The Registration Situation and Use of Mycopesticides in the World
Abstract
:1. Introduction
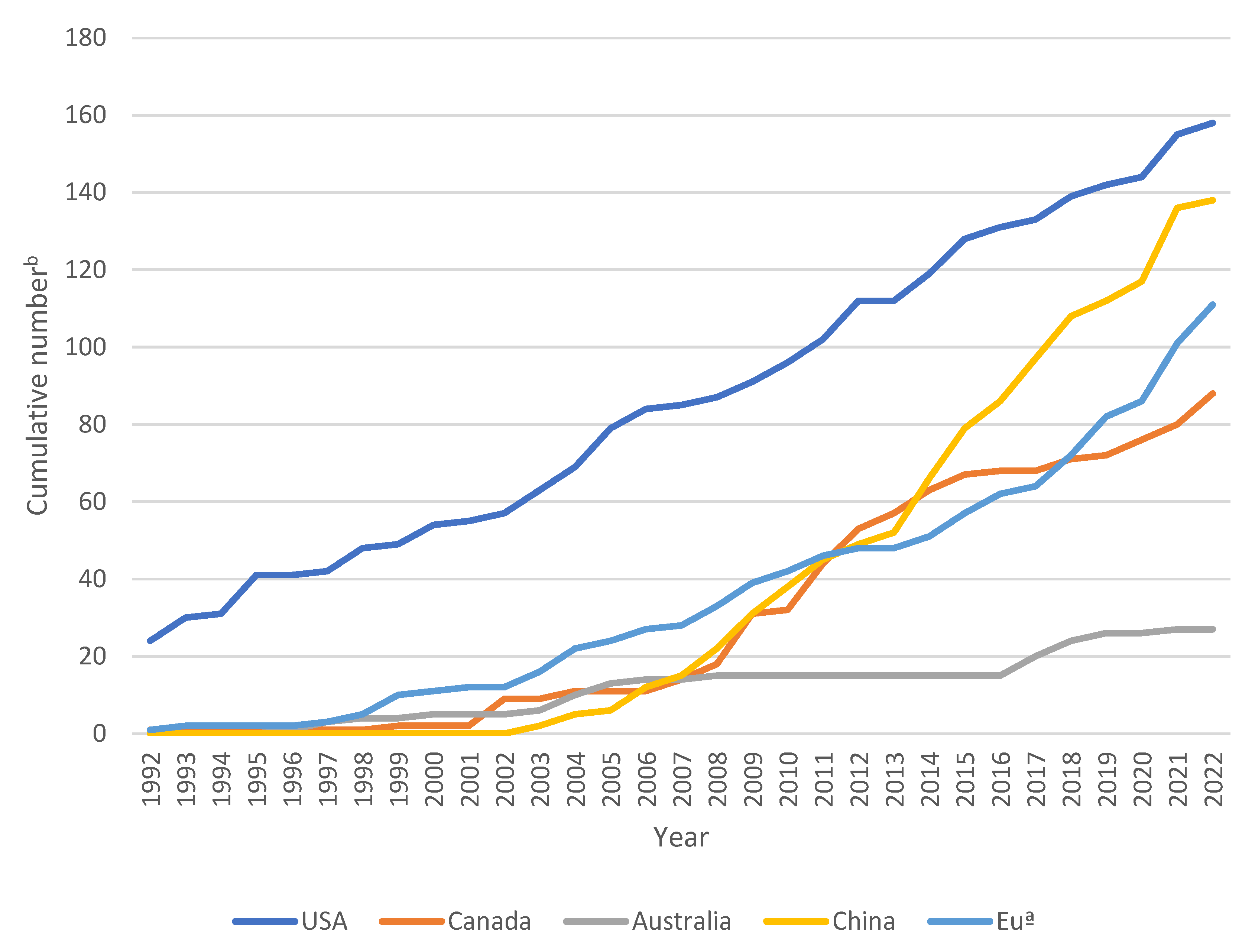
2. Registration of Mycopesticide Products
3. The Development History and Application of Mycopesticides
3.1. Mycoinsecticides
3.1.1. Beauveria
3.1.2. Metarhizium
3.1.3. Cordyceps
3.1.4. Akanthomyces lecanii
3.1.5. Hirsutella thompsonii
3.2. Mycofungicides and Nematophagous Fungi
3.2.1. Trichoderma
3.2.2. Ampelomyces quisqualis
3.2.3. Paraphaeosphaeria minitans
3.2.4. Paecilomyces
3.3. Mycoherbicides
3.3.1. Phytophthora palmivora
3.3.2. Colletotrichum gloeosporioides
3.3.3. Chondrostereum purpureum
4. Problems and Development Trend of Mycopesticides
4.1. Problems
4.1.1. Environmental Limitations
4.1.2. Virulence
4.1.3. Difficulties in Promotion
4.2. Development Trend
4.2.1. Virulence Enhancement by Genetic Engineering
4.2.2. Fermentation Improvement
4.2.3. Combined Use
5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Schreinemachers, P.; Tipraqsa, P. Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 2012, 37, 616–626. [Google Scholar] [CrossRef]
- Jennings, A.A.; Li, Z. Scope of the worldwide effort to regulate pesticide contamination in surface soils. J. Environ. Manag. 2014, 146, 420–443. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.A.; Foppen, R.P.B.; van Turnhout, C.A.M.; de Kroon, H.; Jongejans, E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 2014, 511, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.C. How effective are common household preparations on removing pesticide residues from fruit and vegetables? A review. J. Sci. Food Agric. 2018, 98, 2857–2870. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Mascarin, G.M.; Lopes, R.B.; Delalibera, I., Jr.; Fernandes, E.K.K.; Luz, C.; Faria, M. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr. Pathol. 2019, 165, 46–53. [Google Scholar] [CrossRef]
- Institute for the Control of Agrochemicals, Ministry of Agriculture and Rural Affairs. List of Prohibited and Restricted Pesticides. Available online: http://www.chinapesticide.org.cn/zgnyxxw/zwb/detail/13081 (accessed on 10 March 2023).
