Global Change Factors Influence Plant-Epichloë Associations
Abstract
1. Introduction
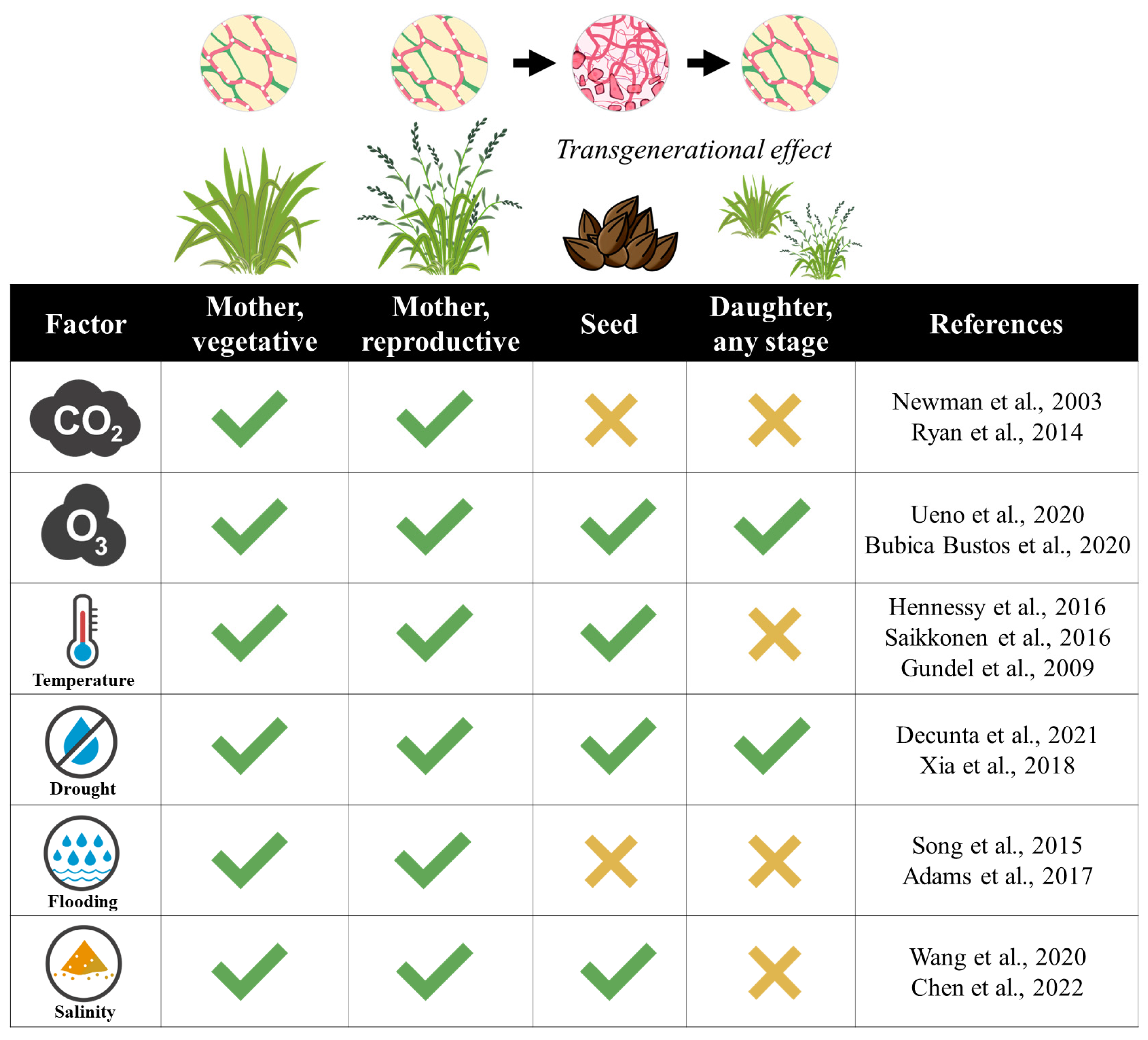
2. Effects of Global Change Factors on Plant–Epichloë Associations
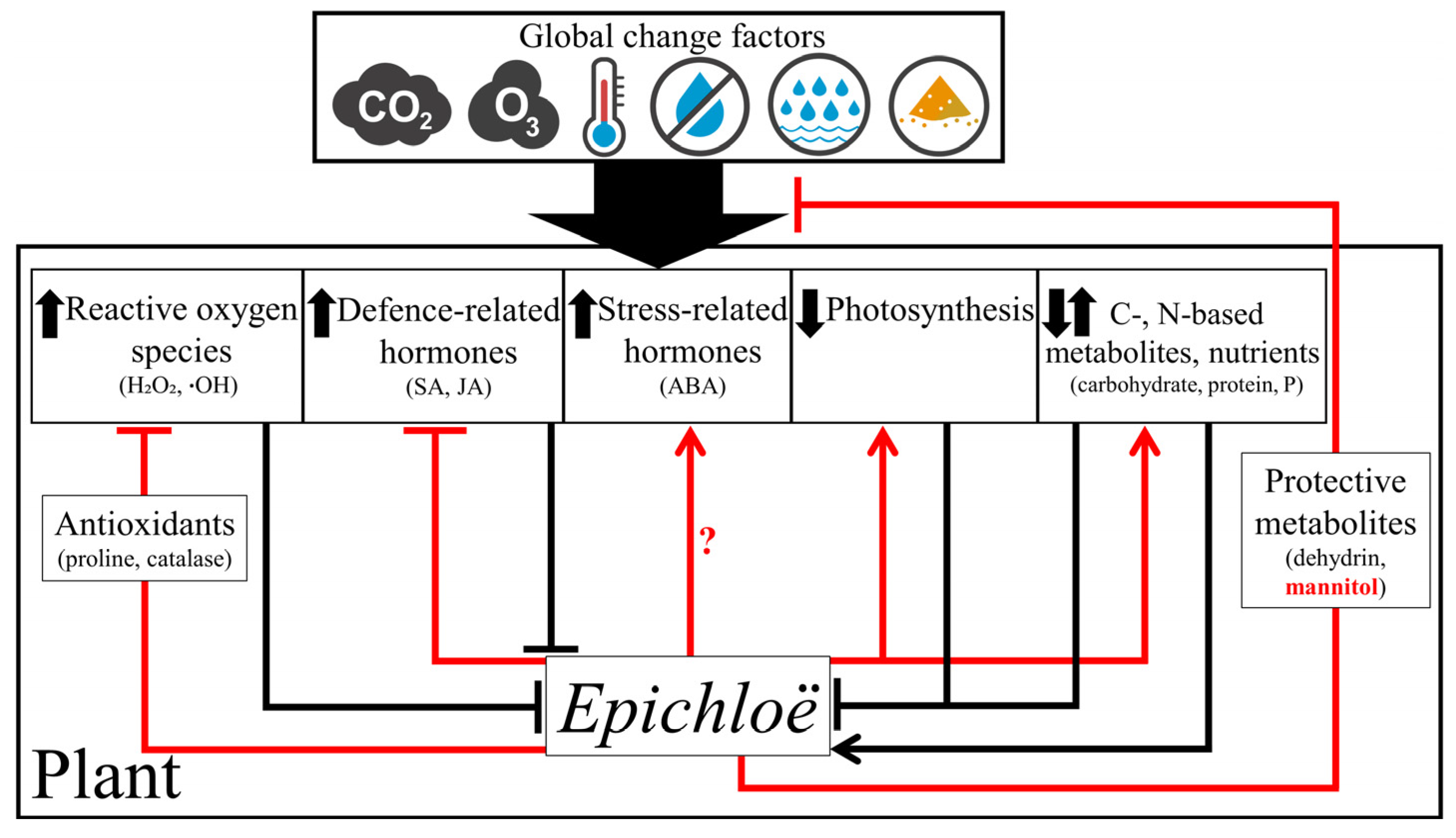
3. Mechanisms Underlying the Effects of Global Change Factors on Plant–Epichloë Associations
4. Endophyte-Based Mechanisms of Plant Protection against Global Change Factors
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pörtner, H.-O.; Roberts, D.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Changes in Atmospheric Constituents and in Radiative Forcing. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, M.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 129–234. ISBN 978-0-521-70596-7. [Google Scholar]
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Ben-Gal, A.; Assouline, S.; Javaux, M.; Minhas, P.S.; et al. Chapter One—Critical Knowledge Gaps and Research Priorities in Global Soil Salinity. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: New York, NY, USA, 2021; Volume 169, pp. 1–191. ISBN 0065-2113. [Google Scholar]
- Parmesan, C.; Hanley, M.E. Plants and Climate Change: Complexities and Surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature Increase Reduces Global Yields of Major Crops in Four Independent Estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Bastías, D.A.; Applegate, E.R.; Johnson, L.J.; Card, S.D. Factors Controlling the Effects of Mutualistic Bacteria on Plants Associated with Fungi. Ecol. Lett. 2022, 25, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–Microbiome Interactions under a Changing World: Responses, Consequences and Perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Bastías, D.A.; Balestrini, R.; Pollmann, S.; Gundel, P.E. Environmental Interference of Plant-Microbe Interactions. Plant Cell Environ. 2022, 45, 3387–3398. [Google Scholar] [CrossRef]
- Batista, B.D.; Singh, B.K. Next Generation Tools for Crop-Microbiome Manipulation to Mitigate the Impact of Climate Change. Environ. Microbiol. 2022, 25, 105–110. [Google Scholar] [CrossRef]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses of Grasses with Seedborne Fungal Endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef]
- Zhang, W.; Card, S.D.; Mace, W.J.; Christensen, M.J.; McGill, C.R.; Matthew, C. Defining the Pathways of Symbiotic Epichloë Colonization in Grass Embryos with Confocal Microscopy. Mycologia 2017, 109, 153–161. [Google Scholar] [CrossRef]
- Gundel, P.E.; Rudgers, J.A.; Ghersa, C.M. Incorporating the Process of Vertical Transmission into Understanding of Host–Symbiont Dynamics. Oikos 2011, 120, 1121–1128. [Google Scholar] [CrossRef]
- Gundel, P.E.; Batista, W.B.; Texeira, M.; Martínez-Ghersa, M.A.; Omacini, M.; Ghersa, C.M. Neotyphodium Endophyte Infection Frequency in Annual Grass Populations: Relative Importance of Mutualism and Transmission Efficiency. Proc. R. Soc. B Biol. Sci. 2008, 275, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.A.; Gillis, S.; Hager, H.A. Costs, Benefits, Parasites and Mutualists: The Use and Abuse of the Mutualism–Parasitism Continuum Concept for Epichloë Fungi. Philos. Theory Pract. Biol. 2022, 14, 9. [Google Scholar] [CrossRef]
- Bastias, D.A.; Martínez-Ghersa, M.A.; Ballaré, C.L.; Gundel, P.E. Epichloë Fungal Endophytes and Plant Defenses: Not Just Alkaloids. Trends Plant Sci. 2017, 22, 939–948. [Google Scholar] [CrossRef]
- Card, S.D.; Bastías, D.A.; Caradus, J.R. Antagonism to Plant Pathogens by Epichloë Fungal Endophytes—A Review. Plants 2021, 10, 1997. [Google Scholar] [CrossRef]
- Decunta, F.A.; Pérez, L.I.; Malinowski, D.P.; Molina-Montenegro, M.A.; Gundel, P.E. A Systematic Review on the Effects of Epichloë Fungal Endophytes on Drought Tolerance in Cool-Season Grasses. Front. Plant Sci. 2021, 12, 644731. [Google Scholar] [CrossRef]
