A Comparative Study of Lactarius Mushrooms: Chemical Characterization, Antibacterial, Antibiofilm, Antioxidant and Cytotoxic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Lactarius Species
2.2. Extracts Preparation
2.3. Chemical Analysis of the Basidiocarps of the Tested Mushrooms
2.3.1. Hydrophilic Compounds
2.3.2. Lipophilic Compounds
2.3.3. Phenolic Compounds
2.4. Biological Activity of Lactarius Species
2.4.1. Antibacterial Potential
2.4.2. Antibiofilm Potential
2.4.3. Antioxidant Activity Measured by Thiobarbituric Acid Reactive Substances Assay—TBARS
2.4.4. Cytotoxicity Evaluation
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition of Lactarius Species
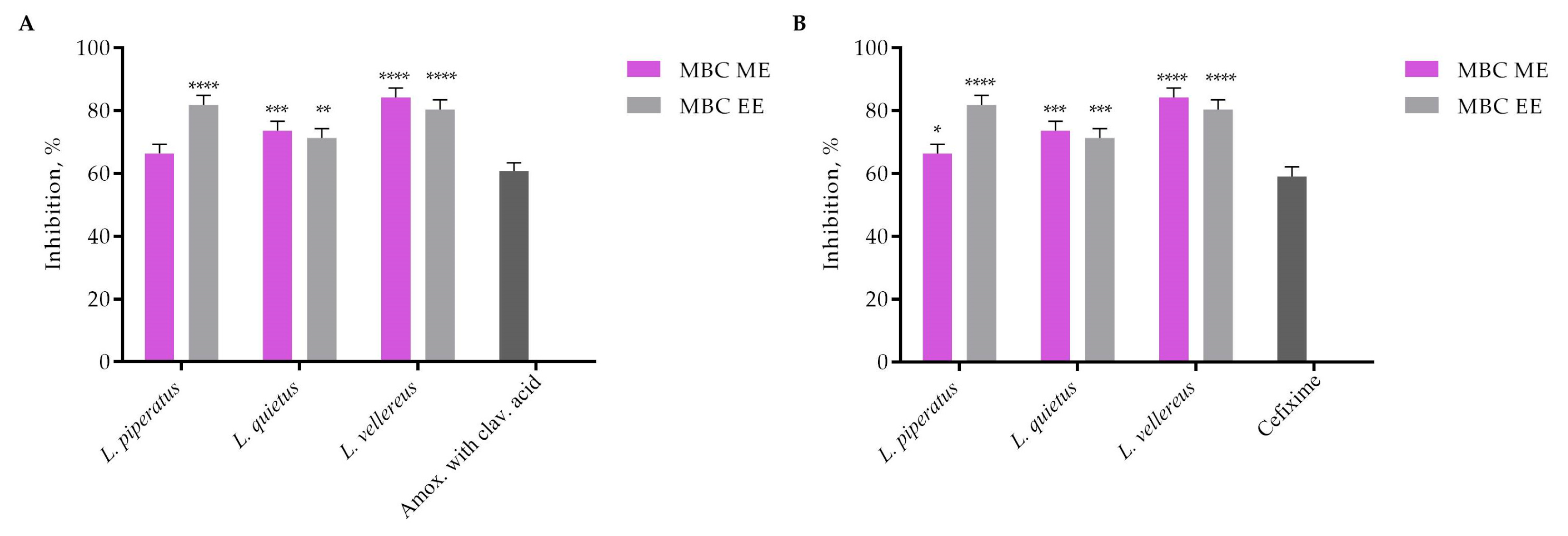
3.2. Biological Activities of Lactarius Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anusiya, G.; Gowthama Prabu, U.; Yamini, N.V.; Sivarajasekar, N.; Rambabu, K.; Bharath, G.; Banat, F. A Review of the Therapeutic and Biological Effects of Edible and Wild Mushrooms. Bioengineered 2021, 12, 11239. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 686. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, J.Y.; Wisitrassameewong, K.; Kim, M.J.; Park, M.S.; Kim, N.K.; Lee, J.K.; Lim, Y.W. First Report of Eight Milkcap Species Belonging to Lactarius and Lactifluus in Korea. Mycobiology 2018, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Effect of Fruiting Body Maturity Stage on Chemical Composition and Antimicrobial Activity of Lactarius Sp. Mushrooms. J. Agric. Food Chem. 2007, 55, 8766–8771. [Google Scholar] [CrossRef]
- Vieira, V.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Expanding Current Knowledge on the Chemical Composition and Antioxidant Activity of the Genus Lactarius. Molecules 2014, 19, 20650–20663. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Song, Z.; Hou, Y. Comparative Study on the Structure Characterization and Immune Activity of Lactarius vellereus Fr. Polysaccharide (LV-1) and Cordyceps militaris (L. Ex Fr.) Link. Polysaccharide (CM-S). J. Food Meas. Charact. 2022, 16, 901–919. [Google Scholar] [CrossRef]
- Niedzielski, P.; Szostek, M.; Budka, A.; Budzyńska, S.; Siwulski, M.; Proch, J.; Kalač, P.; Mleczek, M. Lactarius and Russula Mushroom Genera–Similarities/Differences in Mineral Composition within the Russulaceae Family. J. Food Compos. Anal. 2023, 115, 104970. [Google Scholar] [CrossRef]
- Fogarasi, M.; Diaconeasa, M.; Rodica Pop, C.; Fogarasi, S.; Anamaria Semeniuc, C.; Corina Fărca, A.; Sălăgean, C.-D.; Tofană, M.; Ancut, S. Elemental Composition, Antioxidant and Antibacterial Properties of Some Wild Edible Mushrooms from Romania. Agronomy 2020, 10, 72. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic, Polysaccharidic, and Lipidic Fractions of Mushrooms from Northeastern Portugal: Chemical Compounds with Antioxidant Properties. J. Agric. Food Chem. 2012, 60, 4634–4640. [Google Scholar] [CrossRef]