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Marrone, P.G. Status of the biopesticide market and prospects for new bioherbicides. Pest Manag. Sci. 2023. [Google Scholar] [CrossRef]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the Art and Future Opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, R.K.; Goswami, B.K. Bio-control activity of Purpureocillium lilacinum strains in managing root-knot disease of tomato caused by Meloidogyne incognita. Biocontrol Sci. Technol. 2013, 23, 1469–1489. [Google Scholar] [CrossRef]
- Singh, H.B. Management of Plant Pathogens with Microorganisms. Proc. Indian Natl. Sci. Acad. 2014, 80, 443–454. [Google Scholar] [CrossRef]
- Zaki, O.; Weekers, F.; Thonart, P.; Tesch, E.; Kuenemann, P.; Jacques, P. Limiting factors of mycopesticide development. Biol. Control 2020, 144, 104220. [Google Scholar] [CrossRef]
- Thakore, Y. The biopesticide market for global agricultural use. Ind. Biotechnol. 2006, 2, 194–208. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Hajek, A.E.; St. Leger, R.J. Interactions Between Fungal Pathogens and Insect Hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci. Technol. 2007, 17, 879–920. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef]
- Heydari, A.; Pessarakli, M. A Review on Biological Control of Fungal Plant Pathogens Using Microbial Antagonists. J. Biol. Sci. 2010, 10, 273. [Google Scholar] [CrossRef]
- Helepciuc, F.-E.; Todor, A. EU microbial pest control: A revolution in waiting. Pest Manag. Sci. 2022, 78, 1314–1325. [Google Scholar] [CrossRef]
- Frederiks, C.; Wesseler, J.H.H. A comparison of the EU and US regulatory frameworks for the active substance registration of microbial biological control agents. Pest Manag. Sci. 2019, 75, 87–103. [Google Scholar] [CrossRef]
- Bailey, K.L.; Boyetchko, S.M.; Laengle, T. Social and economic drivers shaping the future of biological control: A Canadian perspective on the factors affecting the development and use of microbial biopesticides. Biol. Control 2010, 52, 221–229. [Google Scholar] [CrossRef]
- Kabaluk, J.T.; Svircev, A.M.; Goettel, M.S.; Woo, S.G. The Use and Regulation of Microbial Pesticides in Representative Jurisdictions Worldwide; International Organization for Biological Control of Noxious Animals and Plants (IOBC): Denver, CO, USA, 2010; pp. 80–88. [Google Scholar]
- Singh, P.; Mazumdar, P. Chapter 5—Microbial pesticides: Trends, scope and adoption for plant and soil improvement. In Biopesticides; Rakshit, A., Meena, V.S., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L., Parihar, M., Singh, A.K., Eds.; Woodhead Publishing: Cambridge, UK, 2022; Volume 2, pp. 37–71. [Google Scholar]
- Singh, S.; Singh, A.; Chaurasiya, D.; Mukherjee, S.; Mondal, G. Entomopathogenic Fungi as Biocontrol Agents in Agriculture. Res. Today 2020, 2, 264–266. [Google Scholar]
- Li, Z.; Alves, S.B.; Roberts, D.W.; Fan, M.; Delalibera, I., Jr.; Tang, J.; Lopes, R.B.; Faria, M.; Rangel, D.E.N. Biological control of insects in Brazil and China: History, current programs and reasons for their successes using entomopathogenic fungi. Biocontrol Sci. Technol. 2010, 20, 117–136. [Google Scholar] [CrossRef]
- Roberts, D.W.; Humber, R.A. 21—Entomogenous Fungi. In Biology of Conidial Fungi; Cole, G.T., Kendrick, B., Eds.; Academic Press: Cambridge, MA, USA, 1981; Volume 2, pp. 201–236. [Google Scholar]
- Rajitha, K.; Savithri, G. Original Research Article Studies on symptomological and economic parameters of silk cocoons of Bombyx mori inoculated with Beauveria Bassiana (Bals.) Vuill. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 44–54. [Google Scholar]
- Sharma, R.; Sharma, P. Fungal entomopathogens: A systematic review. Egypt. J. Biol. Pest Control 2021, 31, 1–13. [Google Scholar] [CrossRef]
- Lord, J.C. From Metchnikoff to Monsanto and beyond: The path of microbial control. J. Invertebr. Pathol. 2005, 89, 19–29. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Klein, M.G. Pest management of soil-inhabiting insects with microorganisms. Agric. Ecosyst. Environ. 1988, 24, 337–349. [Google Scholar] [CrossRef]
- Faria, M.R.d.; Wraight, S.P. Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Chapter Six—Molecular Genetics of Beauveria bassiana Infection of Insects. In Advances in Genetics; Lovett, B., St. Leger, R.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 94, pp. 165–249. [Google Scholar]
- Feng, M.G.; Poprawski, T.J.; Khachatourians, G.G. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: Current status. Biocontrol Sci. Technol. 1994, 4, 3–34. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef] [PubMed]
- van Lenteren, J.C.; Bolckmans, K.; Kohl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. Biocontrol 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Mayerhofer, J.; Enkerli, J.; Zelger, R.; Strasser, H. Biological control of the European cockchafer: Persistence of Beauveria brongniartii after long-term applications in the Euroregion Tyrol. BioControl 2015, 60, 617–629. [Google Scholar] [CrossRef]
- St. Leger, R.J.; Wang, C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Appl. Microbiol. Biotechnol. 2010, 85, 901–907. [Google Scholar] [CrossRef]
