New Ascomycetes from the Mexican Tropical Montane Cloud Forest
Abstract
:1. Introduction
2. Material and Methods
2.1. Morphological Studies
2.2. Amplification and Sequencing
2.3. Phylogenetic Analysis
3. Results
3.1. Taxonomy
3.1.1. Dothideomycetes, Patellariales, Patellariaceae
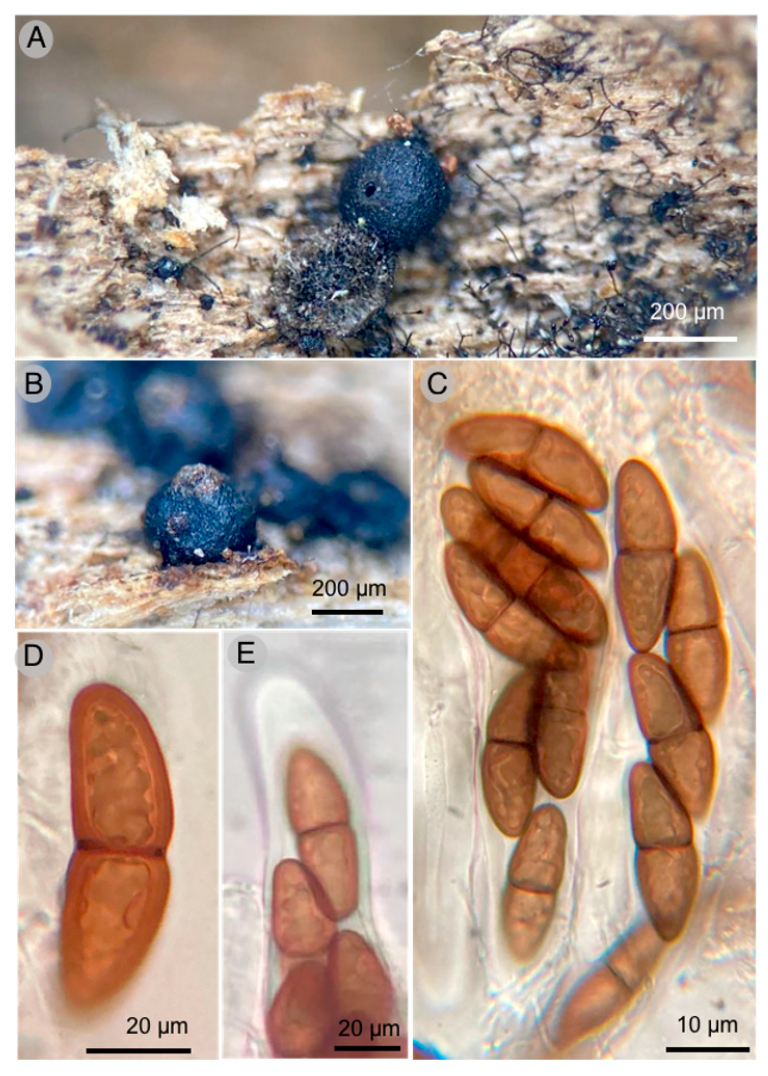
- Holmiella hidalgoensis Raymundo, Martínez-González & R. Valenz. sp. nov.
3.1.2. Pleosporales, Kirschteiniotheliaceae
- 2.
- Kirschsteiniothelia esperanzae Raymundo, Cobos-Villagrán & R. Valenz. sp. nov.
3.1.3. Geoglossomycetes, Geoglossales, Geoglossaceae
- 3.
- Microglossum flavoviride Sánchez-Flores, García-Jiménez & Raymundo sp. nov.
3.1.4. Leotiomycetes, Helotiales, Helotiaceae
- 4.
- Claussenomyces paulinae Raymundo
3.1.5. Pezizomycetes, Pezizales, Chorioactidaceae
- 5.
- Wolfina molangoensis R. Valenz. & Raymundo
3.1.6. Sordariomycetes, Xylariales, Xylariaceae
- 6.
