Hydrocolloids from the Mushroom Auricularia heimuer: Composition and Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Extract Preparation and Deacetylation
2.2. FTIR and NMR Measurements
2.3. Determination of Total Carbohydrates and Glucans
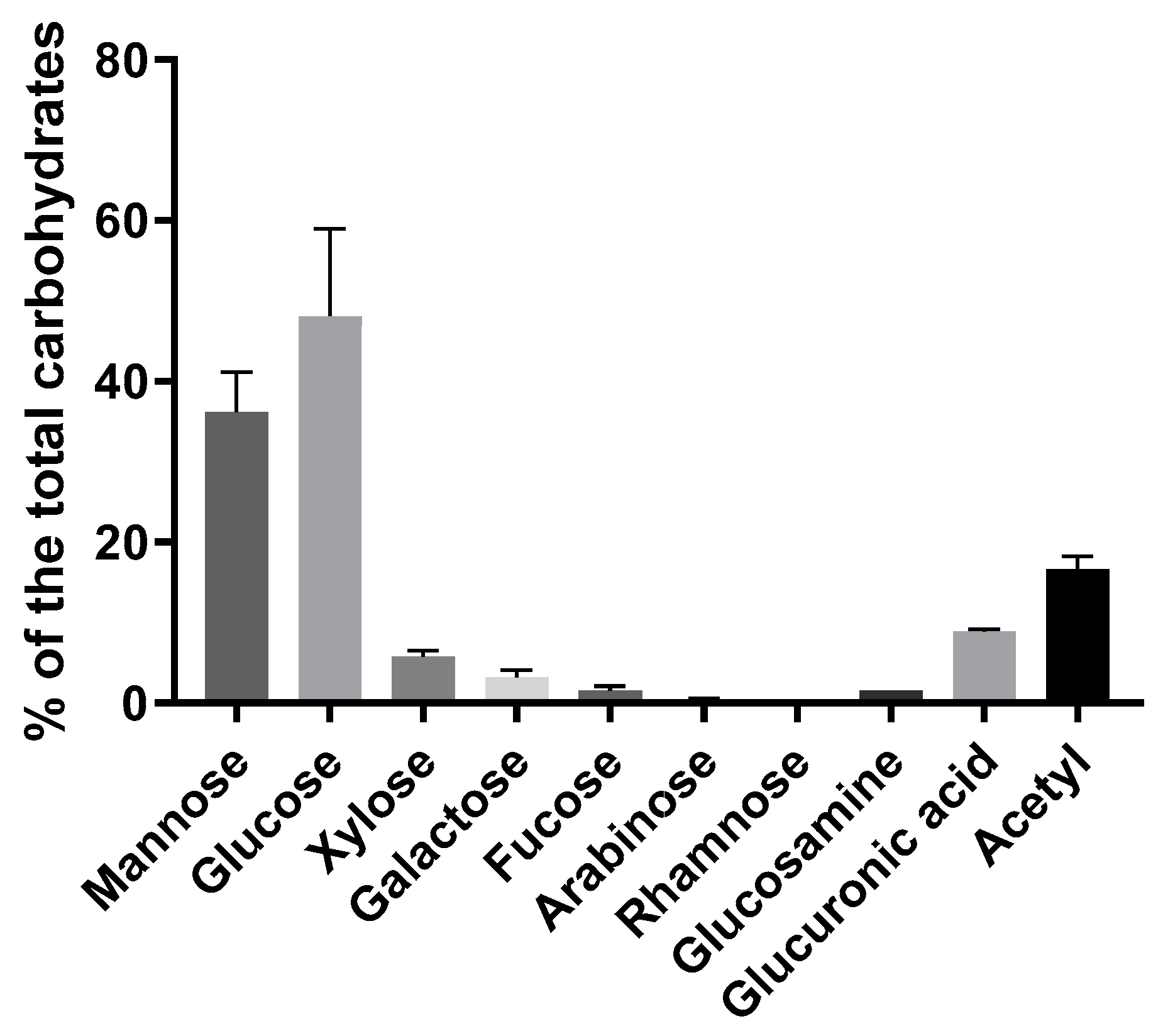
2.4. Carbohydrate Profile
2.5. Lipid Fraction
2.6. Protein Content
2.7. Ash Content and Minerals
2.8. Relative Density and Flow Rate Measurements
2.9. Moisture Retention Capacity
2.10. Statistical Analysis
3. Results and Discussion
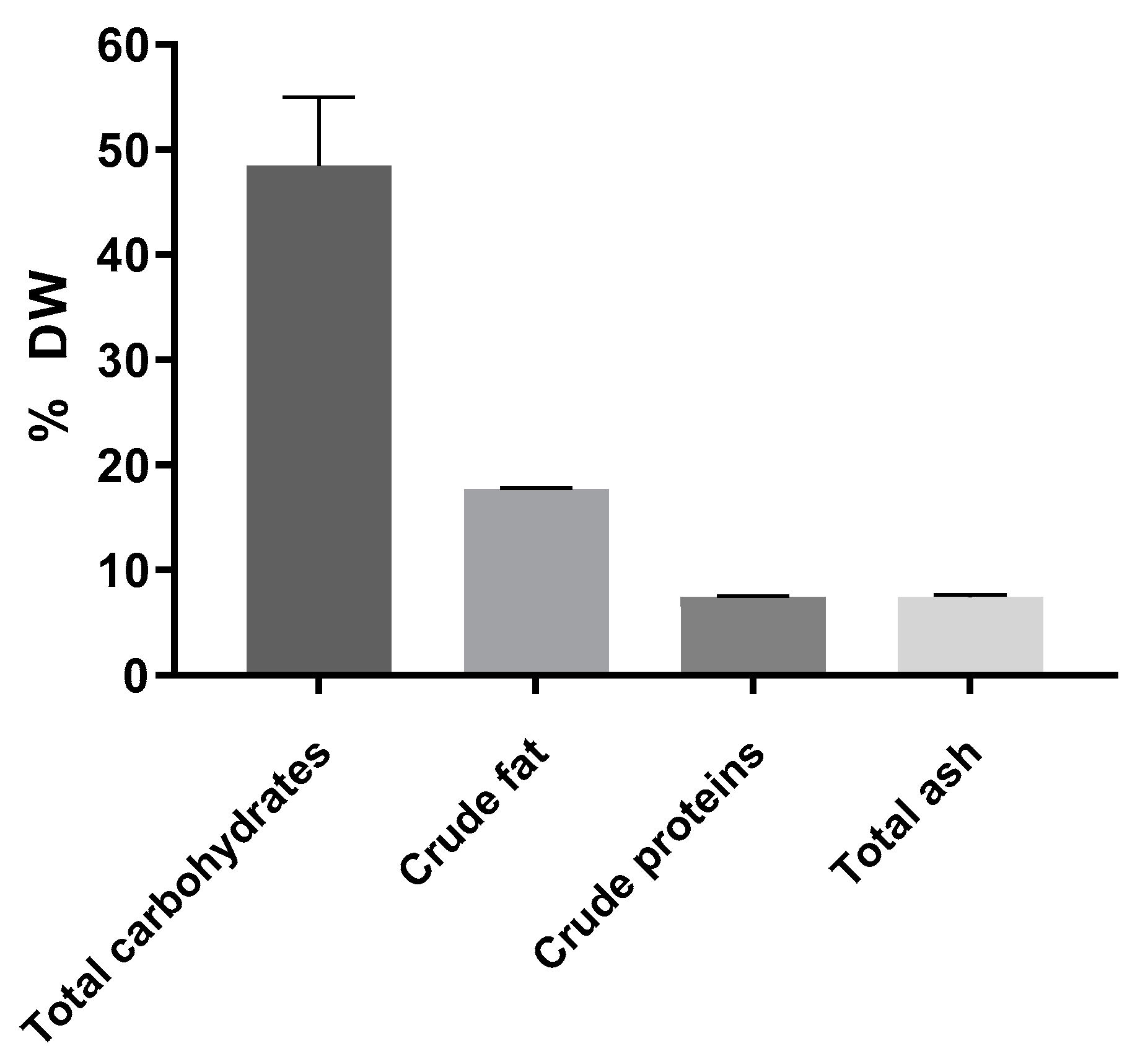
3.1. Composition
3.2. Spectroscopic Analyses
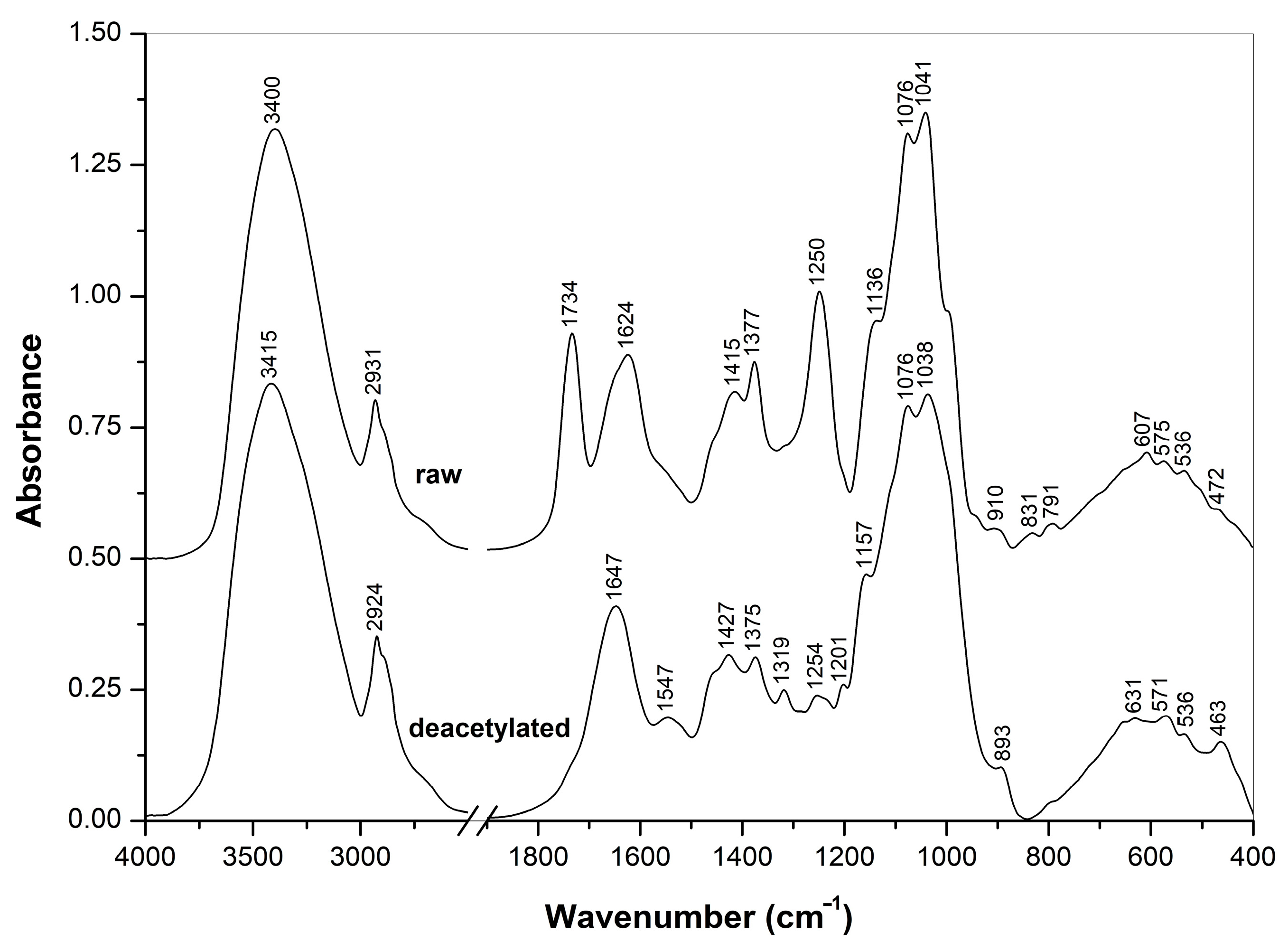
3.2.1. FTIR Spectra
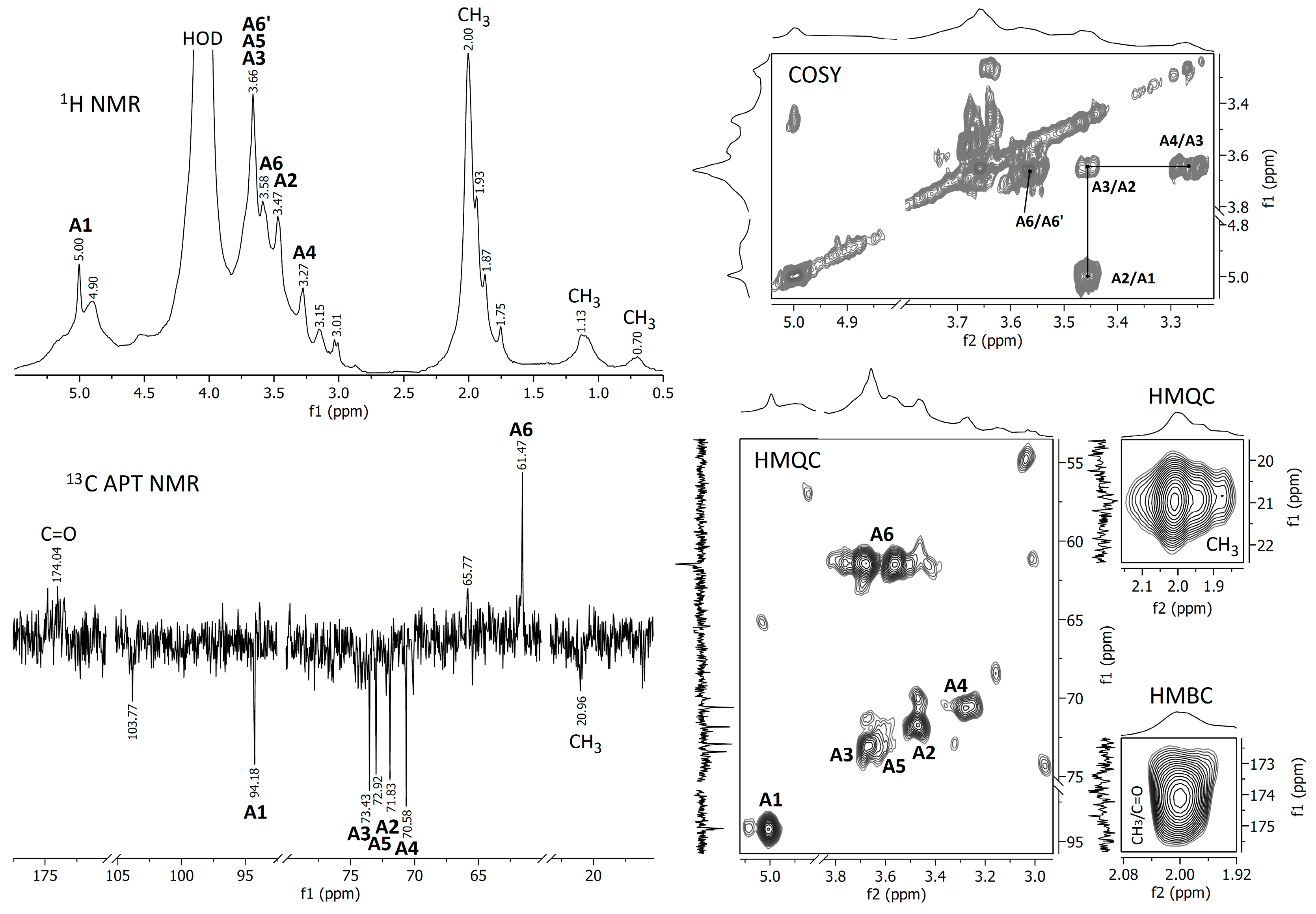
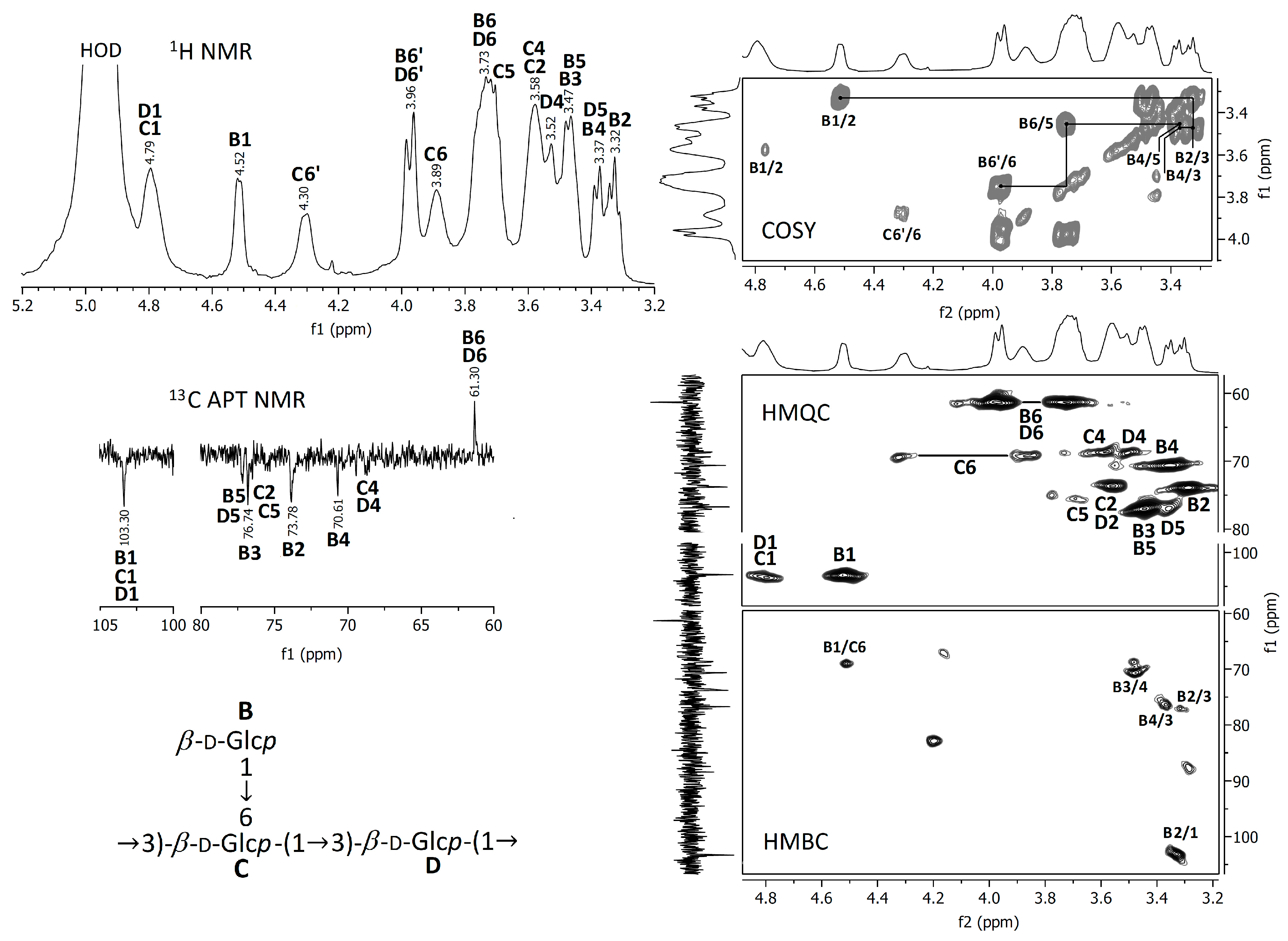
3.2.2. NMR Spectra
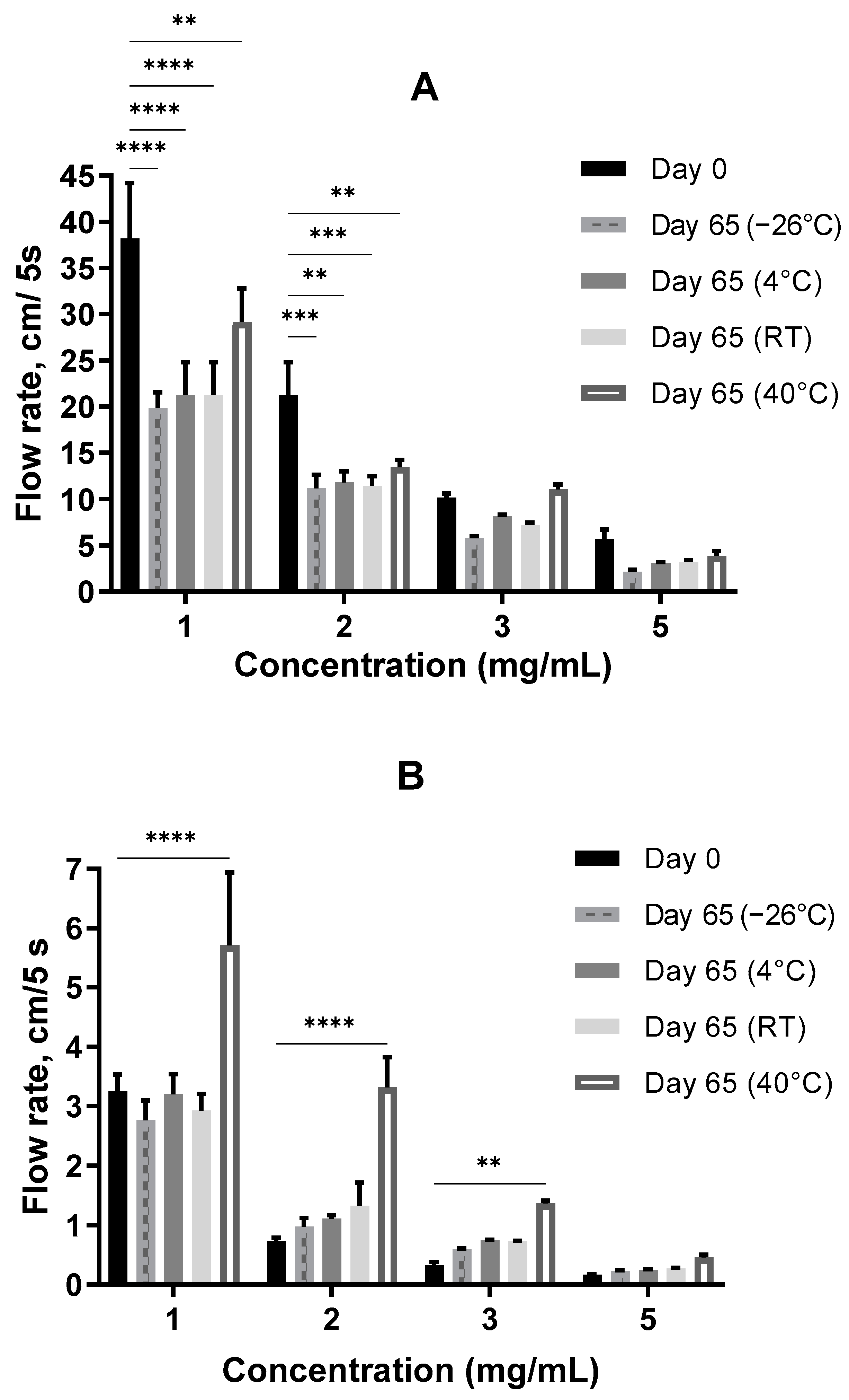
3.3. Storage Stability
3.3.1. Consistency at Neutral pH
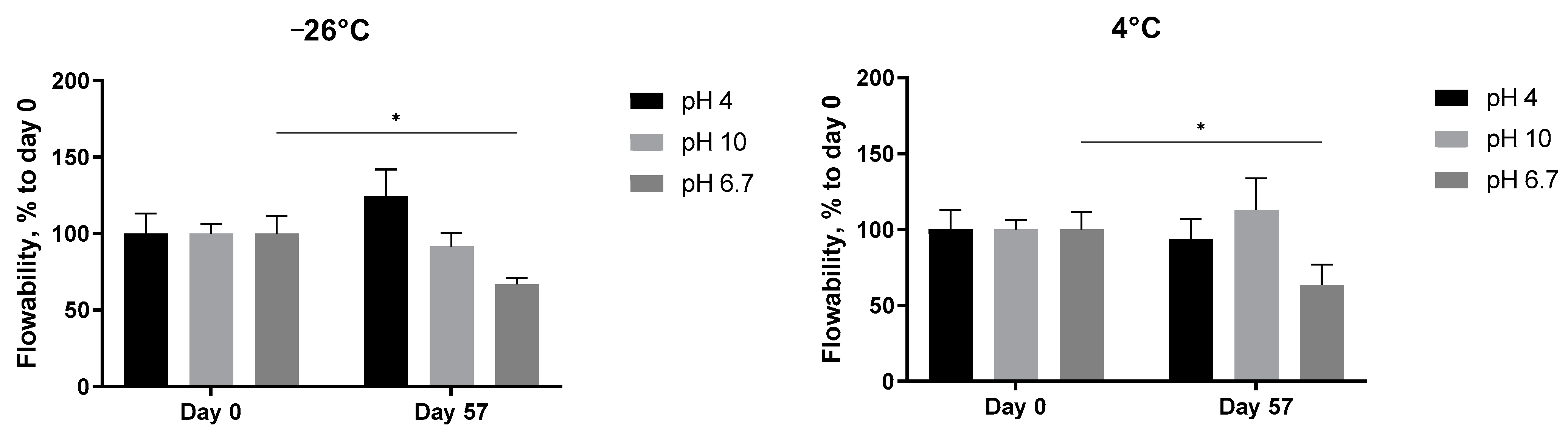
3.3.2. Consistency at Different pH Values
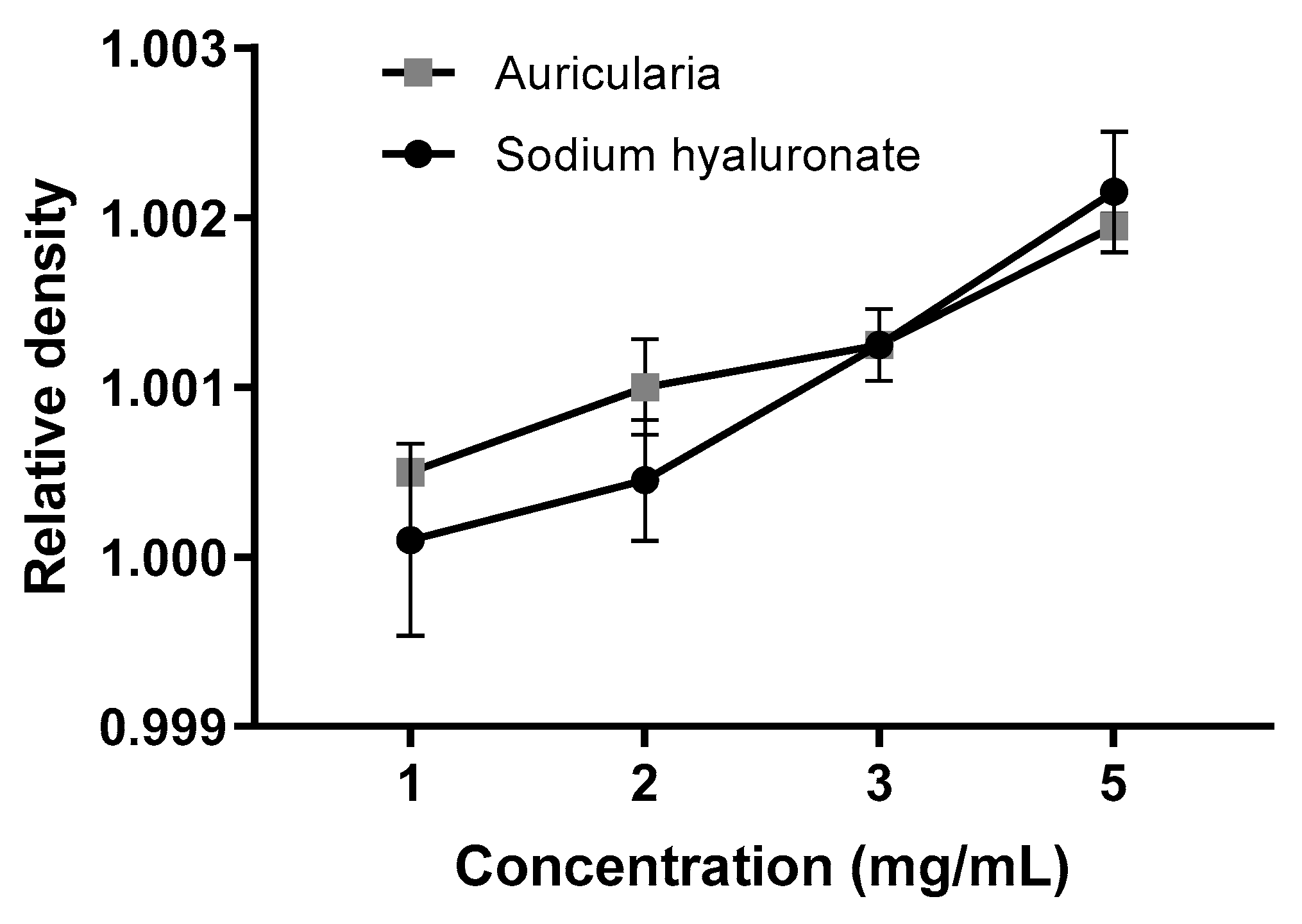
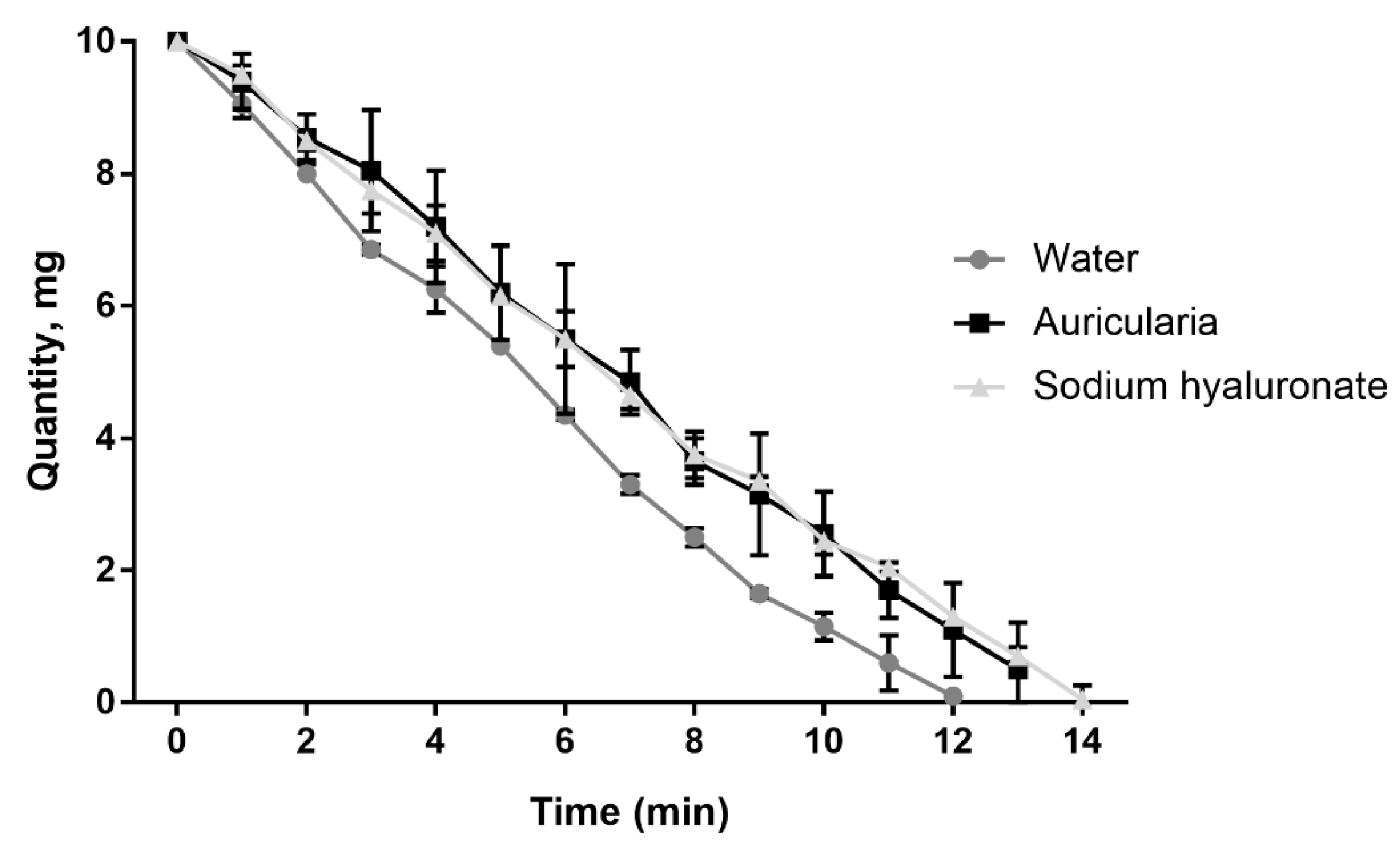
3.4. Moisture Retention Capacity
4. Conclusions
5. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bandara, A.R.; Rapior, S.; Mortimer, P.E.; Kakumyan, P.; Hyde, K.D.; Xu, J. A Review of the Polysaccharide, Protein and Selected Nutrient Content of Auricularia, and their Potential Pharmacological Value. Mycosphere 2019, 10, 579–607. [Google Scholar] [CrossRef]
- Khatua, S.; Sett, S.; Acharya, K. Auricularia spp.: From Farm to Pharmacy. In Biology, Cultivation and Applications of Mushrooms; Arya, A., Rusevska, K., Eds.; Springer Nature: Berlin, Germany; Singapore Pte Ltd.: Singapore, 2022; Chapter 11; pp. 301–355. [Google Scholar] [CrossRef]
- Yao, F.J.; Lu, L.X.; Wang, P.; Fang, M.; Zhang, Y.M.; Chen, Y.; Zhang, W.T.; Kong, X.H.; Lu, J.; Honda, Y. Development of a Molecular Marker for Fruiting Body Pattern in Auricularia auricula-judae. Mycobiology 2018, 46, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kamal, S.; Sharma, V.P. Status and Trends in World Mushroom Production-III. World Production of Different Mushroom Species in 21st Century. Mushroom Res. 2021, 29, 75–111. [Google Scholar] [CrossRef]
- Xu, S.; Xu, X.; Zhang, L. Branching Structure and Chain Conformation of Water-Soluble Glucan Extracted from Auricularia auricula-judae. J. Agric. Food Chem. 2012, 60, 3498–3506. [Google Scholar] [CrossRef] [PubMed]