- Peng, G.; Wang, Z.; Yin, Y.; Zeng, D.; Xia, Y. Field trials of Metarhizium anisopliae var. acridum (Ascomycota: Hypocreales) against oriental migratory locusts, Locusta migratoria manilensis (Meyen) in Northern China. Crop Prot. 2008, 27, 1244–1250. [Google Scholar] [CrossRef]
- Brown, A.H.S.; Smith, G. The genus Paecilomyces Bainier and its perfect stage Byssochlamys Westling. Trans. Br. Mycol. Soc. 1957, 40, 17–89. [Google Scholar] [CrossRef]
- Hodge, K.T.; Gams, W.; Samson, R.A.; Korf, R.P.; Seifert, K.A. Lectotypification and status of Isaria pers. Fr. Taxon 2005, 54, 485–489. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef]
- Kunimi, Y. Current status and prospects on microbial control in Japan. J. Invertebr. Pathol. 2007, 95, 181–186. [Google Scholar] [CrossRef]
- Zimmermann, G. The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): Biology, ecology and use in biological control. Biocontrol Sci. Technol. 2008, 18, 865–901. [Google Scholar] [CrossRef]
- Kang, B.R.; Han, J.H.; Kim, J.J.; Kim, Y.C. Dual Biocontrol Potential of the Entomopathogenic Fungus, Isaria javanica, for Both Aphids and Plant Fungal Pathogens. Mycobiology 2018, 46, 440–447. [Google Scholar] [CrossRef]
- Seo, D.-S.; Kang, J.-K.; Jeong, M.-H.; Kwon, M.; Park, C.-B. Anti-diabetic Effects of Isaria tenuipes in OLETF Rats as an Animal Model of Diabetes Mellitus Type II. J. Food Hyg. Saf. 2013, 28, 152–157. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Weng, Q. Secondary metabolites (SMs) of Isaria cicadae and Isaria tenuipes. Rsc Adv. 2019, 9, 172–184. [Google Scholar] [CrossRef]
- Zhang, X.-N.; Guo, J.-J.; Zou, X.; Jin, D.-C. Pathogenic differences of the entomopathogenic fungus Isaria cateniannulata to the spider mite Tetranychus urticae (Trombidiformes: Tetranychidae) and its predator Euseius nicholsi (Mesostigmata: Phytoseiidae). Exp. Appl. Acarol. 2018, 75, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.Q.; Han, Y.F.; Chu, H.L.; Liu, A.Y. Studies on the genus Paecilomyces in China. I. Fungal Divers. 2005, 20, 83–101. [Google Scholar] [CrossRef]
- Shimazu, M. Paecilomyces cateniannulatus Liang, a commonly found, but an unrecorded entomogenous fungus in Japan. Appl. Entomol. Zool. 2001, 36, 283–288. [Google Scholar] [CrossRef]
- Xie, Y.P.; Xue, J.L.; Zhang, Z.J.; Liu, W.M.; Yang, Q.; Fan, J.H. Entomopathogenic fungal parasites of scale insects and their potential in biological control. MYCOSYSTEMA 2012, 31, 307–321. [Google Scholar] [CrossRef]
- Hayden, T.P.; Bidochka, M.J.; Khachatourians, G.G. Entomopathogenicity of Several Fungi toward the English Grain Aphid (Homoptera: Aphididae) and Enhancement of Virulence with Host Passage of Paecilomyces farinosus. J. Econ. Entomol. 1992, 85, 58–64. [Google Scholar] [CrossRef]
- Annamalai, M.; Kaushik, H.; Krishnan, S. Pathogenicity of Beauveria bassiana (Balsamo) Vuillemin and Lecanicillium lecanii Zimmerman against Onion Thrips, Thrips tabaci Lindeman. Biopestic. Int. 2013, 9, 148–156. [Google Scholar]
- Diaz, B.M.; Oggerin, M.; Lastra, C.C.L.; Rubio, V.; Fereres, A. Characterization and virulence of Lecanicillium lecanii against different aphid species. Biocontrol 2009, 54, 825–835. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Blackburn, L.F.; Eyre, D.P.; Cannon, R.J.C.; Miller, J.; Northing, P. Bemisia tabaci: The current situation in the UK and the prospect of developing strategies for eradication using entomopathogens. Insect Sci. 2011, 18, 1–10. [Google Scholar] [CrossRef]
- Kiss, L. A review of fungal antagonists of powdery mildews and their potential as biocontrol agents. Pest Manag. Sci. 2003, 59, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Gerson, U.; Gafni, A.; Paz, Z.; Sztejnberg, A. A tale of three acaropathogenic fungi in Israel: Hirsutella, Meira and Acaromyces. Exp. Appl. Acarol. 2008, 46, 183–194. [Google Scholar] [CrossRef]
- McCoy, C.W. Chapter 2.4 Pathogens of eriophyoid mites. In World Crop Pests; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 6, pp. 481–490. [Google Scholar]
- Kumar, K.K.; Sridhar, J.; Murali-Baskaran, R.K.; Senthil-Nathan, S.; Kaushal, P.; Dara, S.K.; Arthurs, S. Microbial biopesticides for insect pest management in India: Current status and future prospects. J. Invertebr. Pathol. 2019, 165, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Acevedo, J.L.; Boucias, D.G.; Lezama, R.; Sims, K.; Pescador, A. Exudate from sporulating cultures of Hirsutella thompsonii inhibit oviposition by the two-spotted spider mite Tetranychus urticae. Exp. Appl. Acarol. 2003, 29, 213–225. [Google Scholar] [CrossRef]
- Baker, K.F. Evolving Concepts of Biological Control of Plant Pathogens. Annu. Rev. Phytopathol. 1987, 25, 67–85. [Google Scholar] [CrossRef]
- Khan, M.R.; Anwer, M.A. Fungal Bioinoculants for Plant Disease Management. In Microbes and Microbial Technology; Ahmad, I., Ahmad, F., Pichtel, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 447–488. [Google Scholar]
- Adams, P.B. The Potential of Mycoparasites for Biological Control of Plant Diseases. Annu. Rev. Phytopathol. 1990, 28, 59–72. [Google Scholar] [CrossRef]