- Dematophora oaxacana Sánchez-Flores, R. Valenz. & Raymundo sp. nov.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decon, J.W. Fungal Biology; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2006; p. 371. [Google Scholar]
- Rzedowski, J. La Vegetación de México; Limusa: Mexico City, Mexico, 1978; p. 432. [Google Scholar]
- Luna-Vega, I.; Alcántara-Ayala, O.; Espinosa, D.; Morrone, J.J. Historical relationships of the Mexican cloud forests: A preliminary vicariance model applying Parsimony Analysis of Endemicity to vascular plant taxa. J. Biogeogr. 1999, 26, 1299–1305. [Google Scholar] [CrossRef]
- Guzmán, G. Análisis de los estudios de los macromicetos de México. Rev. Mex. Micol. 2008, 28, 7–15. [Google Scholar]
- González, M.; Hanlin, R.T. Distribution and occurrence of Ascomycetes in Mexico. North Amer. Fungi 2008, 3, 139–145. [Google Scholar] [CrossRef]
- Heredia-Abarca, G.; Arias-Mota, R.M.; Becerra-Hernández, C.I. Análisis de conocimiento de los hongos anamorfos saprobios de México. In Tópicos Sobre Diversidad Ecología y Uso de Los Hongos Microscópicos; Heredia-Abarca, G., Ed.; Programa Iberomaricano de Ciencia y tecnología para el Desarrollo (CYTED) e Instituto de Ecología, A.C. Xalapa: Xalapa, Mexico, 2008; 386p. [Google Scholar]
- Aguirre-Acosta, E.; Ulloa, M.; Aguilar, S.; Cifuentes, J.; Valenzuela, R. Biodiversidad de hongos en México. Rev. Mex. Biodivers. 2014, 85, 76–81. [Google Scholar] [CrossRef]
- Cifuentes, J. Hongos. Catálogo taxonómico de especies de México. In Capital Natural de México, Vol. 1; Conocimiento actual de la biodiversidad Conabio: Mexico City, Mexico, 2008. [Google Scholar]
- Del Olmo-Ruiz, M.; García-Sandoval, R.; Alcántara-Ayala, O.; Véliz, M.; Luna-Vega, I. Current knowledge of fungi from neotropical montane cloud forests: Distributional patterns and composition. Biodiv. Conserv. 2017, 26, 1919–1942. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Eyre Methuen: London, UK, 1978; p. 252. [Google Scholar]
- Bessette, A.E.; Roody, W.C.; Bessette, A.R. North American Boletes: A Color Guide to the Fleshy PORED Mushrooms, 1st ed.; Syracuse University Press: Syracuse, NY, USA, 2000; p. 396. [Google Scholar]
- Ulloa, M.; Hanlin, R.T. Illustrated Dictionary of Mycology, 2nd ed.; APS Press: St. Paul, MI, USA, 2012; p. 762. [Google Scholar]
- Martínez-González, C.R.; Ramírez-Mendoza, R.; Jiménez-Ramírez, J.; Gallegos-Vázquez, C.; Luna-Vega, I. Improved method for genomic DNA extraction for Opuntia Mill. (Cactaceae). Plant Methods 2017, 13, 82. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- GenBank. National Center of Biotechnology Information. National Library of Medicine. 2020. Available online: https://www.ncbi.nlm.nih.gov/genbank (accessed on 1 March 2023).
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Pem, D.; Gafforv, Y.; Jeewon, R.; Hongsanan, S.; Promputtha, I.; Doilom, M.; Hyde, K.D. Multigene phylogeny coupled with morphological characterization reveal two new species of Holiella and taxonomic insights within Patellariaceae. Cryptogam. Mycol. 2018, 39, 193–209. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7, improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Müller, K. SeqState. Appl. Bioinform. 2005, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, Version 3.70; 2021. Available online: http://mesquiteproject.org (accessed on 1 March 2023).
- Sun, Y.R.; Jayawardena, R.S.; Hyde, K.D.; Wang, Y. Krischsteiniothelia thailandica sp. nov. (Krischsteiniotheliaceae) from Thailandia. Phytotaxa 2021, 490, 172–182. [Google Scholar] [CrossRef]
- Healy, A.H.; Arnold, A.E.; Bonito, G.; Huang, Y.L.; Lemmond, B.; Pfister, D.H.; Smith, M.E. Endophytism and endolichenism in Pezizomycetes: The exception of the rule? New Phytol. 2021, 233, 1974–1983. [Google Scholar] [CrossRef]
- Boonmee, S.; Wanasinghe, D.N.; Calabon, M.S.; Huanraluek, N.; Chandrasiri, S.K.U.; Jones, G.E.B.; Rossi, W.; Leonardi, M.; Singh, S.K.; Rana, S.; et al. Fungal diversity notes 1387–1511, taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2021, 111, 1–335. [Google Scholar] [CrossRef]
- Argnello, C.; Carbone, M.; Braaten, C. Wolfina aurantiopsis, a rare species in the family Chorioactidaceae (Pezizales). Ascomycete.Org 2013, 5, 39–45. [Google Scholar] [CrossRef]
- Wittstein, K.; Cordsmeier, A.; Lambert, C.; Wendt, L.; Sir, E.B.; Weber, J.; Wurzler, N.; Petrini, L.E.; Stadler, M. Identification of Rosellinia species as producers of cyclodepsipeptide PF1022 A and resurrection of the genus Dematophora as inferred from polythetic taxonomy. Stud. Mycol. 2020, 96, 1–16. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8, a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Frandsen, P.B.; Calcott, B.; Mayer, C.; Lanfear, R. Automatic selection of partitioning schemes for phylogenetic analyses using iterative k-means clustering of site rates. BMC Evol. Biol. 2015, 15, 13. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Kainer, D.; Mayer, C.; Stamatakis, A. Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evol. Biol. 2014, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. Partition Finder 2, new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree, version 1.4.2. 2014. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 1 March 2023).