- Sone, Y.; Kakuta, M.; Misaki, A. Isolation and Characterization of Polysaccharides of “Kikurage”, Fruit Body of Auricularia auricula-judae. Agric. Biol.Chem. 1978, 42, 417–425. [Google Scholar] [CrossRef]
- Bao, H.; You, S.; Cao, L.; Zhou, R.; Wang, Q.; Cui, S.W. Chemical and Rheological Properties of Polysaccharides from Fruit Body of Auricularia auricular-judae. Food Hydrocoll. 2016, 57, 30–37. [Google Scholar] [CrossRef]
- Chang, A.K.T.; Frias, R.R.; Alvarez, L.V.; Bigol, U.G.; Guzman, J.P.M. Comparative Antibacterial Activity of Commercial Chitosan and Chitosan Extracted from Auricularia sp. Biocatal. Agric. Biotechnol. 2019, 17, 189–195. [Google Scholar] [CrossRef]
- Commission Implementing Decision (EU) 2022/677 of 31 March 2022 Laying Down Rules for the Application of Regulation (EC) No 1223/2009 of the European Parliament and of the Council as Regards the Glossary of Common Ingredient Names for Use in the Labelling of Cosmetic Products (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2022.127.01.0001.01.ENG&toc=OJ%3AL%3A2022%3A127%3ATOC (accessed on 6 January 2023).