- Samuels, G.J. Trichoderma: A review of biology and systematics of the genus. Mycol. Res. 1996, 100, 923–935. [Google Scholar] [CrossRef]
- Bissett, J.; Szakacs, G.; Nolan, C.A.; Druzhinina, I.; Gradinger, C.; Kubicek, C.P. New species of Trichoderma from Asia. Can. J. Bot. 2003, 81, 570–586. [Google Scholar] [CrossRef]
- Rajesh, R.W.; Rahul, M.S.; Ambalal, N.S. Trichoderma: A significant fungus for agriculture and environment. Afr. J. Agric. Res. 2016, 11, 1952–1965. [Google Scholar] [CrossRef]
- Keswani, C.; Mishra, S.; Sarma, B.K.; Singh, S.P.; Singh, H.B. Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Appl. Microbiol. Biotechnol. 2014, 98, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Kapat, A. The Role of Trichoderma harzianum Protease in the Biocontrol of Botrytis cinerea. Eur. J. Plant Pathol. 1999, 105, 177–189. [Google Scholar] [CrossRef]
- Woo, S.L.; Scala, F.; Ruocco, M.; Lorito, M. The molecular biology of the interactions between Trichoderma spp., phytopathogenic fungi, and plants. Phytopathology 2006, 96, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Costa Carvalho, D.D.; Marques de Mello, S.C.; Martins, I.; Lobo, M., Jr. Biological control of Fusarium wilt on common beans by in-furrow application of Trichoderma harzianum. Trop. Plant Pathol. 2015, 40, 375–381. [Google Scholar] [CrossRef]
- de Aguiar, R.A.; da Cunha, M.G.; Lobo Junior, M. Management of white mold in processing tomatoes by Trichoderma spp. and chemical fungicides applied by drip irrigation. Biol. Control 2014, 74, 1–5. [Google Scholar] [CrossRef]
- Kiss, L.; Russell, J.C.; Szentiványi, O.; Xu, X.; Jeffries, P. Biology and biocontrol potential of Ampelomyces mycoparasites, natural antagonists of powdery mildew fungi. Biocontrol Sci. Technol. 2004, 14, 635–651. [Google Scholar] [CrossRef]
- Sztejnberg, A.; Galper, S.; Mazar, S.; Lisker, N. Ampelomyces quisqualis for Biological and Integrated Control of Powdery Mildews in Israel. J. Phytopathol. 1989, 124, 285–295. [Google Scholar] [CrossRef]
- Legler, S.E.; Pintye, A.; Caffi, T.; Gulyás, S.; Bohár, G.; Rossi, V.; Kiss, L. Sporulation rate in culture and mycoparasitic activity, but not mycohost specificity, are the key factors for selecting Ampelomyces strains for biocontrol of grapevine powdery mildew (Erysiphe necator). Eur. J. Plant Pathol. 2016, 144, 723–736. [Google Scholar] [CrossRef]
- Campbell, W.A. A New Species of Coniothyrium Parasitic on Sclerotia. Mycologia 1947, 39, 190–195. [Google Scholar] [CrossRef]
- Sandys-Winsch, C.; Whipps, J.M.; Gerlagh, M.; Kruse, M. World distribution of the sclerotial mycoparasite Coniothyrium minitans. Mycol. Res. 1993, 97, 1175–1178. [Google Scholar] [CrossRef]
- Verkley, G.J.M.; Dukik, K.; Renfurm, R.; Goker, M.; Stielow, J.B. Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota). Persoonia 2014, 32, 25–51. [Google Scholar] [CrossRef]
- de Vrije, T.; Antoine, N.; Buitelaar, R.M.; Bruckner, S.; Dissevelt, M.; Durand, A.; Gerlagh, M.; Jones, E.E.; Luth, P.; Oostra, J.; et al. The fungal biocontrol agent Coniothyrium minitans: Production by solid-state fermentation, application and marketing. Appl. Microbiol. Biotechnol. 2001, 56, 58–68. [Google Scholar] [CrossRef]
- Al-Sheikh, H.; Abdelzaher, H.M.A. Isolation of Aspergillus sulphureus, Penicillium islandicum and Paecilomyces variotii from Agricultural Soil and their Biological Activity Against Pythium spinosum, the Damping-Off Organism of Soybean. J. Biol. Sci. 2010, 10, 178–189. [Google Scholar] [CrossRef]
- Wahid, O.A.; Moustafa, A.F.; Ibrahim, M.E. Integrated control of tomato Fusarium-wilt through implementation of soil solarization and filamentous fungi. J. Plant Dis. Prot. 2001, 108, 345–355. [Google Scholar]
- Rodrigo, S.; Santamaria, O.; Halecker, S.; Lledó, S.; Stadler, M. Antagonism between Byssochlamys spectabilis (anamorph Paecilomyces variotii) and plant pathogens: Involvement of the bioactive compounds produced by the endophyte. Ann. Appl. Biol. 2017, 171, 464–476. [Google Scholar] [CrossRef]
- Moreno-Gavíra, A.; Huertas, V.; Diánez, F.; Sánchez-Montesinos, B.; Santos, M. Paecilomyces and Its Importance in the Biological Control of Agricultural Pests and Diseases. Plants 2020, 9, 1746. [Google Scholar] [CrossRef]
- Ahmad, R.Z.; Sidi, B.B.; Endrawati, D.; Ekawasti, F. Paecilomyces lilacinus and P. variotii as a predator of nematode and trematode eggs. IOP Conf. Ser. Earth Environ. Sci. 2019, 299, 012056. [Google Scholar] [CrossRef]
- Flores Francisco, B.G.; Ponce, I.M.; Plascencia Espinosa, M.Á.; Mendieta Moctezuma, A.; López y López, V.E. Advances in the biological control of phytoparasitic nematodes via the use of nematophagous fungi. World J. Microbiol. Biotechnol. 2021, 37, 180. [Google Scholar] [CrossRef]
- Fullaway, D.T. Biological Control of Cactus in Hawaii. J. Econ. Entomol. 1954, 47, 696–700. [Google Scholar] [CrossRef]
- Wilson, C.L. Use of Plant Pathogens in Weed Control. Annu. Rev. Phytopathol. 1969, 7, 411–434. [Google Scholar] [CrossRef]
