Synchrospora gen. nov., a New Peronosporaceae Genus with Aerial Lifestyle from a Natural Cloud Forest in Panama
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolate Collection and Maintenance
2.2. DNA Extraction, Amplification and Sequencing
2.3. Phylogenetic Analyses
| Locus | Primer Names | Primer Sequences (5′-3′) | Orientation | Annealing Temperature (°C); Extension Time (s) | Reference for Primer Sequences |
|---|---|---|---|---|---|
| βtub 1,2 | TUBUF2 TUBUR1 | CGGTAACAACTGGGCCAAGG CCTGGTACTGCTGGTACTCAG | Forward Reverse | 68; 12 | [12] |
| Btub_F1A | GCCAAGTTCTGGGARGTSAT | Forward | 66; 15 | [109] | |
| Btub_R1A | CCTGGTACTGCTGGTAYTCMGA | Reverse | |||
| cox1 | OomCoxI-Levup 1OomCoxI-Levlo 1 | TCAWCWMGATGGCTTTTTTCAAC CYTCHGGRTGWCCRAAAAACCAAA | Forward Reverse | 60; 10 | [110] |
| COXF4N 3COXR4N 3 | GTATTTCTTCTTTATTAGGTGC CGTGAACTAATGTTACATATAC | Forward Reverse | 50; 65 | [12] | |
| cox2 | FM35 1,4 OomCoxI-Levlo | CAGAACCTTGGCAATTAGG CYTCHGGRTGWCCRAAAAACCAAA | Forward Reverse | 60; 20 | [110,111] |
| FM35_Oom2 3,4 OomCoxI-Levlo | SCNKWACCTTGGCAAWTRGG CYTCHGGRTGWCCRAAAAACCAAA | Forward Reverse | 50; 80 | [this study] [110] | |
| cox1 and cox2 3,4 | FM35 COXR4N OomCoxI-Levlo 5 FM83_Oom 5 FM80_RC 5 COX2-R 5 | CAGAACCTTGGCAATTAGG CGTGAACTAATGTTACATATAC CYTCHGGRTGWCCRAAAAACCAAA CHCCNATAAARAATAACCARAARTG TTTCAACAAATCATAAAGATAT CCATGATTAATACCACAAATTTCACTAC | Forward Reverse Reverse Reverse Forward Reverse | 50; 150 | [12,31,110,111,112,113] |
| ITS 1 | ITS1 ITS4 6 ITS6 6 | TCCGTAGGTGAACCTGCGG TCCTCCGCTTATTGATATGC GAAGGTGAAGTCGTAACAAGG | Forward Reverse Forward | 63–65; 12 | [10,114] |
| LSU 1,7 | CTB6 LR3 5 LR3R 5 LR7 | GCATATCAATAAGCGGAGG CCGTGTTTCAAGACGGG GTCTTGAAACACGGACC TACTACCACCAAGATCT | Forward Reverse Forward Reverse | 53; 20 | [115,116] |
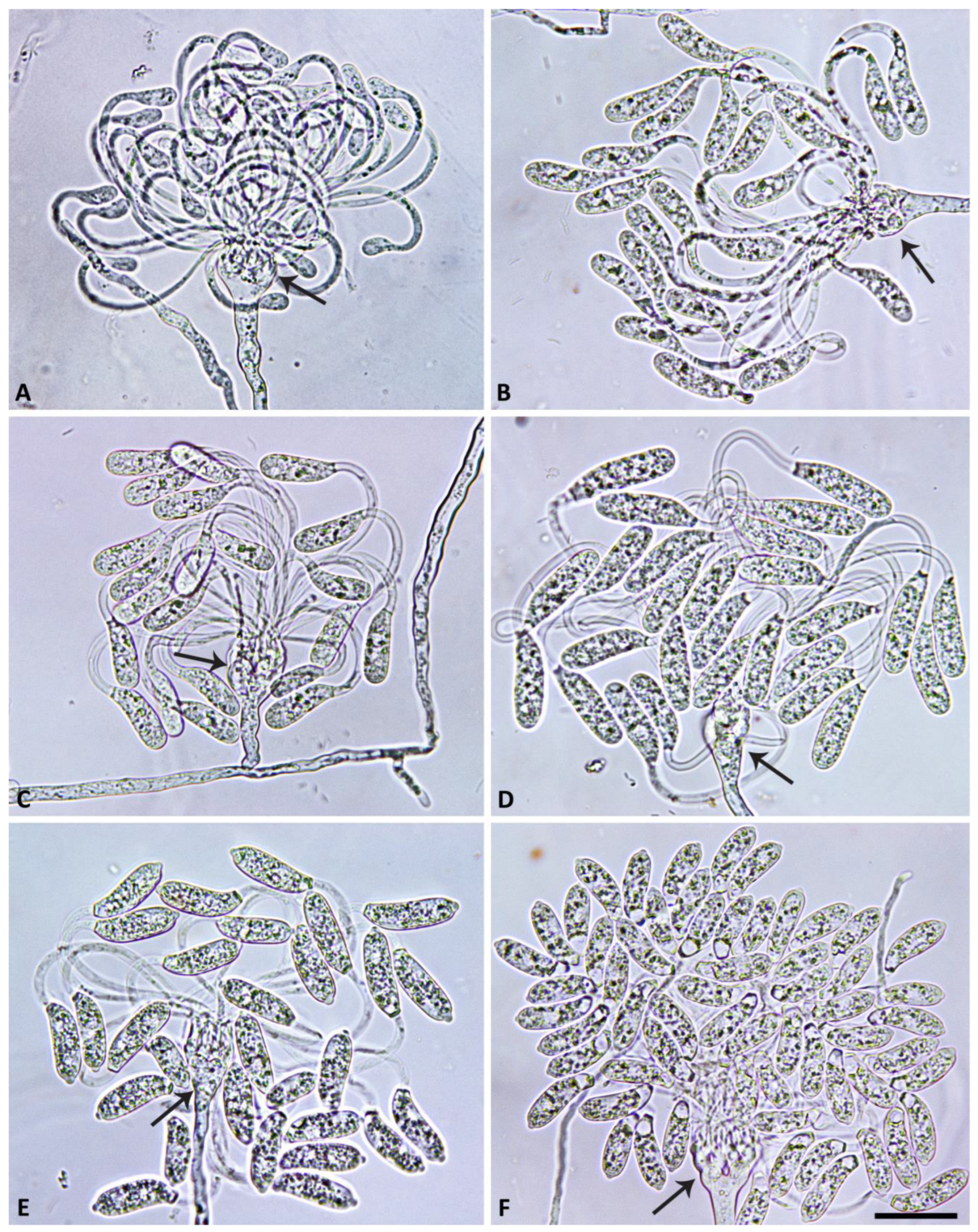
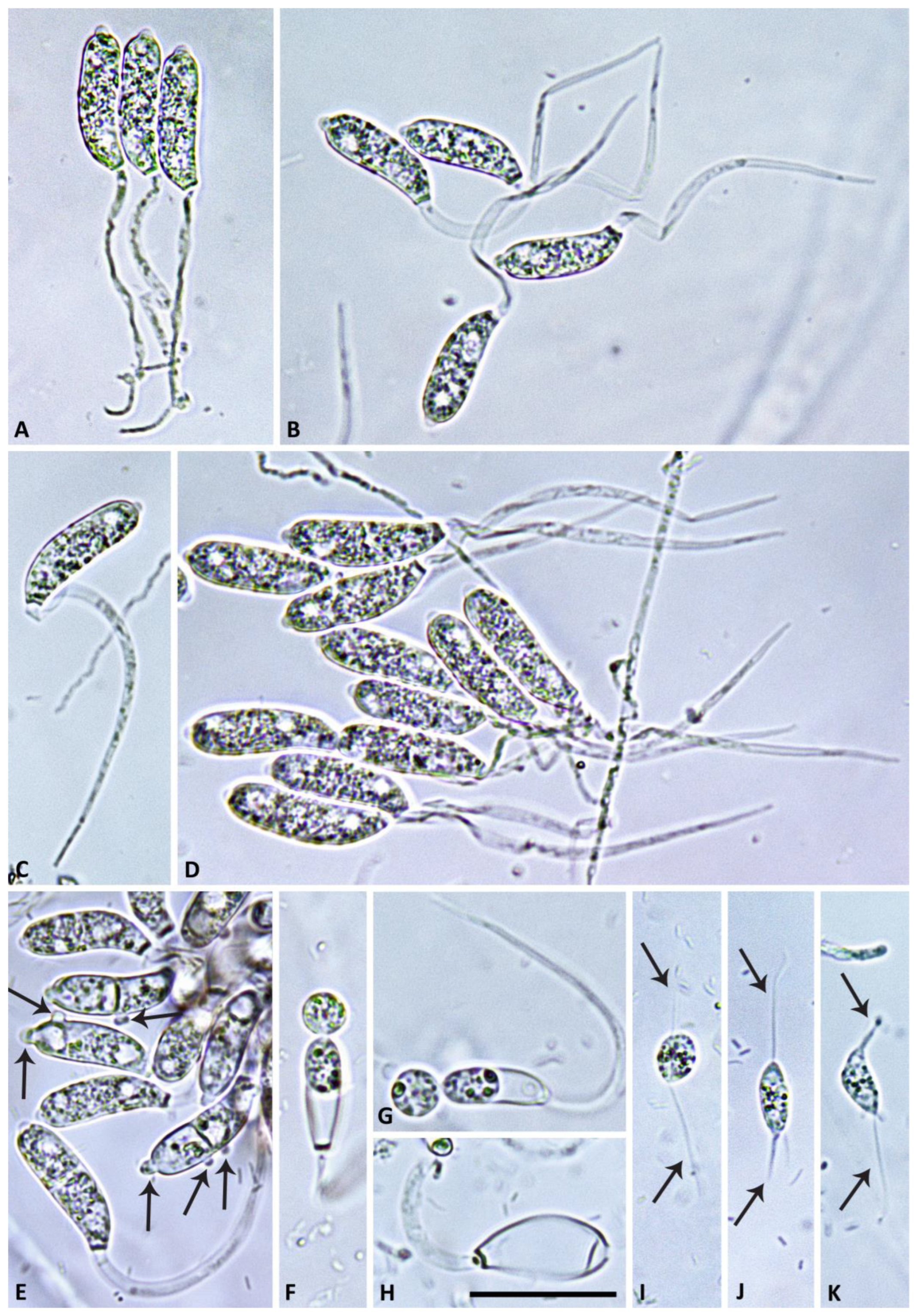
2.4. Morphology of Asexual and Sexual Structures
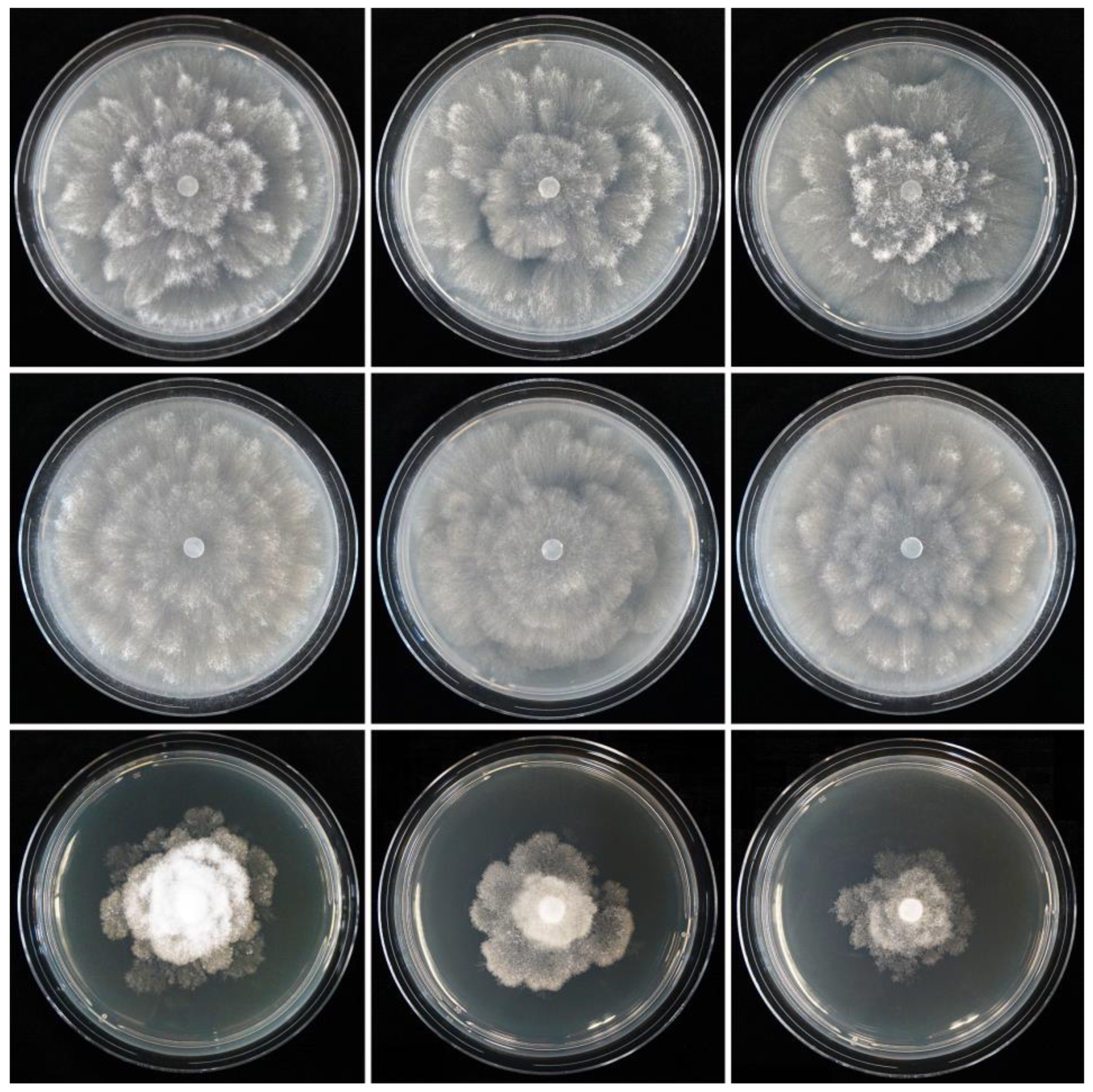
2.5. Colony Morphology, Growth Rates and Cardinal Temperatures
3. Results
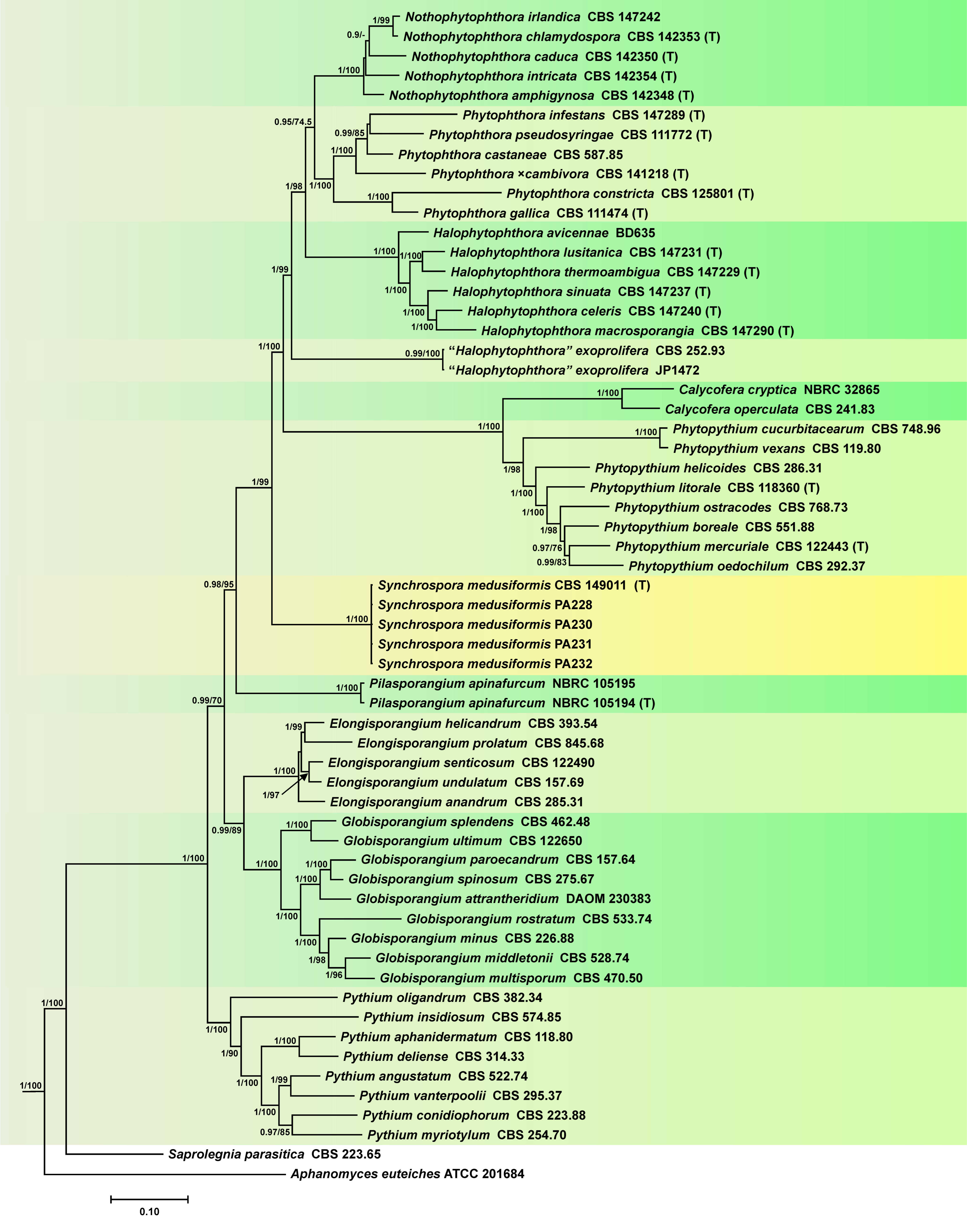
3.1. Phylogeny
3.2. Taxonomy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dick, M.W. Straminipilous fungi: Systematics of the Peronosporomycetes Including Accounts of the Marine Straminipilous Protists, the Plasmodiophorids and Similar Organisms; Kluwer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Hulvey, J.; Telle, S.; Nigrelli, L.; Lamour, K.; Thines, M. Salisapiliaceae—A new family of oomycetes from marsh grass litter of southeastern North America. Persoonia 2010, 25, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Beakes, G.W.; Honda, T.; Thines, M. Systematics of the Stramenipila: Labyrinthulomycota, Hyphochytridiomycota, and Oomycota. In Systematics and Evolution; McLaughlin, D.J., Spatafora, J., Eds.; Springer: New York, NY, USA, 2014; pp. 39–97. [Google Scholar]
- Thines, M.; Choi, Y.-J. Evolution, diversity and taxonomy of the Peronosporaceae, with focus on the genus Peronospora. Phytopathology 2016, 106, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.M.; de Cock, A.W.A.M.; Lévesque, A.; Thines, M. Calycofera gen. nov., an estuarine sister taxon to Phytopythium, Peronosporaceae. Mycol. Prog. 2017, 16, 947–954. [Google Scholar] [CrossRef]
- Jung, T.; Scanu, B.; Bakonyi, J.; Seress, D.; Kovács, G.M.; Durán, A.; Sanfuentes von Stowasser, E.; Schena, L.; Mosca, S.; Thu, P.Q.; et al. Nothophytophthora gen. nov., a new sister genus of Phytophthora from natural and semi-natural ecosystems. Persoonia 2017, 39, 143–174. [Google Scholar] [CrossRef]
- Bourret, T.B.; Choudhury, R.A.; Mehl, H.K.; Blomquist, C.L.; McRoberts, N.; Rizzo, D.M. Multiple origins of downy mildews and mito-nuclear discordance within the paraphyletic genus Phytophthora. PLoS ONE 2018, 13, e0192502. [Google Scholar] [CrossRef]
- Scanu, B.; Jung, T.; Masigol, H.; Linaldeddu, B.T.; Horta Jung, M.; Brandano, A.; Mostowfizadeh-Ghalamfarsa, R.; Janoušek, J.; Riolo, M.; Cacciola, S.O. Phytophthora heterospora sp. nov., a new pseudoconidia-producing sister species of P. palmivora. J. Fungi 2021, 7, 870. [Google Scholar] [CrossRef]
