Characterization of Lung Inflammatory Response to Aspergillus fumigatus Spores
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Transcript Levels of Different Inflammatory Mediators in AFsp-Treated Monocultures
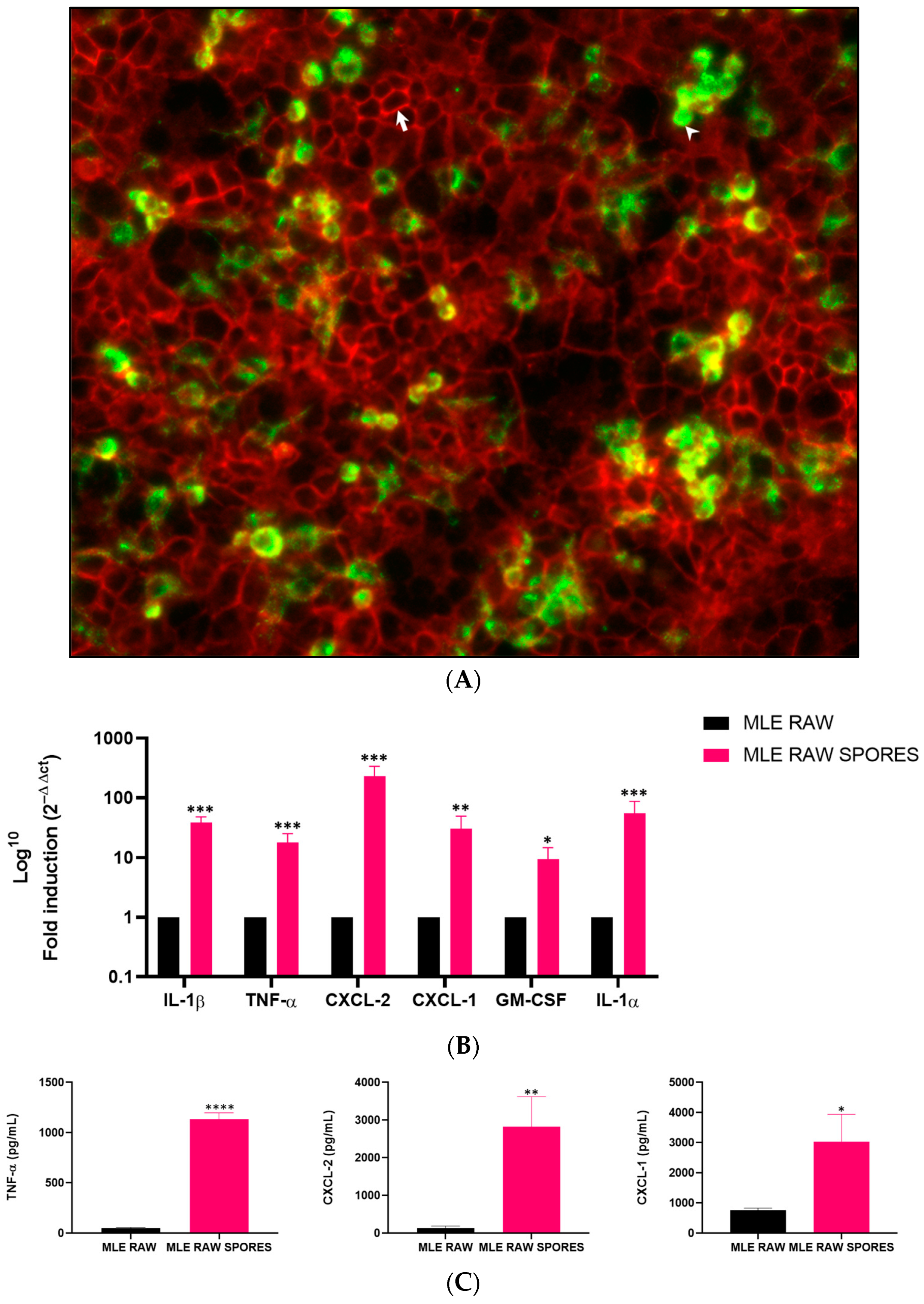
3.2. Induced Gene Expression of Cytokines in RAW 264.7 and MLE-15 Cell Co-Culture
3.3. Involvement of TNF-α, CXCL-2 and CXCL-1 in Anti-Aspergillus Response in Co-Culture Model
3.4. Effects on Mouse Body Weight
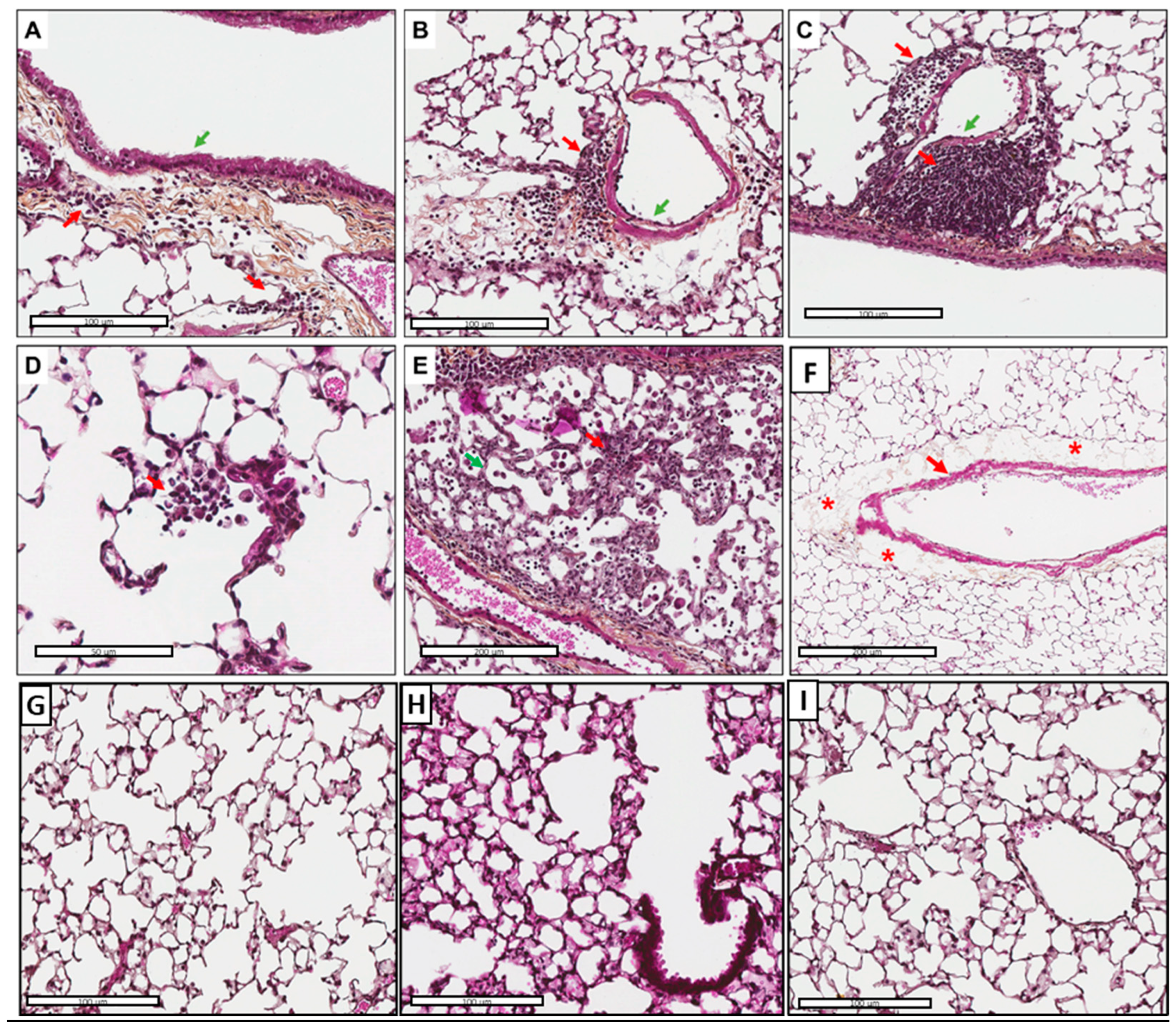
3.5. Tissue Inflammation and Perivascular Edema Induced via AFsp Exposure
3.6. Inflammatory Cell Recruitment, Especially Macrophages in Response to AFsp
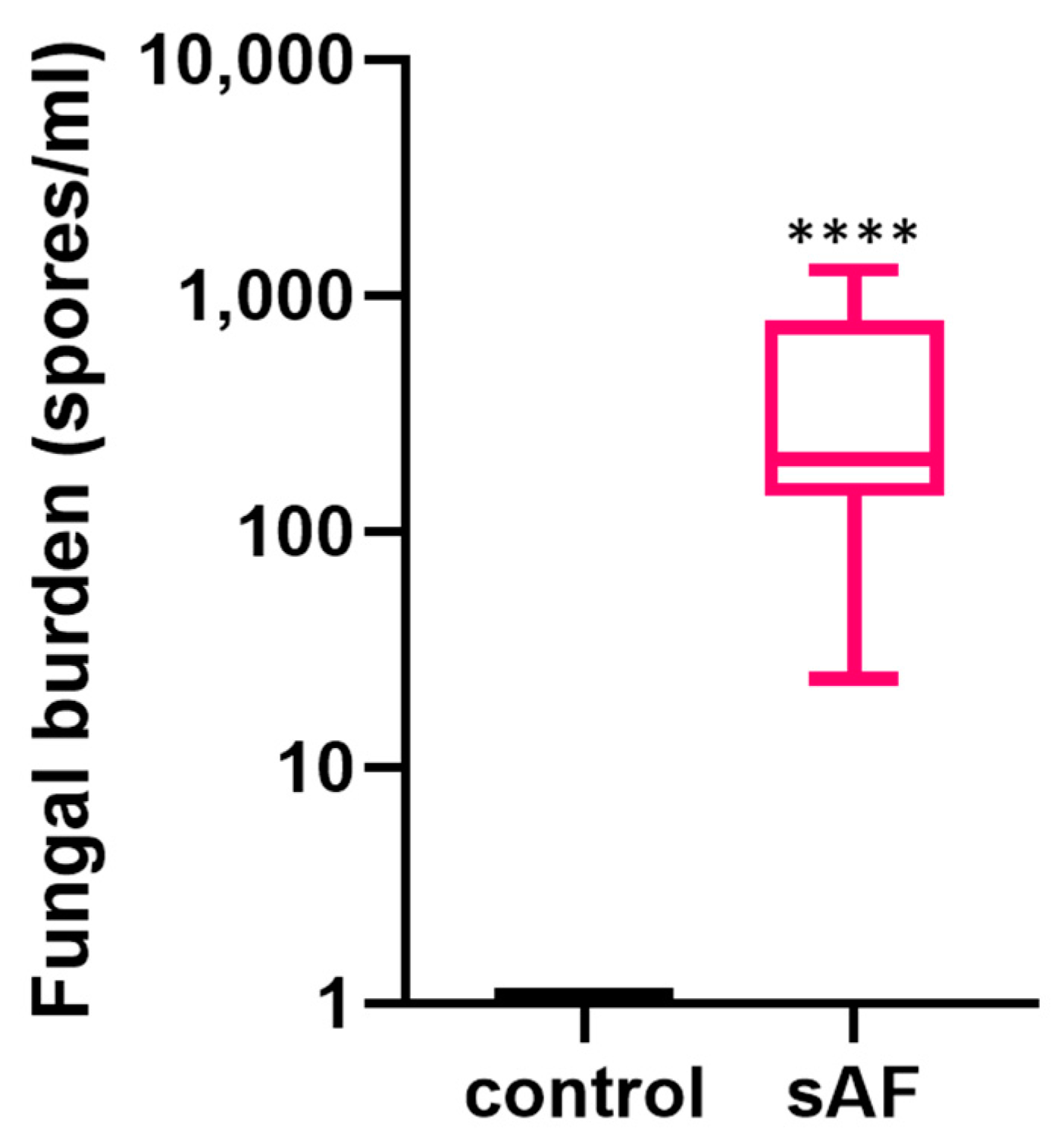
3.7. Quantification of Lung Fungal Burden
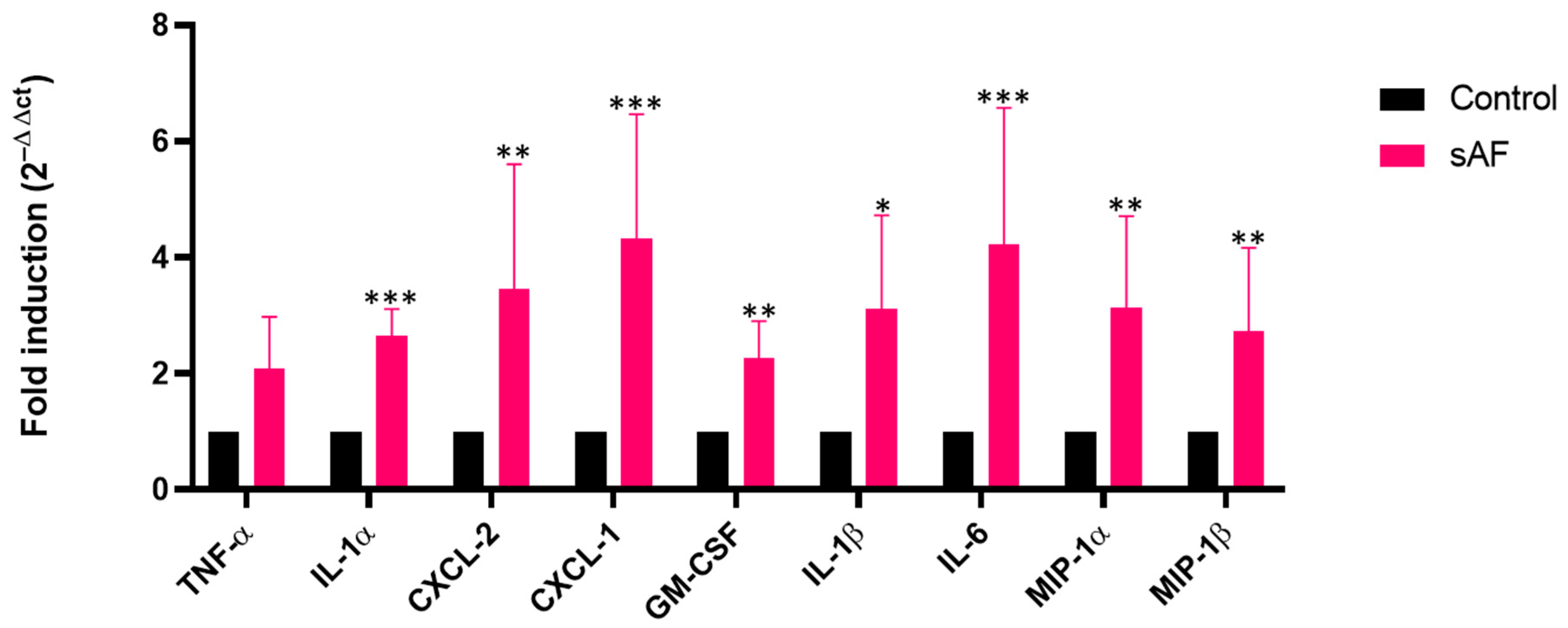
3.8. Increased Transcript Levels of Some Inflammatory Mediators in AFsp-Exposed Lungs
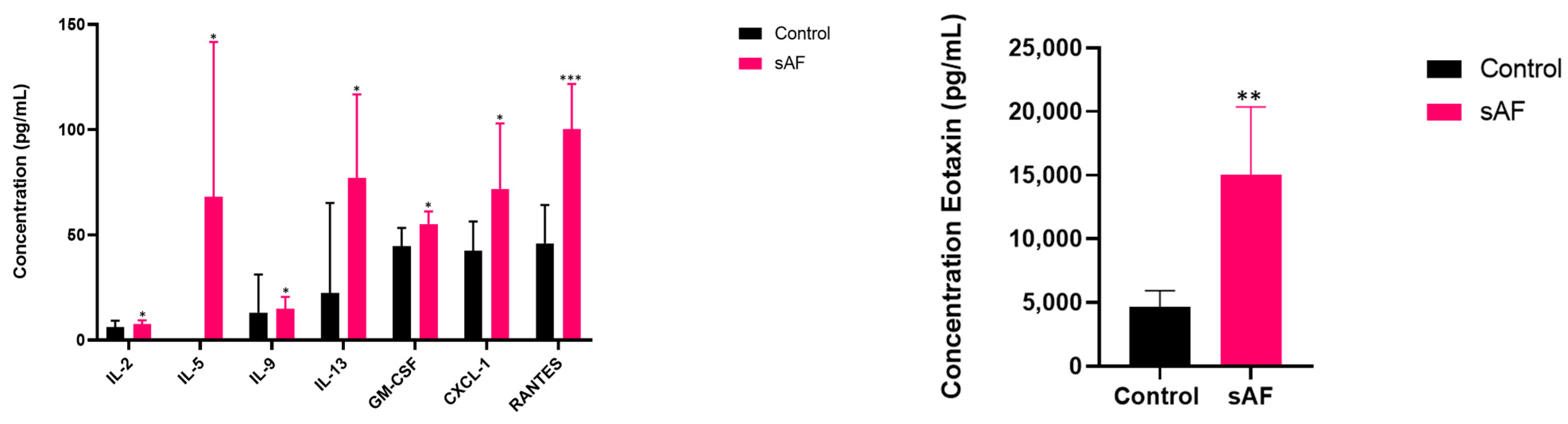
3.9. Pattern of Several Inflammatory Mediators in BAL from Challenged Mice
3.10. Increased Protein Levels of Inflammatory Mediators in Sera from Challenged Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, N.L.; Denning, D.W. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2011, 37, 865–872. [Google Scholar] [CrossRef]
- Lauruschkat, C.D.; Etter, S.; Schnack, E.; Ebel, F.; Schäuble, S.; Page, L.; Rümens, D.; Dragan, M.; Schlegel, N.; Panagiotou, G.; et al. Chronic Occupational Mold Exposure Drives Expansion of Aspergillus-Reactive Type 1 and Type 2 T-Helper Cell Responses. J. Fungi 2021, 7, 698. [Google Scholar] [CrossRef] [PubMed]