- Aneja, K.R.; Kumar, V.; Jiloha, P.; Kaur, M.; Sharma, C.; Surain, P.; Dhiman, R.; Aneja, A. Potential Bioherbicides: Indian Perspectives. In Biotechnology: Prospects and Applications; Salar, R.K., Gahlawat, S.K., Siwach, P., Duhan, J.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 197–215. [Google Scholar]
- HASAN, S. Specificity and host specialization of Puccinia chondrillina. Ann. Appl. Biol. 1972, 72, 257–263. [Google Scholar] [CrossRef]
- Watson, A.K. The Classical Approach with Plant Pathogens. In Microbial Control of Weeds; TeBeest, D.O., Ed.; Springer US: Boston, MA, USA, 1991; pp. 3–23. [Google Scholar]
- Auld, B.A.; Morin, L. Constraints in the Development of Bioherbicides. Weed Technol. 1995, 9, 638–652. [Google Scholar] [CrossRef]
- Aneja, K.R.; Khan, S.A.; Aneja, A. Bioherbicides: Strategies, Challenges and Prospects. In Developments in Fungal Biology and Applied Mycology; Satyanarayana, T., Deshmukh, S.K., Johri, B.N., Eds.; Springer Singapore: Singapore, 2017; pp. 449–470. [Google Scholar]
- Boyette, C.D.; Quimby, P.C.; Connick, W.J.; Daigle, D.J.; Fulgham, F.E. Progress in the Production, Formulation, and Application of Mycoherbicides. In Microbial Control of Weeds; TeBeest, D.O., Ed.; Springer US: Boston, MA, USA, 1991; pp. 209–222. [Google Scholar]
- Bailey, K.L. Chapter 13—The Bioherbicide Approach to Weed Control Using Plant Pathogens. In Integrated Pest Management; Abrol, D.P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 245–266. [Google Scholar]
- Mortensen, K.; Makowski, R.M.D. Effects of Colletotrichum gloeosporioides f. sp. malvae on plant development and blomass of non-target field crops under controlled and field conditions. Weed Res. 1997, 37, 351–360. [Google Scholar] [CrossRef]
- Scheepens, P.C.; Hoogerbrugge, A. Control of Prunus serotina in forests with the endemic fungus Chondrostereum purpureum. In Proceedings of the VIII International Symposium on Biological Control of Weeds, Rome, Italy, 2–7 February 1992. [Google Scholar]
- De Jong, M.D. The BioChon story: Deployment of Chondrostereum purpureum to suppress stump sprouting in hardwoods. Mycologist 2000, 14, 58–62. [Google Scholar] [CrossRef]
- Vartiamäki, H.; Uotila, A.; Vasaitis, R.; Hantula, J. Genetic diversity in Nordic and Baltic populations of Chondrostereum purpureum: A potential herbicide biocontrol agent. For. Pathol. 2008, 38, 381–393. [Google Scholar] [CrossRef]
- Vartiamaki, H.; Hantula, J.; Uotila, A. Susceptibility of Silver Birch Pruning Wounds to Infection by White-Rot Fungus (Chondrostereum purpureum), a Potential Bioherbicide. Silva Fenn. 2009, 43, 537–547. [Google Scholar] [CrossRef]
- Simpson, R.M.; van_Hekezen, R.; van_Lune, F.; Brewster, D.; Christeller, J.T.; Spiers, A.G. Extracellular enzymes of Chondrostereum purpureum causal fungus of silverleaf disease. New Zealand Plant Prot. 2001, 54, 202–208. [Google Scholar] [CrossRef]
- Senda, M.; Narita, T.; Akada, S.; Okuno, T.; Miyairi, K. Characterization of an endopolygalacturonase Gene cppg1 from Phytopathogenic Fungus Chondrostereum purpureum. J. Gen. Plant Pathol. 2001, 67, 41–44. [Google Scholar] [CrossRef]
- Becker, E.; Shamoun, S.F.; Hintz, W.E. Efficacy and environmental fate of Chondrostereum purpureum used as a biological control for red alder (Alnus rubra). Biol. Control 2005, 33, 269–277. [Google Scholar] [CrossRef]
- Bellgard, S.E.; Johnson, V.W.; Than, D.J.; Anand, N.; Winks, C.J.; Ezeta, G.; Dodd, S.L. Use of the silverleaf fungus Chondrostereum purpureum for biological control of stump-sprouting, riparian weedy tree species in New Zealand. Australas. Plant Pathol. 2014, 43, 321–326. [Google Scholar] [CrossRef]
- Sancar, A. Structure and Function of DNA Photolyase and Cryptochrome Blue-Light Photoreceptors. Chem. Rev. 2003, 103, 2203–2238. [Google Scholar] [CrossRef] [PubMed]
- Yasui, A.; Eker, A.P.; Yasuhira, S.; Yajima, H.; Kobayashi, T.; Takao, M.; Oikawa, A. A new class of DNA photolyases present in various organisms including aplacental mammals. EMBO J. 1994, 13, 6143–6151. [Google Scholar] [CrossRef] [PubMed]
- de Laat, W.L.; Jaspers, N.G.; Hoeijmakers, J.H. Molecular mechanism of nucleotide excision repair. Genes Dev. 1999, 13, 768–785. [Google Scholar] [CrossRef]
- Jaronski, S.T. Ecological factors in the inundative use of fungal entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Luz, C.; Fargues, J. Temperature and moisture requirements for conidial germination of an isolate of Beauveria bassiana, pathogenic to Rhodnius prolixus. Mycopathologia 1997, 138, 117–125. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Liu, K.; Dong, C. Influence of Strain Preservation Methods on Fruiting Body Growth and Metabolite Production by the Medicinal Mushroom Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2018, 20, 1003–1011. [Google Scholar] [CrossRef]
- Zhu, W.; Hu, J.; Chi, J.; Li, Y.; Yang, B.; Hu, W.; Chen, F.; Xu, C.; Chai, L.; Bao, Y. Label-Free Proteomics Reveals the Molecular Mechanism of Subculture Induced Strain Degeneration and Discovery of Indicative Index for Degeneration in Pleurotus ostreatus. Molecules 2020, 25, 4920. [Google Scholar] [CrossRef]