- Li, L.; Yang, X.Y.; Pan, L.; Su, Y.; Wang, Y. Comparing Three Methods of Extraction of Auricularia auricula Polysaccharides. Curr. Top. Nutraceutical Res. 2019, 17, 7–10. [Google Scholar]
- Elkhateeb, W.A.; El-Hagrassi, A.M.; Fayad, W.; El-Manawaty, M.A.; Zaghlol, G.M.; Daba, G.M.; Ahmed, E.F. Cytotoxicity and Hypoglycemic Effect of the Japanese Jelly Mushroom Auricularia auricula-judae. Chem. Res. J. 2018, 3, 123–133. [Google Scholar]
- Yuan, Z.; He, P.; Takeuchi, H. Ameliorating Effects of Water-Soluble Polysaccharides from Woody Ear (Auricularia auricula-judae Quel.) in Genetically Diabetic KK-Ay Mice. J. Nutr. Sci. Vitaminol. 1998, 44, 829–840. [Google Scholar] [CrossRef]
- Alvarado, P.; Moreno, G.; Vizzini, A.; Consiglio, G.; Manjon, J.L.; Setti, L. Atractosporocybe, Leucocybe and Rhizocybe: Three New Clitocyboid Genera in the Tricholomatoid Clade (Agaricales) with Notes on Clitocybe and Lepista. Mycologia 2015, 107, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yuan, Y.; Malysheva, V.F.; Du, P.; Dai, Y.C. Species Clarification of the Most Important and Cultivated Auricularia Mushroom “Heimuer”: Evidence from Morphological and Molecular Data. Phytotaxa 2014, 186, 241–253. [Google Scholar] [CrossRef]