- Bao, D.F.; Luo, Z.L.; Liu, J.K.; Bhat, D.J.; Sarunya, N.; Li, W.L.; Su, H.Y.; Hyde, K.D. Freshwater fungi in China III: New species and record of Kirshsteiniothelia from northwestern Yunnan province. Mycosphere 2018, 9, 755–768. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Abreu, V.P.; Bazzicalupo, A. Fungal diversity notes 603–708, Taxonomic and phylogenetic notes on genera and species. Fungal Divers. 2017, 87, 1–235. [Google Scholar] [CrossRef]
- Mains, E.B. North American hyaline-spored species of the Geoglosseae. Mycologia 1955, 47, 846–877. [Google Scholar] [CrossRef]
- Iglesias, P.; Arauzo, S. Microglossum cyanobasis un Microglossum nuevo recolectado en Oriñón (Cantabria). Errotari 2013, 10, 13–26. [Google Scholar]
- Kučera, V.; Lizoň, P.; Tomšovský, M.; Kučera, J.; Gaisler, J. Re-evaluation of the morphological variability of Microglossum viride and M. griseoviride sp. nov. Mycologia 2014, 106, 282–290. [Google Scholar] [CrossRef]
- Hosoya, T. Enumeration of remarkable Japanese Discomycetes (1) Three Helotialean Members New to Japan. Bull. Natn Mus. Tokyo Ser B 2004, 30, 155–163. [Google Scholar]
- Raymundo, T.; Valenzuela, R.; García-Martínez, Y.; Bravo-Álvarez, M.A.; Ramírez-Martínez, J.C.; Bautista-Hernández, S.; Palacios-Pacheco, M.; Luna-Vega, I. Ascomycetes (Fungi) from the relic forest of Fagus grandifolia subsp. mexicana in eastern Mexico. Phytotaxa 2019, 418, 1–41. [Google Scholar] [CrossRef]
- Breitenbach, J.; Kränzlin, F. Fungi of Switzerland. Volume 1; Ascomycetes; Verlag Mykologia: Luzern, Switzerland, 1984; p. 310. [Google Scholar]
- Raymundo, T.; Valenzuela, R.; Ramírez-Martínez, J.C.; Martínez-Pineda, M.; Cobos Villagrán, A.; Trejo-Arana, A.; Sánchez Flores, M.; Gay-González, A.D.; Luna-Vega, I. New records of Ascomycetes from the tropical montane cloud forests of eastern Mexico. Phytotaxa 2020, 454, 161–185. [Google Scholar] [CrossRef]
- Petrini, L.E. Rosellinia—A World Monograph; Biblioteca Mycologica: Stuttgart, Germany, 2013; Volume 205, p. 410. [Google Scholar]
- Luna-Vega, I.; Velázquez, A.; Velázquez, E. México. In Bosques Nublados Del Neotrópico; Kappelle, M., Brown, A.D., Eds.; INBio: Santo Domingo de Heredia, Costa Rica, 2001; pp. 183–229. [Google Scholar]
- Sánchez-González, L.A.; Morrone, J.J.; Navarro-Singüenza, A.G. Distributional patterns of the Neotropical humid montane forest avifaunas. Biol. J. Linn. Soc. 2008, 94, 175–194. [Google Scholar] [CrossRef]
- Raymundo, T.; Valenzuela, R.; Esqueda, M. Marthamyces coronadoae sp. nov. in a Fagus grandifolia subsp. mexicana forest from Hidalgo State, México. Mycotaxon 2016, 131, 521–526. [Google Scholar] [CrossRef]
- Raymundo, T.; Escudero-Leyva, E.; Soto-Agudelo, R.; García-Jiménez, J.; Romero-Bautista, L.; Valenzuela, R. Nuevos registros de Hypocreales (Sordariomycetes, Ascomycota) del bosque mesófilo de monatña de la Sierra Alta Hidalguense en México. Acta Bot. Mex. 2017, 120, 39–57. [Google Scholar] [CrossRef]
- Arias, R.M.; Heredia-Abarca, G.; Castañeda-Ruiz, R. Checklist of saprobic asexual microfungi from the tropical montane cloud forest of Veracruz, México. Mycotaxon 2018, 132, 985. [Google Scholar]
- Medel-Ortiz, R.; Loera-Hernández, F.G.; Baeza-Guzmán, Y.; Palestina-Villa, E.N.; Belingheri-Lagunes, M.E. Ascomicetos asociados a angiospermas en el bosque mesófilo de montaña en el centro de Veracruz, México. Acta Bot. Mex. 2019, 126, e1542. [Google Scholar] [CrossRef]
- Sánchez-Flores, M.; Valenzuela, R.; Hernández-Muñoz, M.A.; García-Jiménez, J.; Martínez-Pineda, M.; Raymundo, T. Ascomicetos del bosque mesófilo de montaña de Honey, Puebla de los Ángeles, México. Acta Bot. Mex. 2020, 127, e1719. [Google Scholar] [CrossRef]
