Three New Species of Fusicolla (Hypocreales) from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Morphological Studies
2.2. DNA Extraction, PCR Amplification, Sequencing and Phylogenetic Analyses
3. Results
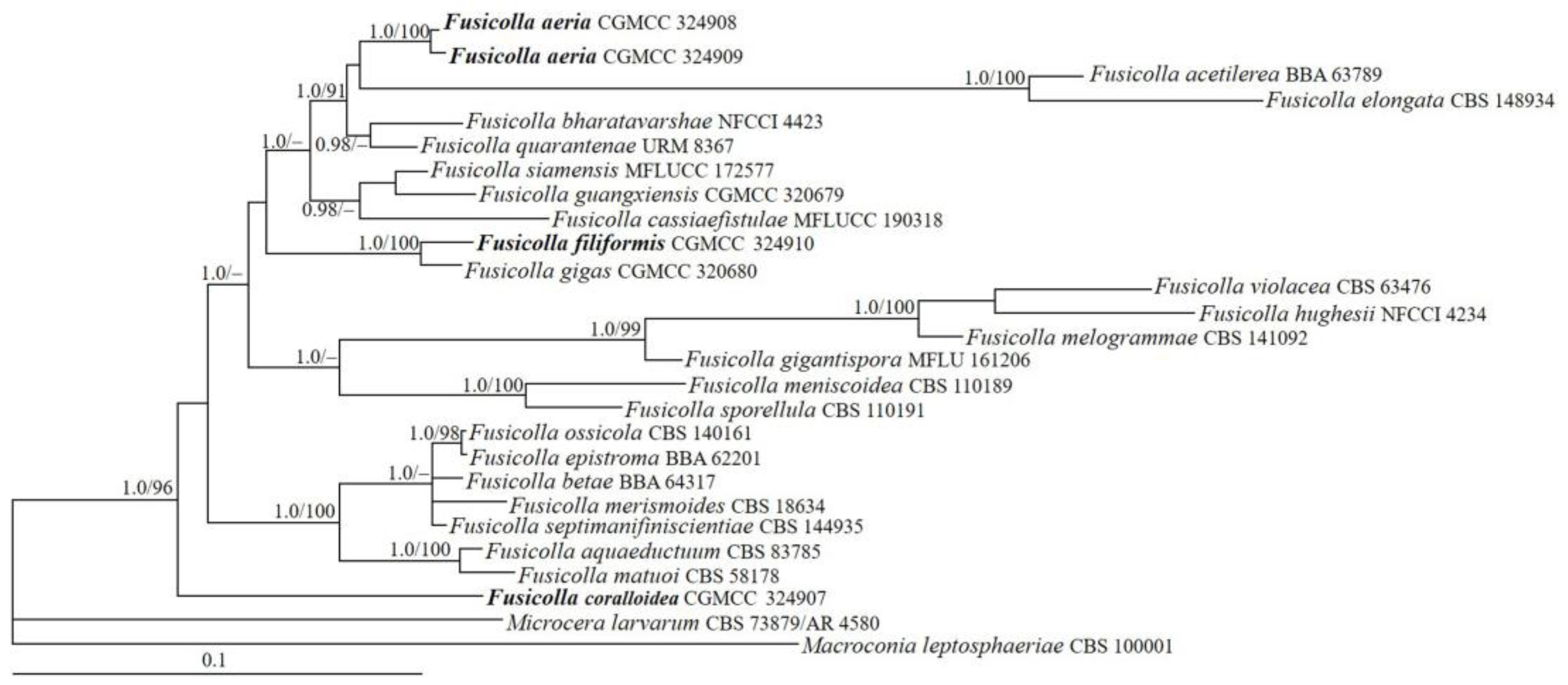
3.1. Phylogeny
3.2. Taxonomy
- Fusicolla aeria Z.Q. Zeng & W.Y. Zhuang, sp. nov., Figure 2.