- Kalitukha, L.; Sari, M.; Lexut, A.; Lexut, P. Gel-Forming Extracts from the Fungi of the Genus Auricularia and Method for their Preparation. Germany Patent DE 102021104013A1, 19 February 2021. [Google Scholar]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, R.A.; Shashkov, A.S.; Nifant’ev, N.E.; Khatuntseva, E.A.; Usov, A.I. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kleber, H.P.; Schlee, D.; Schöpp, W. Biochemisches Praktikum [Practical Course in Biochemistry]; Gustav Fischer Verlag: Jena, Germany, 1997. [Google Scholar]
- Megazyme International Ireland Ltd. Mushroom and Yeast β-glucan Assay Procedure Booklet (K-YBGL 11/19); Megazyme International Ireland Ltd.: Wicklow, Ireland, 2019. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP). In Technical Report No. NREL/TP-510-42618 [Issued April 2008; Revised July 2011]; Contract No. DE-AC36-08GO28308; National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- Ekblad, A.; Näsholm, T. Determination of Chitin in Fungi and Mycorrhizal Roots by an Improved HPLC Analysis of Glucosamine. Carbohydr. Res. 1996, 178, 29–35. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP). In Technical Report No. NREL/TP-510-42619 [Issued 17 July 2005]; Contract No. DE-AC36-99-GO10337; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- German Society of Fats Science. German Standard Methods for the Analysis of Fats, Fat Products, Surfactants and Related Substances, 2nd ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2019. [Google Scholar]
- Algermissen, B.; Nündel, M.; Riedel, E. Analysis of Amino Acids with Fluorescence HPLC. GIT Fachz. Lab. 1989, 33, 783–790. [Google Scholar]
- Kjeldahl, J. New Method for the Determination of Nitrogen in Organic Bodies. Z. Für Anal. Chemie. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Petrovska, B. Protein Fraction in Edible Macedonian Mushrooms. Eur. Food Res. Technol. 2001, 212, 469–472. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of ash in biomass: Laboratory analytical procedure (LAP). In Technical Report No. NREL/TP-510-42622 [Issued 17 July 2005]; Contract No. DE-AC36-99-GO10337; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Standard DIN EN 1134:1994-12; Fruit and Vegetable Juices—Determination of Sodium, Potassium, Calcium and Magnesium Content by Atomic Absorption Spectrometry (AAS). Deutsches Institut für Normung: Berlin, Germany, 1994.
