Phenolic Compounds Accumulation and Cell Death Degree Induced by Fusaric Acid in Agroforestry Hosts Plants of Fusarium Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Fusaric Acid Leaf Exposure Bioassay
2.2. Assessment of Biochemical Parameters in Leaves Under FA Exposure
2.2.1. Evans Blue Staining for Cell Death Measurement
2.2.2. Hydrogen Peroxide Detection by DAB Staining
2.2.3. Electrolyte Leakage from Cells
2.2.4. Chlorophyll Quantitation
2.3. Metabolomics on Plant Leaves After FA Exposure
2.3.1. Extraction
2.3.2. Quantification of FA in Leaf Tissue
2.3.3. Untargeted Metabolomics Analysis
2.3.4. Targeted Metabolomics for Phenolic Compounds
3. Results
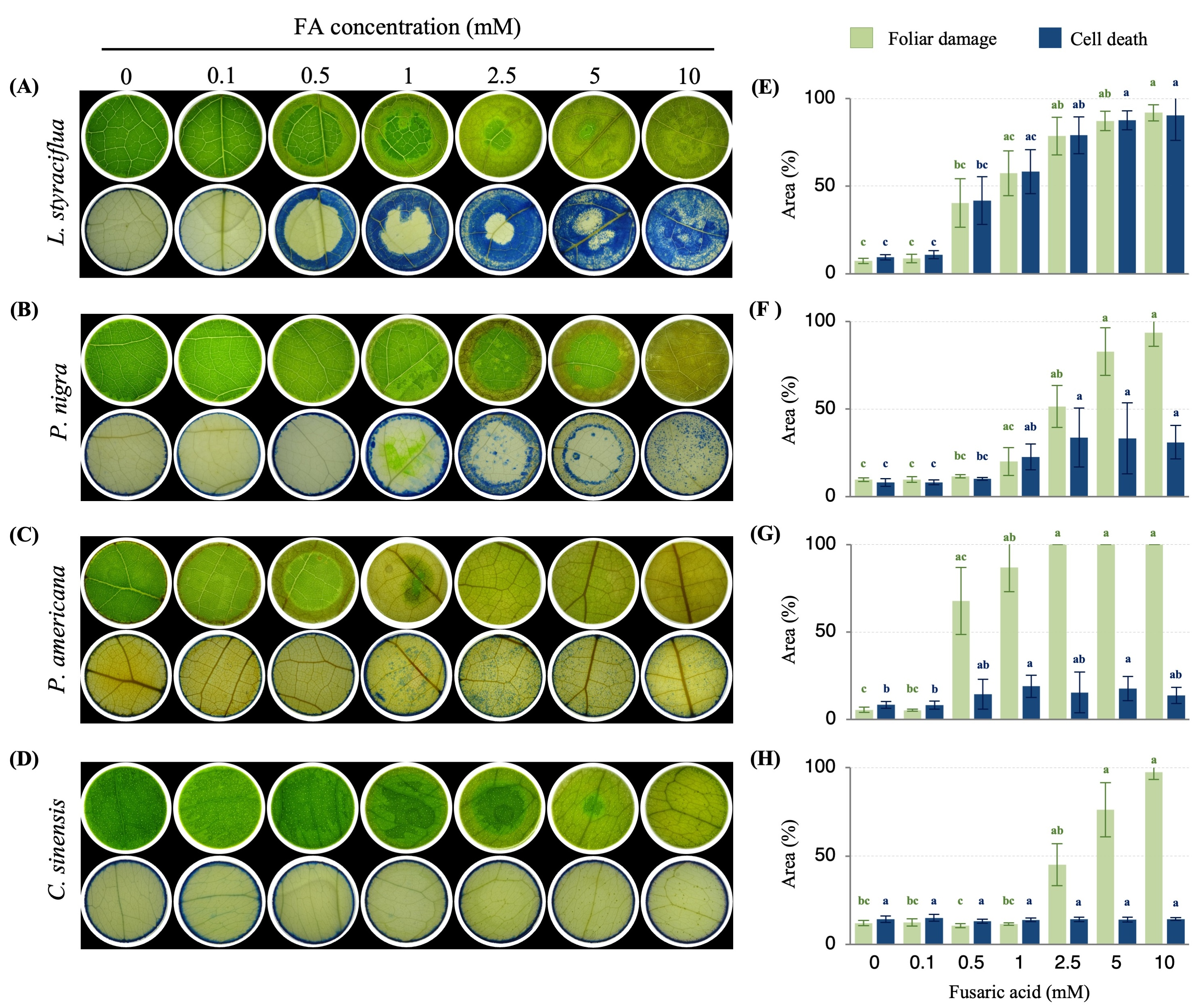
3.1. Fusaric Acid Treatment Leads to Cell Death in Different Plant Species
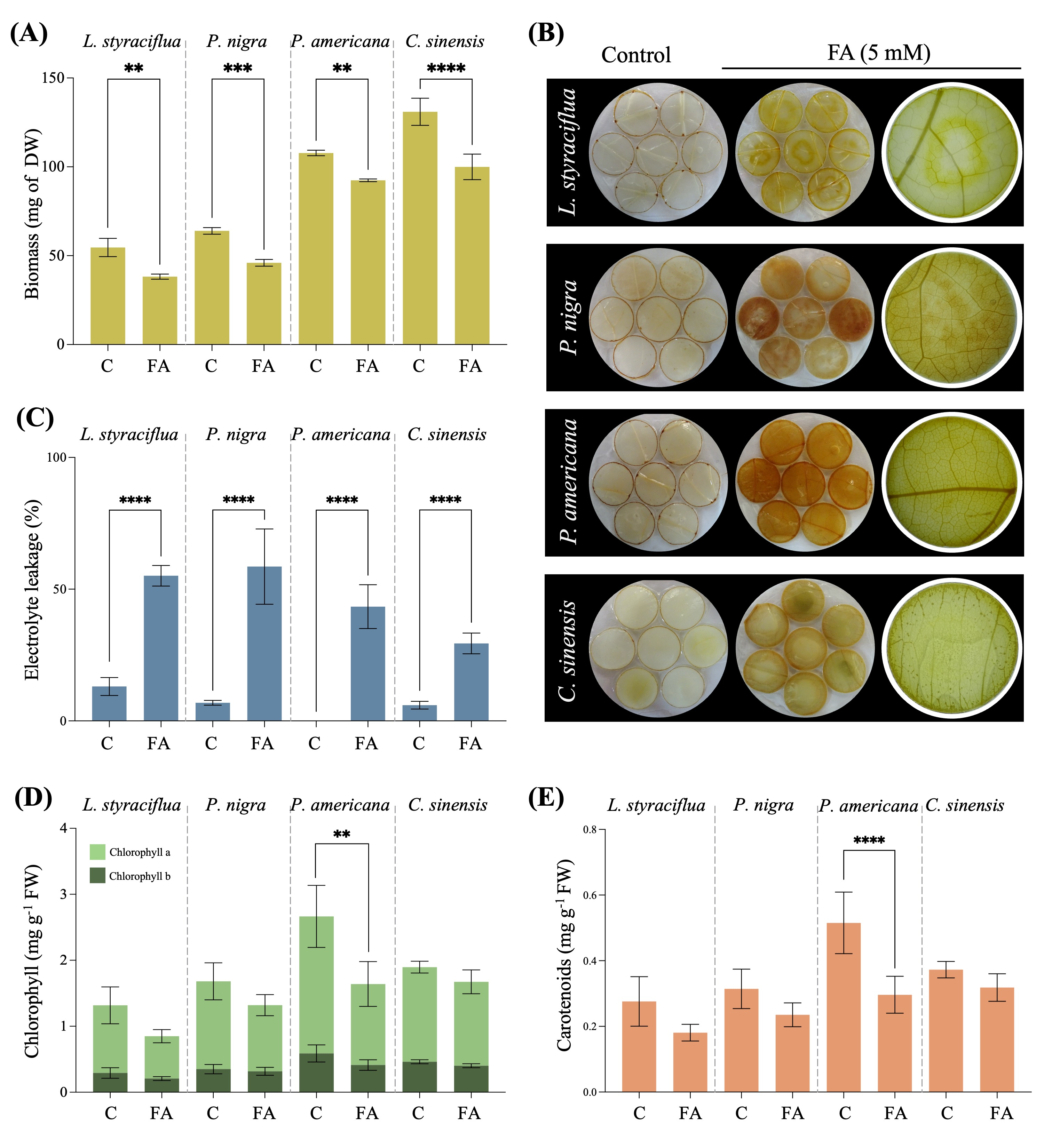
3.2. FA Induces Biochemical Disturbances Within the Cells
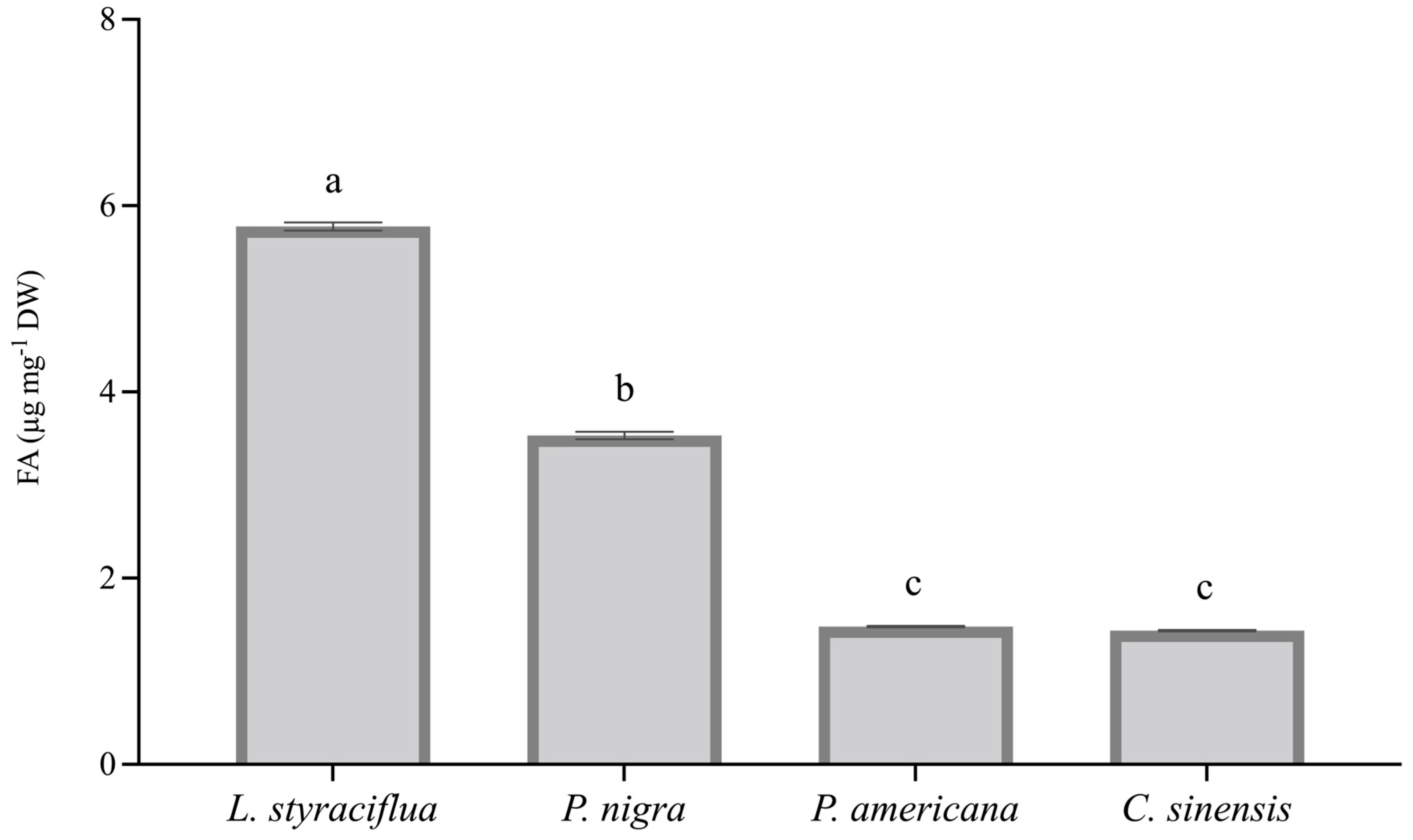
3.3. Fusaric Acid Toxicity Is Dependent of Its Foliar Content
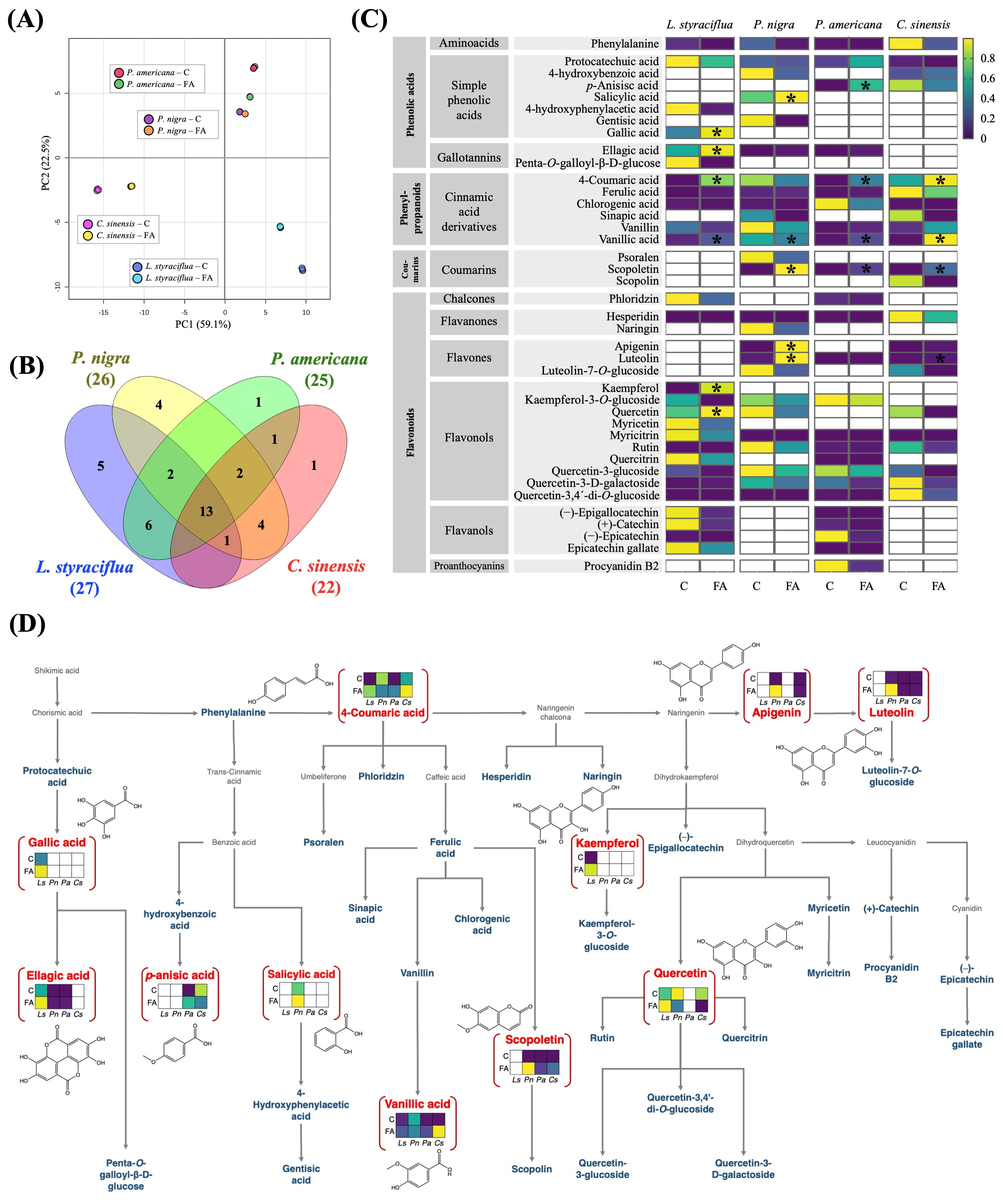
3.4. FA Provoked Changes in the Specialized Metabolism in the Different Plant Species
3.5. FA Alters the Content of Phenolic Compounds in Leaf Tissue
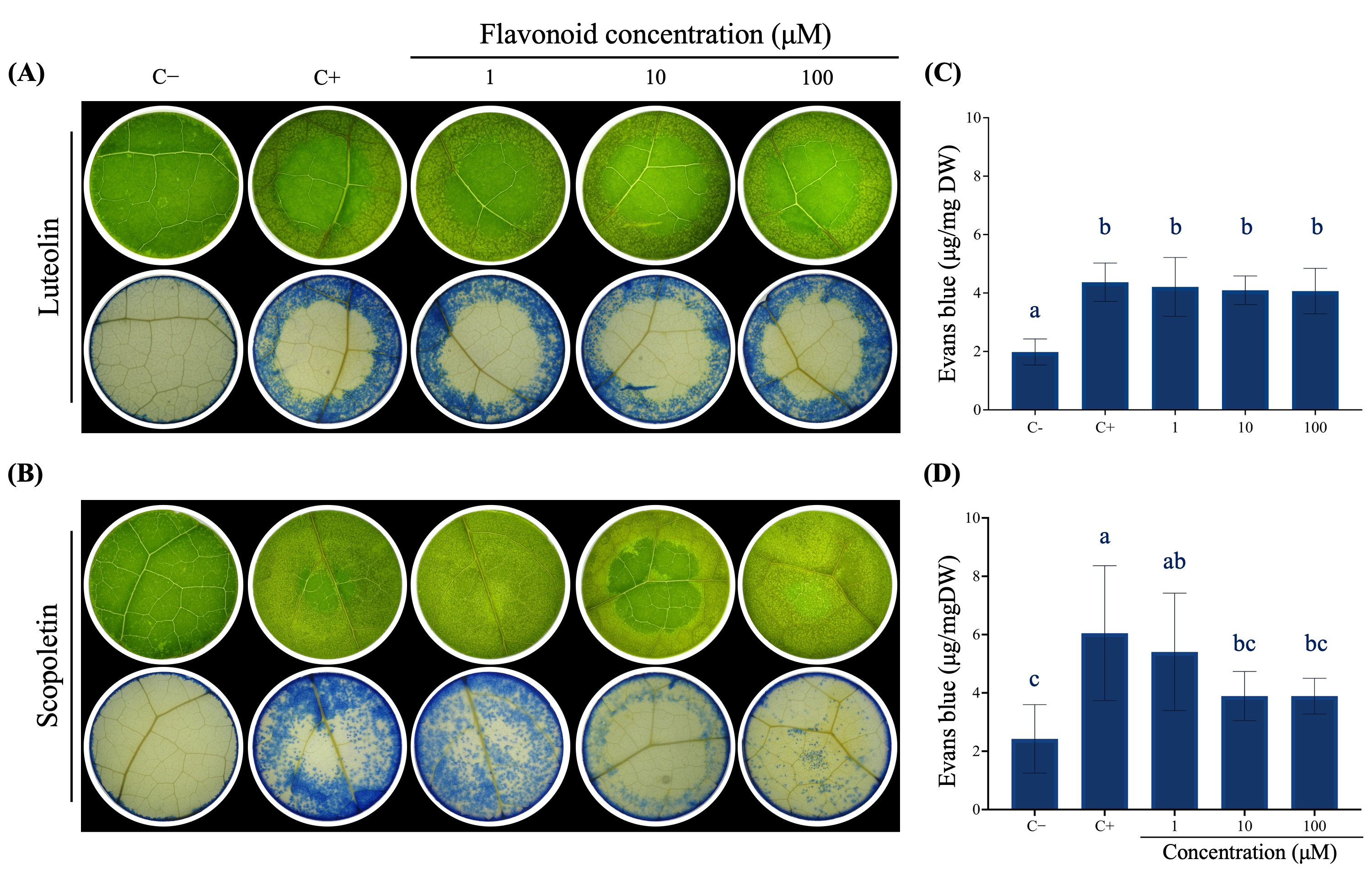
3.6. Scopoletin Exerts a Protective Effect on the Leaves of Liquidambar Against FA Treatment
4. Discussion
4.1. Selection of Woody Plants and Biochemical Disturbance After FA Exposure
4.2. Differential Metabolic Response Against FA in Woody Plants
4.3. Scopoletin Pretreatment Reduce FA-Induced Cell Death in L. styraciflua Leaves
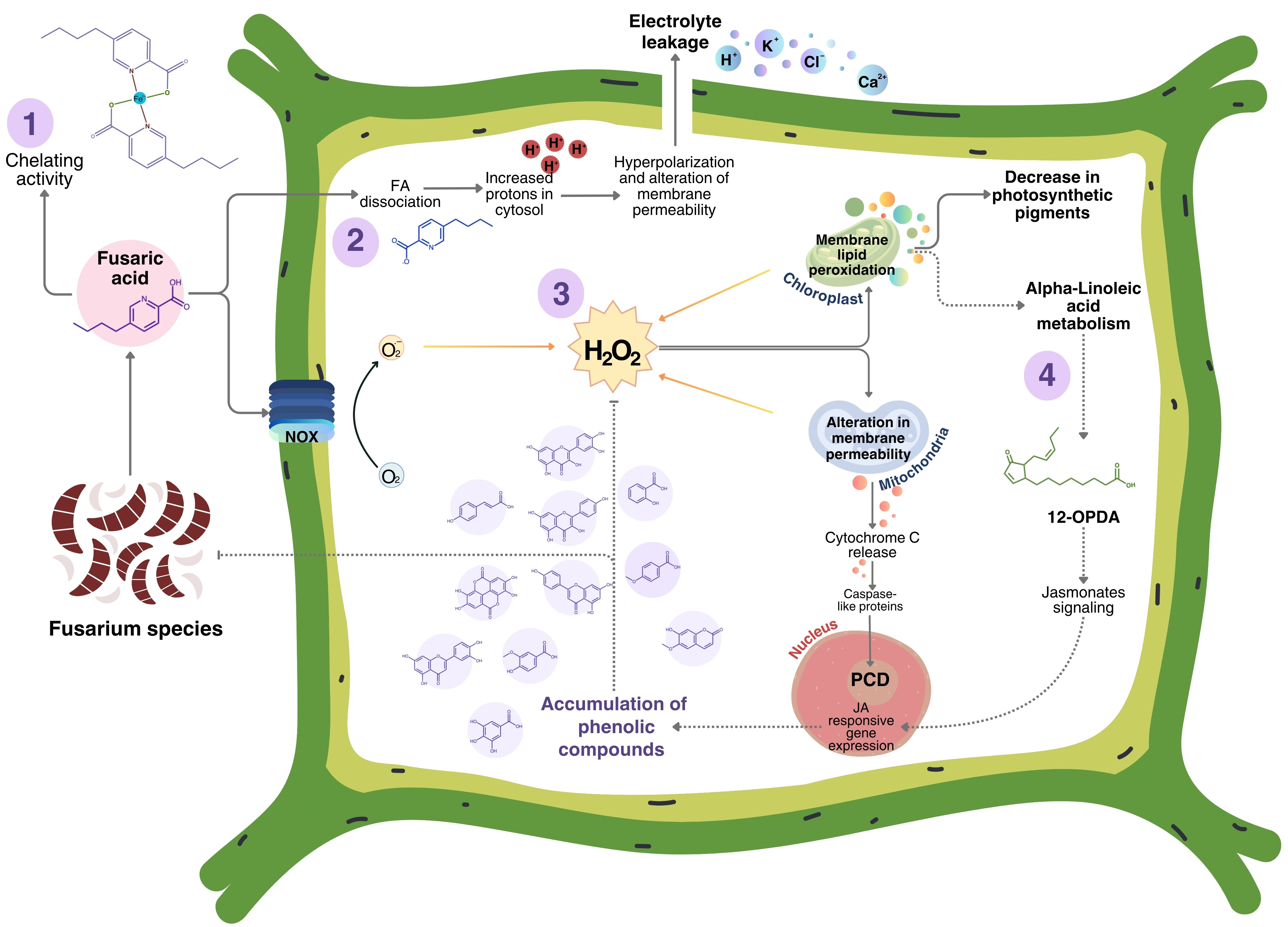
4.4. Proposed Model FA Action in Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Li, J.; Zhang, Y.; Liu, N.; Viljoen, A.; Mostert, D.; Zuo, C.; Hu, C.; Bi, F.; Gao, H.; et al. Fusaric acid instigates the invasion of banana by Fusarium oxysporum f. sp. cubense TR 4. New Phytol. 2020, 225, 913–929. [Google Scholar] [CrossRef]
- Bacon, C.W.; Porter, J.K.; Norred, W.P.; Leslie, J.F. Production of fusaric acid by Fusarium species. Appl. Environ. Microbiol. 1996, 62, 4039–4043. [Google Scholar] [CrossRef]
- Venter, S.L.; Steyn, P.J. Correlation between fusaric acid production and virulence of isolates of Fusarium oxysporum that causes potato dry rot in South Africa. Potato Res. 1998, 41, 289–294. [Google Scholar] [CrossRef]
- Palmieri, D.; Segorbe, D.; López-Berges, M.S.; De Curtis, F.; Lima, G.; Di Pietro, A.; Turrà, D. Alkaline pH, low iron availability, poor nitrogen sources and CWI MAPK signaling are associated with increased fusaric acid production in Fusarium oxysporum. Toxins 2023, 15, 50. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, H.B.; Upadhyay, R.S. Role of fusaric acid in the development of ‘fusarium wilt’ symptoms in tomato: Physiological, biochemical and proteomic perspectives. Plant Physiol. Biochem. 2017, 118, 320–332. [Google Scholar] [CrossRef]
- Kuzniak, E. Effects of fusaric acid on reactive oxygen species and antioxidants in tomato cell cultures. J. Phytopathol. 2001, 149, 575–582. [Google Scholar] [CrossRef]
- D’Alton, A.; Etherton, B. Effects of fusaric acid on tomato root hair membrane potentials and ATP levels. Plant Physiol. 1984, 74, 39–42. [Google Scholar] [CrossRef]
- Marrè, M.T.; Vergani, P.; Albergoni, F.G. Relationship between fusaric acid uptake and its binding to cell structures by leaves of Egeria densa and its toxic effects on membrane permeability and respiration. Physiol. Mol. Plant Pathol. 1993, 42, 141–157. [Google Scholar] [CrossRef]