- Cobos-Villagrán, A.; Valenzuela, R.; Hernández-Rodríguez, C.; Calvillo-Medina, R.P.; Villa-Tanaca, L.; Mateo-Cid, L.E.; Pérez-Valdespino, A.; Martínez-González, C.R.; Raymundo, T. Three new species of Rhytidhysteron (Dothideomycetes, Ascomycota) from Mexico. MycoKeys 2021, 83, 123–144. [Google Scholar] [CrossRef]
- Barbosa-Reséndiz, A.; Valenzuela, R.; Sánchez-Flores, M.; Bautista-Hernández, S.; Cobos-Villagrán, A.; Pérez-Valdespino, A.; Espinoza-Mellado, R.; Martínez-Pineda, M.; Raymundo, T. El género Daldinia (Sordariomycetes, Ascomycota) en México. Acta Bot. Mex. 2020, 127, e1600. [Google Scholar] [CrossRef]
- Raymundo, T.; Martínez-Pineda, M.; Cobos-Villagrán, A.; Sánchez-Flores, M.; Valenzuela, R. Primer registro de Unguiculariopsis ravenelii (Leotiomycetes, Ascomycota) en México. Acta Bot. Mex. 2020, 127, e1666. [Google Scholar] [CrossRef]
- Sánchez-Flores, M.; Martínez-Pineda, M.; Raymundo, T. Ionomidotis mesophila (Ascomycota, Cordieritidaceae), una especie nueva del bosque de niebla en México. Acta Bot. Mex. 2021, 28, e1812. [Google Scholar] [CrossRef]
- Raymundo, T.; Valenzuela, R. Smardaea isoldae sp. nov. from a tropical cloud forest in Mexico. Mycotaxon 2021, 136, 97–106. [Google Scholar] [CrossRef]
- Valenzuela, R.; Raymundo, T.; Reyes, P.; Guzmán-Guillermo, J.; Acosta, S.; Ramírez-Martínez, J.C.; Luna-Vega, I. Ascomycetes from the relic forest of Oreomunnea mexicana, Oaxaca, Mexico. Phyotaxa 2021, 528, 19–44. [Google Scholar] [CrossRef]
- Chacón-Zapata, S.; González, D. Descripción de la segunda especie del género Euacanthe (Scortechiniaceae, Coronophorales), de áreas verdes urbanas y periurbanas de Xalapa, México. Acta Bot. Mex. 2021, 128, e1835. [Google Scholar] [CrossRef]
- Guzmán-Guillermo, J.; Raymundo, T.; Sorcia-Navarrete, P.; Carvajal-Hernández, C.I. Primer registro del hongo briofilo Paruephaedria heimerlii (Dactylosporaceae, Ascomycota) para México. Acta Bot. Mex. 2022, 129, e2006. [Google Scholar] [CrossRef]
- Chacón-Zapata, S.; Ramirez-Guillén, F. Especies conocidas y nuevos registros de Coronophorales (Ascomycota) en México. Acta Bot. Mex. 2022, 129, e2051. [Google Scholar] [CrossRef]
- De la Fuente, J.I.; García-Jiménez, J.; Raymundo, T.; Sánchez-Flores, M.; Valenzuela, R.; Guevara-Guerrero, G.; Pérez-Ovando, E.C.; Ramírez-González, C.R. Elaphomyces castilloi (Elaphomycetaceae, Ascomycota) and Entoloma secotioides (Entolomataceae, Basidiomycota), two new sequestrate fungi from tropical montane cloud forest from south Mexico. Mycokeys 2023, 96, 127–142. [Google Scholar] [CrossRef]
- Churchill, S.P.; Balslev, H.; Forero, E.; Luteyn, J.L. (Eds.) Biodiversity and Conservation of Neotropical Montane Forests. In Proceedings of the Neotropical Montane Forest Biodiversity and Conservation Symposium, New York, NY, USA, 21–26 June 1993; Volume 21. No. 26. [Google Scholar]
- García-Guzmán, O.M.; Garibay-Orijel, R.; Hernández, E.; Arellano-Torres, E.; Oyama, K. World-wide meta-analysis of Quercus forests ectomycorrhizal fungal diversity reveals southwestern Mexico as a hotspot. Mycorrhiza 2017, 27, 811–822. [Google Scholar] [CrossRef]













| Species Name | Isolate/Voucher/Strain | GenBank Accessions | ||
|---|---|---|---|---|
| ITS | nrLSU | SSU | ||
| Anisomeridium ubianum (Vain.) R.C. Harris | MPN94 | KY486750 | GU327709 | JN887379 |
| Cryomyces antarcticus Selbmann, de Hoog, Mazzaglia, Friedman & Onofri | CCFEE 536 | ----- | GU250365 | GU250321 |
| Cryomyces minteri Selbmann, de Hoog, Mazzaglia, Friedman & Onofri | CCFEE 5187 | ----- | KC315869 | KC315858 |
