Modifying the Resistant Starch Content and the Retrogradation Characteristics of Potato Starch Through High-Dose Gamma Irradiation
Abstract
1. Introduction
2. Results and Discussion
2.1. Amylose Content, RS
2.2. Hydration Properties
2.3. Thermal Properties
2.4. Pasting Properties and Gel Texture
2.5. Starch Granule and Crystalline Structure
2.6. Fine Structure of Amylopectin
2.7. Retrogradation Properties
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Determination of Amylose and RS Content
4.3. Measurement of Water Solubility, Water Absorption, and Swelling Power
4.4. Differential Scanning Calorimetry (DSC)
4.5. X-Ray Diffraction (XRD)
4.6. Fourier Transform Infrared Spectroscopy (FTIR)
4.7. Rapid Viscosity Analysis (RVA) and Texture Parameters Measurement
4.8. Scanning Electron Microscopy (SEM)
4.9. Amylopectin Branch Chain Length Distribution
4.10. Retrogradation
4.11. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhat, R.; Karim, A.A. Impact of Radiation Processing on Starch. Compr. Rev. Food. Sci. Food Saf. 2009, 8, 44–58. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, structural and functional properties of native and irradiated starch: A review. J. Food Sci. Technol. 2019, 56, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Liu, Q. Molecular structure and physicochemical properties of potato and bean starches as affected by gamma-irradiation. Int. J. Biol. Macromol. 2010, 47, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Othman, Z.; Lee, J.S. Gamma irradiation of corn starches with different amylose-to-amylopectin ratio. J. Food Sci. Technol. 2015, 52, 6218–6229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhiguang, C.; Rui, Z.; Qi, Y.; Haixia, Z. The effects of gamma irradiation treatment on starch structure and properties: A review. Int. J. Food Sci. Technol. 2023, 58, 4519–4528. [Google Scholar] [CrossRef]
- Kong, X. Chapter 5: Gamma Irradiation of Starch. In Physical Modifications of Starch; Sui, Z., Kong, X., Eds.; Springer Nature: Singapore, 2023; pp. 63–96. [Google Scholar]
