Molecular and Morphological Identification and Pathogenicity of Fusarium Species Causing Melon Wilt in Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material and Isolation of Fusarium Fungi
2.2. DNA Isolation and PCR
2.3. Phylogenetic Analysis
2.4. Macro- and Micromorphology of Fusarium Isolates
2.5. Assessment of Pathogenic Properties of Fusarium Isolates for Cucurbitaceae Crops at Different Stages of Plant Development
2.5.1. Pathogenicity Test at the Sprout Stage
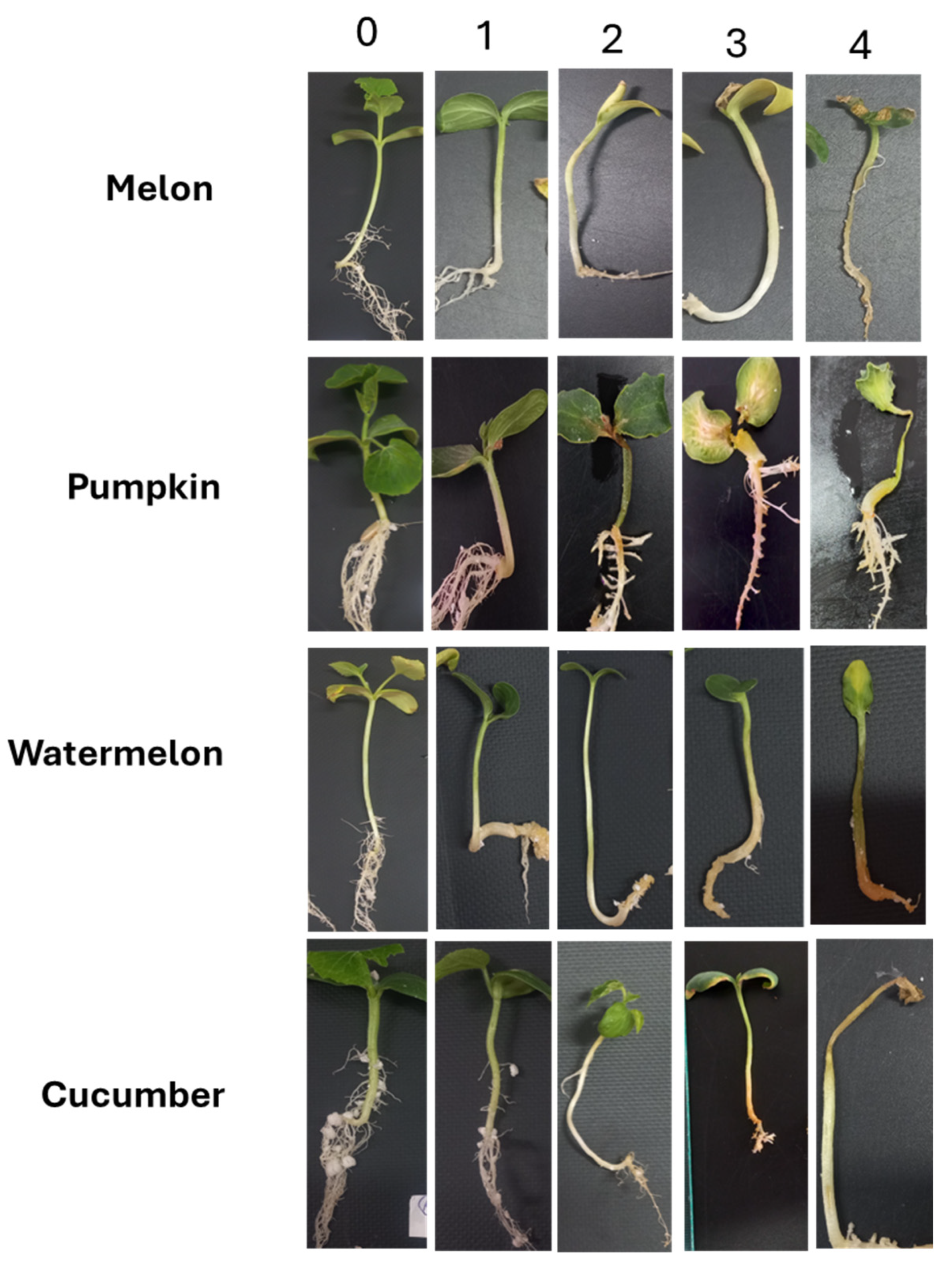
- •
- Non-pathogenic (n/p)—plants are affected by 0–0.4 points.
- •
- Weakly aggressive (WA)—plants are affected by 0.5–0.8 points.
- •
- Moderately aggressive (MA)—50% of lines are affected by 0.9–1.8 points.
- •
- Highly aggressive (HA)—more than 75% of lines are affected by 1.8–4.0 points.