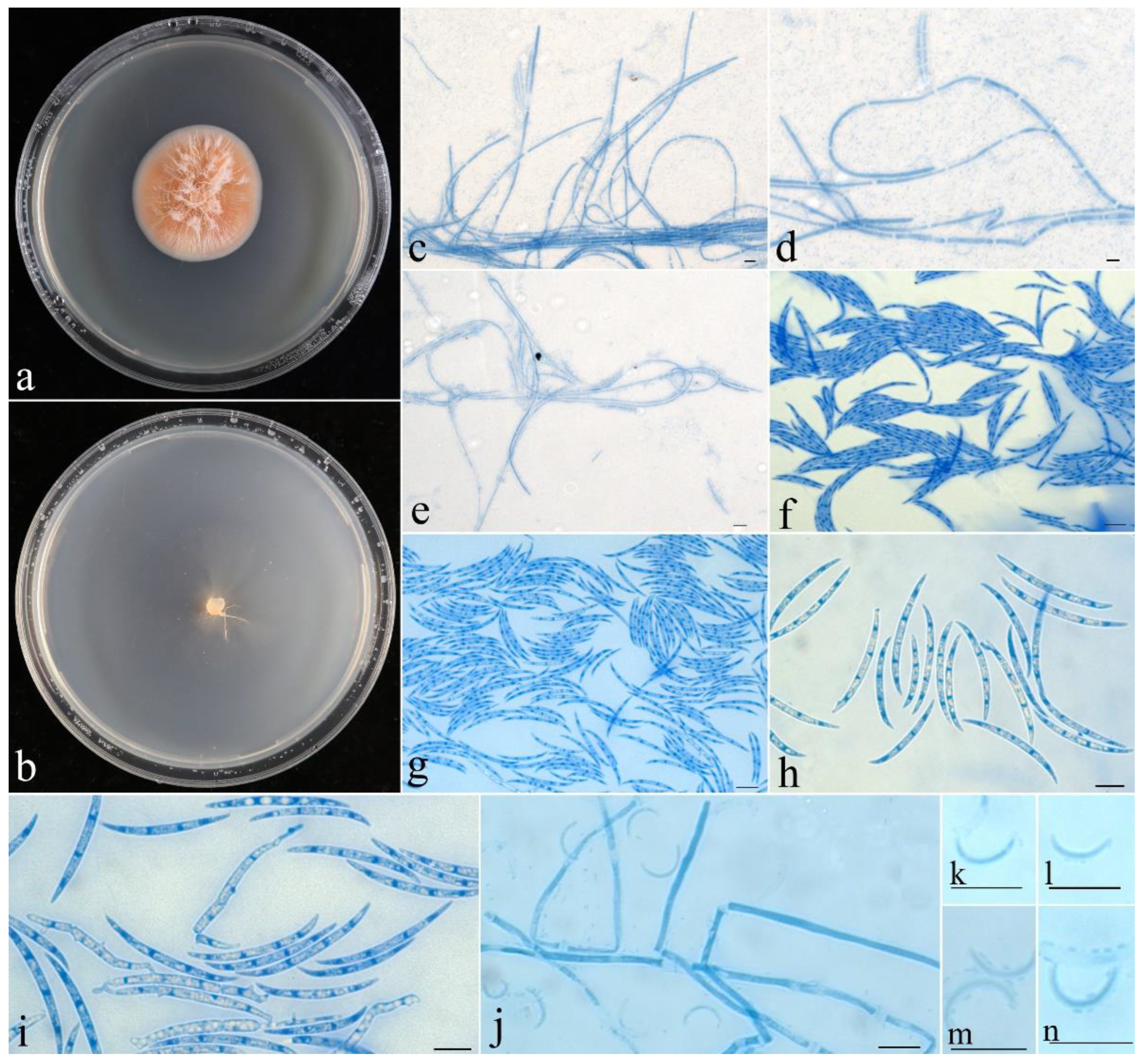
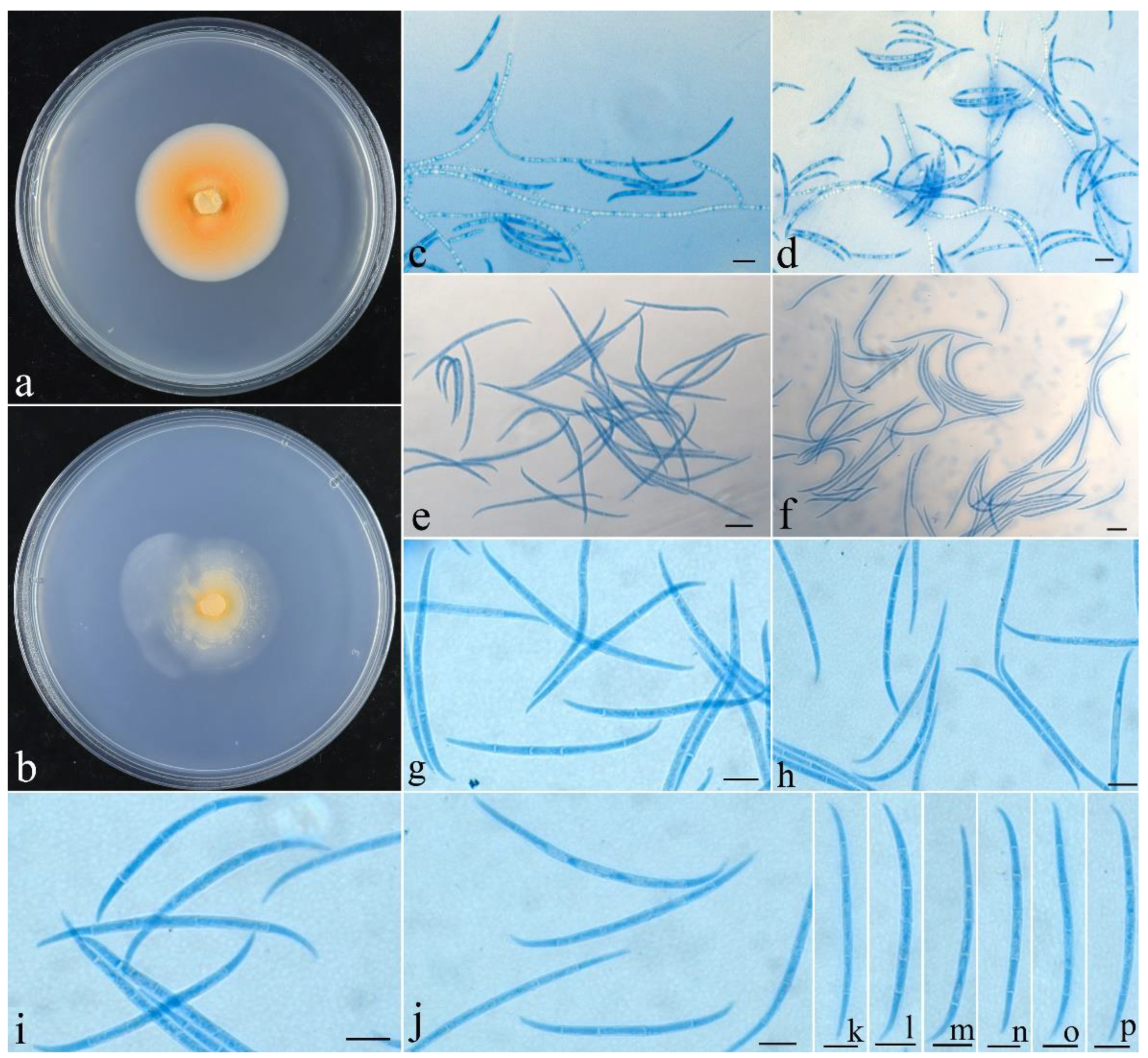
- Fusicolla coralloidea Z.Q. Zeng & W.Y. Zhuang, sp. nov., Figure 3.