- Mayne, R.Y.; Bennett, J.W.; Tallant, J. Instability of an Aflatoxin-Producing Strain of Aspergillus parasiticus. Mycologia 1971, 63, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Guarro, J.; Suarez, G.; Suñe, N.; Calvo, M.A.; Ramírez, C. Morphological changes in strains of Aspergillus flavus link ex fries and Aspergillus parasiticus speare related with aflatoxin production. Mycopathologia 1980, 72, 171–174. [Google Scholar] [CrossRef]
- Bennett, J.W. Loss of Norsolorinic Acid and Aflatoxin Production by a Mutant of Aspergillus parasiticus. Microbiology 1981, 124, 429–432. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Liu, X.; Cui, B.; Miao, W.; Cheng, W.; Zhao, F. Characteristics Analysis Reveals the Progress of Volvariella volvacea Mycelium Subculture Degeneration. Front. Microbiol. 2019, 10, 2045. [Google Scholar] [CrossRef] [PubMed]
- Santoro, P.H.; Zorzetti, J.; Constanski, K.; Neves, P.M.O.J. Conidial production, virulence, and stress tolerance of Beauveria bassiana conidia after successive in vitro subculturing. Rev. Colomb. De Entomol. 2014, 40, 85–90. [Google Scholar]
- Keyhani, N.O. Using host molecules to increase fungal virulence for biological control of insects. Virulence 2012, 3, 415–417. [Google Scholar] [CrossRef]
- Morin, L.; Derby, J.-A.L.; Kokko, E.G. Infection process of Colletotrichum gloeosporioides f. sp. malvae on Malvaceae weeds. Mycol. Res. 1996, 100, 165–172. [Google Scholar] [CrossRef]
- Turgeon, B.G.; Condon, B.; Liu, J.; Zhang, N. Protoplast Transformation of Filamentous fungi. In Molecular and Cell Biology Methods for Fungi; Sharon, A., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 3–19. [Google Scholar]
- Huang, H.; Wang, Z.; Cheng, J.; Zhao, W.; Li, X.; Wang, H.; Zhang, Z.; Sui, X. An efficient cucumber (Cucumis sativus L.) protoplast isolation and transient expression system. Sci. Hortic. 2013, 150, 206–212. [Google Scholar] [CrossRef]
- Fang, W.G.; Leng, B.; Xiao, Y.H.; Jin, K.; Ma, J.C.; Fan, Y.H.; Feng, J.; Yang, X.Y.; Zhang, Y.J.; Pei, Y. Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl. Environ. Microbiol. 2005, 71, 363–370. [Google Scholar] [CrossRef]
- Wang, C.; St. Leger, R.J. A scorpion neurotoxin increases the potency of a fungal insecticide. Nat. Biotechnol. 2007, 25, 1455–1456. [Google Scholar] [CrossRef]
- Fan, Y.; Pereira, R.M.; Kilic, E.; Casella, G.; Keyhani, N.O. Pyrokinin β-Neuropeptide Affects Necrophoretic Behavior in Fire Ants (S. invicta), and Expression of β-NP in a Mycoinsecticide Increases Its Virulence. PLoS ONE 2012, 7, e26924. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Iwanicki, N.S.; Ramirez, J.L.; Delalibera, Í., Jr.; Dunlap, C.A. Transcriptional Responses of Beauveria bassiana Blastospores Cultured Under Varying Glucose Concentrations. Front. Cell. Infect. Microbiol. 2021, 11, 644372. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jackson, M.A.; Kobori, N.N.; Behle, R.W.; Delalibera, I. Liquid culture fermentation for rapid production of desiccation tolerant blastospores of Beauveria bassiana and Isaria fumosorosea strains. J. Invertebr. Pathol. 2015, 127, 11–20. [Google Scholar] [CrossRef]
- Jackson, M.A.; McGuire, M.R.; Lacey, L.A.; Wraight, S.P. Liquid culture production of desiccation tolerant blastospores of the bioinsecticidal fungus Paecilomyces fumosoroseus. Mycol. Res. 1997, 101, 35–41. [Google Scholar] [CrossRef]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef]
- Zhang, C.; Ali Khan, R.A.; Wei, H.; Wang, R.; Hou, J.; Liu, T. Rapid and mass production of biopesticide Trichoderma Brev T069 from cassava peels using newly established solid-state fermentation bioreactor system. J. Environ. Manag. 2022, 313, 114981. [Google Scholar] [CrossRef]
- Pereira, A.I.A.; da Silva, C.M.; Curvelo, C.R.S.; Pontes, N.D.; Pereira, J.L.; Tavares, W.D.; Zanuncio, J.C.; Luz, J.M.Q. Mixtures between Beauveria bassiana and potassium silicate to manage thrips in tomato plants for industrial processing. Hortic. Bras. 2020, 38, 415–420. [Google Scholar] [CrossRef]
- Wu, J.; Yang, B.; Zhang, X.; Cuthbertson, A.G.S.; Ali, S. Synergistic Interaction between the Entomopathogenic Fungus Akanthomyces attenuatus (Zare & Gams) and the Botanical Insecticide Matrine against Megalurothrips usitatus (Bagrall). J. Fungi 2021, 7, 536. [Google Scholar] [CrossRef]
- Shi, W.B.; Jiang, Y.; Feng, M.G. Compatibility of ten acaricides with Beauveria bassiana and enhancement of fungal infection to Tetranychus cinnabarinus (Acari: Tetranychidae) eggs by sublethal application rates of pyridaben. Appl. Entomol. Zool. 2005, 40, 659–666. [Google Scholar] [CrossRef]
- Wakil, W.; Kavallieratos, N.G.; Eleftheriadou, N.; Yaseen, T.; Rasool, K.G.; Husain, M.; Aldawood, A.S. Natural Warriors against Stored-Grain Pests: The Joint Action of Beauveria bassiana and Steinernema carpocapsae. J. Fungi 2023, 9, 835. [Google Scholar] [CrossRef]
- Rizwan, M.; Atta, B.; Rizwan, M.; Sabir, A.M.; Shah, Z.U.; Hussain, M. Effect of the entomopathogenic fungus, Beauveria bassiana, combined with diatomaceous earth on the red flour beetle, Tribolium castaneum (Herbst) (Tenebrionidae: Coleoptera). Egypt. J. Biol. Pest Control 2019, 29, 27. [Google Scholar] [CrossRef]