2.5.2. Study of the Aggressiveness of Fusarium Isolates Against Melon at the Stage of 21-Day-Old Seedlings (Phase of the First Pair of True Leaves)
2.6. Statistical Processing
3. Results
3.1. Symptoms of Fusarium Wilt in the Field
3.2. Phylogenetic Analysis of Fungi Fusarium
3.3. Morphological Characteristics of Fusarium Species Associated with Fusarium Wilt of Melon
3.4. Study of Pathogenic Properties of Fusarium Isolates for Melon at the Sprout Stage
3.5. Study of Pathogenic Properties of Fusarium Fungi Against Melon at the Stage of the First Pair of True Leaves
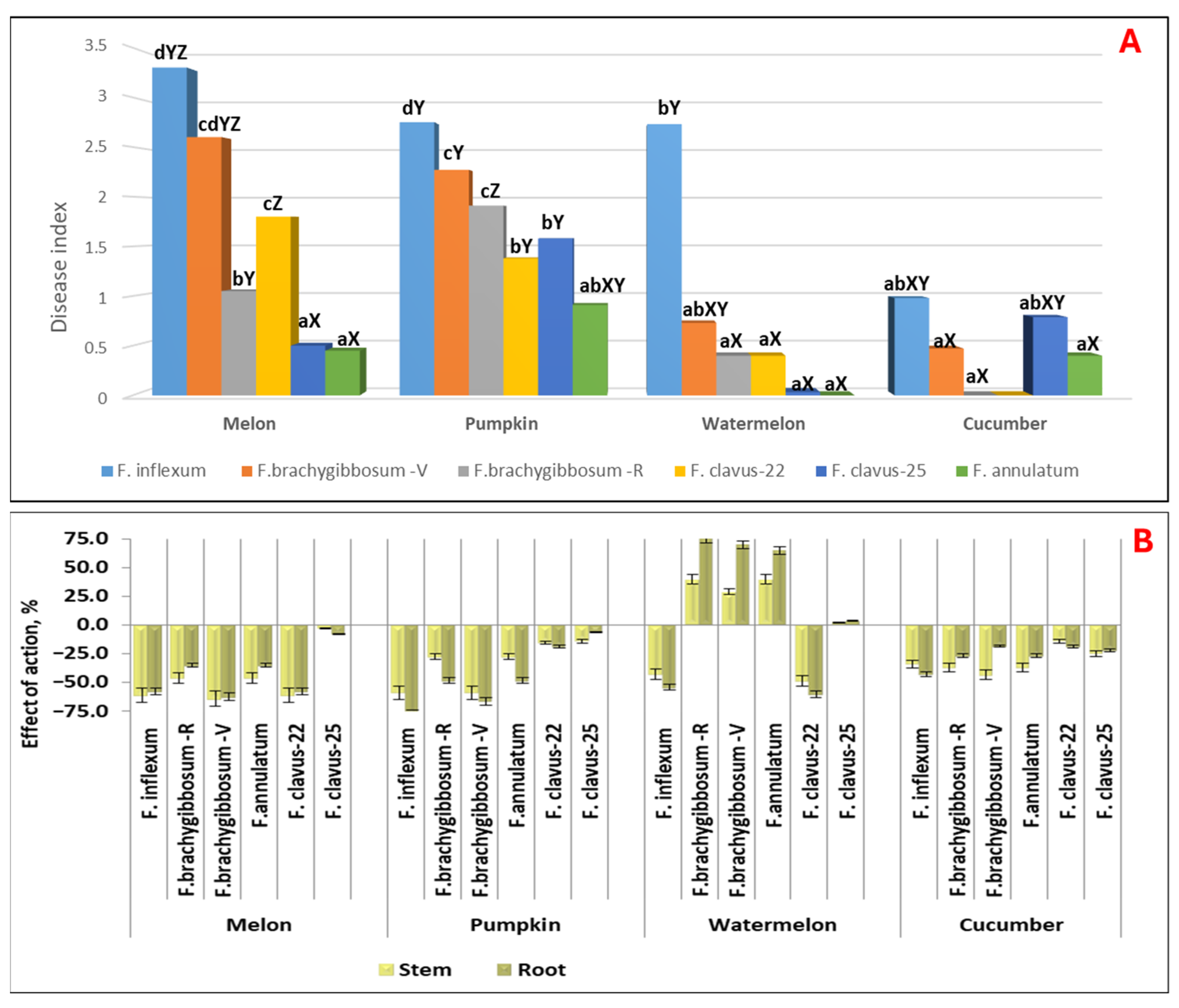
3.6. Study of Aggressiveness of Fusarium Species Against Melon at the Stage of First Pair of True Leaves
3.7. Study of the Pathogenicity of Fusarium Species Against Cucurbitaceae Crops at the Sprout Stage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varivoda, E.A. Priority areas of breeding work on melons. Veg. Crops Russ. 2025, 4, 48–51. [Google Scholar] [CrossRef]
- Kornilova, M.S.; Ryabchikova, N.B. The Source Material for the Creation of Promising Varieties of Melon (Cucumis melo L.) in the Conditions of the Volgograd Trans-Volga Region. News FSVC 2025, 1, 31–40. [Google Scholar] [CrossRef]
- Koleboshina, T.G.; Varivoda, E.A. Melon Growing Industry Analysis in Modern Economic Conditions. IOP Conf. Ser. Earth Environ. Sci. 2020, 459, 062075. [Google Scholar] [CrossRef]
- Varivoda, E.A.; Kornilova, M.S.; Varivoda, G.V. The results of variety trials of new varieties of melons in conditions of the Volgograd Trans-Volga region. Veg. Crops Russ. 2018, 2, 61–64. [Google Scholar] [CrossRef][Green Version]
- Kobkova, N.V. Change in seed productivity of melons of different maturation periods depending on the area of nutrition. News FSVC 2022, 2, 62–69. [Google Scholar] [CrossRef]
- Khakimov, R.A.; Khalimova, M.U. Melon breeding for disease resistance in the Republic of Uzbekistan. Veg. Crops Russ. 2022, 4, 28–32. [Google Scholar] [CrossRef]
- Kurepin, A.V.; Pershin, A.F. «Lighting Price» of Cucumber Yield in the Winter-Spring Turnover of Greenhouses. Veg. Crops Russ. 2021, 2, 34–38. [Google Scholar] [CrossRef]
- Deol, J.K.; Sharma, S.P.; Rani, R.; Kalia, A.; Chhuneja, P.; Sarao, N.K. Inheritance Analysis and Identification of SSR Markers Associated with Fusarium Wilt Resistance in Melon. J. Hortic. Sci. Biotechnol. 2022, 97, 66–74. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Gómez-Guillamón, M.L.; González, V.; Sta-Baba, R.; Garcés-Claver, A. Cucumis melo L. Germplasm in Tunisia: Unexploited Sources of Resistance to Fusarium Wilt. Horticulturae 2021, 7, 208. [Google Scholar] [CrossRef]
- Luongo, L.; Ferrarini, A.; Haegi, A.; Vitale, S.; Polverari, A.; Belisario, A. Genetic Diversity and Pathogenicity of Fusarium Oxysporum f. Sp. Melonis Races from Different Areas of I Taly. J. Phytopathol. 2015, 163, 73–83. [Google Scholar] [CrossRef]
- Ficcadenti, N.; Sestili, S.; Annibali, S.; Campanelli, G.; Belisario, A.; Maccaroni, M.; Corazza, L. Resistance to Fusarium oxysporum f. sp. melonis Race 1, 2 in Muskmelon Lines Nad-1 and Nad-2. Plant Dis. 2002, 86, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Oster, A.; Silva, E.; Borballo, P.; Zocolo, G.; Silveira, M.; Oiram Filho, F.; de Araújo, A. Luz Ultravioleta Pulsada No Controle de Podridão Pós-Colheita e Na Qualidade de Melão Para Exportação. Embrapa Agroindústria Tropical–Boletim de Pesquisa e Desenvolvimento (INFOTECA-E). Bol. Pesqui. 2018, 173, 1–26. [Google Scholar]
- Ates, G.O. Molecular Identification of Fusarium Isolates from Bozcaada Cavus and Karalahna Grapes in Turkiye. J. Fungi 2025, 11, 373. [Google Scholar] [CrossRef]
- Zargaryan, N.Y.; Kekalo, A.Y.; Nemchenko, V.V. Infection of Grain Crops with Fungi of the Genus Fusarium. BIO Web Conf. 2021, 36, 04008. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.; Mokoena, M.; Xu, J.; Shi, J. Occurrence, Toxicity, Production and Detection of Fusarium Mycotoxin: A Review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- O’Donnell, K.; Humber, R.A.; Geiser, D.M.; Kang, S.; Park, B.; Robert, V.A.; Crous, P.W.; Johnston, P.R.; Aoki, T.; Rooney, A.P. Phylogenetic Diversity of Insecticolous Fusaria Inferred from Multilocus DNA Sequence Data and Their Molecular Identification via FUSARIUM-ID and Fusarium MLST. Mycologia 2012, 104, 427–445. [Google Scholar] [CrossRef]