- Ao, T.; Deb, C.R. Nutritional and Antioxidant Potential of Some Wild Edible Mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, M.; Ancu¸ta, S.; Socaci, A.; Dulf, F.V.; Diaconeasa, M.; Fărcas, A.C.; Tofană, M.; Semeniuc, C.A. Molecules Bioactive Compounds and Volatile Profiles of Five Transylvanian Wild Edible Mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef] [PubMed]
- Dogan, H.H.; Aydin, S. Some Biological Activities of Lactarius vellereus (Fr.) Fr. in Turkey. Pakistan J. Biol. Sci. 2013, 16, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Doǧan, H.H.; Duman, R.; Özkalp, B.; Aydin, S. Antimicrobial Activities of Some Mushrooms in Turkey. Pharm. Biol. 2013, 51, 707–711. [Google Scholar] [CrossRef]
- Ozen, T.; Darcan, C.; Aktop, O.; Turkekul, I. Screening of Antioxidant, Antimicrobial Activities and Chemical Contents of Edible Mushrooms Wildly Grown in the Black Sea Region of Turkey. Comb. Chem. High Throughput Screen. 2011, 14, 72–84. [Google Scholar] [CrossRef]
- Reis, F.S.; Heleno, S.A.; Barros, L.; Jo, M.; Martins, A.; Santos-buelga, C. Toward the Antioxidant and Chemical Characterization of Mycorrhizal Mushrooms from Northeast Portugal. J. Food Sci. 2011, 76, 824–830. [Google Scholar] [CrossRef]
- Kosanić, M.; Petrović, N.; Milošević-Djordjević, O.; Grujičić, D.; Tubić, J.; Marković, A.; Stanojković, T. The Health Promoting Effects of the Fruiting Bodies Extract of the Peppery Milk Cap Mushroom Lactarius piperatus (Agaricomycetes) from Serbia. Int. J. Med. Mushrooms 2020, 22, 347–357. [Google Scholar] [CrossRef]
- Stanković, M.; Mitić, V.; Stankov Jovanović, V.; Dimitrijević, M.; Nikolić, J.; Stojanović, G. Selected Fungi of the Genus Lactarius-Screening of Antioxidant Capacity, Antimicrobial Activity, and Genotoxicity. J. Toxicol. Environ. Health Part A 2022, 85, 699–714. [Google Scholar] [CrossRef]
- Basso, M.T. Lactarius Pers., 1st ed.; Candusso Edizioni: Alassio, Italy, 1999; p. 845. [Google Scholar]
- Kostić, M.; Smiljković, M.; Petrović, J.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Ćirić, A.; Soković, M. Chemical, Nutritive Composition and a Wide Range of Bioactive Properties of Honey Mushroom: Armillaria mellea (Vahl: Fr.) Kummer. Food Funct. 2017, 8, 3239–3249. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barros, L.; Antonio, A.L. Using Gamma Irradiation to Attenuate the Effects Caused by Drying or Freezing in Macrolepiota procera Organic Acids and Phenolic Compounds. Food Bioprocess Technol. 2014, 7, 3012–3021. [Google Scholar] [CrossRef][Green Version]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Wild and Commercial Mushrooms as Source of Nutrients and Nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized Analysis of Organic Acids in Edible Mushrooms from Portugal by Ultra Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols Composition of Portuguese Wild Mushrooms with Antioxidant Capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L. EUCAST Definitive Document EDef 7.1: Method for the Determination of Broth Dilution MICs of Antifungal Agents for Fermentative Yeasts. Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.; Ivanov, M.; Babić, S.S.; Tepavčević, Z.; Radanović, O.; Soković, M.; Ćirić, A. Analysis of Tonsil Tissues from Patients Diagnosed with Chronic Tonsillitis—Microbiological Profile, Biofilm-Forming Capacity and Histology. Antibiotics 2022, 11, 1747. [Google Scholar] [CrossRef]
- Kostić, M.; Ivanov, M.; Fernandes, Â.; Pinela, J.; Calhelha, R.C.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Soković, M.; Ćirić, A. Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity. Molecules 2020, 25, 4336. [Google Scholar] [CrossRef] [PubMed]
- Smiljkovic, M.; Dias, M.I.; Stojkovic, D.; Barros, L.; Bukvički, D.; Ferreira, I.C.F.R.; Sokovic, M. Characterization of Phenolic Compounds in Tincture of Edible Nepeta nuda: Development of Antimicrobial Mouthwash. Food Funct. 2018, 9, 5417–5425. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Correia, D.M.; Morais, J.S.; Ferreira, I.C.F.R. Effects of Conservation Treatment and Cooking on the Chemical Composition and Antioxidant Activity of Portuguese Wild Edible Mushrooms. J. Agric. Food Chem. 2007, 55, 4781–4788. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.V.C.; Fernandes, Â.; Barreira, J.C.M.; Verde, S.C.; Antonio, A.L.; Gonzaléz-Paramás, A.M.; Barros, L.; Ferreira, I.C.F.R. Effectiveness of Gamma and Electron Beam Irradiation as Preserving Technologies of Fresh Agaricus bisporus Portobello: A Comparative Study. Food Chem. 2019, 278, 760–766. [Google Scholar] [CrossRef]
- Sławińska, A.; Jabłońska-Ryś, E.; Stachniuk, A. High-Performance Liquid Chromatography Determination of Free Sugars and Mannitol in Mushrooms Using Corona Charged Aerosol Detection. Food Anal. Methods 2021, 14, 209–216. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, C.; Zhao, Y.; Liu, S.; Wang, H.; Wang, C.; Guo, L.; Chen, M. Changes in Mannitol Content, Regulation of Genes Involved in Mannitol Metabolism, and the Protective Effect of Mannitol on Volvariella volvacea at Low Temperature. Biomed Res. Int. 2019, 2019, 1493721. [Google Scholar] [CrossRef] [PubMed]
- Valentão, P.; Lopes, G.; Valente, M.; Barbosa, P.; Andrade, P.B.; Silva, B.M.; Baptista, P.; Seabra, R.M. Quantitation of Nine Organic Acids in Wild Mushrooms. J. Agric. Food Chem. 2005, 53, 3626–3630. [Google Scholar] [CrossRef] [PubMed]
- Magdziak, Z.; Siwulski, M.; Mleczek, M. Characteristics of Organic Acid Profiles in 16 Species of Wild Growing Edible Mushrooms. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2017, 52, 784–789. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Essential Fatty Acids in Health and Chronic Disease. Am. J. Clin. Nutr. 1999, 70, 560–569. [Google Scholar] [CrossRef]