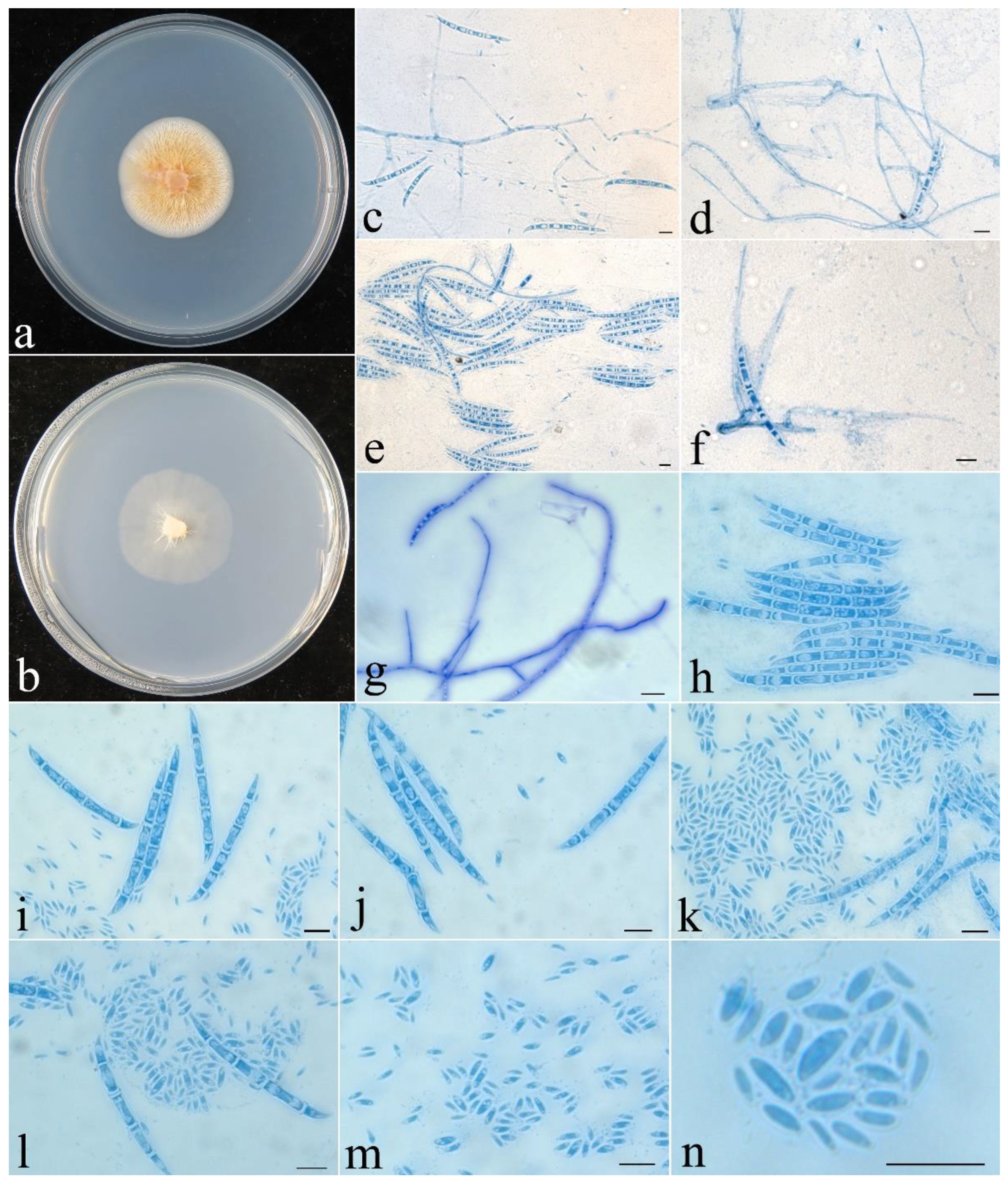
- Fusicolla filiformis Z.Q. Zeng & W.Y. Zhuang, sp. nov., Figure 4.
- Other Fusicolla Species Recorded in China
- Fusicolla aquaeductuum (Radlk. & Rabenh.) Gräfenhan, Seifert & Schroers, in Gräfenhan, Schroers, Nirenberg & Seifert, Stud. Mycol. 68: 100, 2011.