- Jiao, J.; Sun, L.; Zhou, B.; Gao, Z.; Hao, Y.; Zhu, X.; Liang, Y. Hydrogen peroxide production and mitochondrial dysfunction contribute to the fusaric acid-induced programmed cell death in tobacco cells. J. Plant Physiol. 2014, 171, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Samadi, L.; Shahsavan Behboodi, B. Fusaric acid induces apoptosis in saffron root-tip cells: Roles of caspase-like activity, cytochrome C, and H2O2. Planta 2006, 225, 223–234. [Google Scholar] [CrossRef]
- López-Díaz, C.; Rahjoo, V.; Sulyok, M.; Ghionna, V.; Martín-Vicente, A.; Capilla, J.; Di Pietro, A.; López-Berges, M.S. Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Plant Pathol. 2018, 19, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Telles-Pupulin, A.R.; Diniz, S.P.S.S.; Bracht, A.; Ishii-Iwamoto, E.L. Effects of fusaric acid on respiration in maize root mitochondria. Biol. Plant. 1996, 38, 421–429. [Google Scholar] [CrossRef]
- Bouizgarne, B.; El-Maarouf-Bouteau, H.; Frankart, C.; Reboutier, D.; Madiona, K.; Pennarun, A.M.; Monestiez, M.; Trouverie, J.; Amiar, Z.; Briand, J.; et al. Early physiological responses of Arabidopsis thaliana cells to fusaric acid: Toxic and signalling effects. New Phytol. 2005, 169, 209–218. [Google Scholar] [CrossRef]
- Wang, M.; Ling, N.; Dong, X.; Liu, X.; Shen, Q.; Guo, S. Effect of fusaric acid on the leaf physiology of cucumber seedlings. Eur. J. Plant Pathol. 2014, 138, 103–112. [Google Scholar] [CrossRef]
- Eskalen, A.; Gonzalez, A.; Wang, D.H.; Twizeyimana, M.; Mayorquin, S.J.; Lynch, S.C. First report of a Fusarium sp. and its vector tea shot hole borer (Euwallacea fornicatus) causing fusarium dieback on avocado in California. Plant Dis. 2012, 96, 1070. [Google Scholar] [CrossRef]
- Na, F.; Carrillo, J.D.; Mayorquin, J.S.; Ndinga-Muniania, C.; Stajich, J.E.; Stouthamer, R.; Huang, Y.-T.; Lin, Y.-T.; Chen, C.-Y.; Eskalen, A. Two novel fungal symbionts Fusarium kuroshium sp. nov. and Graphium kuroshium sp. nov. of kuroshio shot hole borer (Euwallacea sp. nr. fornicatus) cause fusarium dieback on woody host species in California. Plant Dis. 2018, 102, 1154–1164. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, A.; Plasencia, J.; Monribot-Villanueva, J.L.; Rodríguez-Haas, J.B.; López-Buenfil, J.A.; García-Ávila, C.J.; Ruiz-May, E.; Sánchez-Rangel, D.; Guerrero-Analco, J.A. Characterization of the exo-metabolome of the emergent phytopathogen Fusarium kuroshium sp. nov., a causal agent of fusarium dieback. Toxins 2021, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Eskalen, A.; Stouthamer, R.; Lynch, S.C.; Rugman-Jones, P.F.; Twizeyimana, M.; Gonzalez, A.; Thibault, T. Host range of fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in Southern California. Plant Dis. 2013, 97, 938–951. [Google Scholar] [CrossRef]
- Vijayaraghavareddy, P.; Adhinarayanreddy, V.; Vemanna, R.; Sreeman, S.; Makarla, U. Quantification of membrane damage/cell death using Evan’s blue staining technique. Bio-Protocol 2017, 7, e2519. [Google Scholar] [CrossRef]
- Daudi, A.; O’Brien, J. Detection of hydrogen peroxide by DAB staining in arabidopsis leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as totalcarotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Monribot-Villanueva, J.L.; Altúzar-Molina, A.; Aluja, M.; Zamora-Briseño, J.A.; Elizalde-Contreras, J.M.; Bautista-Valle, M.V.; Arellano De Los Santos, J.; Sánchez-Martínez, D.E.; Rivera-Reséndiz, F.J.; Vázquez-Rosas-Landa, M.; et al. Integrating proteomics and metabolomics approaches to elucidate the ripening process in white Psidium guajava. Food Chem. 2022, 367, 130656. [Google Scholar] [CrossRef]