- Maia, C.; Horta Jung, M.; Carella, G.; Milenković, I.; Janoušek, J.; Tomšovský, M.; Mosca, S.; Schena, L.; Cravador, A.; Moricca, S.; et al. Eight new Halophytophthora species from marine and brackish-water ecosystems in Portugal and an updated phylogeny for the genus. Persoonia 2022, 48, 54–90. [Google Scholar] [CrossRef]
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef]
- Riethmüller, A.; Voglmayr, H.; Göker, M.; Weiß, M.; Oberwinkler, F. Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia 2002, 94, 834–849. [Google Scholar] [CrossRef]
- Kroon, L.P.N.M.; Bakker, F.T.; van den Bosch, G.B.M.; Bonants, P.J.M.; Flier, W.G. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet. Biol. 2004, 41, 766–782. [Google Scholar] [CrossRef]
- Göker, M.; Voglmayer, H.; Riethmüller, A.; Oberwinkler, F. How do obligate parasites evolve? A multi–gene phylogenetic analysis of downy mildews. Fungal Genet. Biol. 2007, 44, 105–122. [Google Scholar] [CrossRef]
- Runge, F.; Telle, S.; Ploch, S.; Savory, E.; Day, B.; Sharma, R.; Thines, M. The inclusion of downy mildews in a multi–locus–dataset and its reanalysis reveals a high degree of paraphyly in Phytophthora. IMA Fungus 2011, 2, 163–171. [Google Scholar] [CrossRef]
- Martin, F.N.; Blair, J.E.; Coffey, M.D. A combined mitochondrial and nuclear multilocus phylogeny of the genus Phytophthora. Fungal Genet. Biol. 2014, 66, 19–32. [Google Scholar] [CrossRef]
- Brasier, C.; Scanu, B.; Cooke, D.; Jung, T. Phytophthora: An ancient, historic, biologically and structurally cohesive and evolutionarily successful generic concept in need of preservation. IMA Fungus 2022, 13, 12. [Google Scholar] [CrossRef]
- Briard, M.; Dutertre, M.; Rouxel, F.; Brygoo, Y. Ribosomal RNA sequence divergence within the Pythiaceae. Mycol. Res. 1995, 99, 1119–1127. [Google Scholar] [CrossRef]
- de Cock, A.W.A.M.; Lévesque, C.A. New species of Pythium and Phytophthora. Stud. Mycol. 2004, 50, 481–487. [Google Scholar]
- Villa, N.O.; Kageyama, K.; Asano, T.; Suga, H. Phylogenetic relationships of Pythium and Phytophthora species based on ITS rDNA, cytochrome oxidase II and beta–tubulin gene sequences. Mycologia 2006, 98, 410–422. [Google Scholar] [PubMed]
- Bala, K.; Robideau, G.P.; Lévesque, C.A.; de Cock, A.W.A.M.; Abad, Z.G.; Lodhi, A.M.; Shahzad, S.; Ghaffar, A.; Coffey, M.D. Phytopythium Abad, de Cock, Bala, Robideau, Lodhi & Lévesque, gen. nov. and Phytopythium sindhum Lodhi, Shahzad & Lévesque, sp. nov. Fungal Planet 49. Persoonia 2010, 24, 136–137. [Google Scholar]
- Uzuhashi, S.; Tojo, M.; Kakishima, M. Phylogeny of the genus Pythium and description of new genera. Mycoscience 2010, 51, 337–365. [Google Scholar] [CrossRef]
- de Cock, A.W.A.M.; Lodhi, A.M.; Rintoul, T.L.; Bala, K.; Robideau, G.P.; Abad, Z.G.; Coffey, M.D.; Shahzad, S.; Lévesque, C.A. Phytopythium: Molecular phylogeny and systematics. Persoonia 2015, 34, 25–39. [Google Scholar] [CrossRef]
- Nguyen, H.D.T.; Dodge, A.; Dadej, K.; Rintoul, T.L.; Ponomareva, E.; Martin, F.N.; de Cock, A.W.A.M.; Lévesque, C.A.; Redhead, S.A.; Spies, C.F.J. Whole genome sequencing and phylogenomic analysis show support for the splitting of genus Pythium. Mycologia 2022, 114, 501–515. [Google Scholar] [CrossRef]
- Gäumann, E.A. The Fungi. A Description of Their Morphological Features and Evolutionary Development; Hafner Publishing: New York, NY, USA; London, UK, 1952. [Google Scholar]
- Newell, S.Y.; Fell, J.W. Do halophytophthoras (marine Pythiaceae) rapidly occupy fallen leaves by intraleaf mycelial growth? Can. J. Bot. 1995, 73, 761–765. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Leaño, E.M.; Jones, E.B.G.; Vrijmoed, L.L.P. Why are Halophytophthora species well adapted to mangrove habitats? Fungal Divers. 2000, 5, 131–151. [Google Scholar]
- Nakagiri, A. Ecology and biodiversity of Halophytophthora species. Fungal Divers. 2000, 5, 153–164. [Google Scholar]
- Brasier, C.M.; Cooke, D.E.L.; Duncan, J.M.; Hansen, E.M. Multiple new phenotypic taxa from trees and riparian ecosystems in Phytophthora gonapodyides–P. megasperma ITS Clade 6, which tend to be high-temperature tolerant and either inbreeding or sterile. Mycol. Res. 2003, 107, 277–290. [Google Scholar] [CrossRef]
- Jung, T.; Stukely, M.J.C.; Hardy, G.E.S.J.; White, D.; Paap, T.; Dunstan, W.A.; Burgess, T.I. Multiple new Phytophthora species from ITS Clade 6 associated with natural ecosystems in Australia: Evolutionary and ecological implications. Persoonia 2011, 26, 13–39. [Google Scholar] [CrossRef]