- Standard DIN EN 13805:2014; Foodstuffs—Determination of Trace Elements—Pressure Digestion. German Version of EN 13805:2014. Deutsches Institut für Normung: Berlin, Germany, 2014.
- Milczarek, R.R.; McCarthy, K.L. Relationship between the Bostwick Measurement and Fluid Properties. J. Texture Stud. 2006, 37, 640–654. [Google Scholar] [CrossRef]
- International ASTM F1080-93(2019); Standard Test Method for Determining the Consistency of Viscous Liquids Using a Consistometer. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2019.
- Malouh, M.A.; Cichero, J.A.Y.; Manrique, Y.J.; Crino, L.; Lau, E.T.L.; Nissen, L.M.; Steadman, K.J. Are Medication Swallowing Lubricants Suitable for Use in Dysphagia? Consistency, Viscosity, Texture, and Application of the International Dysphagia Diet Standardization Initiative (IDDSI) Framework. Pharmaceutics 2020, 12, 924. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Liu, Y.; Ai, S.; Qin, F.; Li, Z.; Zhang, H.; Huang, Z. Antioxidant and Moisture-Retention Activities of the Polysaccharide from Nostoc commune. Carbohyd. Polym. 2011, 83, 1821–1827. [Google Scholar] [CrossRef]
- Pak, S.; Chen, F.; Ma, L.; Hu, X.; Ji, J. Functional perspective of black fungi (Auricularia auricula): Major bioactive components, health benefits and potential mechanisms. Trends Food Sci. Technol. 2021, 114, 245–261. [Google Scholar] [CrossRef]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Yodkeeree, S.; Limtrakul, P. Skin wound-healing potential of polysaccharides from medicinal mushroom Auricularia auricula-judae (Bull.). J. Fungi 2021, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Perera, N.; Yang, F.L.; Chern, J.; Chiu, H.W.; Hsieh, C.Y.; Li, L.H.; Zhang, Y.L.; Hua, K.F.; Wu, S.H. Carboxylic and O-acetyl moieties are essential for the immunostimulatory activity of glucuronoxylomannan: A novel TLR4 specific immunostimulator from Auricularia auricula-judae. Chem. Commun. 2018, 54, 6995–6998. [Google Scholar] [CrossRef]
- Yoon, S.J.; Yu, M.A.; Pyun, Y.R.; Hwang, J.K.; Chu, D.C.; Juneja, L.R.; Mourão, P.A. The nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by antithrombin. Thromb. Res. 2003, 112, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Zhou, R.; You, S.G.; Wu, S.; Wang, Q.; Cui, S.W. Gelation Mechanism of Polysaccharides from Auricularia auricula-judae. Food Hydrocoll. 2018, 76, 35–41. [Google Scholar] [CrossRef]
- Guilhem, G.; Doering, T.L. Biosynthesis and Genetics of the Cryptococcus Capsule. In Cryptococcus: From Human Pathogen to Model Yeast; Heitman, J., Kozel, T.R., Kwon-Chung, K.J., Perfect, J.R., Casadevall, A., Eds.; ASM Press: Washington, DC, USA, 2011; Chapter 3; pp. 27–41. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chien, P.J.; Tong, M.H.; Sheu, F. Mushroom Immunomodulatory Proteins Possess Potential Thermal/Freezing Resistance, Acid/Alkali Tolerance and Dehydration Stability. Food Chem. 2007, 105, 597–605. [Google Scholar] [CrossRef]
- Sheu, F.; Chien, P.J.; Chien, A.L.; Chen, Y.F.; Chin, K.L. Isolation and Characterization of an Immunomodulatory Protein (APP) from the Jew’s Ear Mushroom Auricularia polytricha. Food Chem. 2004, 87, 593–600. [Google Scholar] [CrossRef]
- Oli, A.N.; Edeh, P.A.; Al-Mosawi, R.M.; Mbachu, N.A.; Al-Dahmoshi, H.O.M.; Al-Khafaji, N.S.K.; Ekuma, U.O.; Okezie, U.M.; Saki, M. Evaluation of the Phytoconstituents of Auricularia auricula-judae Mushroom and Antimicrobial Activity of its Protein Extract. Eur. J. Integr. Med. 2020, 38, 101176. [Google Scholar] [CrossRef]
- Cai, M.; Lin, Y.; Luo, Y.L.; Liang, H.H.; Sun, P. Extraction, Antimicrobial, and Antioxidant Activities of Crude Polysaccharides from the Wood Ear Medicinal Mushroom Auricularia auricula-judae (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 591–600. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Investigation of Antioxidant and Antimicrobial Activities of Different Extracts of Auricularia and Termitomyces Species of Mushrooms. Sci. World J. 2019, 2019, 7357048. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Liu, Y.; Ma, Z.F.; Zhang, H.; Fu, T.; Li, Z.; Li, Y.; Hu, W.; Han, S.; Zhao, F.; et al. Analysis of Nutritional Quality of Black Fungus Cultivated with Corn Stalks. J. Food Qual. 2019, 2019, 9590251. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovič, V.J. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal-carboxylate interactions in metal-alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Wellner, N.; Kačuráková, M.; Malovíková, A.; Wilson, R.H.; Belton, P.S. FT-IR study of pectate and pectinate gels formed by divalent cations. Carbohydr. Res. 1998, 308, 123–131. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, X.; Ma, M.; Long, T.; Xiao, C.; Zhang, J.; Liu, J.; Zhao, L. Immunoenhancing glucuronoxylomannan from Tremella aurantialba Bandoni et Zang and its low-molecular-weight fractions by radical depolymerization: Properties, structures and effects on macrophages. Carbohydr. Polym. 2020, 238, 116184. [Google Scholar] [CrossRef]
