Talaromyces amestolkiae Infection in an AIDS Patient with Cryptococcal Meningitis
Abstract
:1. Introduction
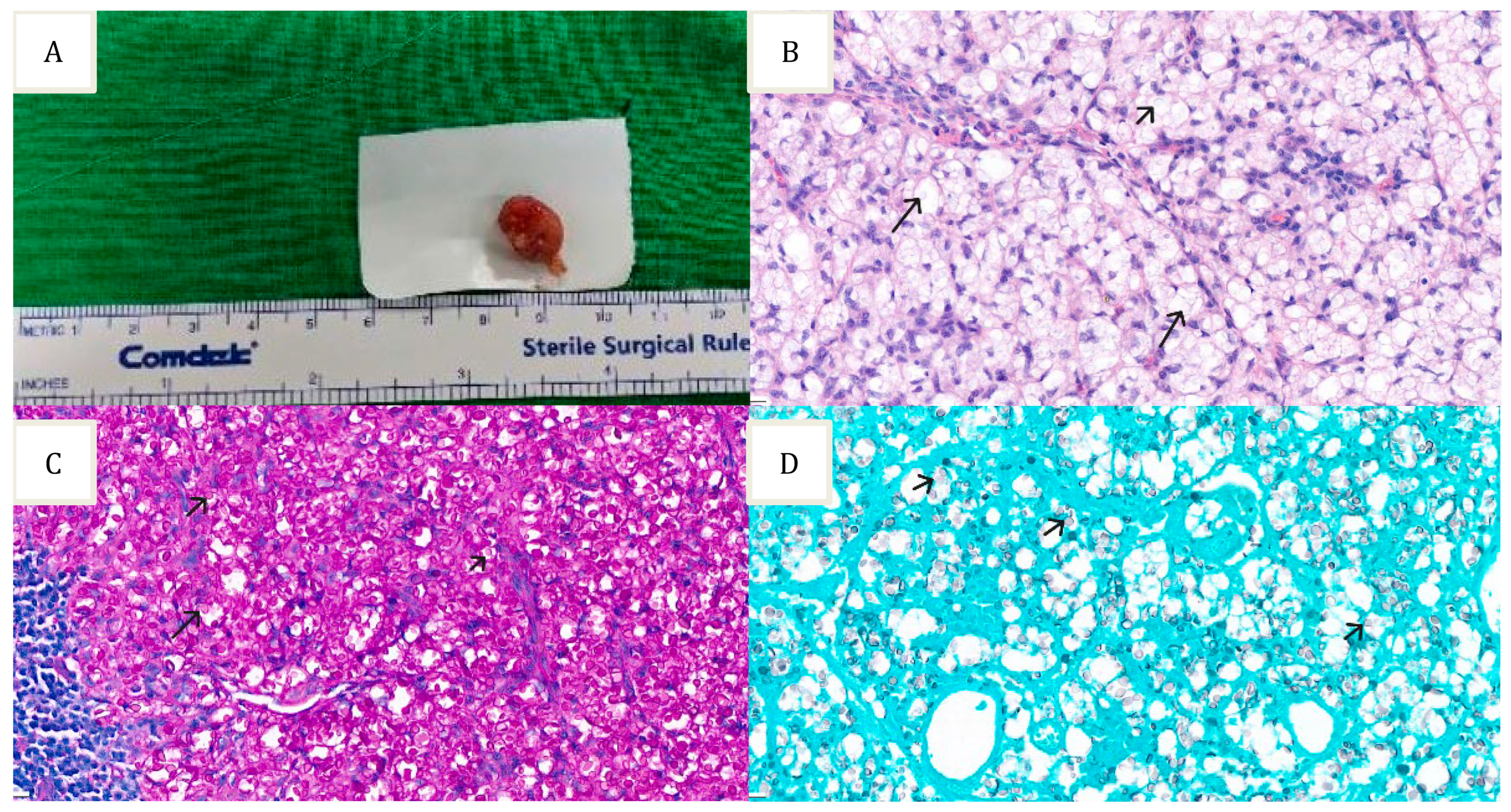
2. Case Report
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, C.R. Ascocarps of Aspergillus and Penicillium. Mycologia 1955, 47, 669–687. [Google Scholar] [CrossRef]
- Yilmaz, N.; Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 2014, 78, 175–341. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, K.; Dhungana, N.; Jang, Y.; Chaturvedi, V.; Desmond, E. Characterization of Clinical Isolates of Talaromyces marneffei and Related Species, California, USA. Emerg. Infect. Dis. 2019, 25, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Houbraken, J.; Hoekstra, E.S.; Frisvad, J.C.; Visagie, C.M.; Samson, R.A. Delimitation and characterisation of Talaromyces purpurogenus and related species. Persoonia 2012, 29, 39–54. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Yilmaz, N.; Houbraken, J.; Spierenburg, H.; Seifert, K.A.; Peterson, S.W.; Varga, J.; Frisvad, J.C. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 2011, 70, 159–183. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Deng, Z.; Ribas, J.L.; Gibson, D.W.; Connor, D.H. Infections caused by Penicillium marneffei in China and Southeast Asia: Review of eighteen published cases and report of four more Chinese cases. Rev. Infect. Dis. 1988, 10, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Supparatpinyo, K.; Khamwan, C.; Baosoung, V.; Nelson, K.E.; Sirisanthana, T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet 1994, 344, 110–113. [Google Scholar] [CrossRef]
- Chiang, C.T.; Leu, H.S.; Wu, T.L.; Chan, H.L. Penicillium marneffei fungemia in an AIDS patient: The first case report in Taiwan. Changgeng Yi Xue Za Zhi 1998, 21, 206–210. [Google Scholar]
- Hien, T.V.; Loc, P.P.; Hoa, N.T.; Duong, N.M.; Quang, V.M.; McNeil, M.M.; Dung, N.T.; Ashford, D.A. First cases of disseminated penicilliosis marneffei infection among patients with acquired immunodeficiency syndrome in Vietnam. Clin. Infect. Dis. 2001, 32, e78–e80. [Google Scholar] [CrossRef] [PubMed]
- Weisenborn, J.L.; Kirschner, R.; Cáceres, O.; Piepenbring, M. Talaromyces indigoticus Takada & Udagawa, the first record for Panama and the American continent. Mycopathologia 2010, 170, 203–208. [Google Scholar] [CrossRef]