- Jung, T.; Milenković, I.; Corcobado, T.; Májek, T.; Janoušek, J.; Kudláček, T.; Tomšovský, M.; Nagy, Z.; Durán, A.; Tarigan, M.; et al. Extensive morphological and behavioural diversity among fourteen new and seven described species in Phytophthora Clade 10 and its evolutionary implications. Persoonia 2022, 49, 1–57. [Google Scholar] [CrossRef]
- Bennett, R.M.; Nam, B.; Dedeles, G.R.; Thines, M. Phytopythium leanoi sp. nov. and Phytopythium dogmae sp. nov., Phytopythium species associated with mangrove leaf litter from the Philippines. Acta Mycol. 2017, 52, 1103. [Google Scholar] [CrossRef]
- Jesus, A.L.; Marano, A.V.; Gonçalves, D.R.; Jerônimo, G.H.; Pires-Zottarelli, C.L.A. Two new species of Halophytophthora from Brazil. Mycol. Prog. 2019, 18, 1411–1421. [Google Scholar] [CrossRef]
- Chen, Q.; Bakhshi, M.; Balci, Y.; Broders, K.D.; Cheewangkoon, R.; Chen, S.F.; Fan, X.L.; Gramaje, D.; Halleen, F.; Horta Jung, M.; et al. Genera of phytopathogenic fungi: GOPHY 4. Stud. Mycol. 2022, 101, 417–564. [Google Scholar] [CrossRef]
- Beakes, G.W.; Glockling, S.L.; Sekimoto, S. The evolutionary phylogeny of the oomycete “fungi”. Protoplasma 2012, 249, 3–19. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, R.; Destefanis, M.; Milenković, I.; Tomšovský, M.; Janoušek, J.; Bellgard, S.E.; Weir, B.S.; Kudláček, T.; Horta Jung, M.; Jung, T. Two new Nothophytophthora species from streams in Ireland and Northern Ireland: Nothophytophthora irlandica and N. lirii sp. nov. PLoS ONE 2021, 16, e0250527. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.M.; Robredo, F.; Ferraz, J.F.P. Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathol. 1993, 42, 140–145. [Google Scholar] [CrossRef]
- Brasier, C.M.; Kirk, S.A.; Delcan, J.; Cooke, D.E.L.; Jung, T.; In’T Veld, W.A.M. Phytophthora alni sp. nov. and its variants: Designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycol. Res. 2004, 108, 1172–1184. [Google Scholar] [CrossRef]
- Jung, T.; Blaschke, H.; Neumann, P. Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. Eur. J. For. Pathol. 1996, 26, 253–272. [Google Scholar] [CrossRef]
- Jung, T.; Blaschke, H.; Osswald, W. Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathol. 2000, 49, 706–718. [Google Scholar] [CrossRef]
- Jung, T.; Vettraino, A.M.; Cech, T.L.; Vannini, A. The impact of invasive Phytophthora species on European forests. In Phytophthora: A Global Perspective; Lamour, K., Ed.; CABI: Wallingford, UK, 2013; pp. 146–158. [Google Scholar]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi–natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef]
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Horta Jung, M.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil- and airborne Phytophthora species in forests and woodlands. Persoonia 2018, 40, 182–220. [Google Scholar] [CrossRef]
- Jung, T.; Durán, A.; von Stowasser, E.S.; Schena, L.; Mosca, S.; Fajardo, S.; González, M.; Navarro Ortega, A.D.; Bakonyi, J.; Seress, D.; et al. Diversity of Phytophthora species in Valdivian rainforests and association with severe dieback symptoms. For. Pathol. 2018, 48, e12443. [Google Scholar] [CrossRef]
- Jung, T.; Scanu, B.; Brasier, C.M.; Webber, J.; Milenković, I.; Corcobado, T.; Tomšovský, T.; Pánek, M.; Bakonyi, J.; Maia, C.; et al. A survey in natural forest ecosystems of Vietnam reveals high diversity of both new and described Phytophthora taxa including P. ramorum. Forests 2020, 11, 93. [Google Scholar] [CrossRef]
- Hansen, E.M.; Goheen, D.J.; Jules, E.S.; Ullian, B. Managing Port–Orford–Cedar and the introduced pathogen Phytophthora lateralis. Plant Dis. 2000, 84, 4–14. [Google Scholar] [CrossRef]
- Hansen, E.M.; Reeser, P.W.; Sutton, W. Phytophthora beyond agriculture. Annu. Rev. Phytopathol. 2012, 50, 359–378. [Google Scholar] [CrossRef]
- Rizzo, D.M.; Garbelotto, M.; Davidson, J.M.; Slaughter, G.W.; Koike, S.T. Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Dis. 2002, 86, 205–214. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Barzanti, G.P.; Bianco, M.C.; Ragazzi, A.; Capretti, P.; Paoletti, E.; Luisi, N.; Anselmi, N.; Vannini, A. Occurrence of Phytophthora species in oak stands in Italy and their association with declining oak trees. For. Pathol. 2002, 32, 19–28. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Morel, O.; Perlerou, C.; Robin, C.; Diamandis, S.; Vannini, A. Occurrence and distribution of Phytophthora species associated with Ink Disease of chestnut in Europe. Eur. J. Plant Pathol. 2005, 111, 169–180. [Google Scholar] [CrossRef]