- Gani, A.; Bashir, M.; Wani, S.M.; Masoodi, F.A. Modification of bean starch by γ-irradiation: Effect on functional and morphological properties. LWT-Food Sci. Technol. 2012, 49, 162–169. [Google Scholar] [CrossRef]
- Gani, A.; Nazia, S.; Rather, S.A.; Wani, S.M.; Shah, A.; Bashir, M.; Gani, A. Effect of γ-irradiation on granule structure and physicochemical properties of starch extracted from two types of potatoes grown in Jammu & Kashmir, India. LWT-Food Sci. Technol. 2014, 58, 239–246. [Google Scholar] [CrossRef]
- Hussain, P.R.; Wani, I.A.; Suradkar, P.P.; Dar, M.A. Gamma irradiation induced modification of bean polysaccharides: Impact on physicochemical, morphological and antioxidant properties. Carbohydr. Polym. 2014, 110, 183–194. [Google Scholar] [CrossRef]
- Sujka, M.; Cieśla, K.; Jamroz, J. Structure and selected functional properties of gamma-irradiated potato starch. Starch-Stärke 2015, 67, 1002–1010. [Google Scholar] [CrossRef]
- Punia, S.; Dhull, S.B.; Kunner, P.; Rohilla, S. Effect of γ-radiation on physico-chemical, morphological and thermal characteristics of lotus seed (Nelumbo nucifera) starch. Int. J. Biol. Macromol. 2020, 157, 584–590. [Google Scholar] [CrossRef]
- Gul, K.; Singh, A.K.; Sonkawade, R.G. Physicochemical, thermal and pasting characteristics of gamma irradiated rice starches. Int. J. Biol. Macromol. 2016, 85, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.; Alzaabi, A.; Robertson, M.; Fielding, B. Starchy Carbohydrates in a Healthy Diet: The Role of the Humble Potato. Nutrients 2018, 10, 1764. [Google Scholar] [CrossRef] [PubMed]
- Birch, P.R.J.; Bryan, G.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- Siddiqui, A.O.; Işık, D.; Jabran, K. Impact of Weed Competition on Morphological and Biochemical Traits of Potato: A Review. Potato Res. 2024, 67, 451–462. [Google Scholar] [CrossRef]