- Su, C.H.; Lai, M.N.; Ng, L.T. Inhibitory Effects of Medicinal Mushrooms on α-Amylase and α-Glucosidase-Enzymes Related to Hyperglycemia. Food Funct. 2013, 4, 644–649. [Google Scholar] [CrossRef]
- Ribeiro, B.; Guedes de Pinho, P.; Andrade, P.B.; Baptista, P.; Valentão, P. Fatty Acid Composition of Wild Edible Mushrooms Species: A Comparative Study. Microchem. J. 2009, 93, 29–35. [Google Scholar] [CrossRef]
- Coyge, B.; Gurbuz, B.; Kiralan, M. Oil Content and Fatty Acid Composition of Some Safflower (Carthamus tinctorius L.) Varieties Sown in Spring and Winter. Int. J. Nat. Eng. Sci. 2007, 1, 11–15. [Google Scholar]
- Wacal, C.; Ogata, N.; Basalirwa, D.; Sasagawa, D.; Kato, M.; Handa, T.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Fatty Acid Composition of Sesame (Sesamum indicum L.) Seeds in Relation to Yield and Soil Chemical Properties on Continuously Monocropped Upland Fields Converted from Paddy Fields. Agronomy 2019, 9, 801. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M.; Zappia, C.; Poiana, M. Influence of High Temperature and Duration of Heating on the Sunflower Seed Oil Properties for Food Use and Bio-Diesel Production. J. Oleo Sci. 2017, 66, 1193–1205. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Bouzgarrou, C.; Amara, K.; Reis, F.S.; Barreira, J.C.M.; Skhiri, F.; Chatti, N.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Incorporation of Tocopherol-Rich Extracts from Mushroom Mycelia into Yogurt. Food Funct. 2018, 9, 3166–3172. [Google Scholar] [CrossRef] [PubMed]
- Alkan, S.; Uysal, A.; Kasik, G.; Vlaisavljevic, S.; Berežni, S.; Zengin, G. Chemical Characterization, Antioxidant, Enzyme Inhibition and Antimutagenic Properties of Eight Mushroom Species: A Comparative Study. J. Fungi 2020, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic Acids Determination by HPLC-DAD-ESI/MS in Sixteen Different Portuguese Wild Mushrooms Species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A Comprehensive Review on Phenolic Compounds from Edible Mushrooms: Occurrence, Biological Activity, Application and Future Prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef]
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of Antioxidant Activity and Antioxidant Compounds in Wild Edible Mushrooms. J. Food Compos. Anal. 2007, 20, 337–345. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial Activity of Phenolic Compounds Identified in Wild Mushrooms, SAR Analysis and Docking Studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Ferreira, I.; Barros, L.; Abreu, R. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evid. Based Complement. Altern. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef]
- An, L.J.; Guan, S.; Shi, G.F.; Bao, Y.M.; Duan, Y.L.; Jiang, B. Protocatechuic Acid from Alpinia oxyphylla against MPP+-Induced Neurotoxicity in PC12 Cells. Food Chem. Toxicol. 2006, 44, 436–443. [Google Scholar] [CrossRef]