- Balci, Y.; Halmschlager, E. Incidence of Phytophthora species in oak forests in Austria and their possible involvement in oak decline. For. Pathol. 2003, 33, 157–174. [Google Scholar] [CrossRef]
- Balci, Y.; Halmschlager, E. Phytophthora species in oak ecosystems in Turkey and their association with declining oak trees. Plant Pathol. 2003, 52, 694–702. [Google Scholar] [CrossRef]
- Jönsson, U.; Lundberg, L.; Sonesson, K.; Jung, T. First records of soilborne Phytophthora species in Swedish oak forests. For. Pathol. 2003, 33, 175–179. [Google Scholar] [CrossRef]
- Jung, T.; Blaschke, M. Phytophthora root and collar rot of alders in Bavaria: Distribution, modes of spread and possible management strategies. Plant Pathol. 2004, 53, 197–208. [Google Scholar] [CrossRef]
- Hardham, A.R. Phytophthora cinnamomi. Mol. Plant Pathol. 2005, 6, 589–604. [Google Scholar] [CrossRef]
- Balci, Y.; Balci, S.; Eggers, J.; MacDonald, W.L.; Juzwik, J.; Long, R.P.; Gottschalk, K.W. Phytophthora spp. associated with forest soils in Eastern and North–Central U.S. oak ecosystems. Plant Dis. 2007, 91, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Balci, Y.; Long, R.P.; Mansfield, M.; Balser, D.; MacDonald, W.L. Involvement of Phytophthora species in white oak (Quercus alba) decline in southern Ohio. For. Pathol. 2010, 40, 430–442. [Google Scholar] [CrossRef]
- Greslebin, A.; Hansen, E.M.; Sutton, W. Phytophthora austrocedrae sp. nov., a new species associated with Austrocedrus chilensis mortality in Patagonia (Argentina). Mycol. Res. 2007, 111, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.; Gryzenhout, M.; Slippers, B.; Ahumada, R.; Rotella, A.; Flores, F.; Wingfield, B.D.; Wingfield, M.J. Phytophthora pinifolia sp. nov. associated with a serious needle disease of Pinus radiata in Chile. Plant Pathol. 2008, 57, 715–727. [Google Scholar] [CrossRef]
- Jung, T. Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. For. Pathol. 2009, 39, 73–94. [Google Scholar] [CrossRef]
- Jung, T.; Burgess, T.I. Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species, Phytophthora plurivora sp. nov. Persoonia 2009, 22, 95–110. [Google Scholar] [CrossRef]
- Brasier, C.; Webber, J. Sudden larch death. Nature 2010, 466, 824–825. [Google Scholar] [CrossRef]
- Green, S.; Brasier, C.M.; Schlenzig, A.; McCracken, A.; MacAskill, G.A.; Wilson, M.; Webber, J.F. The destructive invasive pathogen Phytophthora lateralis found on Chamaecyparis lawsoniana across the UK. For. Pathol. 2013, 43, 19–28. [Google Scholar]
- Green, S.; Elliot, M.; Armstrong, A.; Hendry, S.J. Phytophthora austrocedrae emerges as a serious threat to juniper (Juniperus communis) in Britain. Plant Pathol. 2015, 64, 456–466. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; López-García, C.; León, M.; García-Jiménez, J.; Abad-Campos, P.; Jung, T. Previously unrecorded low temperature Phytophthora species associated with Quercus decline in a Mediterranean forest in Eastern Spain. For. Pathol. 2013, 43, 331–339. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; Chitty, R.; Eacock, A.; Jones, B.; Biddle, M.; Crampton, M.; Lewis, A.; Olivieri, L.; Webber, J.F. First report of Phytophthora pluvialis in Europe causing resinous cankers on western hemlock. New Dis. Rep. 2022, 45, e12064. [Google Scholar] [CrossRef]
- Ginetti, B.; Moricca, S.; Squires, J.N.; Cooke, D.E.L.; Ragazzi, A.; Jung, T. Phytophthora acerina sp. nov., a new species causing bleeding cankers and dieback of Acer pseudoplatanus trees in planted forests in Northern Italy. Plant Pathol. 2014, 63, 858–876. [Google Scholar] [CrossRef]
- Scanu, B.; Linaldeddu, B.T.; Deidda, A.; Jung, T. Diversity of Phytophthora species from declining Mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE 2015, 10, e0143234. [Google Scholar] [CrossRef]