- Aller, E.E.J.G.; Abete, I.; Astrup, A.; Martinez, J.A.; van Baak, M.A. Starches, Sugars and Obesity. Nutrients. 2011, 3, 341–369. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; George, J.; Kumar, S.; Vikas; Sajeevkumar, V.A. Impact of γ− irradiation on the physico-chemical, rheological properties and in vitro digestibility of kithul (Caryota urens) starch; a new source of nonconventional stem starch. Radiat. Phys. Chem. 2019, 162, 54–65. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Y.; Sun, B.; Cai, C.; Ma, R.; Jin, Z. Measurement and characterization of external oil in the fried waxy maize starch granules using ATR-FTIR and XRD. Food Chem. 2018, 242, 131–138. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food. Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Shu, X.; Xu, J.; Wang, Y.; Rasmussen, S.K.; Wu, D. Effects of gamma irradiation on starch digestibility of rice with different resistant starch content. Int. J. Food Sci. Technol. 2013, 48, 35–43. [Google Scholar] [CrossRef]
- Verma, R.; Jan, S.; Rani, S.; Jan, K.; Swer, T.L.; Prakash, K.S.; Dar, M.Z.; Bashir, K. Physicochemical and functional properties of gamma irradiated buckwheat and potato starch. Radiat. Phys. Chem. 2018, 144, 37–42. [Google Scholar] [CrossRef]
- Chung, H.J.; Lee, S.Y.; Kim, J.H.; Lee, J.W.; Byun, M.W.; Lim, S.T. Pasting characteristics and in vitro digestibility of γ-irradiated RS4 waxy maize starches. J. Cereal Sci. 2010, 52, 53–58. [Google Scholar] [CrossRef]
- Dar, M.Z.; Deepika, K.; Jan, K.; Swer, T.L.; Kumar, P.; Verma, R.; Verma, K.; Prakash, K.S.; Jan, S.; Bashir, K. Modification of structure and physicochemical properties of buckwheat and oat starch by γ-irradiation. Int. J. Biol. Macromol. 2018, 108, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.A.; Jabeen, M.; Geelani, H.; Masoodi, F.A.; Saba, I.; Muzaffar, S. Effect of gamma irradiation on physicochemical properties of Indian Horse Chestnut (Aesculus indica Colebr.) starch. Food Hydrocoll. 2014, 35, 253–263. [Google Scholar] [CrossRef]