- Camara, B.; Reymond, E.; Saint-Raymond, C.; Roth, H.; Brenier-Pinchart, M.-P.; Pinel, C.; Cadranel, J.; Ferretti, G.; Pelloux, H.; Pison, C.; et al. Characteristics and outcomes of chronic pulmonary aspergillosis: A retrospective analysis of a tertiary hospital registry: Chronic pulmonary aspergillosis in non-immunocompromised patients. Clin. Respir. J. 2015, 9, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef]
- Denning, D.W. The link between fungi and severe asthma: A summary of the evidence. Eur. Respir. J. 2006, 27, 615–626. [Google Scholar] [CrossRef]
- Bulpa, P.; Duplaquet, F.; Dimopoulos, G.; Vogelaers, D.; Blot, S. Invasive Pulmonary Aspergillosis in Chronic Obstructive Pulmonary Disease Exacerbations. Semin. Respir. Crit. Care Med. 2020, 41, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med. Mycol. 2013, 51, 361–370. [Google Scholar] [CrossRef]
- Ledoux, M.-P.; Guffroy, B.; Nivoix, Y.; Simand, C.; Herbrecht, R. Invasive Pulmonary Aspergillosis. Semin. Respir. Crit. Care Med. 2020, 41, 080–098. [Google Scholar] [CrossRef]
- Dewi, I.M.; Janssen, N.A.; Rosati, D.; Bruno, M.; Netea, M.G.; Brüggemann, R.J.; Verweij, P.E.; van de Veerdonk, F.L. Invasive pulmonary aspergillosis associated with viral pneumonitis. Curr. Opin. Microbiol. 2021, 62, 21–27. [Google Scholar] [CrossRef]
- Dupont, D.; Menotti, J.; Turc, J.; Miossec, C.; Wallet, F.; Richard, J.-C.; Argaud, L.; Paulus, S.; Wallon, M.; Ader, F.; et al. Pulmonary aspergillosis in critically ill patients with Coronavirus Disease 2019 (COVID-19). Med. Mycol. 2021, 59, 110–114. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Gangneux, J.-P.; Camus, C.; Philippe, B. Epidemiology of invasive aspergillosis and risk factors in non neutropaenic patients. Rev. Mal. Respir. 2010, 27, e34–e46. [Google Scholar] [CrossRef]