- Scanu, B.; Webber, J.F. Dieback and mortality of Nothofagus in Britain: Ecology, pathogenicity and sporulation potential of the causal agent Phytophthora pseudosyringae. Plant Pathol. 2016, 65, 26–36. [Google Scholar] [CrossRef]
- Milenković, I.; Keča, N.; Karadžić, D.; Radulović, Z.; Nowakowska, J.A.; Oszako, T.; Sikora, K.; Corcobado, T.; Jung, T. Isolation and pathogenicity of Phytophthora species from poplar plantations in Serbia. Forests 2018, 9, 330. [Google Scholar] [CrossRef]
- Corcobado, T.; Cech, T.L.; Brandstetter, M.; Daxer, A.; Hüttler, C.; Kudláček, T.; Horta Jung, M.; Jung, T. Decline of European beech in Austria: Involvement of Phytophthora spp. and contributing biotic and abiotic factors. Forests 2020, 11, 895. [Google Scholar] [CrossRef]
- Jung, T.; Hansen, E.M.; Winton, L.; Oßwald, W.; Delatour, C. Three new species of Phytophthora from European oak forests. Mycol. Res. 2002, 106, 397–411. [Google Scholar] [CrossRef]
- Jung, T.; Chang, T.T.; Bakonyi, J.; Seress, D.; Pérez-Sierra, A.; Yang, X.; Hong, C.; Scanu, B.; Fu, C.H.; Hsueh, K.-L.; et al. Diversity of Phytophthora species in natural ecosystems of Taiwan and association with disease symptoms. Plant Pathol. 2017, 66, 194–211. [Google Scholar] [CrossRef]
- Jung, T.; Horta Jung, M.; Scanu, B.; Seress, D.; Kovács, D.M.; Maia, C.; Pérez-Sierra, A.; Chang, T.-T.; Chandelier, A.; Heungens, A.; et al. Six new Phytophthora species from ITS Clade 7a including two sexually functional heterothallic hybrid species detected in natural ecosystems in Taiwan. Persoonia 2017, 38, 100–135. [Google Scholar] [CrossRef]
- Jung, T.; Horta Jung, M.; Cacciola, S.O.; Cech, T.; Bakonyi, J.; Seress, D.; Mosca, S.; Schena, L.; Seddaiu, S.; Pane, A.; et al. Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy and Portugal. IMA Fungus 2017, 8, 219–244. [Google Scholar] [CrossRef]
- Jung, T.; La Spada, F.; Pane, A.; Aloi, F.; Evoli, M.; Horta Jung, M.; Scanu, B.; Faedda, R.; Rizza, C.; Puglisi, I.; et al. Diversity and distribution of Phytophthora species in protected natural areas in Sicily. Forests 2019, 10, 259. [Google Scholar] [CrossRef]
- Zeng, H.-C.; Ho, H.-H.; Zheng, F.-C. A survey of Phytophthora species on Hainan Island of South China. J. Phytopathol. 2009, 157, 33–39. [Google Scholar] [CrossRef]
- Brasier, C.M.; Vettraino, A.M.; Chang, T.T.; Vannini, A. Phytophthora lateralis discovered in an old growth Chamaecyparis forest in Taiwan. Plant Pathol. 2010, 59, 595–603. [Google Scholar] [CrossRef]
- Rea, A.J.; Burgess, T.I.; Hardy, G.E.S.J.; Stukely, M.J.C.; Jung, T. Two novel and potentially endemic species of Phytophthora associated with episodic dieback of kwongan vegetation in the south–west of Western Australia. Plant Pathol. 2011, 60, 1055–1068. [Google Scholar] [CrossRef]
- Reeser, P.W.; Sutton, W.; Hansen, E.M.; Remigi, P.; Adams, G.C. Phytophthora species in forest streams in Oregon and Alaska. Mycologia 2011, 103, 22–35. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Brasier, C.M.; Brown, A.V.; Vannini, A. Phytophthora himalsilva sp. nov. an unusually phenotypically variable species from a remote forest in Nepal. Fungal Biol. 2011, 115, 275–287. [Google Scholar] [CrossRef]
- Huai, W.X.; Tian, G.; Hansen, E.M.; Zhao, W.-X.; Goheen, E.M.; Grünwald, N.J.; Cheng, C. Identification of Phytophthora species baited and isolated from forest soil and streams in northwestern Yunnan province, China. For. Pathol. 2013, 43, 87–103. [Google Scholar] [CrossRef]
- Huberli, D.; Hardy, G.E.S.J.; White, D.; Williams, N.; Burgess, T.I. Fishing for Phytophthora from Western Australia’ s waterways: A distribution and diversity survey. Australas. Plant Pathol. 2013, 42, 251–260. [Google Scholar] [CrossRef]
- Oh, E.; Gryzenhout, M.; Wingfield, B.D.; Wingfield, M.J.; Burgess, T.I. Surveys of soil and water reveal a goldmine of Phytophthora diversity in South African natural ecosystems. IMA Fungus 2013, 4, 123–131. [Google Scholar] [CrossRef]
- Shrestha, S.K.; Zhou, Y.; Lamour, K. Oomycetes baited from streams in Tennessee 2010–2012. Mycologia 2013, 105, 1516–1523. [Google Scholar] [CrossRef]
- Català, S.; Peréz–Sierra, A.; Abad-Campos, P. The use of genus–specific amplicon pyrosequencing to assess Phytophthora species diversity using eDNA from soil and water in Northern Spain. PLoS ONE 2015, 10, e0119311. [Google Scholar] [CrossRef]