| Glyphium elatum (Grev.) H. Zogg | EB 0388 | KM220946 | KM220940 | ----- |
| EB 0342 | KM220945 | KM220938 | KM220935 | |
| EB 0329 | ----- | KM220937 | KM220934 | |
| EB 0365 | ----- | KM220939 | KM220936 | |
| Glyphium grisonense Math. | EB 0376 | ----- | KM220942 | ----- |
| Holmiella hidalgoensis | T. Raymundo 4608 Holotype ENCB | OQ877252 | OQ880481 | OQ878242 |
| Holmiella junipericola Pem, Gafforov, Jeewon & K.D. Hyde | MFLUCC 18-0503 | MH188902 | MH188900 | MH188901 |
| Holmiella juniperi-semiglobosae Pem, Gafforov, Jeewon & K.D. Hyde | MFLUCC 17-1955 | MH188905 | MH188903 | MH188904 |
| Holmiella Sabina (De Not.) Petrini, Samuels & E. Müll. | G.M. 2015-04-29.2 | KY486750 | ----- | ----- |
| Hysteropatella clavispora (Peck.) Hönh. | CBS 247.34 | ----- | AY541493 | AY511483 |
| Hysteropatella elliptica (Fr.) Rehm | G.M. 2013-05-06 01 | ----- | KM220948 | KM220948 |
| CBS 935.97 | ----- | DQ767657 | EF495114 | |
| Hysteropatella prostii (Duby) Rehm | H.B. 9934b | KT876980 | KT876980 | ----- |
| G.M. 2014-05-20 01 | ----- | KM220949 | ----- | |
| Lichenothelia calcarea Henssen | L1324 | ----- | KC015062 | KR045803 |
| Lichenothelia convexa Henssen | L1609 | ----- | KC015071 | KR045805 |
| Patellaria atrata (Hedw.) Fr. | BCC 28877 | KM220950 | GU371829 | ----- |
| BCC 28876 | ----- | KM220950 | ----- | |
| CBS 958.97 | ----- | GU301855 | ----- | |
| Yuccamyces citri Crous | CBS 143161 | MG386043 | MG386096 | |
| Yuccamyces pilosus (R.F. Castañeda) R.F. Castañeda | CBS 579.92 | ----- | MG386097 | ----- |
| Species Name | Isolate/Voucher/strain | GenBank Accessions | |
|---|---|---|---|
| ITS | nLSU | ||
| Acrospermum adeanum Höhn. | M133 | EU940180 | EU940104 |
| Acrospermum compressum Tode | M151 | EU940161 | EU940084 |
| Acrospermum graminum Lib. | M152 | EU940162 | EU940085 |
| Kirschsteiniothelia aethiops (Sacc.) D. Hawksw. | CBS 109.53 | ----- | AY016361 |
| MFLUCC 16–1104 | MH182583 | MH182589 | |
| S–783 | MH182586 | MH182595 | |
| MFLUCC 15–0424 | KU500571 | KU500578 | |
| Kirschsteiniothelia aquatica Z.L. Luo, K.D. Hyde & H.Y. Su | MFLUCC 17–1685 | MH182587 | MH182594 |
| Kirschsteiniothelia arasbaranica Mehrabi, Hemmati & Asgari | IRAN 2509C | KX621986 | KX621987 |
| IRAN 2508C | KX621983 | KX621984 | |
| Kirschsteiniothelia cangshanensis Z.L. Luo, D.F. Bao, K.D. Hyde & H.Y. Su | MFLUCC 16–1350 | MH182584 | MH182592 |
| Kirschsteiniothelia esperanzae | T. Raymundo 6581 Holotype ENCB | OQ877253 | OQ880482 |
| Kirschsteiniothelia fluminicola Z.L. Luo, K.D. Hyde & H.Y. Su | MFLUCC 16–1263 | MH182582 | MH182588 |
| Kirschsteiniothelia lignicola Boonmee & K.D. Hyde | MFLUCC 10–0036 | HQ441567 | HQ441568 |
| Kirschsteiniothelia nabanheensis Jing W. Liu & Jian Ma | HJAUP C2006 | OQ023274 | OQ023275 |
| HJAUP C2004 | OQ023197 | OQ023273 | |
| Kirschsteiniothelia phoenicis S. N. Zhang & K.D. Hyde | MFLUCC 18–0216 | MG859978 | MG860484 |
| Kirschsteiniothelia rostrata Jing Yang & K.D. Hyde | MFLUCC 15–0619 | KY697280 | KY697276 |
| MFLUCC 16–1124 | ----- | MH182590 | |
| Kirschsteiniothelia submersa Hong Y. Su & K.D. Hyde | MFLUCC 15–0427 | KU500570 | KU500577 |
| S–481 | ----- | MH182591 | |
| S–601 | MH182585 | MH182593 | |
| Kirschsteiniothelia tectonae Doilom, Bhat & K.D. Hyde | MFLUCC 12–0050 | KU144916 | KU764707 |
| Kirschsteiniothelia thailandica Y.R. Su, Yong Wang bis & K.D. Hyde | MFLUCC 20–0116 | MT985633 | MT984443 |
| Kirschsteiniothelia thujina (Peck.) D. Hawksw. | JF 13210 | KM982716 | KM982718 |
| Megalotremis verrucosa (Makhija & Patw.) Aptroot | MPN104 | ----- | GU327718 |
| Phyllobathelium anomalum Lücking | MPN 242 | ----- | GU327722 |
| Phyllobathelium firmum (Stirt.) Věsda | ERP 3175 | ----- | GU327723 |