- Akbar, W.; Lord, J.C.; Nechols, J.R.; Howard, R.W. Diatomaceous earth increases the efficacy of Beauveria bassiana against Tribolium castaneum larvae and increases Conidia attachment. J. Econ. Entomol. 2004, 97, 273–280. [Google Scholar] [CrossRef]
- Wakil, W.; Gulzar, S.; Prager, S.M.; Ghazanfar, M.U.; Shapiro-Ilan, D.I. Efficacy of entomopathogenic fungi, nematodes and spinetoram combinations for integrated management of Thrips tabaci. Pest Manag. Sci. 2023, 79, 3227–3238. [Google Scholar] [CrossRef]
| Mycoinsecticides a | Country/Region b Where Approved/Registered | Target(s) |
|---|---|---|
| Akanthomyces muscarius Ve6 (formerly Lecanicillium muscarium) | EU, CA | Whiteflies, thrips |
| Beauveria bassiana | CHN, AUS | Rice leaf folder Cnaphalocrocis medinalis, aphids, termites |
| Beauveria bassiana strain 147 | EU | Paysandisia archon, Rhynchophorus ferrugineus |
| Beauveria bassiana strain 203 | EU | Rhynchophorus ferrugineus |
| Beauveria bassiana strain 447 | USA | Ants |
| Beauveria bassiana strain ANT-03 | USA, CA | Foliar-feeding pests and certain grubs |
| Beauveria bassiana strain ATCC 74040 | USA, EU | Ants, aphids, armyworms, whiteflies |
| Beauveria bassiana strain CFL-A | CA | Annual bluegrass weevil larvae Listronotus maculicollis, asiatic garden beetle Maladera castanea |
| Beauveria bassiana strain GHA | USA, EU, CA | Scarab beetles, leaf-feeding beetles, whiteflies, aphids, thrips |
| Beauveria bassiana strain HF23 | USA, CA | Houseflies |
| Beauveria bassiana strain PPRI 5339 | USA, EU, CA | Certain piercing, sucking, and chewing pests (insects and mites) |
| Beauveria bassiana strain R444 | CA | Black cutworm, corn flea beetle, nematodes |
| Beauveria bassiana strain IMI389521 | EU | Coleoptera pests Oryzaephilus surinamensis, Sitophilus granaries, Cryptolestes ferrugineus |
| Beauveria bassiana strain NPP111B005 | EU | Cosmopolites sordidus, Rhynchophorus ferrugineus |
| Beauveria bassiana strain ZJU435 | CHN | Fall armyworm Spodoptera frugiperda, whitefly Trialeurodes vaporariorum |
| Conidiobolus major | CHN | Whiteflies, aphids |
| Cordyceps javanica Ij01 (formerly Isaria javanica, Paecilomyces javanicus) | CHN | Spodoptera litura Fabricius |
| Cordyceps javanica JS001 (formerly Isaria javanica, Paecilomyces javanicus) | CHN | Whitefly Bemisia tabaci |
| Cordyceps fumosorosea strain Apopka 97 (formerly Isaria fumosorosea, Paecilomyces fumosoroseus) | USA, EU | Whiteflies, thrips, aphids, spider mites |
| Cordyceps fumosorosea strain FE 9901 (formerly Isaria fumosorosea, Paecilomyces fumosoroseus) | USA, EU, CA | Aphids, weevils, whiteflies |
| Metarhizium anisopliae | CHN | Thrips, locusts, Carposina niponensisi, Spodoptera exigua |
| Metarhizium anisopliae strain CQMa421 | CHN | Chilo suppressalis, Spodoptera frugiperda |
| Metarhizium anisopliae strain ESF1 | USA | Termites |
| Metarhizium acridum (formerly Metarhizium anisopliae var. acridum) | AUS | Australian plague locust—nymphs, grasshoppers |
| Metarhizium brunneum strain Ma 43 (formerly Metarhizium anisopliae var. anisopliae) | EU | Japanese beetle Popillia japonica, Garden chafer Phyllopertha horticola, Summer chafer Amphimallon solstitialis, European chafer Amphimallon majalis |
| Metarhizium brunneum strain F52 (formerly known as Metarhizium anisopliae strain F52) | USA, CA | Mites, thrips, ticks, weevils and whiteflies |
| Nosema locustae | USA, CA, CHN | Grasshoppers, Mormon cricket |
| Mycofungicides or Nematophagous Fungi | Country/Region Where Approved/Registered | Target(s) |
|---|---|---|
| Ampelomyces quisqualis strain AQ10 | USA, EU | Powdery mildew |
| Aspergillus flavus strain AF36 | USA | Strains of the fungus Aspergillus flavus that produce aflatoxin |
| Aspergillus flavus strain NRRL 21882 | USA | Strains of the fungus A. flavus that produce aflatoxin |
| Aureobasidium pullulans strains DSM 14940 and DSM 14941 | USA, EU, CA, AUS | Bacterial and fungal flower and foliar diseases |
| Candida oleophila isolate I-182 | USA | Post-harvest fungicide |
| Candida oleophila strain O | USA, EU | For post-harvest control of gray mold Botrytis cinerea and blue mold Penicillium expansum |
| Clonostachys rosea strain CR-7 | USA | Botrytis, Colletotrichum, Monilinia, Sclerotinia, Alternaria, Fusarium, and Didymella |
| Clonostachys rosea strain J1446 | USA, EU, CA | Seed borne and soil borne fungi, such as Fusarium, Pythium and Phytophtora, foliar fungal diseases |
| Paraphaeosphaeria minitans (formerly Coniothyrium minitans) strain CON/M/91-08 | USA, EU, CA | Sclerotinia spp. |
| Paraphaeosphaeria minitans (formerly Coniothyrium minitans) strain ZB-1SB | CHN | Sclerotinia spp. |
| Paraphaeosphaeria minitans (formerly Coniothyrium minitans) Campbell CGMCC8325 | CHN | Sclerotinia spp. |
| Duddingtonia flagrans strain IAH 1297 | USA | Nematodes |
| Gliocladium virens GL-21 | USA | Fungi that cause “damping off” disease and root rot. |
| Muscodor albus strain QST 20799 | USA | Bacteria, fungi, and nematodes |
| Muscodor albus strain SA-13 | USA | Soil-borne plant diseases and plant-parasitic nematodes |