- Brazee, N.J.; Wick, R.L.; Hulvey, J.P. Phytophthora species recovered from the Connecticut River Valley in Massachusetts, USA. Mycologia 2016, 108, 6–19. [Google Scholar] [CrossRef]
- Dunstan, W.A.; Howard, K.; Hardy, G.E.S.J.; Burgess, T.I. An overview of Australia’s Phytophthora species assemblage in natural ecosystems recovered from a survey in Victoria. IMA Fungus 2016, 7, 47–58. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Choiseul, J.; Corrigan, M.; Catarame, T.; Destefanis, M. Diversity and detections of Phytophthora species from trade and nontrade environments in Ireland. Bull. OEPP 2016, 46, 594–602. [Google Scholar] [CrossRef]
- Burgess, T.I.; White, D.; McDougall, K.M.; Garnas, J.; Dunstan, W.A.; Català, S.; Carnegie, A.J.; Worboys, S.; Cahill, D.; Vettraino, A.-M.; et al. Distribution and diversity of Phytophthora across Australia. Pac. Conserv. Biol. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Burgess, T.I.; Simamora, A.V.; White, D.; Williams, B.; Schwager, M.; Stukely, M.J.C.; Hardy, G.E.S.J. New species from Phytophthora Clade 6a: Evidence for recent radiation. Persoonia 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Burgess, T.I.; Dang, Q.N.; Le, B.V.; Pham, N.Q.; White, D.; Pham, T.Q. Phytophthora acaciivora sp. nov. associated with dying Acacia mangium in Vietnam. FUSE 2020, 6, 243–252. [Google Scholar] [CrossRef]
- Bose, T.; Hulbert, J.M.; Burgess, T.I.; Paap, T.; Roets, F.; Wingfield, M.J. Two novel Phytophthora species from the southern tip of Africa. Mycol. Prog. 2021, 20, 755–767. [Google Scholar] [CrossRef]
- Dang, Q.N.; Pham, T.Q.; Arentz, F.; Hardy, G.E.S.J.; Burgess, T.I. New Phytophthora species in clade 2a from the Asia-Pacific region including a re-examination of P. colocasiae and P. meadii. Mycol. Prog. 2021, 20, 111–129. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.; Wright, A.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Pattengale, N.; Alipour, M.; Bininda-Emonds, O.; Moret, B.; Stamatakis, A. How many bootstrap replicates are necessary? J. Comput. Biol. 2010, 17, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, F.; Domelevo Entfellner, J.; Wilkinson, E.; Correia, D.; Dávila Felipe, M.; De Oliveira, T.; Gascuel, O. Renewing Felsenstein’s phylogenetic bootstrap in the era of big data. Nature 2018, 556, 452–456. [Google Scholar] [CrossRef]
- Sukumaran, J.; Holder, M.T. DendroPy: A Python library for phylogenetic computing. Bioinformatics 2010, 26, 1569–1571. [Google Scholar] [CrossRef]
- Müller, N.; Bouckaert, R. Adaptive parallel tempering for BEAST 2. bioRxiv 2019, 603514. [Google Scholar] [CrossRef]
- Kone, A.; Kofke, D.A. Selection of temperature intervals for parallel-tempering simulations. J. Chem. Phys. 2005, 122, 1–2. [Google Scholar] [CrossRef]
- Atchadé, Y.F.; Roberts, G.O.; Rosenthal, J.S. Towards optimal scaling of metropolis-coupled Markov chain Monte Carlo. Stat. Comput. 2011, 21, 555–568. [Google Scholar] [CrossRef]
- Bouckaert, R.; Drummond, A. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017, 17, 42. [Google Scholar] [CrossRef]
- Drummond, A.J.; Ho, S.Y.W.; Phillips, M.J.; Rambaut, A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006, 4, 699–710. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.; Xie, D.; Baele, G.; Suchard, M. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Stöver, B.C.; Müller, K.F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform. 2010, 11, 7. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Blair, J.E.; Coffey, M.D.; Park, S.-Y.; Greiser, D.M.; Kang, S. A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genet. Biol. 2008, 45, 266–277. [Google Scholar] [CrossRef]
- Robideau, G.P.; de Cock, A.W.A.M.; Coffey, M.D.; Voglmayr, H.; Brouwer, H.; Bala, K.; Chitty, D.W.; Désaulniers, N.; Eggertson, Q.A.; Gachon, C.M.M.; et al. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Mol. Ecol. Resour. 2011, 11, 1002–1011. [Google Scholar] [CrossRef]
- Martin, F.N. Phylogenetic relationships among some Pythium species inferred from sequence analysis of the mitochondrially encoded cytochrome oxidase II gene. Mycologia 2000, 92, 711–727. [Google Scholar] [CrossRef]
- Martin, F.N.; Tooley, P.W. Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia 2003, 95, 269–284. [Google Scholar] [CrossRef]