- Black, P.N.; Udy, A.A.; Brodie, S.M. Sensitivity to fungal allergens is a risk factor for life-threatening asthma: Fungal allergens and asthma. Allergy 2000, 55, 501–504. [Google Scholar] [CrossRef]
- Zureik, M. Sensitisation to airborne moulds and severity of asthma: Cross sectional study from European Community respiratory health survey. BMJ 2002, 325, 411. [Google Scholar] [CrossRef]
- Goh, K.J.; Yii, A.; Lapperre, T.; Chan, A.; Chew, F.; Chotirmall, S.; Koh, M. Sensitization to Aspergillus species is associated with frequent exacerbations in severe asthma. J. Asthma Allergy 2017, 10, 131–140. [Google Scholar] [CrossRef]
- Margalit, A.; Kavanagh, K. The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol. Rev. 2015, 39, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Persoz, C.; Leleu, C.; Achard, S.; Fasseu, M.; Menotti, J.; Meneceur, P.; Momas, I.; Derouin, F.; Seta, N. Sequential air-liquid exposure of human respiratory cells to chemical and biological pollutants. Toxicol. Lett. 2011, 207, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Guyot, N.; Wartelle, J.; Malleret, L.; Todorov, A.A.; Devouassoux, G.; Pacheco, Y.; Jenne, D.E.; Belaaouaj, A. Unopposed Cathepsin G, Neutrophil Elastase, and Proteinase 3 Cause Severe Lung Damage and Emphysema. Am. J. Pathol. 2014, 184, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Alanio, A.; Menotti, J.; Gits-Muselli, M.; Hamane, S.; Denis, B.; Rafoux, E.; Peffault de la Tour, R.; Touratier, S.; Bergeron, A.; Guigue, N.; et al. Circulating Aspergillus fumigatus DNA Is Quantitatively Correlated to Galactomannan in Serum. Front. Microbiol. 2017, 8, 2040. [Google Scholar] [CrossRef] [PubMed]