- Santos, P.E.; Piontelli, E.; Shea, Y.R.; Galluzzo, M.L.; Holland, S.M.; Zelazko, M.E.; Rosenzweig, S.D. Penicillium piceum infection: Diagnosis and successful treatment in chronic granulomatous disease. Med. Mycol. 2006, 44, 749–753. [Google Scholar] [CrossRef]
- Horré, R.; Gilges, S.; Breig, P.; Kupfer, B.; de Hoog, G.S.; Hoekstra, E.; Poonwan, N.; Schaal, K.P. Case report. Fungaemia due to Penicillium piceum, a member of the Penicillium marneffei complex. Mycoses 2001, 44, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Lozano, H.; Treviño-Rangel, R.d.J.; Renpenning-Carrasco, E.W.; González, G.M. Successful treatment of Talaromyces amestolkiae pulmonary infection with voriconazole in an acute lymphoblastic leukemia patient. J. Infect. Chemother. 2017, 23, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Chariyalertsak, S.; Supparatpinyo, K.; Sirisanthana, T.; Nelson, K.E. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clin. Infect. Dis. 2002, 34, 277–284. [Google Scholar] [CrossRef]
- Tsang, D.N.; Chan, J.K.; Lau, Y.T.; Lim, W.; Tse, C.H.; Chan, N.K. Penicillium marneffei infection: An underdiagnosed disease? Histopathology 1988, 13, 311–318. [Google Scholar] [CrossRef]
- Clezy, K.; Sirisanthana, T.; Sirisanthana, V.; Brew, B.; Cooper, D.A. Late manifestations of HIV in Asia and the Pacific. Aids 1994, 8 (Suppl. 2), S35–S43. [Google Scholar]
- Shi, N.; Kong, J.; Wang, K.; Cao, C. Coinfection with Talaromyces marneffei and Other Pathogens Associated with Acquired Immunodeficiency. JAMA Dermatol. 2019, 155, 1195–1197. [Google Scholar] [CrossRef]
- Kawila, R.; Chaiwarith, R.; Supparatpinyo, K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: A retrospective study. BMC Infect. Dis. 2013, 13, 464. [Google Scholar] [CrossRef]
- Browne, S.K.; Burbelo, P.D.; Chetchotisakd, P.; Suputtamongkol, Y.; Kiertiburanakul, S.; Shaw, P.A.; Kirk, J.L.; Jutivorakool, K.; Zaman, R.; Ding, L.; et al. Adult-onset immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 2012, 367, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Clinicalinfo.HIV.gov. Talaromycosis (Formerly Penicilliosis). Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/talaromycosis?view=full (accessed on 8 November 2022).