- Hamilton, C.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic Mediation of Reactive Oxygen Species and Antioxidant Activity in Plants: A Review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Bastías, D.A.; Gundel, P.E. Plant Stress Responses Compromise Mutualisms with Epichloë Endophytes. J. Exp. Bot. 2022, 74, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Rodríguez, I.S.; Newsham, K.K.; Gundel, P.E.; Torres-Díaz, C.; Molina-Montenegro, M.A. Functional Roles of Microbial Symbionts in Plant Cold Tolerance. Ecol. Lett. 2020, 23, 1034–1048. [Google Scholar] [CrossRef]
- Compant, S.; Van Der Heijden, M.G.A.; Sessitsch, A. Climate Change Effects on Beneficial Plant–Microorganism Interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing Climate-Resilient Crops: Improving Plant Tolerance to Stress Combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Marks, S.; Clay, K. Effects of CO2 Enrichment, Nutrient Addition, and Fungal Endophyte-Infection on the Growth of Two Grasses. Oecologia 1990, 84, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.A.; Abner, M.L.; Dado, R.G.; Gibson, D.J.; Brookings, A.; Parsons, A.J. Effects of Elevated CO2, Nitrogen and Fungal Endophyte-Infection on Tall Fescue: Growth, Photosynthesis, Chemical Composition and Digestibility. Glob. Chang. Biol. 2003, 9, 425–437. [Google Scholar] [CrossRef]
- Geddes-McAlister, J.; Sukumaran, A.; Patchett, A.; Hager, H.A.; Dale, J.C.M.; Roloson, J.L.; Prudhomme, N.; Bolton, K.; Muselius, B.; Powers, J.; et al. Examining the Impacts of CO2 Concentration and Genetic Compatibility on Perennial Ryegrass—Epichloë festucae Var. lolii Interactions. J. Fungi 2020, 6, 360. [Google Scholar] [CrossRef]
- Meijer, G.; Leuchtmann, A. The Effects of Genetic and Environmental Factors on Disease Expression (Stroma Formation) and Plant Growth in Brachypodium sylvaticum Infected by Epichloë Sylvatica. Oikos 2000, 91, 446–458. [Google Scholar] [CrossRef]
- Hunt, M.G.; Rasmussen, S.; Newton, P.C.D.; Parsons, A.J.; Newman, J.A. Near-Term Impacts of Elevated CO2, Nitrogen and Fungal Endophyte-Infection on Lolium perenne L. Growth, Chemical Composition and Alkaloid Production. Plant Cell Environ. 2005, 28, 1345–1354. [Google Scholar] [CrossRef]
- Ryan, G.D.; Rasmussen, S.; Xue, H.; Parsons, A.J.; Newman, J.A. Metabolite Analysis of the Effects of Elevated CO2 and Nitrogen Fertilization on the Association between Tall Fescue (Schedonorus arundinaceus) and Its Fungal Symbiont Neotyphodium coenophialum. Plant Cell Environ. 2014, 37, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Brosi, G.B.; McCulley, R.L.; Bush, L.P.; Nelson, J.A.; Classen, A.T.; Norby, R.J. Effects of Multiple Climate Change Factors on the Tall Fescue–Fungal Endophyte Symbiosis: Infection Frequency and Tissue Chemistry. New Phytol. 2011, 189, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, H.; Wurihan; Gao, Y.; Card, S.D.; Ren, A. The Advantages of Endophyte-Infected over Uninfected Tall Fescue in the Growth and Pathogen Resistance Are Counteracted by Elevated CO2. Sci. Rep. 2017, 7, 6952. [Google Scholar] [CrossRef]
- Ueno, A.C.; Gundel, P.E.; Ghersa, C.M.; Demkura, P.V.; Card, S.D.; Mace, W.J.; Martínez-Ghersa, M.A. Ontogenetic and Trans-Generational Dynamics of a Vertically Transmitted Fungal Symbiont in an Annual Host Plant in Ozone-Polluted Settings. Plant Cell Environ. 2020, 43, 2540–2550. [Google Scholar] [CrossRef]
- Ueno, A.C.; Gundel, P.E.; Ghersa, C.M.; Agathokleous, E.; Martínez-Ghersa, M.A. Seed-Borne Fungal Endophytes Constrain Reproductive Success of Host Plants under Ozone Pollution. Environ. Res. 2021, 202, 111773. [Google Scholar] [CrossRef]
- Ueno, A.C.; Gundel, P.E.; Omacini, M.; Ghersa, C.M.; Bush, L.P.; Martínez-Ghersa, M.A. Mutualism Effectiveness of a Fungal Endophyte in an Annual Grass Is Impaired by Ozone. Funct. Ecol. 2016, 30, 226–232. [Google Scholar] [CrossRef]