- Challier, S.; Boyer, S.; Abachin, E.; Berche, P. Development of a Serum-Based Taqman Real-Time PCR Assay for Diagnosis of Invasive Aspergillosis. J. Clin. Microbiol. 2004, 42, 844–846. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rider, P.; Carmi, Y.; Guttman, O.; Braiman, A.; Cohen, I.; Voronov, E.; White, M.R.; Dinarello, C.A.; Apte, R.N. IL-1α and IL-1β Recruit Different Myeloid Cells and Promote Different Stages of Sterile Inflammation. J. Immunol. 2011, 187, 4835–4843. [Google Scholar] [CrossRef]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef]
- Jones, M.R.; Simms, B.T.; Lupa, M.M.; Kogan, M.S.; Mizgerd, J.P. Lung NF-κB Activation and Neutrophil Recruitment Require IL-1 and TNF Receptor Signaling during Pneumococcal Pneumonia. J. Immunol. 2005, 175, 7530–7535. [Google Scholar] [CrossRef]
- Laan, M.; Prause, O.; Miyamoto, M.; Sjöstrand, M.; Hytönen, A.M.; Kaneko, T.; Lötvall, J.; Lindén, A. A role of GM-CSF in the accumulation of neutrophils in the airways caused by IL-17 and TNF-α. Eur. Respir. J. 2003, 21, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Cramer, R.A.; Rivera, A.; Hohl, T.M. Immune responses against Aspergillus fumigatus: What have we learned? Curr. Opin. Infect. Dis. 2011, 24, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Pylkkänen, L.; Gullstén, H.; Majuri, M.-L.; Andersson, U.; Vanhala, E.; Määttä, J.; Meklin, T.; Hirvonen, M.