- Tong, A.C.; Wong, M.; Smith, N.J. Penicillium marneffei infection presenting as oral ulcerations in a patient infected with human immunodeficiency virus. J. Oral Maxillofac. Surg. 2001, 59, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Kronauer, C.M.; Schär, G.; Barben, M.; Bühler, H. HIV-associated Penicillium marneffei infection. Schweiz. Med. Wochenschr. 1993, 123, 385–390. [Google Scholar] [PubMed]
- Leung, R.; Sung, J.Y.; Chow, J.; Lai, C.K. Unusual cause of fever and diarrhea in a patient with AIDS. Penicillium marneffei infection. Dig. Dis. Sci. 1996, 41, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Kinh, N.V.; Cuc, N.T.K.; Tung, N.L.N.; Lam, N.T.; Thuy, P.T.T.; Cuong, D.D.; Phuc, P.T.H.; Vinh, V.H.; Hanh, D.T.H.; et al. A Trial of Itraconazole or Amphotericin B for HIV-Associated Talaromycosis. N. Engl. J. Med. 2017, 376, 2329–2340. [Google Scholar] [CrossRef]
- Supparatpinyo, K.; Perriens, J.; Nelson, K.E.; Sirisanthana, T. A controlled trial of itraconazole to prevent relapse of Penicillium marneffei infection in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 1998, 339, 1739–1743. [Google Scholar] [CrossRef]
- Ouyang, Y.; Cai, S.; Liang, H.; Cao, C. Administration of Voriconazole in Disseminated Talaromyces (Penicillium) Marneffei Infection: A Retrospective Study. Mycopathologia 2017, 182, 569–575. [Google Scholar] [CrossRef]
- Supparatpinyo, K.; Schlamm, H.T. Voriconazole as therapy for systemic Penicillium marneffei infections in AIDS patients. Am. J. Trop. Med. Hyg. 2007, 77, 350–353. [Google Scholar] [CrossRef]
- Valente-Acosta, B.; Padua-Garcia, J.; Tame-Elorduy, A. Pulmonary coinfection by Pneumocystis jirovecii and Cryptococcus species in a patient with undiagnosed advanced HIV. BMJ Case Rep. 2020, 13, e233607. [Google Scholar] [CrossRef]
- Subahi, E.A.; Aljafar, M.S.; Barjas, H.H.; Abdelrazek, M.; Rasoul, F.A. Co-infection by Cryptococcus neoformans fungaemia and non-tuberculous mycobacteria with Pneumocystis jiroveci pneumonia in a newly diagnosed HIV-infected patient. Clin. Case Rep. 2021, 9, e04191. [Google Scholar] [CrossRef]
- Gregoire, E.; Pirotte, B.F.; Moerman, F.; Altdorfer, A.; Gaspard, L.; Firre, E.; Moonen, M.; Darcis, G. Mycobacterium avium complex and Cryptococcus neoformans co-infection in a patient with acquired immunodeficiency syndrome: A case report. Acta Clin. Belg. 2022, 77, 679–684. [Google Scholar] [CrossRef]
- Aronis, M.L.; dos Santos, R.P.; Goldani, L.Z. Disseminated Histoplasma capsulatum and Cryptococcus neoformans co-infection in patients with AIDS. Mycopathologia 2011, 172, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Saeed, U.; Wei, S.S.; Wang, L.; Kuang, Y.Q. Both coinfections of Penicillium marneffei and Cryptococcus neoformans in AIDS patient: A report of rare case. Aids 2017, 31, 2171–2172. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lv, D.; Xu, Y.; Wu, X.; Lin, L. Concurrent infection with Talaromyces marneffei and Cryptococcus neoformans in a patient without HIV infection. Exp. Ther. Med. 2020, 19, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Awari, D.W.; Shah, A.S.; Sexton, A.M.; Sexton, M.A. Coinfection of Aspergillus and Cryptococcus in Immunocompromised Host: A Case Report and Review of Literature. Case Rep. Infect. Dis. 2020, 2020, 8888270. [Google Scholar] [CrossRef]
- Van Tongeren, L.; Shaipanich, T.; Fleetham, J.A. Coinfection with Cryptococcus gattii and Mycobacterium tuberculosis in an otherwise healthy 18-year-old woman. Can. Respir. J. 2011, 18, e62–e63. [Google Scholar] [CrossRef]