- Gundel, P.E.; Sorzoli, N.; Ueno, A.C.; Ghersa, C.M.; Seal, C.E.; Bastías, D.A.; Martínez-Ghersa, M.A. Impact of Ozone on the Viability and Antioxidant Content of Grass Seeds Is Affected by a Vertically Transmitted Symbiotic Fungus. Environ. Exp. Bot. 2015, 113, 40–46. [Google Scholar] [CrossRef]
- Ueno, A.C.; Gundel, P.E.; Seal, C.E.; Ghersa, C.M.; Martínez-Ghersa, M.A. The Negative Effect of a Vertically-Transmitted Fungal Endophyte on Seed Longevity Is Stronger than that of Ozone Transgenerational Effect. Environ. Exp. Bot. 2020, 175, 104037. [Google Scholar] [CrossRef]
- Bubica Bustos, L.M.; Ueno, A.C.; Di Leo, T.D.; Crocco, C.D.; Martínez-Ghersa, M.A.; Molina-Montenegro, M.A.; Gundel, P.E. Maternal Exposure to Ozone Modulates the Endophyte-Conferred Resistance to Aphids in Lolium multiflorum Plants. Insects 2020, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Gundel, P.E.; Biganzoli, F.; Freitas, P.P.; Landesmann, J.B.; Martínez-Ghersa, M.A.; Ghersa, C.M. Plant Damage, Seed Production and Persistence of the Fungal Endophyte Epichloë occultans in Lolium multiflorum Plants under an Herbivore Lepidopteran Attack and Ozone Pollution. Ecol. Austral 2020, 30, 321–330. [Google Scholar] [CrossRef]
- Ju, H.-J.; Hill, N.S.; Abbott, T.; Ingram, K.T. Temperature Influences on Endophyte Growth in Tall Fescue. Crop Sci. 2006, 46, 404–412. [Google Scholar] [CrossRef]
- Kennedy, C.W.; Bush, L.P. Effect of Environmental and Management Factors on the Accumulation of N-Acetyl and N-Formyl Loline Alkaloid in Tall Fescue. Crop Sci. 1983, 23, 547–552. [Google Scholar] [CrossRef]
- Ryan, G.D.; Rasmussen, S.; Parsons, A.J.; Newman, J.A. The Effects of Carbohydrate Supply and Host Genetic Background on Epichloë Endophyte and Alkaloid Concentrations in Perennial Ryegrass. Fungal Ecol. 2015, 18, 115–125. [Google Scholar] [CrossRef]
- Hennessy, L.M.; Popay, A.J.; Finch, S.C.; Clearwater, M.J.; Cave, V.M. Temperature and Plant Genotype Alter Alkaloid Concentrations in Ryegrass Infected with an Epichloë Endophyte and This Affects an Insect Herbivore. Front. Plant Sci. 2016, 7, 1097. [Google Scholar] [CrossRef]
- Freitas, P.P.; Hampton, J.G.; Rolston, M.P.; Glare, T.R.; Miller, P.P.; Card, S.D. A Tale of Two Grass Species: Temperature Affects the Symbiosis of a Mutualistic Epichloë Endophyte in Both Tall Fescue and Perennial Ryegrass. Front. Plant Sci. 2020, 11, 530. [Google Scholar] [CrossRef]
- Breen, J.P. Temperature and Seasonal Effects on Expression of Acremonium Endophyte-Enhanced Resistance to Schizaphis graminum (Homoptera: Aphididae). Environ. Entomol. 1992, 21, 68–74. [Google Scholar] [CrossRef]
- Bourguignon, M.; Nelson, J.A.; Carlisle, E.; Ji, H.; Dinkins, R.D.; Phillips, T.D.; McCulley, R.L. Ecophysiological Responses of Tall Fescue Genotypes to Fungal Endophyte Infection, Elevated Temperature, and Precipitation. Crop Sci. 2015, 55, 2895–2909. [Google Scholar] [CrossRef]
- Eerens, J.P.J.; Lucas, R.J.; Easton, S.; White, J.G.H. Influence of the Endophyte (Neotyphodium lolii) on Morphology, Physiology, and Alkaloid Synthesis of Perennial Ryegrass during High Temperature and Water Stress. N. Z. J. Agric. Res. 1998, 41, 219–226. [Google Scholar] [CrossRef]
- Salminen, S.O.; Richmond, D.S.; Grewal, S.K.; Grewal, P.S. Influence of Temperature on Alkaloid Levels and Fall Armyworm Performance in Endophytic Tall Fescue and Perennial Ryegrass. Entomol. Exp. Appl. 2005, 115, 417–426. [Google Scholar] [CrossRef]
- McCulley, R.L.; Bush, L.P.; Carlisle, A.E.; Ji, H.; Nelson, J.A. Warming Reduces Tall Fescue Abundance but Stimulates Toxic Alkaloid Concentrations in Transition Zone Pastures of the U.S. Front. Chem. 2014, 2, 88. [Google Scholar] [CrossRef]
- Shi, Q.; Matthew, C.; Liu, W.; Nan, Z. Alkaloid Contents in Epichloë Endophyte-Infected Elymus tangutorum Sampled along an Elevation Gradient on the Qinghai-Tibetan Plateau. Agronomy 2020, 10, 1812. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; White, J.F.; Chen, T.; Shi, Q.; Jin, Y.; Li, X.; Li, C. Ergot Alkaloid and Endogenous Hormones Quantities and Relationship in Epichloë Endophyte: Drunken Horse Grass Are Affected by Altitude. J. Plant Growth Regul. 2022, 1–12. [Google Scholar] [CrossRef]