- Zuleta, A.; Dyner, L.; Sambucetti, M.E.; de Francisco, A. Effect of Gamma Irradiation on the Functional and Nutritive Properties of Rice Flours from Different Cultivars. Cereal Chem. 2006, 83, 76–79. [Google Scholar] [CrossRef]
- Rombo, G.O.; Taylor, J.R.N.; Minnaar, A. Effect of irradiation, with and without cooking of maize and kidney bean flours, on porridge viscosity and in vitro starch digestibility. J. Sci. Food Agric. 2001, 81, 497–502. [Google Scholar] [CrossRef]
- Rombo, G.O.; Taylor, J.R.N.; Minnaar, A. Irradiation of maize and bean flours: Effects on starch physicochemical properties. J. Sci. Food Agric. 2004, 84, 350–356. [Google Scholar] [CrossRef]
- Castanha, N.; Miano, A.C.; Sabadoti, V.D.; Augusto, P.E.D. Irradiation of mung beans (Vigna radiata): A prospective study correlating the properties of starch and grains. Int. J. Biol. Macromol. 2019, 129, 460–470. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Ezekiel, R.; Kaur, A. Effects of gamma-irradiation on the morphological, structural, thermal and rheological properties of potato starches. Carbohydr. Polym. 2011, 83, 1521–1528. [Google Scholar] [CrossRef]
- Reddy, C.K.; Suriya, M.; Vidya, P.V.; Vijina, K.; Haripriya, S. Effect of γ-irradiation on structure and physico-chemical properties of Amorphophallus paeoniifolius starch. Int. J. Biol. Macromol. 2015, 79, 309–315. [Google Scholar] [CrossRef]
- Abu, J.O.; Duodu, K.G.; Minnaar, A. Effect of γ-irradiation on some physicochemical and thermal properties of cowpea (Vigna unguiculata L. Walp) starch. Food Chem. 2006, 95, 386–393. [Google Scholar] [CrossRef]
- Ishiguro, K.; Noda, T.; Kitahara, K.; Yamakawa, O. Retrogradation of Sweetpotato Starch. Starch-Stärke 2000, 52, 13–17. [Google Scholar] [CrossRef]