- Juárez-Trujillo, N.; Monribot-Villanueva, J.L.; Alvarado-Olivarez, M.; Luna-Solano, G.; Guerrero-Analco, J.A.; Jiménez-Fernández, M. Phenolic profile and antioxidative properties of pulp and seeds of Randia monantha Benth. Ind. Crops Prod. 2018, 124, 53–58. [Google Scholar] [CrossRef]
- Zhen, X.-T.; Zhu, S.-C.; Shi, M.-Z.; Yu, Y.-L.; Yan, T.-C.; Yue, Z.-X.; Gu, Y.-X.; Zheng, H.; Cao, J. Analysis of flavonoids in citrus fruits by capillary zone electrophoresis coupled with quadrupole time-of-flight mass spectrometry using chemometrics. J. Food Compos. Anal. 2022, 106, 104275. [Google Scholar] [CrossRef]
- Sugiyama, S.; Umehara, K.; Kuroyanagi, M.; Ueno, A.; Taki, T. Studies on the differentiation inducers of myeloid leukemic cells from citrus species. Chem. Pharm. Bull. 1993, 41, 714–719. [Google Scholar] [CrossRef]
- Johnson, J.L.; Rupasinghe, S.G.; Stefani, F.; Schuler, M.A.; Gonzalez De Mejia, E. Citrus flavonoids luteolin, apigenin, and quercetin inhibit glycogen synthase kinase-3β enzymatic activity by lowering the interaction energy within the binding cavity. J. Med. Food 2011, 14, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus flavones: An update on sources, biological functions, and health promoting properties. Plants 2020, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Takaishi, Y.; Tanaka, N.; Tsuchiya, K.; Shibata, H.; Higuti, T. Chemical constituents from the peels of Citrus sudachi. J. Nat. Prod. 2006, 69, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.; Kim, M. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef]
- Eberle, R.J.; Olivier, D.S.; Amaral, M.S.; Willbold, D.; Arni, R.K.; Coronado, M.A. Promising natural compounds against flavivirus proteases: Citrus flavonoids hesperetin and hesperidin. Plants 2021, 10, 2183. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (lemon) phenomenon—A review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food and cosmetics industries and biotechnological studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef]
- Gualdani, R.; Cavalluzzi, M.; Lentini, G.; Habtemariam, S. The chemistry and pharmacology of citrus limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, S.H.; Vimala, Y.; Jatav, H.S.; Ahmad, P.; Chen, Y.; Siddique, K.H.M. Abscisic acid: Metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation. Front. Plant Sci. 2022, 13, 972856. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Medina, P.I.; Gutiérrez-Espinosa, M.A.; Febres, V.; Mora-Aguilera, G.; Robledo-Paz, A. Identificación y clonación de tres genes endógenos que pueden conferir resistencia a patógenos cn cítricos incluyendo CLas y CTV. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2019, 37, 399–420. [Google Scholar] [CrossRef]
- Reazai, M.; Mohammadpourfard, I.; Nazmara, S.; Jahanbakhsh, M.; Shiri, L. Physicochemical characteristics of citrus seed oils from Kerman, Iran. J. Lipids 2014, 2014, 174954. [Google Scholar] [CrossRef] [PubMed]
- Valim, M.F.; Killiny, N. Occurrence of free fatty acids in the phloem sap of different citrus varieties. Plant Signal. Behav. 2017, 12, e1327497. [Google Scholar] [CrossRef]