- Saikkonen, K.; Phillips, T.D.; Faeth, S.H.; McCulley, R.L.; Saloniemi, I.; Helander, M. Performance of Endophyte Infected Tall Fescue in Europe and North America. PLoS ONE 2016, 11, e0157382. [Google Scholar] [CrossRef]
- Welty, R.E.; Azevedo, M.D.; Cooper, T.M. Influence of Moisture Content, Temperature, and Length of Storage on Seed Germination and Survival of Endophytic Fungi in Seeds of Tall Fescue and Perennial Ryegrass. Phytopathology 1987, 77, 893–900. [Google Scholar] [CrossRef]
- Gundel, P.E.; Martínez-Ghersa, M.A.; Garibaldi, L.A.; Ghersa, C.M. Viability of Neotyphodium Endophytic Fungus and Endophyte-Infected and Non-infected Lolium multiflorum Seeds. Botany 2009, 87, 88–96. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Adaptations of Endophyte-Infected Cool-Season Grasses to Environmental Stresses: Mechanisms of Drought and Mineral Stress Tolerance. Crop Sci. 2000, 40, 923–940. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G. Influence of Fungal Endophytes on Plant Physiology Is More Pronounced under Stress than Well-Watered Conditions: A Meta-Analysis. Planta 2018, 248, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Nagabhyru, P.; Dinkins, R.D.; Wood, C.L.; Bacon, C.W.; Schardl, C.L. Tall Fescue Endophyte Effects on Tolerance to Water-Deficit Stress. BMC Plant Biol. 2013, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Bastías, D.A.; Zhang, X.; Li, C.; Nan, Z. Vertically Transmitted Epichloë Systemic Endophyte Enhances Drought Tolerance of Achnatherum inebrians Host Plants through Promoting Photosynthesis and Biomass Accumulation. J. Fungi 2022, 8, 512. [Google Scholar] [CrossRef]
- Manzur, M.E.; Garello, F.A.; Omacini, M.; Schnyder, H.; Sutka, M.R.; García-Parisi, P.A. Endophytic Fungi and Drought Tolerance: Ecophysiological Adjustment in Shoot and Root of an Annual Mesophytic Host Grass. Funct. Plant Biol. 2022, 49, 272–282. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Nan, Z. Effects of Salt and Drought Stress on Alkaloid Production in Endophyte-Infected Drunken Horse Grass (Achnatherum inebrians). Biochem. Syst. Ecol. 2011, 39, 471–476. [Google Scholar] [CrossRef]
- Lin, W.; Gao, C.; Wang, J.; Xu, W.; Wang, M.; Li, M.; Ma, B.; Tian, P. Effects of Drought Stress on Peramine and Lolitrem B in Epichloë-Endophyte-Infected Perennial Ryegrass. Life 2022, 12, 1207. [Google Scholar] [CrossRef]
- Hesse, U.; Schöberlein, W.; Wittenmayer, L.; Förster, K.; Warnstorff, K.; Diepenbrock, W.; Merbach, W. Effects of Neotyphodium Endophytes on Growth, Reproduction and Drought-Stress Tolerance of Three Lolium perenne L. Genotypes. Grass Forage Sci. 2003, 58, 407–415. [Google Scholar] [CrossRef]
- Gundel, P.E.; Maseda, P.H.; Vila-Aiub, M.M.; Ghersa, C.M.; Benech-Arnold, R. Effects of Neotyphodium Fungi on Lolium multiflorum Seed Germination in Relation to Water Availability. Ann. Bot. 2006, 97, 571–577. [Google Scholar] [CrossRef]
- Gundel, P.E.; Irisarri, J.G.N.; Fazio, L.; Casas, C.; Pérez, L.I. Inferring Field Performance from Drought Experiments Can Be Misleading: The Case of Symbiosis between Grasses and Epichloë Fungal Endophytes. J. Arid Environ. 2016, 132, 60–62. [Google Scholar] [CrossRef]
- Xia, C.; Christensen, M.J.; Zhang, X.; Nan, Z. Effect of Epichloë gansuensis Endophyte and Transgenerational Effects on the Water Use Efficiency, Nutrient and Biomass Accumulation of Achnatherum inebrians under Soil Water Deficit. Plant Soil 2018, 424, 555–571. [Google Scholar] [CrossRef]
- Arachevaleta, M.; Bacon, C.W.; Hoveland, C.S.; Radcliffe, D.E. Effect of the Tall Fescue Endophyte on Plant Response to Environmental Stress. Agron. J. 1989, 81, 83–90. [Google Scholar] [CrossRef]