- Geiser, D.M.; Al-Hatmi, A.M.; Aoki, T.; Arie, T.; Balmas, V.; Barnes, I.; Bergstrom, G.C.; Bhattacharyya, M.K.; Blomquist, C.L.; Bowden, R.L. Phylogenomic Analysis of a 55.1-Kb 19-Gene Dataset Resolves a Monophyletic Fusarium That Includes the Fusarium solani Species Complex. Phytopathology 2021, 111, 1064–1079. [Google Scholar] [CrossRef]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, PA, USA, 1983. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 0-470-27646-0. [Google Scholar]
- Gräfenhan, T.; Schroers, H.-J.; Nirenberg, H.I.; Seifert, K.A. An Overview of the Taxonomy, Phylogeny, and Typification of Nectriaceous Fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud. Mycol. 2011, 68, 79–113. [Google Scholar] [CrossRef]
- O’Donnell, K.; McCormick, S.P.; Busman, M.; Proctor, R.H.; Ward, T.J.; Doehring, G.; Geiser, D.M.; Alberts, J.F.; Rheeder, J.P. Marasas et al. 1984 “Toxigenic Fusarium Species: Identity and Mycotoxicology” Revisited. Mycologia 2018, 110, 1058–1080. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Sandoval-Denis, M.; Cai, L.; Crous, P.W. Changing the Game: Resolving Systematic Issues in Key Fusarium Species Complexes. Persoonia-Mol. Phylogeny Evol. Fungi 2019, 43, i–ii. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a Node or a Foot-Shaped Basal Cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef]
- Kristensen, R.; Mona, T.; Kosiak, B.; Holst-Jensen, A. Phylogeny and Toxigenic Potential Is Correlated in Fusarium Species as Revealed by Partial Translation Elongation Factor 1 Alpha Gene Sequences. Mycol. Res. 2005, 109, 173–186. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Ward, T.J.; Robert, V.A.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA Sequence-Based Identification of Fusarium: Current Status and Future Directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Loza-Reyes, E.; Atkins, S.L.; Fraaije, B.A. The CYP51C Gene, a Reliable Marker to Resolve Interspecific Phylogenetic Relationships within the Fusarium Species Complex and a Novel Target for Species-Specific PCR. Int. J. Food Microbiol. 2010, 144, 301–309. [Google Scholar] [CrossRef]
- Geiser, D.M.; del Mar Jiménez-Gasco, M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’donnell, K. FUSARIUM-ID v. 1.0: A DNA Sequence Database for Identifying Fusarium. Eur. J. Plant Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- Risser, G. A Proposed Nomenclature of Fusarium oxysporum f. sp. melonis Races and Resistance Genes in Cucumis Melo. Phytopathology 1976, 66, 1105. [Google Scholar] [CrossRef]
- Oumouloud, A.; El-Otmani, M.; Chikh-Rouhou, H.; Claver, A.G.; Torres, R.G.; Perl-Treves, R.; Álvarez, J.M. Breeding Melon for Resistance to Fusarium Wilt: Recent Developments. Euphytica 2013, 192, 155–169. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Sta-Baba, R.; Ayed, C.; Belgacem, S.; Boughalleb, N.; Cherif, M. Physiological Races of Fusarium oxysporum f. sp. melonis in Tunisia. Phytoparasitica 2013, 41, 593–596. [Google Scholar] [CrossRef]
- Cohen, R.; Horev, C.; Burger, Y.; Shriber, S.; Hershenhorn, J.; Katan, J.; Edelstein, M. Horticultural and Pathological Aspects of Fusarium Wilt Management Using Grafted Melons. HortScience 2002, 37, 1069–1073. [Google Scholar] [CrossRef]
- Haegi, A.; Catalano, V.; Luongo, L.; Vitale, S.; Scotton, M.; Ficcadenti, N.; Belisario, A. A Newly Developed Real-Time PCR Assay for Detection and Quantification of Fusarium oxysporum and Its Use in Compatible and Incompatible Interactions with Grafted Melon Genotypes. Phytopathology 2013, 103, 802–810. [Google Scholar] [CrossRef]