- Niemetz, R.; Gross, G.G. Oxidation of pentagalloylglucose to the ellagitannin, tellimagrandin II, by a phenol oxidase from Tellima grandiflora leaves. Phytochemistry 2003, 62, 301–306. [Google Scholar] [CrossRef]
- Hatano, T.; Kira, R.; Yoshizaki, M.; Okuda, T. Seasonal changes in the tannins of Liquidambar formosana reflecting their biogenesis. Phytochemistry 1986, 25, 2787–2789. [Google Scholar] [CrossRef]
- Çetinkaya, S.; Çınar Ayan, İ.; Süntar, İ.; Dursun, H.G. The phytochemical profile and biological activity of Liquidambar orientalis Mill. Var. orientalis via NF-κB and apoptotic pathways in human colorectal cancer. Nutr. Cancer 2022, 74, 1457–1473. [Google Scholar] [CrossRef]
- Loeffler, C.; Berger, S.; Guy, A.; Durand, T.; Bringmann, G.; Dreyer, M.; Von Rad, U.; Durner, J.; Mueller, M.J. B1-phytoprostanes trigger plant defense and detoxification responses. Plant Physiol. 2005, 137, 328–340. [Google Scholar] [CrossRef]
- Durand, T.; Bultel-Poncé, V.; Guy, A.; El Fangour, S.; Rossi, J.-C.; Galano, J.-M. Isoprostanes and phytoprostanes: Bioactive lipids. Biochimie 2011, 93, 52–60. [Google Scholar] [CrossRef]
- Liu, W.; Park, S.-W. 12-oxo-phytodienoic acid: A fuse and/or switch of plant growth and defense responses? Front. Plant Sci. 2021, 12, 724079. [Google Scholar] [CrossRef]
- Hsu, P.-S.; Wu, T.-H.; Huang, M.-Y.; Wang, D.-Y.; Wu, M.-C. Nutritive value of 11 bee pollen samples from major floral sources in Taiwan. Foods 2021, 10, 2229. [Google Scholar] [CrossRef]
- Baiocchi, C.; Marengo, E.; Roggero, M.A.; Giacosa, D.; Giorcelli, A.; Toccori, S. Chromatographic and chemometric investigation of the chemical defense mechanism of poplar tree genotypes against a bark fungine parasite. J. Chromatogr. A 1995, 715, 95–104. [Google Scholar] [CrossRef]
- Zhong, L.; Zhou, L.; Zhou, Y.; Chen, Y.; Sui, P.; Wang, J.; Wang, M. Antimicrobial flavonoids from the twigs of Populus nigra × Populus deltoides. Nat. Prod. Res. 2012, 26, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Pichette, A.; Eftekhari, A.; Georges, P.; Lavoie, S.; Mshvildadze, V.; Legault, J. Cytotoxic phenolic compounds in leaf buds of Populus tremuloides. Can. J. Chem. 2010, 88, 104–110. [Google Scholar] [CrossRef]
- Si, C.-L.; Xu, Q.; Li, S.-M.; Liu, Z. Phenolic compounds from Populus davidiana wood. Chem. Nat. Compd. 2009, 45, 634–636. [Google Scholar] [CrossRef]
- Sokolov, I.V.; Torgov, I.V. Flavonoid aglycons in propolis and its sources. Chem. Nat. Compd. 1991, 26, 468. [Google Scholar] [CrossRef]
- Zhang, X.; Hung, T.M.; Phuong, P.T.; Ngoc, T.M.; Min, B.-S.; Song, K.-S.; Seong, Y.H.; Bae, K. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch. Pharm. Res. 2006, 29, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- English, S.; Greenaway, W.; Whatley, F.R. Analysis of phenolics of bud exudate of Populus tristis by GC/MS. Z. Naturforschung C 2014, 47, 512–515. [Google Scholar] [CrossRef]
- Greenaway, W.; English, S.; May, J.; Whatley, F.R. Analysis of phenolics of bud exudates of Populus cathayana and Populus szechuanica by GC-MS. Z. Naturforschung C 1992, 47, 308–312. [Google Scholar] [CrossRef]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in systemic plant immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Abreu, I.N.; Johansson, A.I.; Sokołowska, K.; Niittylä, T.; Sundberg, B.; Hvidsten, T.R.; Street, N.R.; Moritz, T. A Metabolite roadmap of the wood-forming tissue in Populus tremula. New Phytol. 2020, 228, 1559–1572. [Google Scholar] [CrossRef]