- Di Bella, C.E.; Grimoldi, A.A.; Striker, G.G. A Quantitative Revision of the Waterlogging Tolerance of Perennial Forage Grasses. Crop. Pasture Sci. 2022, 73, 1200–1212. [Google Scholar] [CrossRef]
- Saedi, T.; Mosaddeghi, M.R.; Sabzalian, M.R.; Zarebanadkouki, M. Effect of Epichloë Fungal Endophyte Symbiosis on Tall Fescue to Cope with Flooding-Derived Oxygen-Limited Conditions Depends on the Host Genotype. Plant Soil 2021, 468, 353–373. [Google Scholar] [CrossRef]
- Song, M.; Li, X.; Saikkonen, K.; Li, C.; Nan, Z. An Asexual Epichloë Endophyte Enhances Waterlogging Tolerance of Hordeum brevisubulatum. Fungal Ecol. 2015, 13, 44–52. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Lin, W.; Li, M.; Wang, M.; Wang, Z.; Kuang, Y.; Tian, P. Effect of an Epichloë Endophyte on Adaptability to Water Stress in Festuca sinensis. Fungal Ecol. 2017, 30, 39–47. [Google Scholar] [CrossRef]
- Adams, A.E.; Kazenel, M.R.; Rudgers, J.A. Does a Foliar Endophyte Improve Plant Fitness under Flooding? Plant Ecol. 2017, 218, 711–723. [Google Scholar] [CrossRef]
- Kazenel, M.R.; Debban, C.L.; Ranelli, L.; Hendricks, W.Q.; Chung, Y.A.; Pendergast, T.H., IV; Charlton, N.D.; Young, C.A.; Rudgers, J.A. A Mutualistic Endophyte Alters the Niche Dimensions of Its Host Plant. AoB Plants 2015, 7, plv005. [Google Scholar] [CrossRef]
- Chen, T.; Johnson, R.; Chen, S.; Lv, H.; Zhou, J.; Li, C. Infection by the Fungal Endophyte Epichloë bromicola Enhances the Tolerance of Wild Barley (Hordeum brevisubulatum) to Salt and Alkali Stresses. Plant Soil 2018, 428, 353–370. [Google Scholar] [CrossRef]
- Wang, J.; Tian, P.; Christensen, M.J.; Zhang, X.; Li, C.; Nan, Z. Effect of Epichloë gansuensis Endophyte on the Activity of Enzymes of Nitrogen Metabolism, Nitrogen Use Efficiency and Photosynthetic Ability of Achnatherum inebrians under Various NaCl Concentrations. Plant Soil 2019, 435, 57–68. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; White, J. Effects of Epichloë Endophyte Infection on Growth, Physiological Properties and Seed Germination of Wild Barley under Saline Conditions. J. Agron. Crop Sci. 2020, 206, 43–51. [Google Scholar] [CrossRef]
- Yin, L.; Wei, M.; Wu, G.; Ren, A. Epichloë Endophytes Improved Leymus chinensis Tolerance to Both Neutral and Alkali Salt Stresses. Front. Plant Sci. 2022, 13, 968774. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, H.; Ni, X.; Zhang, Y.; Wang, Y. Effects of the Endophyte Epichloë coenophiala on the Root Microbial Community and Growth Performance of Tall Fescue in Different Saline-Alkali Soils. Fungal Ecol. 2022, 57–58, 101159. [Google Scholar] [CrossRef]
- Ahmad, R.Z.; Khalid, R.; Aqeel, M.; Ameen, F.; Li, C.J. Fungal Endophytes Trigger Achnatherum inebrians Germination Ability against Environmental Stresses. S. Afr. J. Bot. 2020, 134, 230–236. [Google Scholar] [CrossRef]
- Ju, Y.; Kou, M.; Zhong, R.; Christensen, M.J.; Zhang, X. Alleviating Salt Stress on Seedings Using Plant Growth Promoting Rhizobacteria Isolated from the Rhizosphere Soil of Achnatherum inebrians Infected with Epichloë gansuensis Endophyte. Plant Soil 2021, 465, 349–366. [Google Scholar] [CrossRef]
- Chen, T.; Simpson, W.R.; Nan, Z.; Li, C. NaCl Stress Modifies the Concentrations of Endophytic Fungal Hyphal and Peramine in Hordeum brevisubulatum Seedlings. Crop. Pasture Sci. 2022, 73, 214–221. [Google Scholar] [CrossRef]
- Laing, W.A.; Greer, D.H.; Campbell, B.D. Strong Responses of Growth and Photosynthesis of Five C3 Pasture Species to Elevated CO2 at Low Temperatures. Funct. Plant Biol. 2002, 29, 1089–1096. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What Have We Learned from 15 Years of Free-Air CO2 Enrichment (FACE)? A Meta-Analytic Review of the Responses of Photosynthesis, Canopy Properties and Plant Production to Rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Robinson, E.A.; Ryan, G.D.; Newman, J.A. A Meta-Analytical Review of the Effects of Elevated CO2 on Plant–Arthropod Interactions Highlights the Importance of Interacting Environmental and Biological Variables. New Phytol. 2012, 194, 321–336. [Google Scholar] [CrossRef]