-R.; Alenius, H.; Savolainen, K. Exposure to Aspergillus fumigatus spores induces chemokine expression in mouse macrophages. Toxicology 2004, 200, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, A.-P.; Millon, L.; Khoufache, K.; Rivollet, D.; Bièche, I.; Laurendeau, I.; Vidaud, M.; Botterel, F.; Bretagne, S. Aspergillus fumigatus germ tube growth and not conidia ingestion induces expression of inflammatory mediator genes in the human lung epithelial cell line A549. J. Med. Microbiol. 2009, 58, 174–179. [Google Scholar] [CrossRef]
- Hohl, T.M.; Van Epps, H.L.; Rivera, A.; Morgan, L.A.; Chen, P.L.; Feldmesser, M.; Pamer, E.G. Aspergillus fumigatus Triggers Inflammatory Responses by Stage-Specific β-Glucan Display. PLoS Pathog. 2005, 1, e30. [Google Scholar] [CrossRef] [PubMed]
- Leleu, C.; Menotti, J.; Meneceur, P.; Choukri, F.; Sulahian, A.; Garin, Y.J.-F.; Derouin, F. Efficacy of liposomal amphotericin B for prophylaxis of acute or reactivation models of invasive pulmonary aspergillosis: Prophylaxis of invasive aspergillosis. Mycoses 2013, 56, 241–249. [Google Scholar] [CrossRef]
- Hoselton, S.A.; Samarasinghe, A.E.; Seydel, J.M.; Schuh, J.M. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Med. Mycol. 2010, 48, 1056–1065. [Google Scholar] [CrossRef]
- Kurup, V.P.; Xia, J.-Q.; Crameri, R.; Rickaby, D.A.; Choi, H.Y.; Flückiger, S.; Blaser, K.; Dawson, C.A.; Kelly, K.J. Purified Recombinant A. fumigatus Allergens Induce Different Responses in Mice. Clin. Immunol. 2001, 98, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Mcmillan, S.J.; Lloyd, C.M. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin. Htmlent Glyphamp Asciiamp Exp. Allergy 2004, 34, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Hogaboam, C.M.; Blease, K.; Mehrad, B.; Steinhauser, M.L.; Standiford, T.J.; Kunkel, S.L.; Lukacs, N.W. Chronic Airway Hyperreactivity, Goblet Cell Hyperplasia, and Peribronchial Fibrosis during Allergic Airway Disease Induced by Aspergillus fumigatus. Am. J. Pathol. 2000, 156, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Haczku, A.; Atochina, E.N.; Tomer, Y.; Chen, H.; Scanlon, S.T.; Russo, S.; Xu, J.; Panettieri, R.A.; Beers, M.F. Aspergillus fumigatus -Induced Allergic Airway Inflammation Alters Surfactant Homeostasis and Lung Function in BALB/c Mice. Am. J. Respir. Cell Mol. Biol. 2001, 25, 45–50. [Google Scholar] [CrossRef]
- Carr, C.; Aykent, S.; Kimack, N.M.; Levine, A.D. Disulfide assignments in recombinant mouse and human interleukin 4. Biochemistry 1991, 30, 1515–1523. [Google Scholar] [CrossRef]
- Yokota, T.; Otsuka, T.; Mosmann, T.; Banchereau, J.; DeFrance, T.; Blanchard, D.; De Vries, J.E.; Lee, F.; Arai, K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc. Natl. Acad. Sci. USA 1986, 83, 5894–5898. [Google Scholar] [CrossRef]
- Dietschmann, A.; Schruefer, S.; Krappmann, S.; Voehringer, D. Th2 cells promote eosinophil-independent pathology in a murine model of allergic bronchopulmonary aspergillosis. Eur. J. Immunol. 2020, 50, 1044–1056. [Google Scholar] [CrossRef]
- Clutterbuck, E.; Hirst, E.; Sanderson, C. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: Comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood 1989, 73, 1504–1512. [Google Scholar] [CrossRef]