| Metschnikowia fructicola strain NRRL Y-27328 | USA, EU | Monilinia fructigena, Monilia laxa, Botrytis cinerea |
| Myrothecium verrucaria dried fermentation solids and solubles | USA | Nematodes |
| Purpureocillium lilacinum [formerly Paecilomyces lilacinus (Thom) Samson] | CHN | Root-knot nematodes Meloidogyne spp. |
| Purpureocillium lilacinum strain 251 (formerly Paecilomyces lilacinus strain 251) | USA, EU | Root-knot nematodes Meloidogyne spp., cyst nematodes Geterodera spp. and Globodera spp. |
| Purpureocillium lilacinum strain PL 11 | USA, EU | Root-knot nematodes Meloidogyne spp. |
| Pseudozyma flocculosa strain PF-A22 UL | USA | Powdery mildew |
| Pseudozyma flocculosa | CA | Soil-borne diseases caused by fungus |
| Phlebiopsis gigantea strain VRA 1992 | USA, CA | Heterobasidion spp. |
| Phlebiopsis gigantea strain VRA 1835, VRA 1984 and FOC PG 410.3 | EU | Heterobasidion spp. |
| Saccharomyces cerevisiae extract hydrolysate | USA | Bacterial diseases |
| Saccharomyces cerevisiae strain LAS02 | EU | Storage diseases Monilinia spp., Botrytis cinerea |
| Trichoderma asperellum strain ICC 012 | USA, EU, CA | Fungal soil diseases in vegetables and ornamentals |
| Trichoderma asperellum strain T25 | EU | Phythophthora sp. Fusarium sp. Pythium sp. |
| Trichoderma asperellum strain TV1 | EU | Pythium spp. Rhizoctonia spp. Fusarium spp. |
| Trichoderma asperellum strain T34 | USA, EU, CA | Fusarium oxysporum f.sp. dianthi |
| Trichoderma asperelloides strain JM41R | USA | Rhizoctonia spp. Fusarium spp. |
| Trichoderma atroviridestrain SC1 | USA, EU | Wood and canker diseases |
| Trichoderma atrobrunneum (formerly Trichoderma harzianum) strain ITEM 908 | EU | Pythium spp., Rhizoctonia spp., Fusarium spp. |
| Trichoderma atroviride strain IMI 206040 | EU | Pythium spp., Rhizoctonia spp., Fusarium spp. |
| Trichoderma atroviride strain T11 | EU | Pythium spp., Rhizoctonia spp., Fusarium spp. |
| Trichoderma atroviride strain I-1237 | EU | Wood decay diseases |
| Trichoderma gamsii strain ICC 080 | USA, EU, CA | Fungal soil diseases in vegetables and ornamentals |
| Trichoderma harzianum | CHN, AUS | Clubroot disease, Botrytis cinerea, Rhizoctonia spp., downy mildew |
| Trichoderma harzianum LTR-2 | CHN | Brown spot, grey mould Botrytis cinerea |
| Trichoderma harzianum DS-10 | CHN | Grey mould Botrytis cinerea |
| Trichoderma harzianum T-39 | USA | Botrytis cinerea |
| Trichoderma harzianum strain T78 | USA | Fusarium, Phytophthora spp., Pythium spp., Rhizoctonia, Sclerotium spp. |
| Trichoderma hamatum isolate 382 | USA | Diseases caused by soil borne plant pathogens |
| Trichoderma harzianum rifai strain T-22 | USA, EU, CA | Various fungi that cause seed rot, diseases of plant roots, and other plant diseases |
| Trichoderma harzianum rifai strain KRL-AG2 | USA, CA | Root pathogens in greenhouse tomatoes, cucumbers, and ornamentals |
| Trichoderma polysporum ATCC 20475 | USA | Fungi that infect tree wounds |
| Trichoderma viride ATCC 20476 | USA | Fungi that infect tree wounds |
| Trichoderma virens strain G-41 | USA, CA | Fungal soil diseases in vegetables, ornamentals |
| Trichoderma spp. | CHN | Various fungi that cause seed rot, diseases of plant roots, and other plant diseases |
| Typhula phacorrhiza strain 94671 | USA, CA | Snow molds in turf |
| Ulocladium oudemansii strain U3 | USA | Botrytis cinerea and Sclerotinia sclerotiorum |
| Verticillium dahliae strain WCS850 | USA, EU, CA | Dutch elm disease |
| Verticillium chlamydosporium Goddard | CHN | Root-knot nematodes |
| Mycoherbicides | Country/Region Where Approved/Registered | Target(s) |
|---|---|---|
| Alternaria destruens strain 059 | USA | Dodder Cuscuta spp. |
| Chondrostereum purpureum strain PFC 2139 | USA, CA | Inhibits the sprouting and regrowth of shrubs and hardwood trees |
| Chondrostereum purpureum strain HQ1 | USA | Inhibits the sprouting and regrowth of shrubs and hardwood trees |
| Colletotrichum gloeosporioides f. sp aeschynomene | USA | Northern jointvetch Aeschynomene virginica |
| Phoma macrostoma | CA | Broadleaved weeds like dandelion, Canada thistle, and clover |
| Phytophthora palmivora MWV | USA | Morenia orderata, commonly known as strangler vine or milkweed vine |
| Puccinia thlaspeos strain woad (dyer’s woad rust) | USA | Dyer’s woad |
| Lasiodiplodia pseudotheobromae NT039, Macrophomina phaseolina NT094, Neoscytalidium novaehollandiae QLD 003 | AUS | Parkinsonia spp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Wang, J. The Registration Situation and Use of Mycopesticides in the World. J. Fungi 2023, 9, 940. https://doi.org/10.3390/jof9090940
Jiang Y, Wang J. The Registration Situation and Use of Mycopesticides in the World. Journal of Fungi. 2023; 9(9):940. https://doi.org/10.3390/jof9090940
Chicago/Turabian StyleJiang, Yali, and Jingjing Wang. 2023. "The Registration Situation and Use of Mycopesticides in the World" Journal of Fungi 9, no. 9: 940. https://doi.org/10.3390/jof9090940
APA StyleJiang, Y., & Wang, J. (2023). The Registration Situation and Use of Mycopesticides in the World. Journal of Fungi, 9(9), 940. https://doi.org/10.3390/jof9090940