- Sestili, S.; Polverari, A.; Luongo, L.; Ferrarini, A.; Scotton, M.; Hussain, J.; Delledonne, M.; Ficcadenti, N.; Belisario, A. Distinct Colonization Patterns and cDNA-AFLP Transcriptome Profiles in Compatible and Incompatible Interactions between Melon and Different Races of Fusarium oxysporum f. sp. melonis. BMC Genom. 2011, 12, 122. [Google Scholar] [CrossRef][Green Version]
- González, V.; Armijos, E.; Garcés-Claver, A. Fungal Endophytes as Biocontrol Agents against the Main Soil-Borne Diseases of Melon and Watermelon in Spain. Agronomy 2020, 10, 820. [Google Scholar] [CrossRef]
- González, V.; Armengol, J.; Garcés-Claver, A. First Report of Fusarium petroliphilum Causing Fruit Rot of Butternut Squash in Spain. Plant Dis. 2018, 102, 1662. [Google Scholar] [CrossRef]
- González, V.; García-Martínez, S.; Ruiz, J.J.; Flores-León, A.; Picó, B.; Garcés-Claver, A. First Report of Neocosmospora falciformis Causing Wilt and Root Rot of Muskmelon in Spain. Plant Dis. 2020, 104, 1256. [Google Scholar] [CrossRef]
- González, V.; García-Martínez, S.; Flores-León, A.; Ruiz, J.J.; Picó, B.; Garcés-Claver, A. Neocosmospora keratoplastica, a Relevant Human Fusarial Pathogen Is Found to Be Associated with Wilt and Root Rot of Muskmelon and Watermelon Crops in Spain: Epidemiological and Molecular Evidences. Eur. J. Plant Pathol. 2020, 156, 1189–1196. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, S.; Liu, X.; Ren, X.; Wang, S.; Gao, Z. The Effects of Trichoderma Viride T23 on Rhizosphere Soil Microbial Communities and the Metabolomics of Muskmelon Under Continuous Cropping. Agronomy 2023, 13, 1092. [Google Scholar] [CrossRef]
- Freeman, S.; Zveibil, A.; Vintal, H.; Maymon, M. Isolation of Nonpathogenic Mutants of Fusarium oxysporum f. sp. melonis for Biological Control of Fusarium Wilt in Cucurbits. Phytopathology 2002, 92, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Lal, D.; Dev, D.; Kumari, S.; Pandey, S.; Aparna; Sharma, N.; Nandni, S.; Jha, R.K.; Singh, A. Fusarium Wilt Pandemic: Current Understanding and Molecular Perspectives. Funct. Integr. Genom. 2024, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gu, X.; Lu, H.; Liu, P.; Miao, H.; Bai, Y.; Zhang, S. Identification of Novel Loci and Candidate Genes for Resistance to Powdery Mildew in a Resequenced Cucumber Germplasm. Genes 2021, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple Evolutionary Origins of the Fungus Causing Panama Disease of Banana: Concordant Evidence from Nuclear and Mitochondrial Gene Genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 31 October 2025).
- Fusarium. Available online: https://www.fusarium.org/ (accessed on 26 January 2024).
- Home. Available online: https://www.megasoftware.net/ (accessed on 1 November 2025).
- Seo, Y.; Kim, Y.H. Potential Reasons for Prevalence of Fusarium Wilt in Oriental Melon in Korea. Plant Pathol. J. 2017, 33, 249–263. [Google Scholar] [CrossRef]
- Rabaaoui, A.; Dall’Asta, C.; Righetti, L.; Susca, A.; Logrieco, A.F.; Namsi, A.; Gdoura, R.; Werbrouck, S.P.O.; Moretti, A.; Masiello, M. Phylogeny and Mycotoxin Profile of Pathogenic Fusarium Species Isolated from Sudden Decline Syndrome and Leaf Wilt Symptoms on Date Palms (Phoenix dactylifera) in Tunisia. Toxins 2021, 13, 463. [Google Scholar] [CrossRef]
- Parra, M.Ä.; Gómez, J.; Aguilar, F.W.; Martinez, J.A. Fusarium annulatum Causes Fusarium Rot of Cantaloupe Melons in Spain. Phytopathol. Mediterr. 2022, 16, 269–277. [Google Scholar] [CrossRef]
- Chistyakova, L.A.; Sokolova, L.M.; Baklanova, O.V.; Egorova, A.A. Evaluation of Fusarium Fungus Strains on Affection of Cucumber Plants. Kartof. Ovoshi 2020, 3, 32–36. [Google Scholar] [CrossRef]
- Elmer, W.H.; Covert, S.F.; O’Donnell, K. Investigation of an Outbreak of Fusarium Foot and Fruit Rot of Pumpkin Within the United States. Plant Dis. 2007, 91, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.C.; Crous, P.W. Epitypification of Fusarium oxysporum—Clearing the Taxonomic Chaos. Persoonia 2019, 43, 1–47. [Google Scholar] [CrossRef]