- Conde, E.; Bertrand, R.; Balbino, B.; Bonnefoy, J.; Stackowicz, J.; Caillot, N.; Colaone, F.; Hamdi, S.; Houmadi, R.; Loste, A.; et al. Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice. Nat. Commun. 2021, 12, 2574. [Google Scholar] [CrossRef]
- Gonzalo, J.-A.; Jia, G.-Q.; Aguirre, V.; Friend, D.; Coyle, A.J.; Jenkins, N.A.; Lin, G.-S.; Katz, H.; Lichtman, A.; Copeland, N.; et al. Mouse Eotaxin Expression Parallels Eosinophil Accumulation during Lung Allergic Inflammation but It Is Not Restricted to a Th2-Type Response. Immunity 1996, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Uguccioni, M.; Baggiolini, M.; Clark-Lewis, I.; Dahinden, C.A. Deletion of the NH2-terminal residue converts monocyte chemotactic protein 1 from an activator of basophil mediator release to an eosinophil chemoattractant. J. Exp. Med. 1996, 183, 681–685. [Google Scholar] [CrossRef]
- Rose, C.E.; Lannigan, J.A.; Kim, P.; Lee, J.J.; Fu, S.M.; Sung, S.J. Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell Mol. Immunol. 2010, 7, 361–374. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Konno, Y.; Kanda, A.; Yamada, Y.; Yasuba, H.; Sakata, Y.; Fukuchi, M.; Tomoda, K.; Iwai, H.; Ueki, S. Critical role of CCL4 in eosinophil recruitment into the airway. Clin. Exp. Allergy 2019, 49, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Stafford, S.; Forsythe, P.; Harrison, R.; Faubion, D.; Lett-Brown, M.A.; Grant, J.A. RANTES is a chemotactic and activating factor for human eosinophils. J. Immunol. Baltim. Md 1950 1993, 150, 3442–3448. [Google Scholar] [CrossRef]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef]