| Pseudorobillarda eucalypti Tangthir. & K.D. Hyde | MFLUCC 12–0422 | KF827451 | KF827457 |
| Pseudorobillarda phragmitis (Cunnell) M. Morelet | CBS 398.61 | MH858101 | EU754203 |
| Strigula guangxiensis S.H. Jiang, X.L. Wei & J.C. Wei | HMAS-L0138040 | NR146255 | MK206256 |
| Strigula macrocarpa Vain. | HMAS-L0141394 | ----- | MK206240 |
| Strigula nemathora Mont. | MPN 72 | ----- | JN887405 |
| Strigula nitidula Mont. | HMAS-L0139358 | ----- | MN788374 |
| Strigula sinoaustralis S.H. Jiang, X.L. Wei & J.C. Wei | HMAS-L0137204 | ----- | MK206249 |
| Strigula univelbiserialis S.H. Jiang, X.L. Wei & J.C. Wei | HMAS-L0137657 | ----- | MK206243 |
| Tenuitholiascus porinoides S.H. Jiang & J.C. Wei | HMAS-L0139638 | ----- | MK206259 |
| HMAS-L0139639 | ----- | MK206258 | |
| HMAS-L0139640 | ----- | MK206260 | |
| Species Name | Isolate/Voucher/Strain | GenBank Accessions | ||
|---|---|---|---|---|
| ITS | nLSU | rpb2 | ||
| Microglossum clavatum V. Kučera, Lizoň & Tomšovský | SAV F-11276 | KX382864 | KX382864 | KX382884 |
| SAV F-11272 | KX382841 | ----- | ----- | |
| SAV F-11074 | KX382865 | KX382865 | KX382885 | |
| Microglossum cyanobasis P. Iglesias & Arauzo | AH 43985 | KX371850 | ----- | ----- |
| Microglossum flavoviride | García 18649 Holotype ITCV | OQ877254 | OQ880483 | |
| García 18686 | OQ877255 | OQ880484 | ||
| Microglossum fuscorubens Boud. | ERRO 2012120704 | KX371856 | ----- | ----- |
| ERRO 2012120705 | KX371857 | ----- | ----- | |
| ERRO 2012120706 | KX371858 | ----- | ----- | |
| SAV F-11275 | KX382834 | KX382834 | KX382883 | |
| Microglossum griseoviride V. Kučera, Lizoň & Tomšovský | SAV F-9920 | KX595249 | KC595250 | KX382872 |
| SAV F-10699 | KC595261 | ----- | ----- | |
| SAV F-10696 | KX382857 | ----- | ----- | |
| Microglossum nudipes Boud. | SAV F-11053 | KX382838 | KX382867 | ----- |
| SAV F-11051 | KX382856 | ----- | ----- | |
| SAV F-11274 | KX382836 | KX382836 | KX382888 | |
| SAV F-11285 | KX382859 | KX382869 | KX382887 | |
| SAV F-11271 | KX382837 | ----- | ----- | |
| Microglossum olivaceum (Pers.) Gillet | KM135962 | EU784374 | ----- | ----- |
| KM135599 | EU784373 | ----- | ----- | |
| ERRO 2004110702 | KX371853 | ----- | ----- | |
| Microglossum parvisporum V. Kučera, Lizoň & Tomšovský | SAV F-10998 | KM114901 | KM114901 | KX382879 |
| LE 291852 | KX382839 | ----- | ----- | |
| SAV F-11283 | KM114901 | KM114901 | KX382879 | |
| Microglossum pratense V. Kučera, Tomšovský & Lisoš | SAV F-10024 | KC595259 | KC595260 | KX382880 |
| SAV F-11020 | KJ513006 | KJ513006 | KX382881 | |
| O 64797 | KJ513004 | ----- | ----- | |
| O 294564 | KJ513005 | ----- | ----- | |
| O 170878 | KJ513002 | ----- | ----- | |
| O 270070 | KJ513003 | ----- | ----- | |
| SAV F-11062 | KX382848 | ----- | ----- | |
| LE 294492 | KX382849 | ----- | ----- | |
| LE 294489 | KX382850 | ----- | ----- | |
| SAV F-10568 | KX382851 | ----- | ----- | |
| SAV F-11056 | KX382847 | ----- | ----- | |
| SAV F-11052 | KX382852 | ----- | ----- | |
| Microglossum rufescens (Grelet) Bon | SAV F-9921 | KC595257 | ----- | ----- |
| ERRO 2004110703 | KX371854 | ----- | ----- | |
| ERRO 2011122601 | KX371855 | ----- | ----- | |
| SAV F-11282 | KX382858 | KX382868 | KX382892 | |
| SAV F-11204 | KX382835 | KX382866 | KX382893 | |
| Microglossum rufum (Schwein.) Underw. | Ingo 163 | DQ257360 | ----- | ----- |
| Microglossum tenebrosum V. Kučera, Tomšovský, Lisoš & F. Hampe | SAV F-11273 | KX382842 | ----- | ----- |
| SAV F-11278 | KX382845 | KX382845 | KX382891 | |
| SAV F-11279 | KX382843 | ----- | ----- | |
| SAV F-11070 | KX382846 | KX382846 | KX382890 | |