- Fusicolla gigas Chang Liu, Z.Q. Zeng & W.Y. Zhuang, in Crous et al., Fungal Systematics and Evolution 9: 192, 2022.
- Fusicolla guangxiensis Z.Q. Zeng, Chang Liu & W.Y. Zhuang, in Crous et al., Fungal Systematics and Evolution 9: 192, 2022.
- Fusicolla matuoi (Hosoya & Tubaki) Gräfenhan & Seifert, in Gräfenhan, Schroers, Nirenberg & Seifert, Stud. Mycol. 68: 101, 2011.
- Fusicolla violacea Gräfenhan & Seifert, in Gräfenhan, Schroers, Nirenberg & Seifert, Stud. Mycol. 68: 101, 2011.
- Key to the Known Species of Fusicolla in China
| 1. Forming macroconidia and microconidia on PDA | 2 |
| 1. Only forming macroconidia on PDA | 6 |
| 2. Microconidia ellipiosoid, rod-shaped to falcate | 3 |
| 2. Microconidia subcylindrical, curved to C-shaped | 4 |
| 3. Producing pale orange-yellow pigment on PDA | F. coralloidea |
| 3. Producing purple pigment on PDA | F. violacea |
| 4. Aerial mycelium abundant on PDA | F. aeria |
| 4. Aerial mycelium absent to spare on PDA | 5 |
| 5. Colony on PDA light yellow to deep orange | F. matuoi |
| 5. Colony on PDA pinkish orange | F. gigas |
| 6. Macroconidia filiform | F. filiformis |
| 6. Macroconidia falcate | 7 |
| 7. Producing orange-yellow pigment on PDA | F. guangxiensis |
| 7. Producing pink pigment on PDA | F. aquaeductuum |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonorden, H.F. Handbuch der Allgemeinen Mykologie; Schweizerbart: Stuttgart, Germany, 1851; pp. 1–336. [Google Scholar]
- Gräfenhan, T.; Schroers, H.J.; Nirenberg, H.I.; Seifert, K.A. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud. Mycol. 2011, 68, 79–113. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef] [PubMed]
- Lechat, C.; Rossman, A.R. A new species of Fusicolla (Hypocreales), F. ossicola, from Belgium. Ascomycete.org 2017, 9, 225–228. [Google Scholar]
- Jones, E.B.G.; Devadatha, B.; Abdel-Wahab, M.A.; Dayarathne, M.C.; Zhang, S.N.; Hyde, K.D.; Liu, J.K.; Bahkali, A.H.; Sarma, V.V.; Tibell, S.; et al. Phylogeny of new marine Dothideomycetes and Sordariomycetes from mangroves and deep-sea sediments. Bot. Mar. 2019, 63, 155–181. [Google Scholar] [CrossRef]
- Dayarathne, M.C.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Devadatha, B.; Sarma, V.V.; Khongphinitbunjong, K.; Chomnunti, P.; Hyde, K.D. Morphomolecular characterization of microfungi associated with marine based habitats. Mycosphere 2020, 11, 1–188. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Jaeduk, G.; Hye, Y.M. Diversity of fungi in brackish water in Korea. Kor. J. Mycol. 2020, 48, 457–473. [Google Scholar]
- Singh, S.K.; Rana, S.; Bhat, J.D.; Singh, P.N. Morphology and phylogeny of a novel species of Fusicolla (Hypocreales, Nectriaceae), isolated from the air in the Western Ghats, India. J. Fungal Res. 2020, 4, 258–265. [Google Scholar]
- Liu, C.; Zhuang, W.Y.; Yu, Z.H.; Zeng, Z.Q. Two new species of Fusicolla (Hypocreales) from China. Phytotaxa 2022, 536, 165–174. [Google Scholar] [CrossRef]