- Schneider, R.; Dalchow, J. Fusarium inflexum Spec. Nov., as the Agent of a Wilt Disease on Vicia faba L. in Germany. Phytopathol. Z. 1975, 82, 70–82. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, X.; Yu, J.; Guo, Z.; Li, Y.; Wu, J.; Chi, Y. First Report of Fusarium ipomoeae Causing Peanut Leaf Spot in China. Plant Dis. 2021, 105, 3754. [Google Scholar] [CrossRef]
- Dobbs, J.T.; Kim, M.-S.; Reynolds, G.J.; Wilhelmi, N.; Dumroese, R.K.; Klopfenstein, N.B.; Fraedrich, S.W.; Cram, M.M.; Bronson, J.; Stewart, J.E. Fusarioid Community Diversity Associated with Conifer Seedlings in Forest Nurseries across the Contiguous USA. Front. Plant Sci. 2023, 14, 1104675. [Google Scholar] [CrossRef]
- Cruz, J.M.F.D.L.; Farias, O.R.D.; Araújo, B.C.L.; Rivera, A.V.; De Souza, C.R.; De Souza, J.T. A New Root and Trunk Rot Disease of Grapevine Plantlets Caused by Fusarium in Four Species Complexes. J. Fungi 2025, 11, 230. [Google Scholar] [CrossRef]
- Tan, D.C.; Flematti, G.R.; Ghisalberti, E.L.; Sivasithamparam, K.; Chakraborty, S.; Obanor, F.; Barbetti, M.J. Mycotoxins Produced by Fusarium Species Associated with Annual Legume Pastures and ‘Sheep Feed Refusal Disorders’ in Western Australia. Mycotox Res. 2011, 27, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.Y.; Cui, W.Y.; Zhang, D.D.; Zhang, J.; Ma, N.N.; Bao, Y.M.; Dai, X.F.; Guo, W. First Report of Fusarium brachygibbosum Causing Maize Stalk Rot in China. Plant Dis. 2017, 101, 837. [Google Scholar] [CrossRef]
- Xia, B.; Hu, J.Y.; Zhu, X.F.; Liang, Y.; Ren, X.; Wu, Y.H.; Chen, D.X. First Report of Sunflower Broomrape Wilt Caused by Fusarium brachygibbosum in China. Plant Dis. 2018, 102, 2372. [Google Scholar] [CrossRef]
- Kostin, N.K.; Kuznetsova, A.A.; Kopina, M.B.; Beloshapkina, O.O. Cultural and Morphological Features of Fusarium oxysporum and Fusarium brachygibbosum Species Associated with Garden Strawberry Plants. Pomic. Small Fruits Cult. Russ. 2023, 71, 69–81. [Google Scholar] [CrossRef]
- Rentería-Martínez, M.E.; Guerra-Camacho, M.Á.; Ochoa-Meza, A.; Moreno-Salazar, S.F.; Meza-Moller, A.D.C.; Guzmán-Ortiz, J.M. Descripción y Comparación Entre Morfotipos de Fusarium brachygibbosum, F. falciforme y F. oxysporum Patogénicos En Sandía Cultivada En Sonora, México. Rev. Mex. Fitopatol. 2018, 37, 16–34. [Google Scholar] [CrossRef]
- Meshram, V.; Elazar, M.; Maymon, M.; Sharma, G.; Shawahna, R.; Belausov, E.; Charuvi, D.; Freeman, S. Endophytic Fusarium clavum Confers Growth and Salt Tolerance in Cucumis melo. Environ. Exp. Bot. 2023, 206, 105153. [Google Scholar] [CrossRef]
- Engalycheva, I.; Kozar, E.; Frolova, S.; Vetrova, S.; Tikhonova, T.; Dzhos, E.; Engalychev, M.; Chizhik, V.; Martynov, V.; Shingaliev, A.; et al. Fusarium Species Causing Pepper Wilt in Russia: Molecular Identification and Pathogenicity. Microorganisms 2024, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, G.; Matic, S.; Guarnaccia, V.; Garibaldi, A.; Gullino, M.L. First Report of Fusarium clavum Causing Leaf Spot and Fruit Rot on Tomato in Italy. Plant Dis. 2021, 105, 2250. [Google Scholar] [CrossRef]
- Azil, N.; Stefańczyk, E.; Sobkowiak, S.; Chihat, S.; Boureghda, H.; Śliwka, J. Identification and Pathogenicity of Fusarium Spp. Associated with Tuber Dry Rot and Wilt of Potato in Algeria. Eur. J. Plant Pathol. 2021, 159, 495–509. [Google Scholar] [CrossRef]
- Edel, V.; Steinberg, C.; Gautheron, N.; Recorbet, G.; Alabouvette, C. Genetic Diversity of Fusarium oxysporum Populations Isolated from Different Soils in France. FEMS Microbiol. Ecol. 2001, 36, 61–71. [Google Scholar] [CrossRef]
- Huang, X.-Q.; Lu, X.-H.; Sun, M.-H.; Guo, R.-J.; Van Diepeningen, A.D.; Li, S.-D. Transcriptome Analysis of Virulence-Differentiated Fusarium oxysporum f. sp. cucumerinum Isolates during Cucumber Colonisation Reveals Pathogenicity Profiles. BMC Genom. 2019, 20, 570. [Google Scholar] [CrossRef]