- Rasmussen, S.; Parsons, A.J.; Bassett, S.; Christensen, M.J.; Hume, D.E.; Johnson, L.J.; Johnson, R.D.; Simpson, W.R.; Stacke, C.; Voisey, C.R.; et al. High Nitrogen Supply and Carbohydrate Content Reduce Fungal Endophyte and Alkaloid Concentration in Lolium perenne. New Phytol. 2007, 173, 787–797. [Google Scholar] [CrossRef]
- Ryan, G.D.; Shukla, K.; Rasmussen, S.; Shelp, B.J.; Newman, J.A. Phloem Phytochemistry and Aphid Responses to Elevated CO2, Nitrogen Fertilization and Endophyte Infection. Agric. For. Entomol. 2014, 16, 273–283. [Google Scholar] [CrossRef]
- Fuchs, B.; Krischke, M.; Mueller, M.J.; Krauss, J. Plant Age and Seasonal Timing Determine Endophyte Growth and Alkaloid Biosynthesis. Fungal Ecol. 2017, 29, 52–58. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic Stress: Interplay between ROS, Hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Kayano, Y.; Tanaka, A.; Takemoto, D. Two Closely Related Rho GTPases, Cdc42 and RacA, of the Endophytic Fungus Epichloë festucae Have Contrasting Roles for ROS Production and Symbiotic Infection Synchronized with the Host Plant. PLoS Pathog. 2018, 14, e1006840. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Christensen, M.J.; Takemoto, D.; Park, P.; Scott, B. Reactive Oxygen Species Play a Role in Regulating a Fungus–Perennial Ryegrass Mutualistic Interaction. Plant Cell 2006, 18, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH Oxidase RBOHD during Plant Immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef]
- Kangasjarvi, J.; Jaspers, P.; Kollist, H. Signalling and Cell Death in Ozone-Exposed Plants. Plant Cell Environ. 2005, 28, 1021–1036. [Google Scholar] [CrossRef]
- Dupont, P.; Eaton, C.J.; Wargent, J.J.; Fechtner, S.; Solomon, P.; Schmid, J.; Day, R.C.; Scott, B.; Cox, M.P. Fungal Endophyte Infection of Ryegrass Reprograms Host Metabolism and Alters Development. New Phytol. 2015, 208, 1227–1240. [Google Scholar] [CrossRef]
- Bernacki, M.J.; Rusaczonek, A.; Czarnocka, W.; Karpiński, S. Salicylic Acid Accumulation Controlled by LSD1 Is Essential in Triggering Cell Death in Response to Abiotic Stress. Cells 2021, 10, 962. [Google Scholar] [CrossRef]
- Kou, M.-Z.; Bastías, D.A.; Christensen, M.J.; Zhong, R.; Nan, Z.-B.; Zhang, X.-X. The Plant Salicylic Acid Signalling Pathway Regulates the Infection of a Biotrophic Pathogen in Grasses Associated with an Epichloë Endophyte. J. Fungi 2021, 7, 633. [Google Scholar] [CrossRef]
- Redkar, A.; Sabale, M.; Zuccaro, A.; Di Pietro, A. Determinants of Endophytic and Pathogenic Lifestyle in Root Colonizing Fungi. Curr. Opin. Plant Biol. 2022, 67, 102226. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Mittler, R. Reactive Oxygen Species and Temperature Stresses: A Delicate Balance between Signaling and Destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Simons, L.; Bultman, T.; Sullivan, T.J. Effects of Methyl Jasmonate and an Endophytic Fungus on Plant Resistance to Insect Herbivores. J. Chem. Ecol. 2008, 34, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Bastías, D.A.; Martínez-Ghersa, M.A.; Newman, J.A.; Card, S.D.; Mace, W.J.; Gundel, P.E. The Plant Hormone Salicylic Acid Interacts with the Mechanism of Anti-Herbivory Conferred by Fungal Endophytes in Grasses. Plant Cell Environ. 2018, 41, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.J.; Ort, D.R. Impacts of Chilling Temperatures on Photosynthesis in Warm-Climate Plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Spiering, M.J.; Lane, G.A.; Christensen, M.J.; Schmid, J. Distribution of the Fungal Endophyte Neotyphodium lolii Is Not a Major Determinant of the Distribution of Fungal Alkaloids in Lolium perenne Plants. Phytochemistry 2005, 66, 195–202. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Medina, V. Drought Adaptation Mechanisms Should Guide Experimental Design. Trends Plant Sci. 2016, 21, 639–647. [Google Scholar] [CrossRef]