- Tang, M.C.; Copeland, L. Investigation of starch retrogradation using atomic force microscopy. Carbohydr. Polym. 2007, 70, 1–7. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline structures of the main components of starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kubo, A.; Shimamune, T.; Matsuda, T.; Harada, K.; Satoh, H. Correlation between activities of starch debranching enzyme and α-polyglucan structure in endosperms of sugary-1 mutants of rice. Plant J. 1997, 12, 143–153. [Google Scholar] [CrossRef]
- Polesi, L.F.; Sarmento, S.B.; Moraes, J.; Franco, C.M.; Canniatti-Brazaca, S.G. Physicochemical and structural characteristics of rice starch modified by irradiation. Food Chem. 2016, 191, 59–66. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Vansoest, J.J.G.; Dewit, D.; Tournois, H.; Vliegenthart, J.F.G. Retrogradation of Potato Starch as Studied by Fourier-Transform Infrared-Spectroscopy. Starch 1994, 46, 453–457. [Google Scholar] [CrossRef]
- Ji, N.; Liu, C.; Zhang, S.; Yu, J.; Xiong, L.; Sun, Q. Effects of chitin nano-whiskers on the gelatinization and retrogradation of maize and potato starches. Food Chem. 2017, 214, 543–549. [Google Scholar] [CrossRef]
- Shu, X.; Jia, L.; Gao, J.; Song, Y.; Zhao, H.; Nakamura, Y.; Wu, D. The Influences of Chain Length of Amylopectin on Resistant Starch in Rice (Oryza sativa L.). Starch-Stärke 2007, 59, 504–509. [Google Scholar] [CrossRef]
- Bao, J.; Shen, S.; Sun, M.; Corke, H. Analysis of Genotypic Diversity in the Starch Physicochemical Properties of Nonwaxy Rice: Apparent Amylose Content, Pasting Viscosity and Gel Texture. Starch-Stärke 2006, 58, 259–267. [Google Scholar] [CrossRef]
- Yun, S.H.; Matheson, N.K. Estimation of Amylose Content of Starches after Precipitation of Amylopectin by Concanavalin-A. Starch-Stärke 2006, 42, 302–305. [Google Scholar] [CrossRef]
- Cornejo, F.; Rosell, C.M. Physicochemical properties of long rice grain varieties in relation to gluten free bread quality. LWT-Food Sci. Technol. 2015, 62, 1203–1210. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Zhang, X.; Rasmussen, S.K.; Jiang, X.; Song, W.; Wu, D.; Shu, X. Dependence of physiochemical, functional and textural properties of high-resistant starch rice on endogenous nonstarch polysaccharides. Int. J. Food Sci. Technol. 2017, 53, 1079–1086. [Google Scholar] [CrossRef]