- Vaz, J.A.; Almeida, G.M.; Ferreira, I.C.F.R.; Martins, A.; Vasconcelos, M.H. Clitocybe alexandri Extract Induces Cell Cycle Arrest and Apoptosis in a Lung Cancer Cell Line: Identification of Phenolic Acids with Cytotoxic Potential. Food Chem. 2012, 132, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Ćirić, A.; Glamočlija, J.; Stojković, D. The Bioactive Properties of Mushrooms. In Wild Plants, Mushroom and Nuts. Functional Food Properties and Applications, 1st ed.; Ferreira, I.C.F.R., Morales, P., Barros, L., Eds.; Wiley Blackwell: New York, NY, USA, 2017; pp. 83–122. [Google Scholar]
- Parastan, R.; Kargar, M.; Solhjoo, K.; Kafilzadeh, F. Staphylococcus aureus Biofilms: Structures, Antibiotic Resistance, Inhibition, and Vaccines. Gene Rep. 2020, 20, 100739. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Lourenço, I.; Costa, E.; Martins, A.; Pintado, M. Wild Mushroom Extracts as Inhibitors of Bacterial Biofilm Formation. Pathogens 2014, 3, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Čuvalová, A.; Strapáč, I.; Handrová, L.; Kmeť, V. Antibiofilm Activity of Mushroom Extracts against Staphylococcus aureus. Ann. Univ. Paedagog. Crac. Stud. Nat. 2018, 3, 17–23. [Google Scholar] [CrossRef]
- Fasciana, T.; Gargano, M.L.; Serra, N.; Galia, E.; Arrigo, I.; Tricoli, M.R.; Diquattro, O.; Graceffa, G.; Vieni, S.; Venturella, G.; et al. Potential Activity of Albino Grifola frondosa Mushroom Extract against Biofilm of Meticillin-Resistant Staphylococcus aureus. J. Fungi 2021, 7, 551. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Recent Developments in Mushrooms as Anti-Cancer Therapeutics: A Review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef]

| Scientific Name | Code Name | Locality | Geographical Coordinates | Collection Year |
|---|---|---|---|---|
| Lactarius piperatus (L.) Pers. | Lp-061-2017 | Kosmaj | 44°28′22″ N 20°34′25″ E | 2017 |
| Lactarius quietus (Fr.) Fr. | Lq-071-2014 | Divčibare | 44°06′52″ N 20°02′15″ E | 2014 |
| Lactarius vellereus (Fr.) Fr. | Lv-082-2016 | Kopaonik | 43°17′30″ N 20°49′06″ E | 2016 |
| Constituent | Lactarius piperatus | Lactarius quietus | Lactarius vellereus |
|---|---|---|---|
| Free Sugars (g/100 g dw) | |||
| Fructose | n.d. | n.d. | n.d. |
| Mannitol | 5.8 ± 0.3 b | 3.67 ± 0.04 c | 13.47 ± 0.04 a |
| Trehalose | 17.8 ± 0.3 a | 0.34 ± 0.01 c | 0.77 ± 0.01 b |
| Total sugars | 23.59 ± 0.04 a | 4.01 ± 0.03 c | 14.2 ± 0.4 b |
| Organic Acids (g/100 g dw) | |||
| Oxalic acid | 1.9 ± 0.2 c | 3.7 ± 0.1 b | 3.9 ± 0.2 a |
| Quinic acid | 0.3 ± 0.1 | n.d. | n.d. |
| Malic acid | 6 ± 1 | n.d. | n.d. |
| Citric acid * | 0.5 ± 0.1 | n.d. | 8.5 ± 3 |
| Fumaric acid | 0.33 ± 0.03 | tr. | tr. |
| Shikimic acid | n.d. | n.d. | 0.49 ± 0.02 |
| Total organic acids | 9 ± 2 b | 3.7 ± 0.1 c | 12.4 ± 0.4 a |
| Lactarius piperatus | Lactarius quietus | Lactarius vellereus | |
|---|---|---|---|
| Fatty acids (%) | |||
| C16:0 | 4.93 ± 0.03 c | 20.1 ± 0.4 a | 10.0 ± 0.2 b |
| C18:0 | 42.1 ± 0.2 b | 10.1 ± 0.1 c | 58.2 ± 0.9 a |
