing oil recovery and its mechanism. Fuel 2019, 237, 1151–1160. [Google Scholar] [CrossRef]
- Radaev, A.V.; Sabirzyanov, A.N. Investigation of the mixing mode of oil displacement using supercritical fluid systems from low-permeability reservoirs of the terrigenic type. Theor. Found. Chem. En. 2022, 56, 1020–1025. [Google Scholar] [CrossRef]
- Cheng, H.; Zheng, X.; Wu, Y.; Zhang, J.; Zhao, X.; Li, C. Experimental and numerical investigation on oil displacement mechanism of weak gel in waterflood reservoirs. Gels 2022, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Yao, E.; Yu, G.; Li, B.; Zhao, L.; Li, Y.; Bai, H.; Zhou, F. High–temperature–resistant, low–concentration water–controlling composite cross–linked polyacrylamide weak gel system prepared from oilfield sewage. ACS Omega 2022, 7, 12570–12579. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajri, S.; Mahmood, S.M.; Akbari, S.; Abdulelah, H. Gelation behavior as a function of concentration of sodium thiosulfate for PAM gels cross-linked with chromium. J. Pet. Explor. Prod. Te. 2019, 9, 1539–1546. [Google Scholar] [CrossRef]
- Yin, H.; Yin, X.; Cao, R.; Zeng, P.; Wang, J.; Wu, D.; Luo, X.; Zhu, Y.; Zheng, Z.; Feng, Y. In situ crosslinked weak gels with ultralong and tunable gelation times for improving oil recovery. Chem. Eng. J. 2022, 432, 134350. [Google Scholar] [CrossRef]
- Kargozarfard, Z.; Riazi, M.; Ayatollahi, S.; Shahnazar, S. Performance of polyacrylamide/Cr(III) gel polymer in oil recovery from heterogeneous porous media: An experimental study. Korean. J. Chem. Eng. 2016, 33, 3350–3358. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Gu, C.; Li, L.; Sun, Y.; Dai, C. Dispersed particle gel-strengthened polymer/surfactant as a novel combination flooding system for enhanced oil recovery. Energy Fuels 2018, 32, 11317–11327. [Google Scholar] [CrossRef]
- Pi, Y.; Liu, J.; Cao, R.; Liu, L.; Ma, Y. Visualized study on a new preformed particle gels (PPG) + polymer system to enhance oil recovery by oil saturation monitoring online flooding experiment. Gels 2023, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Wu, J.; Bai, Y.; Zhang, N.; Xing, P.; Xu, Q.; Li, D.; Cong, X.; Liu, J. Research on the formulation system of weak gel and the influencing factors of gel formation after polymer flooding in Y1 block. Processes 2022, 10, 1405. [Google Scholar] [CrossRef]
- Di, Q.; Zhang, J.; Hua, S.; Chen, H.; Gu, C. Visualization experiments on polymer-weak gel profile control and displacement by NMR technique. Pet. Explor. Dev. 2017, 44, 294–298. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, H.; Li, S.; Liu, L. Dynamic sweep experiments on a heterogeneous phase composite system based on branched-preformed particle gel in high water-cut reservoirs after polymer flooding. Gels 2023, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- P. C. Caplan, S.; B. G. Silva, T.; D. S. Franscisco, A.; R. Lachter, E.; S. V. Nascimento, R. Sulfonated polystyrene nanoparticles as oleic acid diethanolamide surfactant nanocarriers for enhanced oil recovery processes. Polymers 2019, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.; Guo, X.; Zhou, N.; Xu, Q. Shear resistance properties of modified nano-SiO2/AA/AM copolymer oil displacement agent. Energies 2016, 9, 1037. [Google Scholar] [CrossRef]
- Zhi, J.; Liu, Y.; Chen, J.; Bo, L.; Qu, G.; Jiang, N.; He, W. Preparation and performance evaluation of a temperature and salt resistant hydrophobic associative weak polymer gel system. Molecules 2023, 28, 3125. [Google Scholar] [CrossRef] [PubMed]
- Ilyin, S.O. Structural rheology in the development and study of complex polymer materials. Polymers 2024, 16, 2458. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Li, Y.; Li, Z.; Dong, M. Enhanced oil recovery by branched-preformed particle gel injection in parallel-sandpack models. Fuel 2014, 136, 295–306. [Google Scholar] [CrossRef]
- Zhao, G.; Dai, C.; You, Q. Characteristics and displacement mechanisms of the dispersed particle gel soft heterogeneous compound flooding system. Pet. Explor. Dev. 2018, 45, 481–490. [Google Scholar] [CrossRef]


| Test | Permeability (10−3 μm2) | Permeability Ratio | Initial oil Saturation (%) | Recovery of Water Flood (%) | After Polymer Flooding | After Weak Gel Flooding | ||
|---|---|---|---|---|---|---|---|---|
| COR (%) | EOR (%) | COR (%) | EOR (%) | |||||
| 1 | High 4740.82 | 35.64 | 78 | 54 | 80 | 26 | 80 | 0 |
| Low 133.02 | 82 | 3 | 17 | 14 | 66 | 48 | ||
| 2 | High 3278.17 | 21.47 | 78 | 55 | 81 | 26 | 81 | 0 |
| Low 152.69 | 84. | 11 | 36 | 26 | 70 | 34 | ||
| 3 | High 2633.68 | 14.39 | 77 | 56 | 83 | 27 | 83 | 0 |
| Low 183.02 | 88 | 6 | 48 | 42 | 70 | 22 | ||
| Flooding System | Incremental Oil Recovery (%) | Ref |
|---|---|---|
| Weak gel flooding after polymer flooding | 18 | Di et al. [39] |
| ASP flooding after polymer flooding | 19 | Wang et al. [16] |
| Heterogeneous combination flooding after polymer flooding | 25 | Wang et al. [16] |
| HPC flooding after polymer flooding | 14 | Zhang et al. [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Zhang, C.; Yin, D.; Huang, T.; Zhang, H.; Yue, M.; Huang, X. Rheological Properties of Weak Gel System Cross-Linked from Chromium Acetate and Polyacrylamide and Its Application in Enhanced Oil Recovery After Polymer Flooding for Heterogeneous Reservoir. Gels 2024, 10, 784. https://doi.org/10.3390/gels10120784
Long Y, Zhang C, Yin D, Huang T, Zhang H, Yue M, Huang X. Rheological Properties of Weak Gel System Cross-Linked from Chromium Acetate and Polyacrylamide and Its Application in Enhanced Oil Recovery After Polymer Flooding for Heterogeneous Reservoir. Gels. 2024; 10(12):784. https://doi.org/10.3390/gels10120784
Chicago/Turabian StyleLong, Yunqian, Chenkan Zhang, Dandan Yin, Tao Huang, Hailong Zhang, Ming Yue, and Xiaohe Huang. 2024. "Rheological Properties of Weak Gel System Cross-Linked from Chromium Acetate and Polyacrylamide and Its Application in Enhanced Oil Recovery After Polymer Flooding for Heterogeneous Reservoir" Gels 10, no. 12: 784. https://doi.org/10.3390/gels10120784
APA StyleLong, Y., Zhang, C., Yin, D., Huang, T., Zhang, H., Yue, M., & Huang, X. (2024). Rheological Properties of Weak Gel System Cross-Linked from Chromium Acetate and Polyacrylamide and Its Application in Enhanced Oil Recovery After Polymer Flooding for Heterogeneous Reservoir. Gels, 10(12), 784. https://doi.org/10.3390/gels10120784