- Bao, J.; Corke, H. Pasting Properties of γ-Irradiated Rice Starches as Affected by pH. J. Agric. Food Chem. 2001, 50, 336–341. [Google Scholar] [CrossRef]
- Duarte-Correa, Y.; Díaz-Osorio, A.; Osorio-Arias, J.; Sobral, P.J.A.; Vega-Castro, O. Development of fortified low-fat potato chips through Vacuum Impregnation and Microwave Vacuum Drying. Innov. Food Sci. Emerg. Technol. 2020, 64, 102437. [Google Scholar] [CrossRef]
- Kong, X.; Bertoft, E.; Bao, J.; Corke, H. Molecular structure of amylopectin from amaranth starch and its effect on physicochemical properties. Int. J. Biol. Macromol. 2008, 43, 377–382. [Google Scholar] [CrossRef]
- Liu, Q.; Thompson, D.B. Retrogradation of du wx and su2 wx Maize Starches after different gelatinization heat treatments. Cereal Chem. 1998, 75, 868–874. [Google Scholar] [CrossRef]
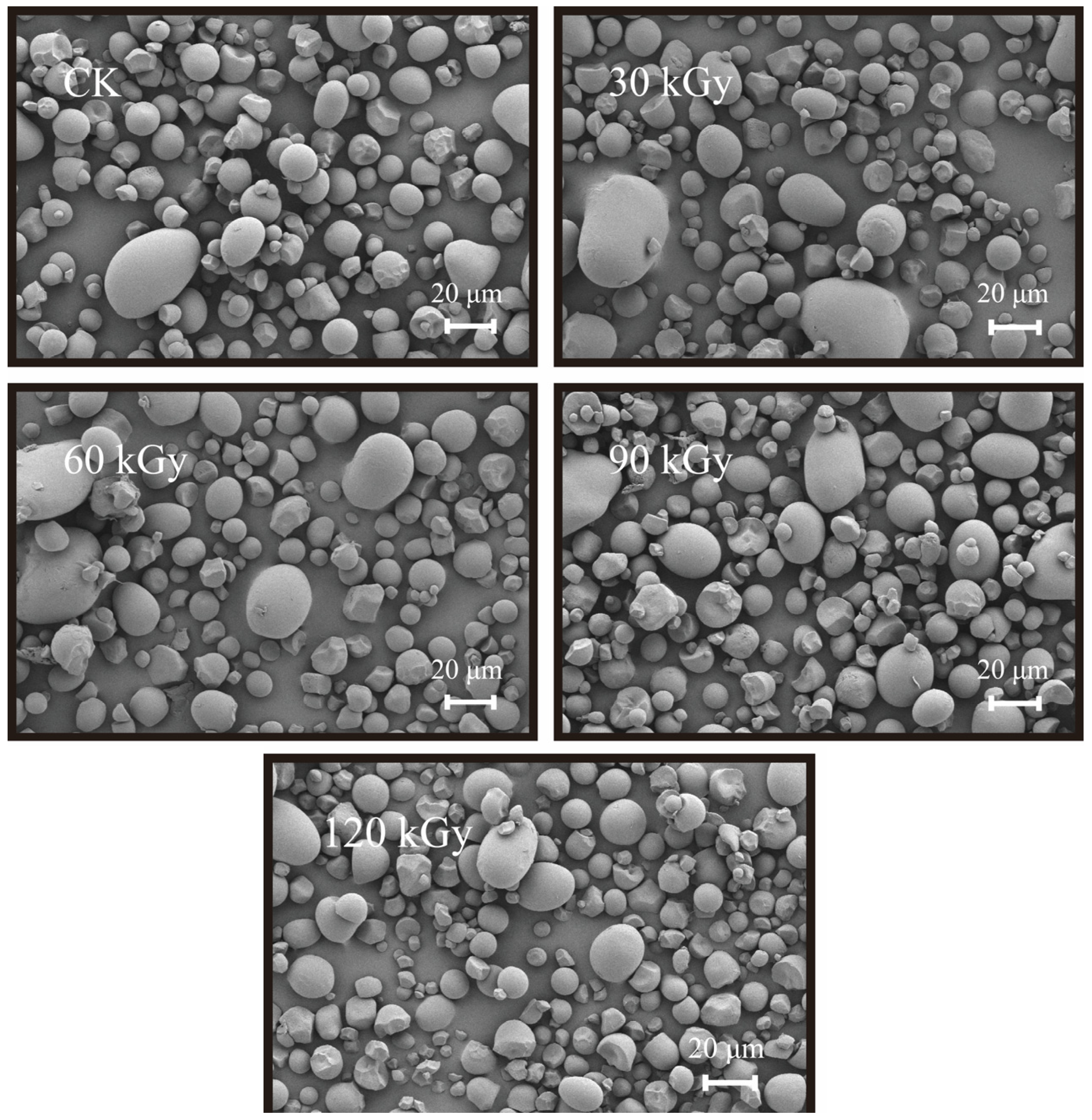

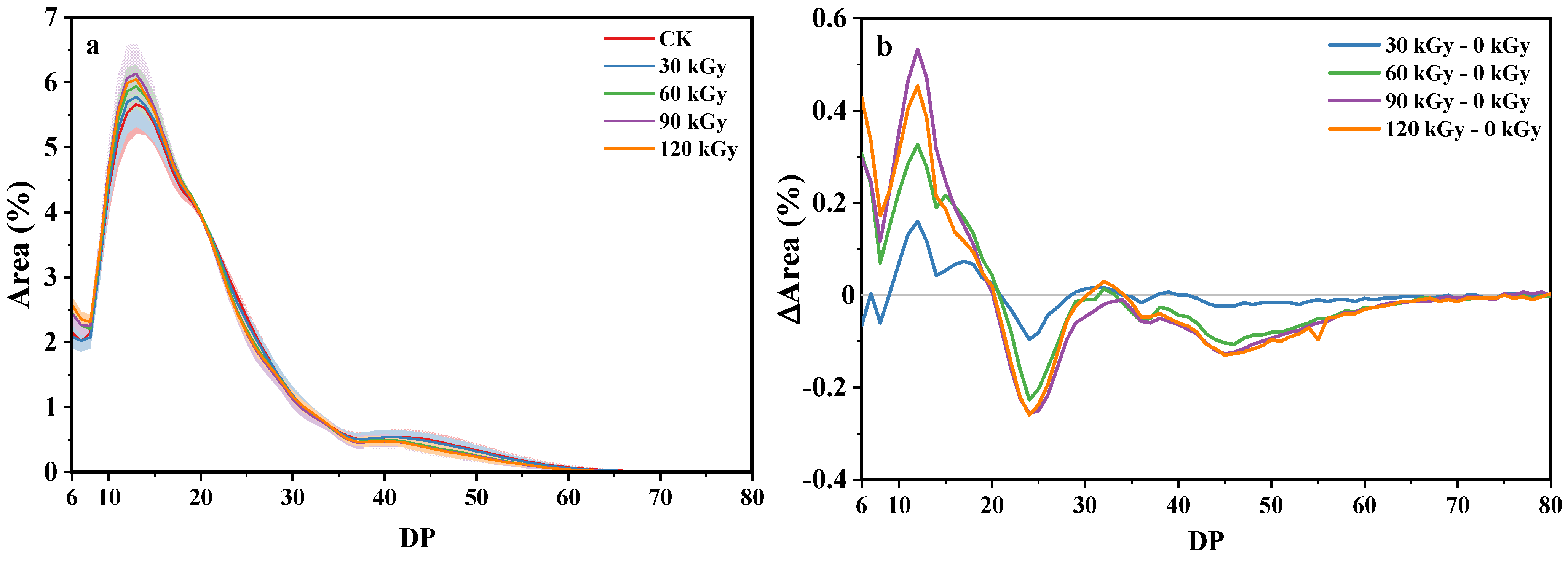
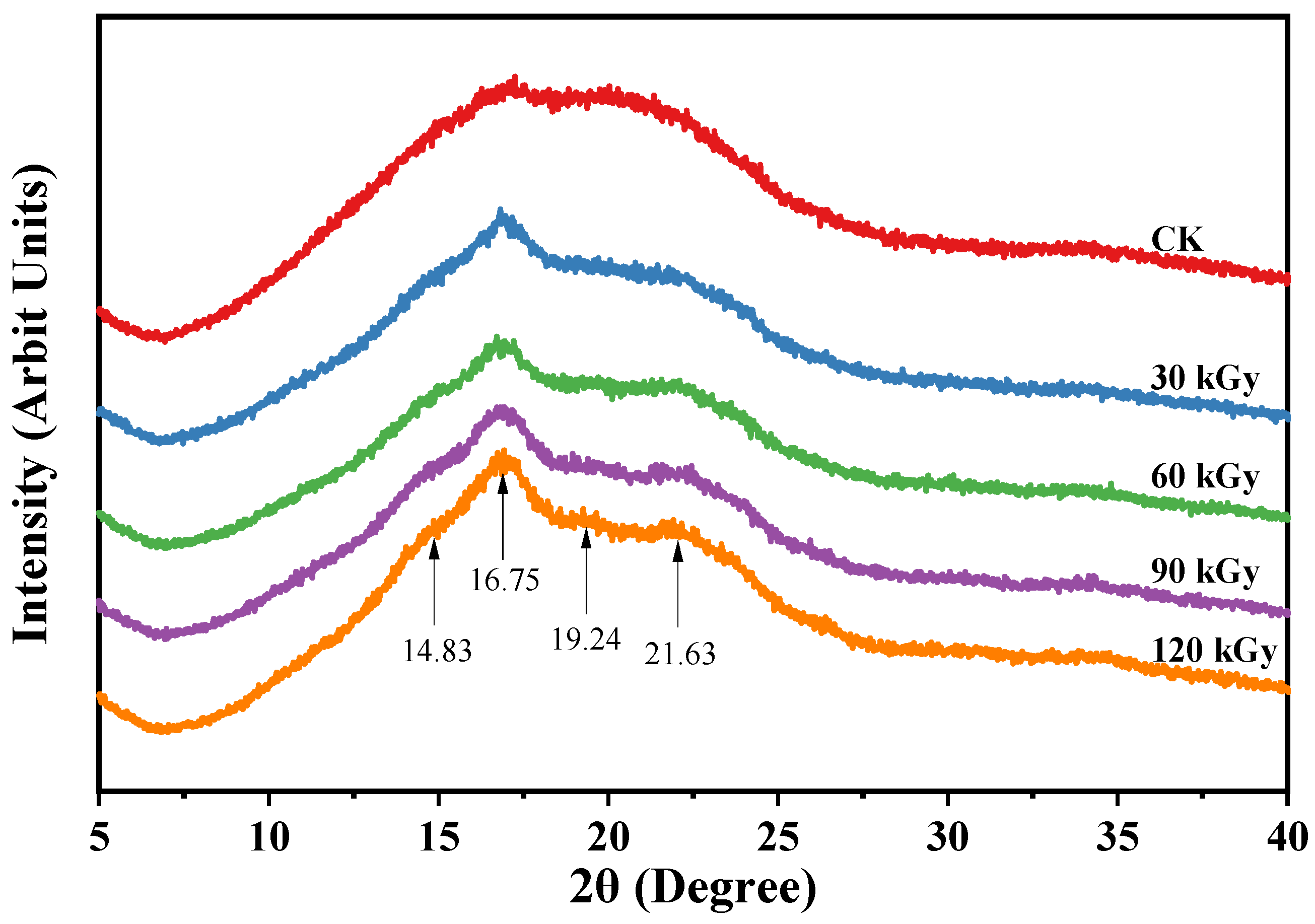
| Sample | AAC (%) | AC-ConA (%) | RS2 (%) | RS3 (%) | WAI (%) | WSI (%) | SP (%) |
|---|---|---|---|---|---|---|---|
| CK (0 kGy) | 31.96 ± 0.84 e | 43.41 ± 0.84 a | 45.29 ± 1.67 a | 4.73 ± 0.14 a | 4.95 ± 0.16 a | 21.32 ± 2.09 a | 6.29 ± 0.07 abc |
| 30 kGy | 27.63 ± 0.38 d | 70.24 ± 0.24 b | 43.18 ± 0.94 a | 5.97 ± 0.42 b | 3.32 ± 0.46 b | 43.78 ± 5.18 b | 5.88 ± 0.30 ab |
| 60 kGy | 22.84 ± 0.75 c | 69.77 ± 0.50 b | 48.35 ± 3.73 b | 6.48 ± 0.27 c | 2.83 ± 0.37 b | 56.68 ± 4.49 cd | 6.51 ± 0.17 bc |
| 90 kGy | 20.62 ± 0.68 b | 71.27 ± 1.19 b | 47.95 ± 3.13 b | 6.77 ± 0.42 c | 3.31 ± 0.14 b | 52.27 ± 1.94 c | 6.93 ± 0.07 c |
| 120 kGy | 16.03 ± 0.78 a | 71.64 ± 1.34 b | 55.42 ± 0.94 c | 7.39 ± 0.31 d | 2.11 ± 0.22 c | 63.24 ± 0.52 d | 5.73 ± 0.53 a |
| Sample | First Scan | Second Scan (7 Days of Retrogradation) | ||||||
|---|---|---|---|---|---|---|---|---|
| To1/°C | Tp1/°C | Tc1/°C | ΔH1/(J/g) | To2/°C | Tp2/°C | Tc2/°C | ΔH2/(J/g) | |