- Jogawat, A.; Yadav, B.; Chhaya; Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between Phytohormones and Secondary Metabolites in the Drought Stress Tolerance of Crop Plants: A Review. Physiol. Plant. 2021, 172, 1106–1132. [Google Scholar] [CrossRef]
- Semmartin, M.; Omacini, M.; Gundel, P.E.; Hernández-Agramonte, I.M. Broad-Scale Variation of Fungal-Endophyte Incidence in Temperate Grasses. J. Ecol. 2015, 103, 184–190. [Google Scholar] [CrossRef]
- Casas, C.; Gundel, P.E.; Deliens, E.; Iannone, L.J.; García Martinez, G.; Vignale, M.V.; Schnyder, H. Loss of Fungal Symbionts at the Arid Limit of the Distribution Range in a Native Patagonian Grass—Resource Eco-Physiological Relations. Funct. Ecol. 2022, 36, 583–594. [Google Scholar] [CrossRef]
- Perata, P.; Armstrong, W.; Voesenek, L.A.C.J. Plants and Flooding Stress. New Phytol. 2011, 190, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of Waterlogging Tolerance in Wheat—A Review of Root and Shoot Physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and Waterlogging Stress in Plants: A Review Highlighting Research Opportunities and Understudied Aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Gundel, P.E.; Garibaldi, L.A.; Tognetti, P.M.; Aragón, R.; Ghersa, C.M.; Omacini, M. Imperfect Vertical Transmission of the Endophyte Neotyphodium in Exotic Grasses in Grasslands of the Flooding Pampa. Microb. Ecol. 2009, 57, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Bastías, D.A.; Martínez-Ghersa, M.A.; Newman, J.A.; Card, S.D.; Mace, W.J.; Gundel, P.E. Jasmonic Acid Regulation of the Anti-Herbivory Mechanism Conferred by Fungal Endophytes in Grasses. J. Ecol. 2018, 106, 2365–2379. [Google Scholar] [CrossRef]
- Cheplick, G.P.; Clay, K.; Marks, S. Interactions between Infection by Endophytic Fungi and Nutrient Limitation in the Grasses Lolium perenne and Festuca arundinacea. New Phytol. 1989, 111, 89–97. [Google Scholar] [CrossRef]
- Song, M.; Chai, Q.; Li, X.; Yao, X.; Li, C.; Christensen, M.J.; Nan, Z. An Asexual Epichloë Endophyte Modifies the Nutrient Stoichiometry of Wild Barley (Hordeum brevisubulatum) under Salt Stress. Plant Soil 2015, 387, 153–165. [Google Scholar] [CrossRef]
- Chen, T.; White, J.F.; Li, C. Fungal Endophyte Epichloë Bromicola Infection Regulates Anatomical Changes to Account for Salt Stress Tolerance in Wild Barley (Hordeum brevisubulatum). Plant Soil 2021, 461, 533–546. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Ueno, A.C.; Gundel, P.E.; Molina-Montenegro, M.A.; Ramos, P.; Ghersa, C.M.; Martínez-Ghersa, M.A. Getting Ready for the Ozone Battle: Vertically Transmitted Fungal Endophytes Have Transgenerational Positive Effects in Plants. Plant Cell Environ. 2021, 44, 2716–2728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nan, Z.B. Growth and Anti-Oxidative Systems Changes in Elymus dahuricus Is Affected by Neotyphodium Endophyte under Contrasting Water Availability. J. Agron. Crop Sci. 2007, 193, 377–386. [Google Scholar] [CrossRef]
- Patel, T.K.; Williamson, J.D. Mannitol in Plants, Fungi, and Plant–Fungal Interactions. Trends Plant Sci. 2016, 21, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Bastías, D.A.; Martínez-Ghersa, M.A.; Newman, J.A.; Card, S.D.; Mace, W.J.; Gundel, P.E. Sipha maydis Sensitivity to Defences of Lolium multiflorum and Its Endophytic Fungus Epichloë occultans. PeerJ 2019, 7, e8257. [Google Scholar] [CrossRef]