- Tone, K.; Tamura, T.; Hagiwara, S.; Kuwano, K.; Makimura, K. Pulmonary Coinfection Due to Mycobacterium abscessus subsp. massiliense and Cryptococcus neoformans var. grubii. Jpn. J. Infect. Dis. 2021, 74, 600–603. [Google Scholar] [CrossRef]
| Species | Country | Underlying Conditions | Treatment | Outcome |
|---|---|---|---|---|
| T. amestolkiae | New Caledonia [4] Taiwan | AML case AIDS patients Cystic fibrosis case ALL case | Not mentioned in article | Not mentioned in article |
| T. marneffei | East Asian countries including China, Taiwan, Thailand, and Vietnam | AIDS | Amphotericin B then Itraconazole | Mortality rate: ~75% in untreated patient ~25% in received treatment patient |
| T. indigoticus | Nepal, Japan, Thailand, and Western Panama [12] | Diabetes mellitus | Not mentioned in article | Not mentioned in article |
| T. piceus | Argentina [13] | X-linked chronic granulomatous disease (X-CGD) patient | Amphotericin B then voriconazole due to renal impairment Surgical resection of affected ribs | Recovered and free from fungal infections |
| Germany [14] | Alcohol-abused and cholangiocarcinoma | Deceased before treatment | ||
| T. stollii | France, Hamburg, and Netherlands [4] | lung transplantation, France AIDS patient; the Netherlands | Not mentioned in article | Not mentioned in article |
| Talaromyces amestolkiae | Talaromyces marneffei | |
|---|---|---|
| Risk factors | Immunocompromised patient. Case reported in an acute lymphoblastic leukemia patient [15] and we found this pathogen in an AIDS patient. | AIDS (majority of cases having a CD4 count <100 cells/mm3) [16,17,18], autoimmune disorders, cancer, diabetes mellitus [19,20,21]. |
| Clinical manifestations | Respiratory symptoms Productive cough, mild dyspnea, and occasional low grade fever [15] Other symptoms Lymphadenopathy | Respiratory symptoms Nonproductive cough, fever, dyspnea, and chest pain Gastrointestinal symptoms Diarrhea and abdominal pain Skin lesions Papules on the face, chest, and extremities. Subsequently, the center of the papule becomes necrotic, giving the appearance of an umbilicated papule, which can resemble molluscum contagiosum [22]. Mucosal lesions Mucosal lesions appear similar to skin lesions. Distributed in the oral cavity, oropharynx, hypopharynx, stomach, colon, and genitalia had been reported [9,23,24,25]. Other symptoms Weight loss, hepatomegaly, splenomegaly, and/or generalized lymphadenopathy [22]. |
| Definitive diagnosis | ||
| Culture | 7 days in CYA at 25 °C [2] | May need 4~7 days to grow |
|
| |
| 7 days in MEA at 25 °C | At 25 °C to 30 °C, yellow-green colonies with sulcate folds and a red diffusible pigment in the medium are produced. At 32 to 37 °C (yeast phase) | |
| Morphological transition from a mold to a yeast, producing colonies without a red diffusible pigment [22] | |
| Molecular diagnostics | PCR amplification Sequence identification of specific regions | PCR amplification Sequence identification of specific regions |
| Treatment | ||
| Pulmonary infection [15] Oral Voriconazole 200 mg every 12 h for 2 months Our patient (co-infected with cryptococcus neoformans meningitis) Amphotericin B for 6 weeks then oral Voriconazole | Recommended induction therapy [22] Amphotericin B, preferably liposomal amphotericin B 3 to 5 mg/kg body weight/day or Deoxycholate amphotericin B 0.7 mg/kg body weight/day, IV for 2 weeks | |
| Consolidation therapy oral itraconazole, 200 mg every 12 h for a subsequent duration of 10 weeks [26] | ||
| Maintenance therapy (or secondary prophylaxis) oral Itraconazole 200 mg/day | ||
| ||