| CK (0 kGy) | 58.07 ± 0.42 a | 65.29 ± 0.33 a | 79.71 ± 0.40 b | 16.12 ± 0.51 a | 38.90 ± 0.64 ab | 57.15 ± 1.13 ab | 73.63 ± 0.29 ab | 4.27 ± 0.20 a |
| 30 kGy | 58.56 ± 0.13 a | 67.35 ± 0.23 c | 80.87 ± 0.17 c | 16.98 ± 0.28 b | 36.71 ± 0.84 a | 54.09 ± 1.14 ab | 73.02 ± 2.61 ab | 5.10 ± 0.06 b |
| 60 kGy | 59.21 ± 0.13 b | 66.84 ± 0.06 b | 78.62 ± 0.29 a | 17.01 ± 0.34 b | 40.42 ± 2.50 b | 57.93 ± 3.14 b | 74.32 ± 2.01 b | 5.28 ± 0.12 b |
| 90 kGy | 59.52 ± 0.25 b | 66.55 ± 0.31 bc | 80.47 ± 0.14 c | 17.21 ± 0.11 b | 37.35 ± 0.80 ab | 53.21 ± 0.95 a | 69.89 ± 0.36 a | 6.02 ± 0.28 c |
| 120 kGy | 59.60 ± 0.19 b | 66.37 ± 0.15 b | 80.30 ± 0.45 bc | 17.53 ± 0.11 b | 38.08 ± 0.60 ab | 54.41 ± 0.80 ab | 70.15 ± 1.38 a | 5.75 ± 0.13 c |
| Sample | PV (RVU) | HPV (RVU) | BV (RVU) | FV (RVU) | SV (RVU) | PT (°C) | Hardness (N) | Gumminess | Springiness |
|---|---|---|---|---|---|---|---|---|---|
| CK (0 kGy) | 4276.33 ± 26.41 e | 2382.00 ± 33.18 c | 1894.33 ± 6.94 e | 3152.67 ± 49.26 c | 770.67 ± 20.00 c | 66.52 ± 0.33 a | 16.05 ± 1.72 d | 9.16 ± 0.58 e | 0.6 ± 0.02 b |
| 30 kGy | 2040.33 ± 25.98 d | 387.67 ± 4.19 b | 1652.67 ± 23.30 d | 444.67 ± 5.79 b | 57.00 ± 1.63 b | 69.22 ± 0.02 b | 15.65 ± 1.59 d | 6.39 ± 0.86 d | 0.74 ± 0.03 c |
| 60 kGy | 1792.33 ± 14.94 c | 214.00 ± 0.82 a | 1578.33 ± 15.17 c | 232.33 ± 1.24 a | 18.33 ± 0.47 a | 71.02 ± 0.38 c | 12.42 ± 0.86 c | 4.20 ± 0.02 c | 0.54 ± 0.02 b |
| 90 kGy | 481.33 ± 25.38 b | 186.67 ± 0.47 a | 294.67 ± 25.10 b | 199.67 ± 0.94 a | 13.00 ± 0.82 a | 69.15 ± 0.00 b | 5.89 ± 0.88 b | 2.08 ± 0.54 b | 0.13 ± 0.10 a |
| 120 kGy | 322.00 ± 6.53 a | 183.33 ± 1.25 a | 138.67 ± 5.79 a | 193.67 ± 0.94 a | 10.33 ± 0.47 a | 69.92 ± 0.61 b | 2.11 ± 0.13 a | 0.64 ± 0.33 a | 0.79 ± 0.08 c |
| Sample | Relative Crystallinity 1 (%) | Relative Crystallinity 2 (%) | FTIR Ratio 995/1024 (cm−1) | FTIR Ratio 1048/1024 (cm−1) | DP6–12 (%) | DP13–24 (%) | DP25–36 (%) | DP ≥ 37 (%) |