- Plett, J.M.; Kemppainen, M.; Kale, S.D.; Kohler, A.; Legué, V.; Brun, A.; Tyler, B.M.; Pardo, A.G.; Martin, F. A Secreted Effector Protein of Laccaria bicolor Is Required for Symbiosis Development. Curr. Biol. 2011, 21, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jiang, H.; Boeren, S.; Dings, H.; Kulikova, O.; Bisseling, T.; Limpens, E. A Nuclear-Targeted Effector of Rhizophagus irregularis Interferes with Histone 2B Mono-Ubiquitination to Promote Arbuscular Mycorrhization. New Phytol. 2021, 230, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Nagabhyru, P.; Schardl, C.L.; Dinkins, R.D. Differential Gene Expression in Tall Fescue Tissues in Response to Water Deficit. Plant Genome 2022, 15, e20199. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, X.; Shi, L.; Christensen, M.J.; Nan, Z.; Xia, C. Effects of Epichloë Endophyte and Transgenerational Effects on Physiology of Achnatherum inebrians under Drought Stress. Agriculture 2022, 12, 761. [Google Scholar] [CrossRef]
- Li, F.; Guo, Y.; Christensen, M.J.; Gao, P.; Li, Y.; Duan, T. An Arbuscular Mycorrhizal Fungus and Epichloë festucae Var. Lolii Reduce Bipolaris sorokiniana Disease Incidence and Improve Perennial Ryegrass Growth. Mycorrhiza 2018, 28, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Krom, N.; Kwon, T.; Li, G.; Saha, M.C. Transcriptome of Endophyte-Positive and Endophyte-Free Tall Fescue under Field Stresses. Front. Plant Sci. 2022, 13, 803400. [Google Scholar] [CrossRef] [PubMed]
- Dinkins, R.D.; Nagabhyru, P.; Young, C.A.; West, C.P.; Schardl, C.L. Transcriptome Analysis and Differential Expression in Tall Fescue Harboring Different Endophyte Strains in Response to Water Deficit. Plant Genome 2019, 12, 180071. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, B.; Guihur, A. Heat Shock Signaling in Land Plants: From Plasma Membrane Sensing to the Transcription of Small Heat Shock Proteins. Front. Plant Sci. 2021, 12, 710801. [Google Scholar] [CrossRef]
- Tiwari, P.; Chakrabarty, D. Dehydrin in the Past Four Decades: From Chaperones to Transcription Co-Regulators in Regulating Abiotic Stress Response. Curr. Res. Biotechnol. 2021, 3, 249–259. [Google Scholar] [CrossRef]
- Reza Sabzalian, M.; Mirlohi, A. Neotyphodium Endophytes Trigger Salt Resistance in Tall and Meadow Fescues. J. Plant Nutr. Soil Sci. 2010, 173, 952–957. [Google Scholar] [CrossRef]
- Gundel, P.E.; Rudgers, J.A.; Whitney, K.D. Vertically Transmitted Symbionts as Mechanisms of Transgenerational Effects. Am. J. Bot. 2017, 104, 787–792. [Google Scholar] [CrossRef]
- Miller, T.A.; Hudson, D.A.; Johnson, R.D.; Singh, J.S.; Mace, W.J.; Forester, N.T.; Maclean, P.H.; Voisey, C.R.; Johnson, L.J. Dissection of the Epoxyjanthitrem Pathway in Epichloë Sp. LpTG-3 Strain AR37 by CRISPR Gene Editing. Front. Fungal Biol. 2022, 3, 944234. [Google Scholar] [CrossRef]
- Nagabhyru, P.; Dinkins, R.D.; Schardl, C.L. Transcriptome Analysis of Epichloë Strains in Tall Fescue in Response to Drought Stress. Mycologia 2022, 114, 697–712. [Google Scholar] [CrossRef]
- Wang, M.; Tian, P.; Gao, M.; Li, M. The Promotion of Festuca sinensis Heavy Metal Stress Tolerance Mediated by Epichloë Endophyte. Agronomy 2021, 11, 2049. [Google Scholar] [CrossRef]
- Aroca, R.; del Mar Alguacil, M.; Vernieri, P.; Ruiz-Lozano, J.M. Plant Responses to Drought Stress and Exogenous ABA Application Are Modulated Differently by Mycorrhization in Tomato and an ABA-Deficient Mutant (Sitiens). Microb. Ecol. 2008, 56, 704–719. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastías, D.A.; Ueno, A.C.; Gundel, P.E. Global Change Factors Influence Plant-Epichloë Associations. J. Fungi 2023, 9, 446. https://doi.org/10.3390/jof9040446
Bastías DA, Ueno AC, Gundel PE. Global Change Factors Influence Plant-Epichloë Associations. Journal of Fungi. 2023; 9(4):446. https://doi.org/10.3390/jof9040446
Chicago/Turabian StyleBastías, Daniel A., Andrea C. Ueno, and Pedro E. Gundel. 2023. "Global Change Factors Influence Plant-Epichloë Associations" Journal of Fungi 9, no. 4: 446. https://doi.org/10.3390/jof9040446
APA StyleBastías, D. A., Ueno, A. C., & Gundel, P. E. (2023). Global Change Factors Influence Plant-Epichloë Associations. Journal of Fungi, 9(4), 446. https://doi.org/10.3390/jof9040446