- Rauwane, M.E.; Ogugua, U.V.; Kalu, C.M.; Ledwaba, L.K.; Woldesemayat, A.A.; Ntushelo, K. Pathogenicity and Virulence Factors of Fusarium graminearum Including Factors Discovered Using Next Generation Sequencing Technologies and Proteomics. Microorganisms 2020, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.H.; Souza, E.A.; Shoji, J.; Connolly, L.; Freitag, M.; Read, N.D.; Roca, M.G. Heterokaryon Incompatibility Is Suppressed Following Conidial Anastomosis Tube Fusion in a Fungal Plant Pathogen. PLoS ONE 2012, 7, e31175. [Google Scholar] [CrossRef] [PubMed]
- Kurian, S.M.; Di Pietro, A.; Read, N.D. Live-Cell Imaging of Conidial Anastomosis Tube Fusion During Colony Initiation in Fusarium oxysporum. PLoS ONE 2018, 13, e0195634. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-H.; Lin, Y.-H.; Wan, Y.-L.; Chen, K.-S.; Huang, J.-W.; Chang, P.-F.L. Degenerated Virulence and Irregular Development of Fusarium oxysporum f. sp. niveum Induced by Successive Subculture. J. Fungi 2020, 6, 382. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Raza, W.; Li, J.; Liu, Y.; Qiu, M.; Zhang, F.; Shen, Q. Trichoderma harzianum SQR-T037 Rapidly Degrades Allelochemicals in Rhizospheres of Continuously Cropped Cucumbers. Appl. Microbiol. Biotechnol. 2011, 89, 1653–1663. [Google Scholar] [CrossRef]
- Jangir, P.; Mehra, N.; Sharma, K.; Singh, N.; Rani, M.; Kapoor, R. Secreted in Xylem Genes: Drivers of Host Adaptation in Fusarium oxysporum. Front. Plant Sci. 2021, 12, 628611. [Google Scholar] [CrossRef]
- López-Orona, C.A.; Hernández-Verdugo, S.; Velarde-Félix, S.; Garzón-Tiznado, J.A.; Sy, O.; Retes-Manjarrez, J.E. Cross Pathogenicity of Fusarium oxysporum Isolated from Peppers. Eur. J. Plant Pathol. 2019, 154, 1111–1123. [Google Scholar] [CrossRef]
- Medeiros Araújo, M.B.; Moreira, G.M.; Nascimento, L.V.; Nogueira, G.D.A.; Nascimento, S.R.D.C.; Pfenning, L.H.; Ambrósio, M.M.D.Q. Fusarium Rot of Melon Is Caused by Several Fusarium Species. Plant Pathol. 2021, 70, 712–721. [Google Scholar] [CrossRef]
- Hasan, H.A.H. Phytotoxicity of Pathogenic Fungi and Their Mycotoxins to Cereal Seedling Viability. Mycopathologia 1999, 148, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Haris, N.; Tijjani, M.; Umar, A.; Bako, A.A.; Buhari, A.S.; Bature, M. Pathogenicity Evaluation of Some Common Soil Borne Fungi on Seeds from Three Local Varieties of Sorghum (Sorghum bicolor L.). UMYU Sci. 2022, 1, 171–177. [Google Scholar] [CrossRef]
- Orina, A.S.; Gavrilova, O.P.; Gagkaeva, T.Y. Physiological and Biochemical Characters of Nigrospora gorlenkoana Novobr. Occurring on Cereals. Biol. Bull. 2023, 5, 463–476. [Google Scholar] [CrossRef]
- Soldatenko, A.V.; Egorova, A.A.; Baklanova, O.V.; Hovrin, A.N.; Chistyakova, L.A.; Razin, O.A. Selection of Cucumis sativus L. for Resistance to Fusarium Wilt Using Filtrate of the Culture Fluid of the Fungus Fusarium oxysporum Schlectend. Veg. Crops Russ. 2019, 4, 50–53. [Google Scholar] [CrossRef]
- Pietro, A.D.; Madrid, M.P.; Caracuel, Z.; Delgado-Jarana, J.; Roncero, M.I.G. Fusarium oxysporum: Exploring the Molecular Arsenal of a Vascular Wilt Fungus. Mol. Plant Pathol. 2003, 4, 315–325. [Google Scholar] [CrossRef]




| Strain | Fusarium Species | Origin | Substrate | Year |
|---|---|---|---|---|
| FV-C-3 | F. annulatum (FFSC) | Volgograd | stem | 2022 |
| FV-C-7 | F. annulatum (FFSC) | Volgograd | root | 2022 |
| FV-C-19 | F. annulatum (FFSC) | Volgograd | leaf | 2025 |
| FV-C-25 | F. annulatum (FFSC) | Volgograd | leaf | 2025 |
| FR-C-28 | F. cf. inflexum (FOSC) | Rostov | stem | 2025 |
| FR-C-15a | F. cf. inflexum (FOSC) | Volgograd | stem | 2025 |
| FV-C-30 | F. cf. inflexum (FOSC) | Volgograd | root | 2025 |
| FV-C-31 | F. cf. inflexum (FOSC) | Volgograd | leaf | 2025 |
| FV-C-11 | F. brachygibbosum (FSAMSC) | Volgograd | stem | 2022 |
| FR-C-18a | F. brachygibbosum (FSAMSC) | Volgograd | leaf | 2022 |
| FR-C-29 | F. brachygibbosum (FSAMSC) | Rostov | stem | 2025 |
| FR-C-24a | F. brachygibbosum (FSAMSC) | Rostov | leaf | 2025 |
| FV-C-12 | F. clavus (FIESC) | Volgograd | stem | 2022 |
| FV-C-13 | F. clavus (FIESC) | Volgograd | leaf | 2022 |
| FV-C-14 | F. clavus (FIESC) | Volgograd | leaf | 2022 |
| FV-C-21 | F. clavus (FIESC) | Volgograd | fruit | 2025 |
| FV-C-26 | F. clavus (FIESC) | Volgograd | stem | 2025 |