- Bacon, B.E.; Cherniak, R.; Kwon-Chung, K.J.; Jacobson, E.S. Structure of the O-deacetylated glucuronoxylomannan from Cryptococcus neoformans Cap70 as determined by 2D NMR spectroscopy. Carbohydr. Res. 1996, 283, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Skelton, M.A.; Cherniak, R.; Poppe, L.; van Halbeek, H. Structure of the De-O-acetylated glucuronoxylomannan from Cryptococcus neoformans serotype D, as determined by 2D NMR spectroscopy. Magn. Reson. Chem. 1991, 29, 786–793. [Google Scholar] [CrossRef]
- Cherniak, R.; Jones, R.G.; Reiss, E. Structure determination of Cryptococcus neoformans serotype A-variant glucuronoxylomannan by 13C-nmr spectroscopy. Carbohydr. Res. 1988, 172, 113–138. [Google Scholar] [CrossRef]
- Synytsya, A.; Novak, M. Structural Analysis of Glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Zhang, L. Structure and chain conformation of beta-glucan isolated from Auricularia auricula-judae. Biopolym. Orig. Res. Biomol. 2008, 89, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic Acid (Hyaluronan): A Review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Scott, J.E.; Cummings, C.; Brass, A.; Chen, Y. Secondary and Tertiary structures of Hyaluronan in Aqueous Solution, Investigated by Rotary Shadowing-Electron Microscopy and Computer Simulation. Hyaluronan is a Very Efficient Network-Forming Polymer. Biochem. J. 1991, 274, 699–705. [Google Scholar] [CrossRef]
- Patel, B.K.; Campanella, O.H.; Janaswamy, S. Impact of Urea on the Three-Dimensional Structure, Viscoelastic and Thermal Behavior of Iota-Carrageenan. Carbohydr. Polym. 2013, 92, 1873–1879. [Google Scholar] [CrossRef]
- Liao, W.C.; Hsueh, C.Y.; Chan, C.F. Antioxidative Activity, Moisture Retention, Film Formation, and Viscosity Stability of Auricularia fuscosuccinea, White Strain Water Extract. Biosci. Biotechnol. Biochem. 2014, 78, 1029–1036. [Google Scholar] [CrossRef]

| Constituent | Mean ± SD (g/100 g DW) |
|---|---|
| Total glucans | 18.77 ± 1.47 |
| α-Glucans | 0.53 ± 0.13 |
| β-Glucans | 18.86 ± 0.71 |
| Amino Acids | Mean, g/kg | SD | % of Total Amount |
|---|---|---|---|
| Alanine | 6.6 | 0.7 | 8 |
| Arginine ** | 3.2 | 0.3 | 4 |
| Aspartic acid | 9.0 | 0.9 | 11 |
| Cysteine ** | 1.4 | 0.1 | 2 |
| Glutamic acid | 8.8 | 0.9 | 11 |
| Glycine ** | 3.9 | 0.4 | 5 |
| Histidine * | 2.4 | 0.2 | 3 |
| Isoleucine * | 2.6 | 0.3 | 3 |
| Leucine * | 5.4 | 0.5 | 7 |
| Lysine * | 2.5 | 0.2 | 3 |
| Methionine * | 0.9 | 0.1 | 1 |
| Phenylalanine * | 4.2 | 0.4 | 5 |
| Proline ** | 3.6 | 0.4 | 4 |
| Serine | 5.5 | 0.5 | 7 |
| Threonine * | 6.2 | 0.6 | 8 |
| Tryptophan * | 1.1 | 0.1 | 1 |
| Tyrosine ** | 10.0 | 1.0 | 12 |
| Valine * | 4.3 | 0.4 | 5 |
| Total amount (18 AAs) | 81.6 | 100 | |
| Essential AAs * | 29.6 | 36 | |
| Essential * + semi-essential ** AAs | 51.7 | 63 | |
| Top 5 AAs | 40.6 | 50 |
| Fatty Acid | Mean ± SD (% of Total Fatty Acid) |
|---|---|
| Capric acid (C 10:0) | 0.06 ± 0.01 |
| Lauric acid (C 12:0) | 0.1 ± 0.01 |
| Myristic acid (C 14:0) | 0.57 ± 0.06 |
| Pentadecanoic acid (C 15:0) | 1.47 ± 0.15 |
| Palmitic acid (C 16:0) | 21.76 ± 2.15 |
| Palmitoleic acid (C 16:1) | 0.48 ± 0.05 |
| Margaric acid (C 17:0) | 0.41 ± 0.04 |
| Stearic acid (C 18:0) | 10.49 ± 1.04 |
| Oleic acid (C 18:1) | 23.55 ± 2.33 |
| Linoleic acid (C 18:2) | 34.05 ± 3.37 |
| gamma-linolenic acid (C 18:3) | 0.21 ± 0.02 |
| alpha-linolenic acid (C 18:3) | 1.09 ± 0.11 |
| Arachidic acid (C 20:0) | 1.18 ± 0.12 |
| Eicosenoic acid (C 20:1) | 0.23 ± 0.02 |
| Eicosadienic acid (C 20:2) | 0.15 ± 0.01 |
| Eicosatrienic acid (C 20:3) | 0.32 ± 0.03 |
| Erucic acid (C 22:1) | 2.97 ± 0.29 |
| Docosadienic acid (C 22:2) | 0.08 ± 0.01 |
| Lignoceric acid (C 24:0) | 0.84 ± 0.08 |
| Unsaturated fatty acids | 63 |
| Top 4 | 90 |
| Mineral Content | Mean ± SD (mg/100 g DW) |
|---|---|
| Macroelements | |
| K | 2560 ± 235 |
| Ca | 527 ± 52 |
| Mg | 203 ± 20 |
| Na | 92 ± 9 |
| Microelements | |
| Cu | 238 ± 24 |
| Fe | 11.5 ± 1.1 |
| Zn | 3.7 ± 0.4 |
| Total | 3635 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalitukha, L.; Bleha, R.; Synytsya, A.; Kraska, J.; Sari, M. Hydrocolloids from the Mushroom Auricularia heimuer: Composition and Properties. J. Fungi 2023, 9, 681. https://doi.org/10.3390/jof9060681
Kalitukha L, Bleha R, Synytsya A, Kraska J, Sari M. Hydrocolloids from the Mushroom Auricularia heimuer: Composition and Properties. Journal of Fungi. 2023; 9(6):681. https://doi.org/10.3390/jof9060681
Chicago/Turabian StyleKalitukha, Liudmila, Roman Bleha, Andriy Synytsya, Janina Kraska, and Miriam Sari. 2023. "Hydrocolloids from the Mushroom Auricularia heimuer: Composition and Properties" Journal of Fungi 9, no. 6: 681. https://doi.org/10.3390/jof9060681
APA StyleKalitukha, L., Bleha, R., Synytsya, A., Kraska, J., & Sari, M. (2023). Hydrocolloids from the Mushroom Auricularia heimuer: Composition and Properties. Journal of Fungi, 9(6), 681. https://doi.org/10.3390/jof9060681