- Al-Takay, T. Utilizing of GLC technique for identification of some fatty acids of Populus nigra L. bark growing in ninavah plantation. Mesop. J. Agric. 2018, 46, 166–171. [Google Scholar] [CrossRef]
- Taki, N.; Sasaki-Sekimoto, Y.; Obayashi, T.; Kikuta, A.; Kobayashi, K.; Ainai, T.; Yagi, K.; Sakurai, N.; Suzuki, H.; Masuda, T.; et al. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in arabidopsis. Plant Physiol. 2005, 139, 1268–1283. [Google Scholar] [CrossRef]
- Yabuta, T.; Kambe, K.; Hayashi, T. Biochemical studies of bakanae fungus. In: Fusarinic acid, a new product of the bakanae fungus. J. Agric. Chem. Soc. Jpn. 1937, 10, 1059–1068. [Google Scholar]
- Pavlovkin, J.; Mistríková, I.; Luxová, M.; Mistrík, I. Effects of beauvericin on root cell transmembrane electric potential, electrolyte leakage and respiration of maize roots with different susceptibility to fusarium. Plant Soil Environ. 2004, 52, 492–498. [Google Scholar] [CrossRef]
- Dong, X.; Ling, N.; Wang, M.; Shen, Q.; Guo, S. Fusaric acid is a crucial factor in the disturbance of leaf water imbalance in fusarium-infected banana plants. Plant Physiol. Biochem. 2012, 60, 171–179. [Google Scholar] [CrossRef]
- Singh, V.K.; Upadhyay, R.S. Fusaric acid induced cell death and changes in oxidative metabolism of Solanum lycopersicum L. Bot. Stud. 2014, 55, 66. [Google Scholar] [CrossRef]
- Hatsugai, N.; Katagiri, F. Quantification of plant cell death by electrolyte leakage assay. Bio-Protocol 2018, 8, e2758. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef]
- Torres, M.A. ROS in biotic interactions. Physiol. Plant. 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Petrov, V.D.; Van Breusegem, F. Hydrogen peroxide—A central hub for information flow in plant cells. AoB PLANTS 2012, 2012, pls014. [Google Scholar] [CrossRef]
- De Quadros, F.M.; Garcés-Fiallos, F.R.; De Borba, M.C.; De Freitas, M.B.; Stadnik, M.J. Fusarium oxysporum affects differently the hydrogen peroxide levels and oxidative metabolism in susceptible and resistant bean roots. Physiol. Mol. Plant Pathol. 2019, 106, 1–6. [Google Scholar] [CrossRef]
- Sherin, G.; Aswathi, K.P.R.; Puthur, J.T. Photosynthetic functions in plants subjected to stresses are positively influenced by priming. Plant Stress 2022, 4, 100079. [Google Scholar] [CrossRef]
- Pshibytko, N.L.; Zenevich, L.A.; Kabashnikova, L.F. Changes in the photosynthetic apparatus during fusarium wilt of tomato. Russ. J. Plant Physiol. 2006, 53, 25–31. [Google Scholar] [CrossRef]
- De Souza Marques, M.M.; Vitorino, L.C.; Rosa, M.; Dário, B.M.M.; Silva, F.G.; Bessa, L.A. Leaf physiology and histopathology of the interaction between the opportunistic phytopathogen Fusarium equiseti and Gossypium hirsutum plants. Eur. J. Plant Pathol. 2024, 168, 329–349. [Google Scholar] [CrossRef]
- Krishnamani, M.R.S.; Lakshmanan, M. Photosynthetic changes in Fusarium-infected cotton. Can. J. Bot. 1976, 54, 1257–1263. [Google Scholar] [CrossRef]
- Wu, H.-S.; Bao, W.; Liu, D.-Y.; Ling, N.; Ying, R.-R.; Raza, W.; Shen, Q.-R. Effect of fusaric acid on biomass and photosynthesis of watermelon seedlings leaves. Caryologia 2008, 61, 258–268. [Google Scholar] [CrossRef]
- Iqbal, N.; Czékus, Z.; Ördög, A.; Poór, P. Ethylene-dependent effects of fusaric acid on the photosynthetic activity of tomato plants. Photosynthetica 2021, 59, 337–348. [Google Scholar] [CrossRef]