- Hudspeth, D.S.S.; Nadler, S.A.; Hudspeth, M.E.S. A COX2 molecular phylogeny of the Peronosporomycetes. Mycologia 2000, 92, 674–684. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Garbelotto, M.M.; Lee, H.K.; Slaughter, G.; Popenuck, T.; Cobb, F.W.; Brunset, T.D. Heterokaryosis is not required for virulence of Heterobasidion annosum. Mycologia 1997, 89, 92–102. [Google Scholar] [CrossRef]
- Hopple, J.S.; Vilgalys, R. Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia 1994, 86, 96–107. [Google Scholar] [CrossRef]
- Dick, M.W. Keys to Pythium; University of Reading Press: Reading, UK, 1990. [Google Scholar]
- Jung, T.; Cooke, D.E.L.; Blaschke, H.; Duncan, J.M.; Oßwald, W. Phytophthora quercina sp. nov., causing root rot of European oaks. Mycol. Res. 1999, 103, 785–798. [Google Scholar] [CrossRef]
- Thines, M. Bridging the Gulf: Phytophthora and downy mildews are connected by rare grass parasites. PLoS ONE 2009, 4, e4790. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.W.; Dick, M.W. Monograph of Basidiophora (Oomycetes) with the description of a new species. Bot. J. Linn. Soc. 1991, 107, 313–332. [Google Scholar] [CrossRef]
- Telle, S.; Thines, M. Reclassification of an enigmatic downy mildew species on lovegrass (Eragrostis) to the new genus Eraphthora, with a key to the genera of the Peronosporaceae. Mycol. Prog. 2012, 11, 121–129. [Google Scholar] [CrossRef]
- Thines, M.; Telle, S.; Choi, Y.-J.; Tan, Y.P.; Shivas, R.G. Baobabopsis, a new genus of graminicolous downy mildews from tropical Australia, with an updated key to the genera of downy mildews. IMA Fungus 2015, 6, 483–491. [Google Scholar] [CrossRef]
- Crouch, J.A.; Davis, W.J.; Shishkoff, N.; Castroagudín, V.L.; Martin, F.; Michelmore, R.; Thines, M. Peronosporaceae species causing downy mildew diseases of Poaceae, including nomenclature revisions and diagnostic resources. FUSE 2022, 9, 43–86. [Google Scholar] [CrossRef]
- Baxter, L.; Tripathy, S.; Ishaque, L.; Boot, N.; Cabral, A.; Kemen, E.; Thines, M.; Ah-Fong, A.; Anderson, R.; Badejoko, W.; et al. Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsis genome. Science 2010, 330, 1549–1551. [Google Scholar] [CrossRef]
- Falloon, R.E.; Sutherland, P.W. Peronospora viciae on Pisum sativum: Morphology of asexual and sexual reproductive structures. Mycologia 1996, 88, 473–483. [Google Scholar] [CrossRef]
- Nordskog, B.; Gadoury, D.M.; Seem, R.C.; Hermansen, A. Impact of diurnal periodicity, temperature, and light on sporulation of Bremia lactucae. Phytopathology 2007, 97, 979–986. [Google Scholar] [CrossRef]
- Kandel, S.L.; Mou, B.; Shishkoff, N.; Shi, A.; Subbarao, K.V.; Klosterman, S.J. Spinach downy mildew: Advances in our understanding of the disease cycle and prospects for disease management. Plant Dis. 2019, 103, 791–803. [Google Scholar] [CrossRef]
- Brown, A.V.; Brasier, C.M. Colonization of tree xylem by Phytophthora ramorum, P. kernoviae and other Phytophthora species. Plant Pathol. 2007, 56, 227–241. [Google Scholar] [CrossRef]
- Dick, M.A.; Williams, N.M.; Bader, M.K.-F.; Gardner, J.F.; Bulman, L.S. Pathogenicity of Phytophthora pluvialis to Pinus radiata and its relation with red needle cast disease in New Zealand. N. Z. J. For. Sci. 2014, 44, 6. [Google Scholar] [CrossRef]
- Scanu, B.; Linaldeddu, B.T.; Peréz–Sierra, A.; Deidda, A.; Franceschini, A. Phytophthora ilicis as a leaf and stem pathogen of Ilex aquifolium in Mediterranean islands. Phytopathol. Mediterr. 2014, 53, 480–490. [Google Scholar]
- Sanfuentes, E.; Fajardo, S.; Sabag, M.; Hansen, E.; González, M. Phytophthora kernoviae isolated from fallen leaves of Drymis winteri in native forest of southern Chile. Australas. Plant Dis. Notes 2016, 11, 19. [Google Scholar] [CrossRef]
- Jung, T.; Horta Jung, M.; Webber, J.F.; Kageyama, K.; Hieno, A.; Masuya, H.; Uematsu, S.; Pérez-Sierra, A.; Harris, A.R.; Forster, J.; et al. The destructive tree pathogen Phytophthora ramorum originates from the Laurosilva forests of East Asia. J. Fungi 2021, 7, 226. [Google Scholar] [CrossRef]
- Brasier, C.M.; Griffin, M.J. Taxonomy of Phytophthora palmivora on cocoa. Trans. Br. Mycol. Soc. 1979, 71, 111–143. [Google Scholar] [CrossRef]