- Crous, P.W.; Sandoval-Denis, M.; Costa, M.M.; Groenewald, J.Z.; van Iperen, A.L.; Starink-Willemse, M.; Hernández-Restrepo, M.; Kandemir, H.; Ulaszewski, B.; de Boer, W.; et al. Fusarium and allied fusarioid taxa (FUSA). 1. Fungal Syst. Evol. 2022, 9, 161–200. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, T.; Qu, Z.; Li, H.; Yang, Z. Contribution of filamentous fungi to the musty odorant 2,4,6-trichloroanisole in water supply reservoirs and associated drinking water treatment plants. Chemosphere 2017, 182, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Jeewon, R.; Hyde, K.D.; Bhat, D.; Wen, T.C. Novel taxa within Nectriaceae: Cosmosporella gen. nov. and Aquanectria sp. nov. from freshwater habitats in China. Cryptogam. Mycol. 2018, 39, 169–192. [Google Scholar] [CrossRef]
- Li, W.; Long, Y.; Mo, F.; Shu, R.; Yin, X.; Wu, X.; Zhang, R.; Zhang, Z.; He, L.; Chen, T.; et al. Antifungal activity and biocontrol mechanism of Fusicolla violacea J-1 against soft rot in Kiwifruit caused by Alternaria alternata. J. Fungi 2021, 7, 937. [Google Scholar] [CrossRef] [PubMed]
- Nirenberg, H.I. Studies on the morphologic and biologic differentiation in Fusarium section Liseola. Mitt. Biol. Bundesanst. Land-Forstw. 1976, 169, 1–117. [Google Scholar]
- Wang, L.; Zhuang, W.Y. Designing primer sets for amplification of partial calmodulin genes from penicillia. Mycosystema 2004, 23, 466–473. [Google Scholar]
- Nowrousian, M.; Kück, U.; Loser, K.; Weltring, K.M. The fungal acl1 and acl2 genes encode two polypeptides with homology to the N- and C-terminal parts of the animal ATP citrate lyase polypeptide. Curr. Genet. 2000, 37, 189–193. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfland, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analyzed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA Polymerase II Subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgin, D.G. The ClustalX windows interface: Flexible strategies for multiple sequences alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest v2, Program distributed by the author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004.
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Mol. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef]
- Page, R.D. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996, 12, 357–358. [Google Scholar] [PubMed]
- Hosoya, T.; Tubaki, K. Fusarium matuoi sp. nov. and its teleomorph Cosmospora matuoi sp. nov. Mycoscience 2004, 45, 261–270. [Google Scholar] [CrossRef]
- O’Donnell, K. Fusarium and its near relatives. In The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematic; Reynolds, D.R., Taylor, J.W., Eds.; CAB International: Wallingford, UK, 1993; pp. 224–233. [Google Scholar]
- Rossman, A.Y.; Samuels, G.J.; Rogerson, C.T.; Lowen, R. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Stud. Mycol. 1999, 42, 1–248. [Google Scholar]
- Summerbell, R.C.; Schroers, H.J. Analysis of phylogenetic relationship of Cylindrocarpon lichenicola and Acremonium falciforme to the Fusarium solani species complex and a review of similarities in the spectrum of opportunistic infections caused by these fungi. J. Clin. Microbiol. 2002, 40, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Schroers, H.J.; Gräfenhan, T.; Nirenberg, H.I.; Seifert, K.A. A revision of Cyanonectria and Geejayessia gen. nov., and related species with Fusarium-like anamorphs. Stud. Mycol. 2011, 68, 115–138. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, S.; Yanagisawa, M.; Katoh, A.; Fujii, T.; Sano, T.; Matsukuma, S.; Furumai, T.; Fujiu, M.; Watanabe, K.; Yokose, K.; et al. Fusarium merismoides CORDA NR 6356, the source of the protein kinase C inhibitor, azepinostatin. taxonomy, yield improvement, fermentation and biological activity. J. Antibiot. 1994, 47, 639–647. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, R.; Koss, M.; Ziegler, D.; De Respinis, S.; Petrini, O. Fungi in water samples of a full-scale water work. Mycol. Prog. 2018, 17, 467–478. [Google Scholar] [CrossRef]