- Mendoza-Vargas, L.A.; Villamarín-Romero, W.P.; Cotrino-Tierradentro, A.S.; Ramírez-Gil, J.G.; Chávez-Arias, C.C.; Restrepo-Díaz, H.; Gómez-Caro, S. Physiological response of cape gooseberry plants to Fusarium oxysporum f. sp. physali, fusaric acid, and water deficit in a hydrophonic system. Front. Plant Sci. 2021, 12, 702842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action mechanisms of effectors in plant-pathogen interaction. Int. J. Mol. Sci. 2022, 23, 6758. [Google Scholar] [CrossRef]
- Wu, L.; Chen, H.; Curtis, C.; Fu, Z.Q. Go in for the kill: How plants deploy effector-triggered immunity to combat pathogens. Virulence 2014, 5, 710–721. [Google Scholar] [CrossRef]
- Shao, D.; Smith, D.L.; Kabbage, M.; Roth, M.G. Effectors of plant necrotrophic fungi. Front. Plant Sci. 2021, 12, 687713. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhan, C.; Yang, C.; Fernie, A.R.; Luo, J. Metabolomics-centered mining of plant metabolic diversity and function: Past decade and future perspectives. Mol. Plant 2023, 16, 43–63. [Google Scholar] [CrossRef]
- Manickam, S.; Rajagopalan, V.R.; Kambale, R.; Rajasekaran, R.; Kanagarajan, S.; Muthurajan, R. Plant metabolomics: Current initiatives and future prospects. Curr. Issues Mol. Biol. 2023, 45, 8894–8906. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Hirai, M.Y.; Nakamura, Y. Plant metabolomics. J. Exp. Bot. 2024, 75, 1651–1653. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- He, M.; Qin, C.-X.; Wang, X.; Ding, N.-Z. Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Begara-Morales, J.C.; Luque, F.; Jiménez-Ruiz, J.; Padilla, M.N.; Fierro-Risco, J.; Valderrama, R.; Fernández-Ocaña, A.; Corpas, F.J.; et al. Transcriptomic profiling of linolenic acid-responsive genes in ROS signaling from RNA-seq data in arabidopsis. Front. Plant Sci. 2015, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Stone, S.R.; Kaur, P. Recent advances in heterologous synthesis paving way for future green-modular bioindustries: A review with special reference to isoflavonoids. Front. Bioeng. Biotechnol. 2021, 9, 673270. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Zhuang, W.-B.; Li, Y.-H.; Shu, X.-C.; Pu, Y.-T.; Wang, X.-J.; Wang, T.; Wang, Z. The Classification, molecular structure and biological biosynthesis of flavonoids, and their roles in biotic and abiotic stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef]
- Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in phenylpropanoid and trichothecene production by Fusarium culmorum and F. graminearum sensu stricto via exposure to flavonoids. Toxins 2018, 10, 110. [Google Scholar] [CrossRef]
- Ahmed, S.; Kovinich, N. Regulation of phytoalexin biosynthesis for agriculture and human health. Phytochem. Rev. 2021, 20, 483–505. [Google Scholar] [CrossRef]
- Shimizu, B.; Miyagawa, H.; Ueno, T.; Sakata, K.; Watanabe, K.; Ogawa, K. Morning glory systemically accumulates scopoletin and scopolin after interaction with Fusarium oxysporum. Z. Naturforschung C 2005, 60, 83–90. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Zhao, T.; Huang, X.; Zong, J.; Luo, Q.; Peng, C.; Wu, X.; Qiu, F.; Zhao, D.; et al. Biosynthesis of scopoletin in sweet potato confers resistance against Fusarium oxysporum. J. Agric. Food Chem. 2024, 72, 7749–7764. [Google Scholar] [CrossRef]