| Special consideration | For patients who cannot tolerate any form of amphotericin induction therapy with IV Voriconazole 6 mg/kg every 12 h on day 1 (loading dose), then 4 mg/kg every 12 h or with oral Voriconazole 600 mg every 12 h on day 1 (loading dose), then 400 mg every 12 h for 2 weeks is recommended [28,29] | |
| Pathogens | Underlying Disease | Specimen of Co-Infectants | Specimens of Cryptococcus | Treatment Strategy | Treatment for Cryptococcus | Treatment for Co-Infectants | Outcome |
|---|---|---|---|---|---|---|---|
| Pneumocystis jiroveci [30] | AIDS | * BAL 1 | BAL 2 and Blood 2 | Pneumocystis jiroveci, then ART, then asymptomatic cryptococcus | Voriconazole | Cotrimoxazol | Complete resolution of the cavitation |
| Non-tuberculous Mycobacteria [31] | AIDS, syphilis | Sputum/BAL 1 (CMV, EBV, Candida albicans also detected) | Blood 2 | Treat NTM only because the culture results are later than the patient’s discharge | No treatment | Trimethoprim-sulfamethoxazole and steroids | Not mentioned |
| Mycobacterium avium complex [32] | AIDS | Lymph node biopsy 2 | Blood 1 and * CSF 1 | Treat Cryptococcus first, then ART, then MAC | Amphotericin B and flucytosine then fluconazole | Azithromycin, ethambutol and rifabutin | Good clinical evolution |
| Histoplasmosis [33] | AIDS | Lymph node biopsy | Blood and Sputum | HARRT then treat Cryptococcus infection | Amphotericin B and flucytosine then fluconazole | Continue * ART and Fluconazole | |
| T. marneffei | AIDS [34] | Skin papules culture | * CSF | HARRT and treat Cryptococcus infection | Amphotericin B then itraconazole | Not mentioned | Skin papule disappeared and Continue * HAART therapy |
| Hemolytic anemia with 8-year steroid history [35] | Blood 1 and lymph node aspiration | Blood culture 2 | Voriconazole for T. marneffei and Cryptoccocus | Voriconazole | Voriconazole | Discharged with oral voriconazole | |
| Aspergillus [36] | Multiple myeloma | * BAL 2 | Pulmonary infection 1, not mentioned about the specimen | Treat Cryptococcus first then Aspergillus | Amphotericin B and flucytosine, then fluconazole | Fluconazole was shifted to voriconazole for additional coverage | Discharged with oral voriconazole |
| Mycobacterium tuberculosis [37] | No remarkable history | Transbronchial biopsy specimen from RUL lung and Sputum 1 | * BAL 1 & CSF 2 | combination of anti-TB and antifungal therapy | Amphotericin B and flucytosine then fluconazole | Isoniazid+ rifampin+pyrazinamide+ ethambutol | Hold fluconaconazole for nephrotoxicity; Discharged |
| Mycobacterium abscessus [38] | Lupus nephritis and 10-year corticosteroid history | Sputum | Sputum | Patient refused inpatient care | Itraconazole | Clarithromycin and faropenem | No recurrence was observed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-A.; Chuang, Y.-C.; Yeh, T.-K.; Lin, K.-P.; Lin, C.-J.; Liu, P.-Y. Talaromyces amestolkiae Infection in an AIDS Patient with Cryptococcal Meningitis. J. Fungi 2023, 9, 932. https://doi.org/10.3390/jof9090932
Wang L-A, Chuang Y-C, Yeh T-K, Lin K-P, Lin C-J, Liu P-Y. Talaromyces amestolkiae Infection in an AIDS Patient with Cryptococcal Meningitis. Journal of Fungi. 2023; 9(9):932. https://doi.org/10.3390/jof9090932
Chicago/Turabian StyleWang, Li-An, Yu-Chuan Chuang, Ting-Kuang Yeh, Kuan-Pei Lin, Chi-Jan Lin, and Po-Yu Liu. 2023. "Talaromyces amestolkiae Infection in an AIDS Patient with Cryptococcal Meningitis" Journal of Fungi 9, no. 9: 932. https://doi.org/10.3390/jof9090932
APA StyleWang, L.-A., Chuang, Y.-C., Yeh, T.-K., Lin, K.-P., Lin, C.-J., & Liu, P.-Y. (2023). Talaromyces amestolkiae Infection in an AIDS Patient with Cryptococcal Meningitis. Journal of Fungi, 9(9), 932. https://doi.org/10.3390/jof9090932