| FV-C-27 | F. clavus (FIESC) | Volgograd | stem | 2025 |
| FV-C-32 | F. clavus (FIESC) | Volgograd | stem | 2025 |
| Characteristic | F. cf. inflexum (FR-C-28) | F. brachygibbosum-V (FV-C-11) | F. brachygibbosum-R (FR-C-29) | F. clavus (FV-C-12) | F. annulatum (FV-C-3) |
|---|---|---|---|---|---|
| Colony: | |||||
| growth rate on PDA, mm/day ± SE | 10.82 ± 0.03 | 10.60 ± 0.19 | 8.18 ± 0.14 | 9.55 ± 0.14 | 7.57 ± 0.06 |
| mycelium | White, sparse, heterogeneous | White, felt | White, felt | White, dense, uniform, | White, loose, heterogeneous |
| pigment | lemon on day 14 | purple on day 7 | pink on day 18 | yellowish on days 18–20 | cream on day 12 |
| type of conidiophores | monophialides | polyphialides and monophialides | monophialides | monophialides | not found |
| Microconidia: | |||||
| size, µm | 9.2 ± 1.8 × 3.7 ± 0.5 | 11.5 ± 4.1 × 3.4 ± 0.7 | 9.65 ± 1.2 × 3.4 ± 0.4 | 4.9 ± 0.6 × 3.4 ± 0.2 | 5.84 ± 1.8 × 2.6 ± 0.4 |
| septation | 0 | 0 | 1 | 0–1 | 1 |
| shape | oval or round | oval | slightly curved | oval to slightly curved | oval |
| Macroconidia: | |||||
| size, µm | 25.9 ± 5.3 × 3.9 ± 0.9 | 23.0 ± 4.4 × 4.5 ± 0.7 | 20.2 ± 5.5 × 3.1 ± 0.5 | 14.1 ± 0.3 × 2.7 ± 0.1 | 25.8 ± 0.9 × 3.9 ± 0.2 |
| septation | 3 | 3–4 | 2–3 | 2–3 | 2–3 |
| shape | slightly curved | slightly curved | straight | fusiform or slightly curved | almost straight |
| Chlamydospores: | |||||
| size, µm | 7.4 ± 1.3 × 7.4 ± 0.9 | 10.2 ± 1.1 × 7.3 ± 1.2 | 9.6 ± 0.8 × 9.6 ± 0.9 | 10.4 ± 0.9 × 10.1 ± 1.1 | 10.6 ± 0.1 × 10.6 ± 0.9 |
| shape | globose | globose or oblong | globose | globose | globose |
| abundance | in abundance, single or in pairs | more often in pairs | single, in pairs, in chains | in abundance, 3–4 in chains | single |
| Strain | Type | Aggressiveness Degree * | Disease Index * | |||||
|---|---|---|---|---|---|---|---|---|
| Variety | Average for Isolate | Average for Fusarium Species | ||||||
| C-IM-7 | C-IM-1 | C-IM-10 | C-IM-6 | |||||
| Control | 0 aX | 0 aX | 0 aX | 0 aX | 0 a | 0 a | ||
| FV-C-3 | F. annulatum (FFSC) | WA | 0.45 ± 0.28 aX | 0.35 ± 0.63 aX | 0.3 ± 0.95 aX | 0 aX | 0.28 ± 0.68 a | 0.08 ± 0.14 a |
| FV-C-7 | F. annulatum (FFSC) | n/p | 0 aX | 0 aX | 0 aX | 0 aX | 0 a | |
| FV-C-19 | F. annulatum (FFSC) | WA | 0.21 ± 0.03 aX | 0 aX | 0 aX | 0 aX | 0.05 a | |
| FV-C-25 | F. annulatum (FFSC) | WA | 0.38 ± 0.02 aX | 0 aX | 0 aX | 0 aX | 0.10 a | |
| FR-C-28 | F. cf. inflexum (FOSC) | HA | 2.7 ± 0.67 cZ | 3.3 ± 1.16 cdZ | 4 dZ | 0 aX | 2.50 ± 0.83 d | 2.01 ± 0.85 d |
| FR-C-15a | F. cf. inflexum (FOSC) | HA | 2.6 ± 0.73 cZ | 3.0 ± 1.39 cdZ | 3.67 ± 0.95 dZ | 0 aX | 2.32 d | |
| FV-C-30 | F. cf. inflexum (FOSC) | HA | 2.4 ± 0.61 cZ | 2.6 ± 1.40 cZ | 3.67 ± 0.95 dZ | 0 aX | 2.17 c | |
| FV-C-31 | F. cf. inflexum (FOSC) | MA | 1.1 ± 0.61 bY | 1.5 bY | 1.64 ± 0.05 bcYZ | 0 aX | 1.06 bc | |
| FV-C-11 | F. brachygibbosum (FSAMSC) | HA | 2.3 ± 1.16 cZ | 2.39 ± 0.81 cYZ | 2.6 ± 1.19 cZ | 0 aX | 1.82 ± 1.15 c | 1.36 ± 1.05 c |
| FV-C-18a | F. brachygibbosum (FSAMSC) | WA | 0.84 ± 0.10 abXY | 2.75 ± 1.39 cdYZ | 0 aX | 0 aX | 0.90 b | |
| FR-C-29 | F. brachygibbosum (FSAMSC) | MA | 1.55 ± 0.26 bcY | 0.35 ± 0.13 aX | 0.8 ± 0.01 abXY | 0 aX | 0.68 ± 1.35 b | 0.35 ± 0.65 b |
| FR-C-24a | F. brachygibbosum (FSAMSC) | n/p | 0.1 aX | 0 aX | 0 aX | 0 aX | 0.03 a | |
| FV-C-12 | F. clavus (FIESC) | HA | 2.0 ± 0.34 cZ | 2.4 ± 1.90 cdZ | 2.9 ± 0.15 cdZ | 0 aX | 1.82 ± 0.45 | 0.66 ± 0.58 |
| FV-C-13 | F. clavus (FIESC) | MA | 1.0 ± 0.35 bY | 1.0 ± 0.35 bY | 1.0 ± 0.35 bY | 0 aX | 0.75 | |
| FV-C-14 | F. clavus (FIESC) | MA | 1.0 ± 0.35 bY | 1.0 ± 0.35 bY | 1.0 ± 0.35 bY | 0 aX | 0.75 | |
| FV-C-21 | F. clavus (FIESC) | WA | 1.05 ± 1.21 | 0.4 ± 0.84 aX | 0.8 ± 1.40 bX | 0 aX | 0.56 ± 0.33 | |
| FV-C-26 | F. clavus (FIESC) | n/p | 0 aX | 0.4 ± 0.84 aX | 0 aX | 0 aX | 0.1 ± 0.23 | |
| FV-C-27 | F. clavus (FIESC) | WA | 0.4 ± 1.26 aX | 0.65 ± 1.20 aX | 1.6 ± 2.07 bcYZ | 0 aX | 0.66 ± 0.63 | |
| FV-C-32 | F. clavus (FIESC) | n/p | 0 aX | 0.14 ± 0.24 | 0 aX | 0 aX | 0.04 ± 0.08 | |