- Rajalakshmy, V.K.; Joseph, A.; Arthassery, S. Occurrence of two mating groups in Phytophthora meadii causing abnormal leaf fall disease of rubber in South India. Trans. Br. Mycol. Soc. 1985, 85, 723–725. [Google Scholar] [CrossRef]
- Drenth, A.; Guest, D.I. Diversity and Management of Phytophthora in Southeast Asia; Australian Centre for International Agricultural Research: Canberra, Australia, 2004. [Google Scholar]
- Cerqueira, A.O.; Luz, E.D.M.N.; De Souza, J.T. First record of Phytophthora tropicalis causing leaf blight and fruit rot on breadfruit in Brazil. Plant Pathol. 2006, 55, 296. [Google Scholar] [CrossRef]
- Guest, D.I. Black pod: Diverse pathogens with a global impact on cocoa yield. Phytopathology 2007, 97, 1650–1653. [Google Scholar] [CrossRef]
- Akrofi, A.Y.; Amoako-Atta, I.; Assuah, M.; Asare, E.K. Black pod disease on cacao (Theobroma cacao, L) in Ghana: Spread of Phytophthora megakarya and role of economic plants in the disease epidemiology. Crop Prot. 2015, 72, 66–75. [Google Scholar] [CrossRef]
- Tri, M.V.; Van Hoa, N.; Minh Chau, N.; Pane, A.; Faedda, R.; De Patrizio, A.; Schena, L.; Olsson, C.H.B.; Wright, S.A.I.; Ramstedt, M.; et al. Decline of jackfruit (Artocarpus heterophyllus) incited by Phytophthora palmivora in Vietnam. Phytopathol. Mediterr. 2015, 54, 275–280. [Google Scholar]
- Puglisi, I.; De Patrizio, A.; Schena, L.; Jung, T.; Evoli, M.; Pane, A.; Hoa, N.V.; Tri, M.V.; Wright, S.; Ramstedt, M.; et al. Two previously unknown Phytophthora species associated with brown rot of Pomelo (Citrus grandis) fruits in Vietnam. PLoS ONE 2017, 12, e0172085. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Ramírez, B.; Rodríguez-Velázquez, N.D.; Chávez-Sánchez, M.E.; Vásquez-Murrieta, M.S.; Hernández-Gallegos, M.A.; Velázquez-Martínez, J.R.; Avendaño-Arrazate, C.H.; Estrada-de los Santos, P. Morphological and molecular identification of Phytophthora tropicalis causing black pod rot in Mexico. Can. J. Plant Pathol. 2021, 43, 670–679. [Google Scholar] [CrossRef]
- Patil, B.; Hedge, V.; Sridhara, S.; Pandian, R.T.P.; Thube, S.H.; Palliath, G.K.; Gangurde, S.S.; Jha, P.K. Multigene phylogeny and haplotype analysis reveals predominance of oomycetous fungus, Phytophthora meadii (McRae) associated with fruit rot disease of arecanut in India. Saudi J. Biol. Sci. 2019, 29, 103341. [Google Scholar] [CrossRef]




| Species | Isolate Codes 1; Status 2 | Host/Habitat | Location; Year; Collectors |
|---|---|---|---|
| Synchrospora medusiformis | CBS 149011 = PA229; T | Fallen leaf, tropical cloud forest | Panama, Volcano Baru; 2019; K.D. Broders andY. Balci |
| S. medusiformis | PA228 | Fallen leaf, tropical cloud forest | Panama, Volcano Baru; 2019; K.D. Broders and Y. Balci |
| S. medusiformis | PA230 | Fallen leaf, tropical cloud forest | Panama, Volcano Baru; 2019; K.D. Broders and Y. Balci |
| S. medusiformis | PA231 | Fallen leaf, tropical cloud forest | Panama, Volcano Baru; 2019; K.D. Broders and Y. Balci |
| S. medusiformis | PA232 | Fallen leaf, tropical cloud forest | Panama, Volcano Baru; 2019; K.D. Broders and Y. Balci |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, T.; Balci, Y.; Broders, K.D.; Milenković, I.; Janoušek, J.; Kudláček, T.; Đorđević, B.; Horta Jung, M. Synchrospora gen. nov., a New Peronosporaceae Genus with Aerial Lifestyle from a Natural Cloud Forest in Panama. J. Fungi 2023, 9, 517. https://doi.org/10.3390/jof9050517
Jung T, Balci Y, Broders KD, Milenković I, Janoušek J, Kudláček T, Đorđević B, Horta Jung M. Synchrospora gen. nov., a New Peronosporaceae Genus with Aerial Lifestyle from a Natural Cloud Forest in Panama. Journal of Fungi. 2023; 9(5):517. https://doi.org/10.3390/jof9050517
Chicago/Turabian StyleJung, Thomas, Yilmaz Balci, Kirk D. Broders, Ivan Milenković, Josef Janoušek, Tomáš Kudláček, Biljana Đorđević, and Marilia Horta Jung. 2023. "Synchrospora gen. nov., a New Peronosporaceae Genus with Aerial Lifestyle from a Natural Cloud Forest in Panama" Journal of Fungi 9, no. 5: 517. https://doi.org/10.3390/jof9050517
APA StyleJung, T., Balci, Y., Broders, K. D., Milenković, I., Janoušek, J., Kudláček, T., Đorđević, B., & Horta Jung, M. (2023). Synchrospora gen. nov., a New Peronosporaceae Genus with Aerial Lifestyle from a Natural Cloud Forest in Panama. Journal of Fungi, 9(5), 517. https://doi.org/10.3390/jof9050517