- Beyer, S.F.; Beesley, A.; Rohmann, P.F.W.; Schultheiss, H.; Conrath, U.; Langenbach, C.J.G. The arabidopsis non-host defence-associated coumarin scopoletin protects soybean from asian soybean rust. Plant J. 2019, 99, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.A.; Bernar, E.M.; Jung, K. Production of siderophores increases resistance to fusaric acid in Pseudomonas protegens Pf-5. PLoS ONE 2015, 10, e0117040. [Google Scholar] [CrossRef]
- Jiao, J.; Zhou, B.; Zhu, X.; Gao, Z.; Liang, Y. Fusaric acid induction of programmed cell death modulated through nitric oxide signalling in tobacco suspension cells. Planta 2013, 238, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Wu, J. Synergistic induction of phytoalexins in Nicotiana attenuata by jasmonate and ethylene signaling mediated by NaWRKY70. J. Exp. Bot. 2024, 75, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, L.; Zhang, B.; Ma, J.; Hettenhausen, C.; Cao, G.; Sun, G.; Wu, J.; Wu, J. Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J. Exp. Bot. 2014, 65, 4305–4315. [Google Scholar] [CrossRef] [PubMed]

| Superclass | Class | Metabolite | m /z | R.t. (min) | Ionization Mode | Aduct | Mass Error (ppm) | Level ID * | Fragments | References |
|---|---|---|---|---|---|---|---|---|---|---|
| C. sinensis | ||||||||||
| Flavonoids | Flavones | Schaftoside | 565.1538 | 4.15 | ESI+ | [M+H]+ | 2.5 | 2 | - | [24] |
| Salvigenin | 327.0870 | 7.79 | ESI− | [M-H]− | 1.2 | 1 | 311.1042; 297.0396 | [25] | ||
| Apigenin | 269.0450 | 8.15 | ESI− | [M-H]− | 1.9 | 1 | 117.0331; 151.0031; 183.0432; 227.0329 | [26] | ||
| Chrysoeriol | 301.0716 | 7.10 | ESI+ | [M+H]+ | −3.0 | 1 | 286.0493; 258.0543; 153.0181 | [27] | ||
| Xanthomicrol | 343.0818 | 8.73 | ESI− | [M-H]− | 1.5 | 1 | 300.0614 | [28] | ||
| Flavanones | Hesperetin | 301.0710 | 5.94 | ESI− | [M-H]− | 2.7 | 1 | 286.0461; 242.0545; 164.0100; 151.0015; 134.0367 | [29,30] | |
| Flavonols | Limocitrin | 347.0760 | 5.18 | ESI+ | [M+H]+ | 0.3 | 1 | 332.0503; 301.0373 | [31] | |
| Triterpenoids | Limonoids | Limonexin | 501.1759 | 6.00 | ESI− | [M-H]− | 1.4 | 1 | 457.1868; 455.1712; 429.1890 | [32] |
| Apocarotenoids | Apocarotenoids-beta | Xanthoxin | 249.1488 | 9.42 | ESI− | [M-H]− | 3.2 | 1 | 221.1171; 205.1595; 217.1605 | [33] |
| Octadecanoids | Phytodienoic acids | 12-Oxo-phytodienoic acid | 293.2117 | 11.73 | ESI+ | [M+H]+ | −2.0 | 2 | - | [34] |
| Fatty acids and conjugates | Dicarboxylic acids | Azelaic acid | 187.0972 | 5.02 | ESI− | [M-H]− | 2.1 | 1 | 125.0956; 97.0647; 123.0796 | [35] |
| Unsaturated fatty acids | Palmitic acid | 255.2321 | 13.42 | ESI− | [M-H]− | 3.5 | 1 | 256.2352; 237.1833 | [36,37] | |
| Linoleic acid | 279.2322 | 13.55 | ESI− | [M-H]− | 2.9 | 1 | 277.2150 | |||
| Oleic acid | 281.2476 | 13.86 | ESI− | [M-H]− | 3.6 | 2 | - | |||
| L. styraciflua | ||||||||||
| Phenolic acids | Gallotanins | 1,2,3,4,6-pentagalloyl glucose | 939.1089 | 4.53 | ESI− | [M-H]− | 2.1 | 1 | 787.0975; 769.0876; 617.0771; 169.0136 | [38] |
| Tellimagrandin II | 937.0924 | 4.56 | ESI− | [M-H]− | 3.1 | 1 | 169.0143; 125.0230 | [39] | ||
| Flavonoids | Flavonols | Quercetin-3-O-glucoside | 463.0873 | 5.07 | ESI− | [M-H]− | 1.9 | 1 | 301.0718; 299.9899 | [40] |
| Octadecanoids | Phyto- prostanes | F1-phytoprostane | 327.2173 | 7.34 | ESI− | [M-H]− | 1.2 | 1 | 309.2061; 291.1953; 283.0642; 155.1073 | [41,42] |
| Phytodienoic acids | 12-Oxo-phytodienoic acid | 291.1958 | 10.27 | ESI− | [M-H]− | 2.7 | 1 | 273.1841; 255.0538; 207.1006 | [43] | |