- You, N.; Xu, J.; Wang, L.; Zhuo, L.; Zhou, J.; Song, Y.; Ali, A.; Luo, Y.; Yang, J.; Yang, W.; et al. Fecal fungi dysbiosis in nonalcoholic fatty liver disease. Obesity 2021, 29, 350–358. [Google Scholar] [CrossRef]
- Zhong, M.Y.; Xiong, Y.B.; Zhao, J.B.; Gao, Z.; Ma, J.S.; Wu, Z.X.; Song, Y.X.; Hong, X.H. Candida albicans disorder is associated with gastric carcinogenesis. Theranostics 2021, 11, 4945–4956. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Yu, D.; Gao, P.; Jiang, Q.; Yang, F. Dynamics and diversity of microbial community succession during fermentation of Suan yu, a Chinese traditional fermented fish, determined by high throughput sequencing. Food Res. Int. 2018, 111, 565–573. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Huang, Y.G. Structure and diversity analysis of mold community in main Maotai-flavor baijiu brewing areas of Maotai town using high-throughput sequencing. Food Sci. 2021, 42, 150–156. [Google Scholar]
- Clocchiatti, A.; Hannula, S.E.; van den Berg, M.; Hundscheid, M.P.J.; de Boer, W. Evaluation of phenolic root exudates as stimulants of saptrophic fungi in the rhizosphere. Front. Microbiol. 2021, 12, 644046. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, N.; Duan, B.; Wang, Q.; Wang, Y. Rhizosphere bacterial and fungal communities succession patterns related to growth of poplar fine roots. Sci. Total Environ. 2021, 756, 143839. [Google Scholar] [CrossRef]
- Hoch, H.C.; Abawi, G.S. Mycoparasitism of oospores of Pythium ultimum by Fusarium merismoides. Mycologia 1979, 71, 621–625. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Zhou, T.; Akkaya, M.S.; Wang, L.; Li, S.; Li, X. Biocontrol of Fusarium wilt disease in strawberries using bioorganic fertilizer fortified with Bacillus licheniformis X-1 and Bacillus methylotrophicus Z-1. 3 Biotech 2020, 10, 80. [Google Scholar] [CrossRef]
- Wang, M.; Xue, J.; Ma, J.; Feng, X.; Ying, H.; Xu, H. Streptomyces lydicus M01 regulates soil microbial community and alleviates foliar disease caused by Alternaria alternata on cucumbers. Front. Microbiol. 2020, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Forin, N.; Vizzini, A.; Nigris, S.; Ercole, E.; Voyron, S.; Girlanda, M.; Baldan, B. Illuminating type collections of nectriaceous fungi in Saccardo’s fungarium. Persoonia 2020, 45, 221–249. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.H.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Jones, E.B.G.; Mckenzie, E.H.C.; Stadler, M.; Lee, H.B.; Samarakoon, M.C.; Ekanayaka, A.H.; Camporesi, E.; et al. Fungi on wild seeds and fruits. Mycosphere 2020, 11, 2108–2480. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.; Crane, C.; Barrett, S.; Cano-Lira, J.F.; Le Roux, J.J.; Thangavel, R.; Guarro, J.; et al. Fungal Planet description sheets: 469–557. Persoonia 2016, 37, 218–403. [Google Scholar] [CrossRef]