| SAV F-11072 | KX382844 | KX382844 | KX382889 | |
| Microglossum truncatum V. Kučera, Tomšovský & Lisoš | SAV F-11023 | KJ513009 | KJ513009 | KX382874 |
| SAV F-10720 | KX382840 | ----- | ----- | |
| O 224247 | KJ513010 | ----- | ----- | |
| SAV F-11280 | KX382861 | KX382861 | KX382875 | |
| SAV F-11022 | KJ513011 | ----- | ----- | |
| SAV F-11064 | KX382855 | ----- | ----- | |
| LE 291847 | KX382863 | KX382871 | KX382876 | |
| SAV F-11262 | KX382862 | KX382862 | KX382877 | |
| SAV F-11261 | KX382853 | ----- | ----- | |
| SAV F-11263 | KX382854 | ----- | ----- | |
| Microglossum viride (Schrad. ex J.F. Gmel.) Gillet | SAV F-10249 | KC595253 | KC595254 | KX382873 |
| SAV F-10697 | KC595265 | ----- | ----- | |
| SAV F-10698 | KC595263 | ----- | ----- | |
| KM90199 | EU784375 | ----- | ----- | |
| Species Name | Isolate/Voucher/Strain | GenBank Accessions |
|---|---|---|
| ITS | ||
| Claussenomyces atrovirens (Pers.) Korf & Abawi | 22FM2A1 | MW709917 |
| Claussenomyces atrovirens (Pers.) Korf & Abawi | LEG25 | MW204926 |
| Claussenomyces atrovirens (Pers.) Korf & Abawi | FC1636 | LC425048 |
| Claussenomyces aff. Atrovirens | GM20144422.1 | MW178207 |
| Claussenomyces aff. Atrovirens | GM20150815.9 | MT949706 |
| Claussenomyces aff. Atrovirens | GM20190817.1 | MT522872 |
| Claussenomyces kirschsteinianus (Kirschst.) G. Marson & Baral | GM20150502.2 | KY689631 |
| Claussenomyces kirschsteinianus (Kirschst.) G. Marson & Baral | GM20141112.2 | KY689629 |
| Claussenomyces kirschsteinianus (Kirschst.) G. Marson & Baral | GM20141108.4 | KY689628 |
| Claussenomyces olivaceus (Fuckel) Sherwood | GM20150423.1 | KY661433 |
| Claussenomyces olivaceus (Fuckel) Sherwood | GM20190729.3 | OP103955 |
| Claussenomyces olivaceus (Fuckel) Sherwood | GM20161231.2 | MW167780 |
| Claussenomyces paulinae | T. Raymundo 7564 Holotype ENCB | OQ877256 |
| Claussenomyces prasinulus (P. Karst.) Korf & Abawi | HB7165a | OM808929 |
| CBS111551 | MN082653 | |
| NBRC 112536 | LC488725 | |
| Collophorina badensis S. Bien & Damm | CBS144833 | NR165902 |
| Collophorina germanica S. Bien & Damm | CBS144831 | NR165903 |
| Collophorina hispanica (Gramaje, Armengol & Damm) Damm & Crous | CBS128569 | MH864962 |
| Collophorina neorubra S. Bien & Damm | CBS144829 | NR165901 |
| Scolecoleotia eriocamporesi H. B. Jiang, Phookamsak & K.D. Hyde | IT3027A | MW981448 |
| Scolecoleotia eriocamporesi H. B. Jiang, Phookamsak & K.D. Hyde | IT3027B | MW981449 |
| Species Name | Isolate/Voucher/Strain | GenBank Accessions |
|---|---|---|
| ITS | ||
| Chorioactis geaster (Peck) Kupfer ex Eckblad | K. Rice s.n. | AY307936 |
| S. Kurogi s.n. | AY307937 | |
| Trichaleurina javanica (Peck) M. Carbone, Agnello & P. Alvarado | KSRF 0019 | MF476196 |
| PL8 | MZ061709 | |
| 20170467 | MK184529 | |
| Trichaleurina sp. | TNS-F-31213 | KF418250 |
| Trichaleurina tenuispora M. Carbone, Yei Z. Wang & Cheng L. Huang | TNM F10376 | NR159000 |
| TNM F20404 | KF418249 | |
| Wolfina aurantiopsis (Ellis) Seaver ex Eckblad | TENN 67128 | KC306744 |
| Wolfina molangoensis | R. Valenzuela 18918 Holotype ENCB | OQ877257 |
| Neournula pouchetii (Berthet & Riousset) Paden | TURA195798 | JX669837 |
| Species Name | Isolate/Voucher/Strain | GenBank Accessions | |
|---|---|---|---|
| ITS | nLSU | ||
| Amphirosellinia fushanensis Y.M. Ju, J.D. Rogers & H.M. Hsieh | HAST 91111209 | GU339496 | ----- |
| Amphirosellinia nigrospora Y.M. Ju, J.D. Rogers & H.M. Hsieh | HAST 91092308 | GU322457 | ----- |
| Coniolarelia limoniispora | MUCL 29409 | MN984615 | MN984624 |
| Dematophora bunodes (Berk. & Broome) C. Lamb., Wittstein & M. Stadler | CBS 123584 | MN984617 | ----- |