| C18:1n9c | 36.9 ± 0.4 b | 40.4 ± 0.4 a | 17.6 ± 0.8 c |
| C18:2n6c | 11.9 ± 0.2 c | 19.2 ± 0.1 a | 12.15 ± 0.02 b,c |
| Total SFA (% of total FA) | 50.8 ± 0.3 b | 38.3 ± 0.5 c | 69.8 ± 0.8 a |
| Total MUFA (% of total FA) | 37.2 ± 0.4 b | 42 ± 1 a | 17.9 ± 0.9 c |
| Total PUFA (% of total FA) | 12.0 ± 0.2 b | 19.8 ± 0.1 a | 12.31 ± 0.02 b |
| Tocopherols (µg/100 g dw) | |||
| α-Tocopherol | 4 ± 1 | n.d. | n.d. |
| β-Tocopherol | 47 ± 2 c | 69.0 ± 0.8 b | 1391 ± 21 a |
| γ-Tocopherol | 26.1 ± 0.1 | n.d. | n.d. |
| δ-Tocopherol | 3.1 ± 0.7 | n.d. | n.d. |
| Total Tocopherols | 80 ± 3 b | 69.0 ± 0.8 c | 1391 ± 21 a |
| Phenolic and Related Compounds (µg/g ext) | LP-ME | LP-EE | LQ-ME | LQ-EE | LV-ME | LV-EE |
|---|---|---|---|---|---|---|
| p-Hydroxybenzoic acid | n.d. | n.d. | 795 ± 3 b | 4735 ± 9 a | 2.0 ± 0.2 d | 2.5 ± 0.2 c |
| Protocatechuic acid * | n.d. | n.d. | n.d. | n.d. | 4.2 ± 0.3 | 11.4 ± 0.5 |
| Total phenolic compounds | - | - | 795 ± 3 b | 4735 ± 9 a | 6.2 ± 0.5 d | 13.9 ± 0.40 c |
| Cinnamic acid | 8.5 ± 0.4 c | 5.2 ± 0.2 d | 23.8 ± 0.1 b | 126 ± 3 a | n.d. | n.d. |
| LP-ME | LP-EE | LQ-ME | LQ-EE | LV-ME | LV-EE | Amoxicillin with Clavulanic Acid | Cefixime | ||
|---|---|---|---|---|---|---|---|---|---|
| Micrococcus luteus | MIC | 0.8 | 6.25 | 3.1 | 3.1 | 1.5 | 1.5 | 0.0002 | 0.002 |
| MBC | 1.6 | 12.5 | 6.2 | 6.25 | 3.1 | 3.1 | 0.0004 | 0.003 | |
| Rothia mucilagenosa | MIC | 0.8 | 3.1 | 6.25 | 0.6 | 1.6 | 0.8 | 0.007 | 0.002 |
| MBC | 1.6 | 6.25 | 12.5 | 3.1 | 3.1 | 1.6 | 0.014 | 0.003 | |
| Streptococcus agalactiae | MIC | 0.4 | 3.1 | 0.2 | 0.6 | 0.8 | 0.8 | 0.007 | 0.002 |
| MBC | 0.8 | 6.25 | 0.4 | 3.1 | 1.6 | 1.6 | 0.014 | 0.004 | |
| Streptococcus anginosus | MIC | 12.5 | 3.1 | 3.1 | 3.1 | 0.8 | 0.8 | 0.028 | 0.0002 |
| MBC | 25 | 6.25 | 6.25 | 6.2 | 1.6 | 1.6 | 0.056 | 0.0004 | |
| Streptococcus constellatus | MIC | 0.8 | 6.25 | 1.56 | 3.1 | 1.56 | 1.56 | 0.0002 | 0.0002 |
| MBC | 1.56 | 12.5 | 3.1 | 6.2 | 3.1 | 3.1 | 0.0004 | 0.0004 | |
| Streptococcus dysgalactiae | MIC | 0.4 | 1.5 | 1.5 | 0.8 | 1.5 | 1.5 | 0.007 | 0.0002 |
| MBC | 0.8 | 3.1 | 3.1 | 1.6 | 3.1 | 3.1 | 0.014 | 0.0004 | |
| Streptococcus oralis | MIC | 6.2 | 3.1 | 0.8 | 3.1 | 6.25 | 3.1 | 0.0004 | 0.002 |
| MBC | 12.5 | 6.25 | 1.6 | 6.2 | 12.5 | 6.25 | 0.001 | 0.004 | |
| Streptococcus parasanquinis | MIC | 0.4 | 3.1 | 0.2 | 0.6 | 0.8 | 0.8 | 0.004 | 0.003 |
| MBC | 0.8 | 6.25 | 0.4 | 3.1 | 1.6 | 1.6 | 0.01 | 0.006 | |
| Streptococcus pseudopneumoniae | MIC | 0.8 | 3.1 | 1.6 | 6.2 | 6.25 | 1.6 | 0.001 | 0.013 |
| MBC | 1.6 | 6.25 | 3.1 | 12.5 | 12.5 | 3.1 | 0.002 | 0.027 | |
| Streptococcus pyogenes | MIC | 3.1 | 0.8 | 0.4 | 6.25 | 3.1 | 0.8 | 0.0004 | 0.0008 |
| MBC | 6.2 | 1.6 | 0.8 | 12.5 | 6.25 | 1.6 | 0.001 | 0.002 | |
| Streptococcus salivarius | MIC | 1.6 | 1.6 | 0.4 | 3.1 | 6.25 | 3.1 | 0.01 | 0.013 |
| MBC | 3.1 | 3.1 | 0.8 | 6.2 | 12.5 | 6.25 | 0.014 | 0.027 | |