- Noverr, M.C.; Falkowski, N.R.; McDonald, R.A.; McKenzie, A.N.; Huffnagle, G.B. Development of Allergic Airway Disease in Mice following Antibiotic Therapy and Fungal Microbiota Increase: Role of Host Genetics, Antigen, and Interleukin-13. Infect. Immun. 2005, 73, 30–38. [Google Scholar] [CrossRef]
- Vieira, P.; Rajewsky, K. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 1988, 18, 313–316. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouyssi, A.; Déméautis, T.; Trecourt, A.; Delles, M.; Agostini, F.; Monneret, G.; Glehen, O.; Wallon, M.; Persat, F.; Devouassoux, G.; et al. Characterization of Lung Inflammatory Response to Aspergillus fumigatus Spores. J. Fungi 2023, 9, 682. https://doi.org/10.3390/jof9060682
Bouyssi A, Déméautis T, Trecourt A, Delles M, Agostini F, Monneret G, Glehen O, Wallon M, Persat F, Devouassoux G, et al. Characterization of Lung Inflammatory Response to Aspergillus fumigatus Spores. Journal of Fungi. 2023; 9(6):682. https://doi.org/10.3390/jof9060682
Chicago/Turabian StyleBouyssi, Alexandra, Tanguy Déméautis, Alexis Trecourt, Marie Delles, Fany Agostini, Guillaume Monneret, Olivier Glehen, Martine Wallon, Florence Persat, Gilles Devouassoux, and et al. 2023. "Characterization of Lung Inflammatory Response to Aspergillus fumigatus Spores" Journal of Fungi 9, no. 6: 682. https://doi.org/10.3390/jof9060682
APA StyleBouyssi, A., Déméautis, T., Trecourt, A., Delles, M., Agostini, F., Monneret, G., Glehen, O., Wallon, M., Persat, F., Devouassoux, G., Bentaher, A., & Menotti, J. (2023). Characterization of Lung Inflammatory Response to Aspergillus fumigatus Spores. Journal of Fungi, 9(6), 682. https://doi.org/10.3390/jof9060682