| Strain | Type | Aggressiveness Degree * | Disease Index | |||||
|---|---|---|---|---|---|---|---|---|
| Variety | Average for Isolate | Average for Fusarium Species | ||||||
| C-IM-7 | C-IM-1 | C-IM-10 | C-IM-6 | |||||
| Control | 0 aW | 0 aW | 0 aW | 0 aW | 0 a | 0 a | ||
| FV-C-3 | F. annulatum(FFSC) | n/p | 0 aW | 0 aW | 0 aW | 0 aW | 0.13 a | 0.03 a |
| FV-C-7 | F. annulatum (FFSC) | WA | 0 aW | 0 aW | 0 aW | 0 aW | 0 a | |
| FV-C-19 | F. annulatum (FFSC) | n/p | 0 aW | 0 aW | 0 aW | 0 aW | 0 a | |
| FV-C-25 | F. annulatum (FFSC) | n/p | 0 aW | 0 aW | 0 aW | 0 aW | 0 a | |
| FR-C-28 | F. cf. inflexum (FOSC) | HA | 2.8 ± 0.89 cZ | 0.7 ± 0.41 bXY | 2.05 ± 0.76 dYZ | 0.45 ± 0.28 bX | 1.5 d | 1.31 c |
| FR-C-15a | F. cf. inflexum (FOSC) | HA | 2.8 ± 0.89 cZ | 0.7 ± 0.41 bXY | 2.05 ± 0.76 dYZ | 0.45 ± 0.28 bX | 1.5 d | |
| FV-C-30 | F. cf. inflexum (FOSC) | HA | 2.8 ± 0.89 cZ | 0.7 ± 0.41 bXY | 2.05 ± 0.76 dYZ | 0.45 ± 0.28 bX | 1.5 d | |
| FV-C-31 | F. cf. inflexum (FOSC) | HA | 1.5 ± 1.08 bcXY | 0 aW | 1.5 ± 1.08 bcXY | 0 aW | 0.75 bc | |
| FV-C-11 | F. brachygibbosum (FSAMSC) | HA | 3.0 ± 0.69 cdZ | 0.48 ± 0.11 bX | 2.7 ± 0.47 eZ | 0.23 ± 0.26 aW | 1.60 d | 1.31 c |
| FR-C-18a | F brachygibbosum (FSAMSC) | MA | 1.05 ± 0.34 cX | 0 aW | 1.05 ± 0.34 dX | 0 aW | 1.03 c | |
| FR-C-29 | F. brachygibbosum (FSAMSC) | n/p | 0 aW | 0 aW | 0 aW | 0 aW | 0 a | 0 a |
| FR-C-24a | F. brachygibbosum (FSAMSC) | n/p | 0 aW | 0 aW | 0 aW | 0 aW | 0 a | |
| FV-C-12 | F. clavus (FIESC) | HA | 2.60 ± 0.55 cY | 2.0 ± 1.74 cdX | 2.0 ± 0.89 dX | 0.5 bW | 1.78 d | 0.505 b |
| FV-C-13 | F. clavus (FIESC) | WA | 0.73 ± 0.15 bX | 0.73 ± 0.15 bX | 0.73 ± 0.15 bX | 0.11 aW | 0.58 b | |
| FV-C-14 | F. clavus (FIESC) | WA | 0.73 ± 0.15 bX | 0.73 ± 0.15 bX | 0.73 ± 0.15 bX | 0 aW | 0.55 b | |
| FV-C-21 | F. clavus (FIESC) | WA | 0.85 ± 1.05 bY | 0.85 ± 1.06 bX | 0.85 ± 1.07 bX | 0 aW | 0.64 b | |
| FV-C-26 | F. clavus (FIESC) | n/p | 0 aW | 0 aW | 0 aW | 0 aW | 0 a | |
| FV-C-27 | F. clavus (FIESC) | n/p | 0 aW | 0 aW | 0 aW | 0 aW | 0 a | |
| FV-C-32 | F. clavus (FIESC) | n/p | 0 aW | 0 aW | 0 aW | 0 aW | 0 a | |
| Mean | 0.99 Y | 0.34 X | 0.85 XY | 0.12 W | ||||
| Strain | Type | Aggressiveness Degree | DSI * | EA **, % | *** Proportion of Susceptible Samples, % | ||
|---|---|---|---|---|---|---|---|
| Range | Average | Root | Stem | ||||
| Control | 0 | 0 a | 0 a | 0 a | |||
| FV-C-3 | F. annulatum (FFSC) | WA | 0–0.5 | 0.21 a | 2.8 a | −4.3 a | 2.1 |
| FR-C-28 | F. cf. inflexum (FOSC) | HA | 0.5–4.0 | 2.94 d | −38.5 c | −16.7 b | 75.8 |
| FV-C-11 | F. brachygibbosum (FSAMSC) | HA | 0.5–4.0 | 2.44 bc | −16.7 bc | −15.5 b | 69.5 |
| FV-C-12 | F. clavus (FIESC) | HA | 0–3.0 | 1.96 b | −20.4 bc | −16.8 b | 20.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engalycheva, I.; Kozar, E.; Kameneva, A.; Sletova, M.; Vetrova, S.; Chizhik, V.; Kornilova, M.; Martynov, V. Molecular and Morphological Identification and Pathogenicity of Fusarium Species Causing Melon Wilt in Russia. J. Fungi 2025, 11, 888. https://doi.org/10.3390/jof11120888
Engalycheva I, Kozar E, Kameneva A, Sletova M, Vetrova S, Chizhik V, Kornilova M, Martynov V. Molecular and Morphological Identification and Pathogenicity of Fusarium Species Causing Melon Wilt in Russia. Journal of Fungi. 2025; 11(12):888. https://doi.org/10.3390/jof11120888
Chicago/Turabian StyleEngalycheva, Irina, Elena Kozar, Alina Kameneva, Maria Sletova, Svetlana Vetrova, Vera Chizhik, Maria Kornilova, and Viktor Martynov. 2025. "Molecular and Morphological Identification and Pathogenicity of Fusarium Species Causing Melon Wilt in Russia" Journal of Fungi 11, no. 12: 888. https://doi.org/10.3390/jof11120888
APA StyleEngalycheva, I., Kozar, E., Kameneva, A., Sletova, M., Vetrova, S., Chizhik, V., Kornilova, M., & Martynov, V. (2025). Molecular and Morphological Identification and Pathogenicity of Fusarium Species Causing Melon Wilt in Russia. Journal of Fungi, 11(12), 888. https://doi.org/10.3390/jof11120888