| Staphylococcus aureus | MIC | 12.5 | 25 | 12.5 | 12.5 | 25 | 12.5 | 0.001 | 0.003 |
| MBC | 25 | 50 | 25 | 25 | 30 | 25 | 0.002 | 0.006 | |
| Staphylococcus hominis | MIC | 15 | 15 | 3.75 | 3.75 | 7.5 | 7.5 | 0.004 | 0.002 |
| MBC | 30 | 30 | 7.5 | 7.5 | 15 | 15 | 0.007 | 0.004 | |
| Staphylococcus warnerii | MIC | 6.25 | 6.25 | 6.25 | 3.1 | 6.25 | 12.5 | 0.001 | 0.003 |
| MBC | 12.5 | 12.5 | 12.5 | 6.25 | 12.5 | 25 | 0.002 | 0.006 | |
| Enterobacter cloacae | MIC | 12.5 | 25 | 6.25 | 3.1 | 6.25 | 1.6 | 0.028 | 0.003 |
| MBC | 25 | 50 | 12.5 | 6.2 | 12.5 | 3.1 | 0.056 | 0.007 | |
| Stenotrophomonas maltophilia | MIC | 25 | 25 | 6.25 | 25 | 25 | 12.5 | 0.003 | 0.003 |
| MBC | 50 | 50 | 12.5 | 50 | 50 | 25 | 0.007 | 0.006 |
| Type of Extract | Antioxidant Activity (EC50, mg/mL) * | Cytotoxicity to Non-Tumor Cell Lines (GI50, μg/mL) ** | Cytotoxicity to Tumor Cell Lines (GI50, μg/mL) ** | |||
|---|---|---|---|---|---|---|
| TBARS | PLP2 | HeLa | HepG2 | MCF-7 | NCI-H460 | |
| (porcine liver primary culture) *** | (cervical carcinoma) | (hepatocellular carcinoma) | (breast carcinoma) | (non-small cell lung cancer) | ||
| LP-ME | 0.41 ± 0.08 b | >400 | 229 ± 10 c | 263 ± 6 c | 216 ± 11 b | >400 |
| LP-EE | 1.39 ± 0.06 a | >400 | 266 ± 17 a | >400 | 276 ± 11 a | >400 |
| LQ-ME | 0.17 ± 0.01 e | 347 ± 12 | 196 ± 3 d | 191 ± 14 d | 202 ± 8 c | 220 ± 6 b |
| LQ-EE | 0.23 ± 0.02 d | >400 | >400 | 312 ± 20 a | >400 | >400 |
| LV-ME | 0.32 ± 0.01 c | >400 | 253 ± 8 b | 271 ± 3 b | >400 | 331 ± 3 a |
| LV-EE | 0.30 ± 0.01 c | 163 ± 7 | 71 ± 3 e | 80 ± 6 e | 87 ± 4 d | 83 ± 3 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, M.; Ivanov, M.; Fernandes, Â.; Calhelha, R.C.; Glamočlija, J.; Barros, L.; Soković, M.; Ćirić, A. A Comparative Study of Lactarius Mushrooms: Chemical Characterization, Antibacterial, Antibiofilm, Antioxidant and Cytotoxic Activity. J. Fungi 2023, 9, 70. https://doi.org/10.3390/jof9010070
Kostić M, Ivanov M, Fernandes Â, Calhelha RC, Glamočlija J, Barros L, Soković M, Ćirić A. A Comparative Study of Lactarius Mushrooms: Chemical Characterization, Antibacterial, Antibiofilm, Antioxidant and Cytotoxic Activity. Journal of Fungi. 2023; 9(1):70. https://doi.org/10.3390/jof9010070
Chicago/Turabian StyleKostić, Marina, Marija Ivanov, Ângela Fernandes, Ricardo C. Calhelha, Jasmina Glamočlija, Lillian Barros, Marina Soković, and Ana Ćirić. 2023. "A Comparative Study of Lactarius Mushrooms: Chemical Characterization, Antibacterial, Antibiofilm, Antioxidant and Cytotoxic Activity" Journal of Fungi 9, no. 1: 70. https://doi.org/10.3390/jof9010070
APA StyleKostić, M., Ivanov, M., Fernandes, Â., Calhelha, R. C., Glamočlija, J., Barros, L., Soković, M., & Ćirić, A. (2023). A Comparative Study of Lactarius Mushrooms: Chemical Characterization, Antibacterial, Antibiofilm, Antioxidant and Cytotoxic Activity. Journal of Fungi, 9(1), 70. https://doi.org/10.3390/jof9010070