- Crous, P.W.; Luangsa-ard, J.J.; Wingfield, M.J.; Carnegie, A.J.; Hernández-Restrepo, M.; Lombard, L.; Roux, J.; Barreto, R.W.; Baseia, I.G.; Cano-Lira, J.F.; et al. Fungal Planet description sheets: 785–867. Persoonia 2018, 41, 238–417. [Google Scholar] [CrossRef]
| Species | Herbarium/Strain Numbers | GenBank Accession Numbers | ||||
|---|---|---|---|---|---|---|
| acl1 | ITS | LSU | rpb2 | tub2 | ||
| F. acetilerea | BBA 63789 T | HQ897839 | HQ897790 | U88108 | HQ897701 | − |
| F. aeria | CGMCC 3.24908 T | OQ134105 a | OQ128334 a | OQ128338 a | OQ134111 a | OQ134100 a |
| CGMCC 3.24909 | OQ134106 a | OQ128335 a | OQ128339 a | OQ134112 a | OQ134101 a | |
| F. aquaeductuum | CBS 837.85 T | HQ897880 | KM231823 | KM231699 | HQ897744 | − |
| F. betae | BBA 64317 T | HQ897917 | − | − | HQ897781 | − |
| F. bharatavarshae | NFCCI 4423 T | − | MK152510 | MK152511 | MK157022 | MK376462 |
| F. cassiae-fistulae | MFLUCC 19-0318 T | − | NR171299 | NG073862 | − | − |
| F. coralloidea | CGMCC 3.24907 T | OQ134104 a | OQ128333 a | OQ128337 a | OQ134110 a | OQ134099 a |
| F. elongata | CBS 148934 T | ON759286 | ON763203 | ON763200 | ON759297 | ON745628 |
| F. epistroma | BBA 62201 T | HQ897901 | − | AF228352 | HQ897765 | − |
| F. filiformis | CGMCC 3.24910 T | OQ134103 a | OQ128332 a | OQ128336 a | OQ134109 a | OQ134098 a |
| F. gigantispora | MFLU 16-1206 T | − | MN047104 | MN017869 | − | − |
| F. gigas | CGMCC 3.20680 T | OQ134107 a | OK465362 | OK465449 | OQ134113 a | OQ134102 a |
| F. guangxiensis | CGMCC 3.20679 T | OQ134108 a | OK465363 | OK465450 | OQ134114 a | − |
| F. hughesii | NFCCI 4234 T | − | MG779450 | MG779452 | − | − |
| F. matuoi | CBS 581.78 T | HQ897858 | KM231822 | KM231698 | HQ897720 | KM232093 |
| F. melogrammae | CBS 141092 T | − | KX897140 | NG058275 | − | MW834305 |
| F. meniscoidea | CBS 110189 T | MW834043 | MW827613 | MW827654 | MW834010 | MW834306 |
| F. merismoides | CBS 186.34 T | − | MH855482 | MH866963 | − | − |
| F. ossicola | CBS 140161 T | − | NR161034 | MF628021 | MW834011 | MW834307 |
| F. quarantenae | URM 8367 T | − | MW553789 | MW553788 | MW556626 | MW556624 |
| F. septimanifiniscientiae | CBS 144935 T | − | MK069422 | MK069418 | − | MK069408 |
| F. siamensis | MFLUCC 172577 T | − | NR171300 | NG073863 | − | − |
| F. sporellula | CBS 110191 T | MW834044 | MW827614 | MW827655 | MW834012 | MW834308 |
| F. violacea | CBS 634.76 T | KM231059 | KM231824 | KM231700 | HQ897696 | KM232095 |
| Macroconia leptosphaeriae | CBS 100001 | HQ897891 | HQ897810 | KC291787 | HQ728164 | KM232097 |
| Microcera larvarum | CBS 738.79/AR 4580 | KM231060 | KM231825 | KM231701 | KM232387 | KC291935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.-Q.; Zhuang, W.-Y. Three New Species of Fusicolla (Hypocreales) from China. J. Fungi 2023, 9, 572. https://doi.org/10.3390/jof9050572
Zeng Z-Q, Zhuang W-Y. Three New Species of Fusicolla (Hypocreales) from China. Journal of Fungi. 2023; 9(5):572. https://doi.org/10.3390/jof9050572
Chicago/Turabian StyleZeng, Zhao-Qing, and Wen-Ying Zhuang. 2023. "Three New Species of Fusicolla (Hypocreales) from China" Journal of Fungi 9, no. 5: 572. https://doi.org/10.3390/jof9050572
APA StyleZeng, Z.-Q., & Zhuang, W.-Y. (2023). Three New Species of Fusicolla (Hypocreales) from China. Journal of Fungi, 9(5), 572. https://doi.org/10.3390/jof9050572