| Fatty acids and conjugates | Unsaturated fatty acids | Linolenic acid | 277.2163 | 12.69 | ESI− | [M-H]− | 3.6 | 1 | 233.2265 | [44] |
| P. nigra | ||||||||||
| Phenolic acids | Simple phenolic acids | Protocatechuic acid | 153.0190 | 5.73 | ESI− | [M-H]− | 2.0 | 1 | 109.0291 | [45] |
| Flavonoids | Flavones | Chrysin | 253.0496 | 7.14 | ESI− | [M-H]− | 4.0 | 1 | 254.0533 | [46] |
| Genkwanin | 283.0599 | 9.85 | ESI− | [M-H]− | 4.6 | 1 | 269.0416; 268.0356 | [47] | ||
| Luteolin | 285.0396 | 6.13 | ESI− | [M-H]− | 3.2 | 1 | 283.0237; 151.0029; 133.0289; 107.0129; 175.0395; 151.0029; 133.0289; 107.0129 | [48] | ||
| Flavonols | Kaempferide | 299.0552 | 7.22 | ESI− | [M-H]− | 3.0 | 1 | 284.0317; 227.0366; 107.0134 | [49] | |
| Galangin | 269.0444 | 7.30 | ESI− | [M-H]− | 4.1 | 1 | 239.129 | [46] | ||
| Flavanones | Naringenin | 271.0602 | 7.13 | ESI− | [M-H]− | 3.7 | 1 | 273.0654; 272.0640 | [48,50] | |
| Chalcones | Naringenin chalcone | 253.0501 | 9.1 | ESI− | [M-H2O-H]− | −0.1 | 1 | 151.0032; 107.0129 | [51] | |
| Pinocembrin chalcone | 255.0653 | 9.32 | ESI− | [M-H]− | 3.9 | 1 | 151.0030; 125.0963; 82.0122 | [52] | ||
| Dihydroflavonols | Aromadendrin | 269.0446 | 6.90 | ESI− | [M-H2O-H]− | 1.5 | 1 | 151.0031; 193.0833; 151.0754; 125.0958; 121.0292; 93.0343 | [47] | |
| Apocarotenoids | Apocarotenoids-beta | Xanthoxin | 249.1488 | 9.73 | ESI− | [M-H]− | 3.2 | 1 | 205.1596; 217.1223; 221.1163 | [33] |
| Fatty acids and conjugates | Dicarboxylic acids | Azelaic acid | 187.0969 | 5.27 | ESI− | [M-H]− | 3.7 | 1 | 125.0966; 97.0647 | [53] |
| Oxo fatty acids | Pyruvic acid | 87.0084 | 0.50 | ESI− | [M-H]− | 4.6 | 2 | - | [54] | |
| Unsaturated fatty acids | Stearic acid | 283.2631 | 14.80 | ESI− | [M-H]− | 2.1 | 1 | 225.0580; 239.0731; 283.2639 | ||
| Palmitic acid | 255.2321 | 13.42 | ESI− | [M-H]− | 1.2 | 1 | 256.2358 | [55] | ||
| P. americana | ||||||||||
| Octadecanoids | Phytodienoic acids | 12-Oxo-phytodienoic acid | 291.1955 | 11.18 | ESI− | [M-H]− | 3.8 | 1 | 273.1844; 247.2053 | [43,56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Sánchez, A.; Plasencia, J.; Monribot-Villanueva, J.L.; Rodríguez-Haas, B.; Ruiz-May, E.; Guerrero-Analco, J.A.; Sánchez-Rangel, D. Phenolic Compounds Accumulation and Cell Death Degree Induced by Fusaric Acid in Agroforestry Hosts Plants of Fusarium Species. J. Fungi 2025, 11, 745. https://doi.org/10.3390/jof11100745
Gutiérrez-Sánchez A, Plasencia J, Monribot-Villanueva JL, Rodríguez-Haas B, Ruiz-May E, Guerrero-Analco JA, Sánchez-Rangel D. Phenolic Compounds Accumulation and Cell Death Degree Induced by Fusaric Acid in Agroforestry Hosts Plants of Fusarium Species. Journal of Fungi. 2025; 11(10):745. https://doi.org/10.3390/jof11100745
Chicago/Turabian StyleGutiérrez-Sánchez, Angélica, Javier Plasencia, Juan L. Monribot-Villanueva, Benjamín Rodríguez-Haas, Eliel Ruiz-May, José A. Guerrero-Analco, and Diana Sánchez-Rangel. 2025. "Phenolic Compounds Accumulation and Cell Death Degree Induced by Fusaric Acid in Agroforestry Hosts Plants of Fusarium Species" Journal of Fungi 11, no. 10: 745. https://doi.org/10.3390/jof11100745
APA StyleGutiérrez-Sánchez, A., Plasencia, J., Monribot-Villanueva, J. L., Rodríguez-Haas, B., Ruiz-May, E., Guerrero-Analco, J. A., & Sánchez-Rangel, D. (2025). Phenolic Compounds Accumulation and Cell Death Degree Induced by Fusaric Acid in Agroforestry Hosts Plants of Fusarium Species. Journal of Fungi, 11(10), 745. https://doi.org/10.3390/jof11100745