| Dematophora bunodes (Berk. & Broome) C. Lamb., Wittstein & M. Stadler | CBS 123585 | MN984618 | ----- |
| Dematophora bunodes (Berk. & Broome) C. Lamb., Wittstein & M. Stadler | CBS 123597 | MN984619 | MN984625 |
| Dematophora buxi (Fabre) C. Lamb., Wittstein & M. Stadler | JDR 99 | GU300070 | ----- |
| Dematophora necatrix R. Hartig | CBS 349.36 | AY909001 | KF719204 |
| Dematophora necatrix R. Hartig | W 97 | DF977487 | DF977487 |
| Dematophora oaxacana | T. Raymundo 6161 Holotype ENCB | OQ877258 | OQ880487 |
| Dematophora oaxacana Sánchez-Flores, R. Valenz. & Raymundo | R. Valenzuela 17218 ENCB | OQ877259 | OQ880488 |
| Dematophora pepo (Pat.) C. Lamb., Wittstein & M. Stadler | CBS 123592 | MN984620 | ----- |
| Entoleuca mammata (Wahlenb.) J.D. Rogers & Y.M. Ju | JDR 100 | GU300072 | ----- |
| Euepixylon sphaeriostomum (Schwein.) Lar.N. Vassiljeva & S.L. Stephenson | JDR 261 | GU292821 | ----- |
| Graphostroma platystomum (Schwein.) Piroz | CBS 270.87 | JX658535 | DQ836906 |
| Hypoxylon fragiforme (Pers.) J. Kickx f. | MUCL 51264 | KC477229 | KM186295 |
| Nemania abortiva J.D. Rogers, Y.M. Ju & Hemmes | BISH 467 | GU292816 | ----- |
| Nemania beaumontii (Berk. & M.A. Curtis) Y.M. Ju & J.D Rogers | HAST 405 | GU292819 | ----- |
| Nemania beaumontii (Berk. & M.A. Curtis) Y.M. Ju & J.D Rogers | FL 0980 | ----- | JQ760608 |
| Nemania bipapillata (Berk. & M.A. Curtis) Pouzar | HAST 90080610 | GU292818 | ----- |
| Podosordaria mexicana Ellis & Holw. | WSP 176 | GU324762 | ----- |
| Podosordaria punctata | CBS 656.78 | KT281904 | KY610496 |
| Rosellinia aquila (Fr.) Ces. & De Not. | MUCL 51703 | KY610392 | KY610460 |
| Rosellinia marcucciana Ces | MUCL 51704 | MN984616 | MN984626 |
| Rosellinia corticium (Schwein.) Sacc. | MUCL 51693 | KY610393 | KY610461 |
| STMA 13324 | MN984621 | MN984627 | |
| STMA 12170-15209 | MN984623 | MN984629 | |
| Rosellinia nectrioides Rehm | CBS 449.89 | MN984622 | MN984628 |
| Xylaria arbuscula Sacc. | CBS 126415 | KY610394 | KY610463 |
| Xylaria hypoxylon (L.) Grev. | CBS 122620 | KY204024 | KY610495 |
| Xylaria bambusicola Y.M. Ju & J.D Rogers | WSP 205 | EF026123 | ----- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raymundo, T.; Valenzuela, R.; Martínez-González, C.R.; García-Jiménez, J.; Cobos-Villagrán, A.; Sánchez-Flores, M.; de la Fuente, J.; Martínez-Pineda, M.; Pérez-Valdespino, A.; Ramírez-Martínez, J.C.; et al. New Ascomycetes from the Mexican Tropical Montane Cloud Forest. J. Fungi 2023, 9, 933. https://doi.org/10.3390/jof9090933
Raymundo T, Valenzuela R, Martínez-González CR, García-Jiménez J, Cobos-Villagrán A, Sánchez-Flores M, de la Fuente J, Martínez-Pineda M, Pérez-Valdespino A, Ramírez-Martínez JC, et al. New Ascomycetes from the Mexican Tropical Montane Cloud Forest. Journal of Fungi. 2023; 9(9):933. https://doi.org/10.3390/jof9090933
Chicago/Turabian StyleRaymundo, Tania, Ricardo Valenzuela, César Ramiro Martínez-González, Jesús García-Jiménez, Aurora Cobos-Villagrán, Marcos Sánchez-Flores, Javier de la Fuente, Michelle Martínez-Pineda, Abigail Pérez-Valdespino, Julio Cesar Ramírez-Martínez, and et al. 2023. "New Ascomycetes from the Mexican Tropical Montane Cloud Forest" Journal of Fungi 9, no. 9: 933. https://doi.org/10.3390/jof9090933
APA StyleRaymundo, T., Valenzuela, R., Martínez-González, C. R., García-Jiménez, J., Cobos-Villagrán, A., Sánchez-Flores, M., de la Fuente, J., Martínez-Pineda, M., Pérez-Valdespino, A., Ramírez-Martínez, J. C., & Luna-Vega, I. (2023). New Ascomycetes from the Mexican Tropical Montane Cloud Forest. Journal of Fungi, 9(9), 933. https://doi.org/10.3390/jof9090933