|---|---|---|---|---|---|---|---|---|
| CK (0 kGy) | 16.63 ± 0.05 b | 5.75 ± 0.20 a | 1.184 ± 0.040 b | 0.829 ± 0.011 b | 24.47 ± 2.09 a | 51.42 ± 1.57 a | 15.10 ± 1.33 a | 9.02 ± 2.36 a |
| 30 kGy | 17.32 ± 0.03 d | 8.40 ± 0.12 b | 1.063 ± 0.021a | 0.787 ± 0.002 a | 24.71 ± 2.12 a | 51.71 ± 1.42 a | 14.98 ± 1.43 a | 8.60 ± 2.11 a |
| 60 kGy | 16.35 ± 0.06 a | 10.62 ± 0.35 c | 1.124 ± 0.008 ab | 0.800 ± 0.007 a | 26.07 ± 1.63 a | 52.24 ± 0.92 a | 14.45 ± 1.18 a | 7.24 ± 1.38 a |
| 90 kGy | 16.92 ± 0.14 c | 12.35 ± 0.19 d | 1.131 ± 0.018 ab | 0.809 ± 0.003 ab | 26.71 ± 2.10 a | 52.24 ± 1.37 a | 14.11 ± 1.43 a | 6.94 ± 2.04 a |
| 120 kGy | 16.54 ± 0.10 b | 10.99 ± 0.25 c | 1.123 ± 0.005 ab | 0.802 ± 0.003 a | 26.80 ± 1.43 a | 51.93 ± 1.01 a | 14.46 ± 0.92 a | 6.81 ± 1.53 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Wang, X.; Liu, Z.; Zhang, L.; Cheng, L.; Nia, J.; Zuo, Y.; Shu, X.; Wu, D. Modifying the Resistant Starch Content and the Retrogradation Characteristics of Potato Starch Through High-Dose Gamma Irradiation. Gels 2024, 10, 763. https://doi.org/10.3390/gels10120763
Peng Z, Wang X, Liu Z, Zhang L, Cheng L, Nia J, Zuo Y, Shu X, Wu D. Modifying the Resistant Starch Content and the Retrogradation Characteristics of Potato Starch Through High-Dose Gamma Irradiation. Gels. 2024; 10(12):763. https://doi.org/10.3390/gels10120763
Chicago/Turabian StylePeng, Zhangchi, Xuwei Wang, Zhijie Liu, Liang Zhang, Linrun Cheng, Jiahao Nia, Youming Zuo, Xiaoli Shu, and Dianxing Wu. 2024. "Modifying the Resistant Starch Content and the Retrogradation Characteristics of Potato Starch Through High-Dose Gamma Irradiation" Gels 10, no. 12: 763. https://doi.org/10.3390/gels10120763
APA StylePeng, Z., Wang, X., Liu, Z., Zhang, L., Cheng, L., Nia, J., Zuo, Y., Shu, X., & Wu, D. (2024). Modifying the Resistant Starch Content and the Retrogradation Characteristics of Potato Starch Through High-Dose Gamma Irradiation. Gels, 10(12), 763. https://doi.org/10.3390/gels10120763







