Transcriptome Analysis Reveals the Function of a G-Protein α Subunit Gene in the Growth and Development of Pleurotus eryngii
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains Materials and Culture Conditions
2.2. Measurement of Mycelial Growth Rate
2.3. Ultrastructure Observation of the Mycelia
2.4. Enzyme Activity Assay of Mycelia
2.5. RNA Sequencing and Data Processing
2.6. Quantification of Gene Expression Levels and Differential Expression Analysis
2.7. GO and KEGG Enrichment Analysis
2.8. Validation of RNA-Seq by Quantitative Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. Effect of GNAI on Mycelial Morphology of P. eryngii
3.2. Effect of GNAI on the Mycelial Enzymatic Activity of P. eryngii
3.3. Effect of GNAI on Primordium Formation and Button of P. eryngii
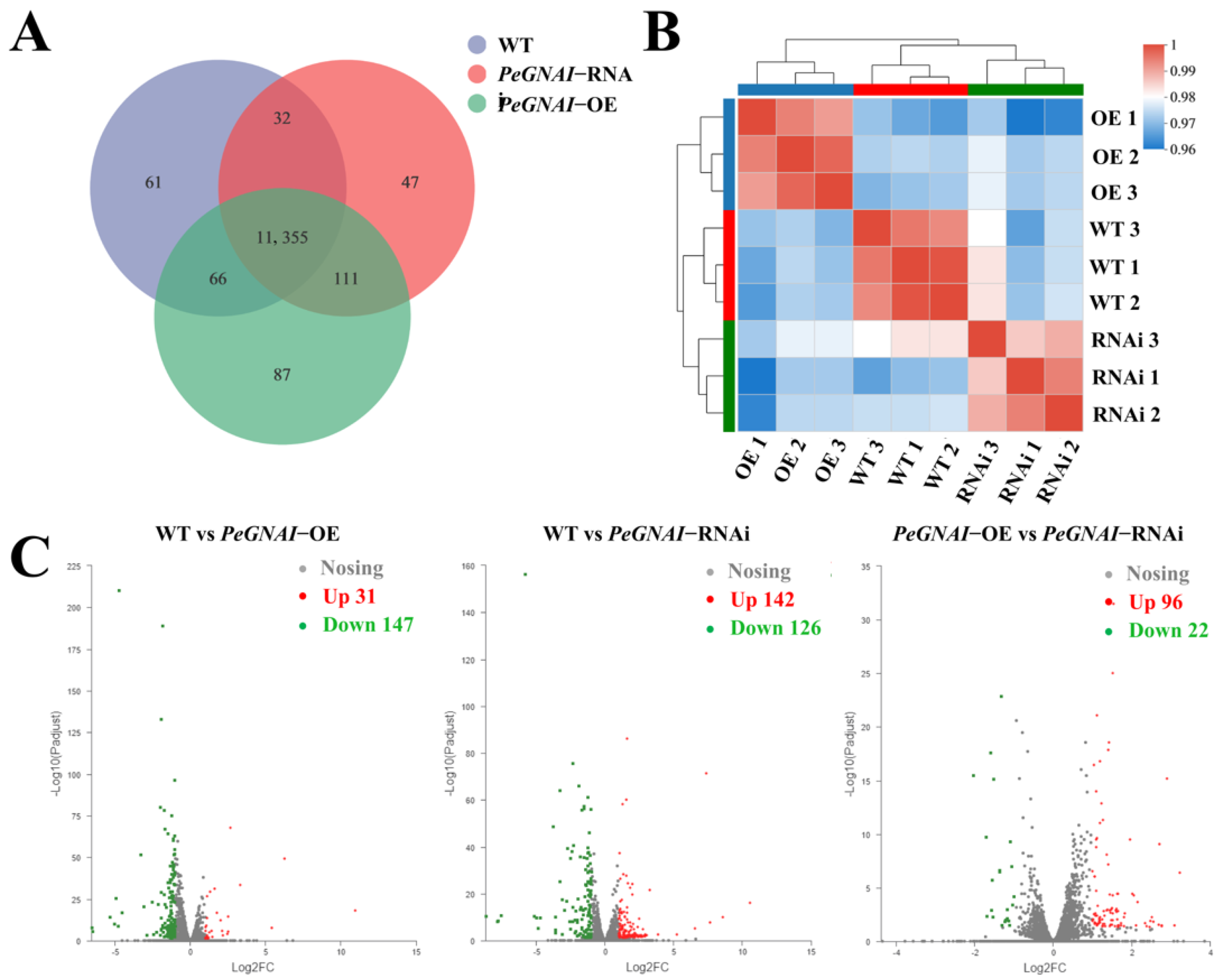
3.4. Analysis of Differentially Expressed Genes (DEGs)
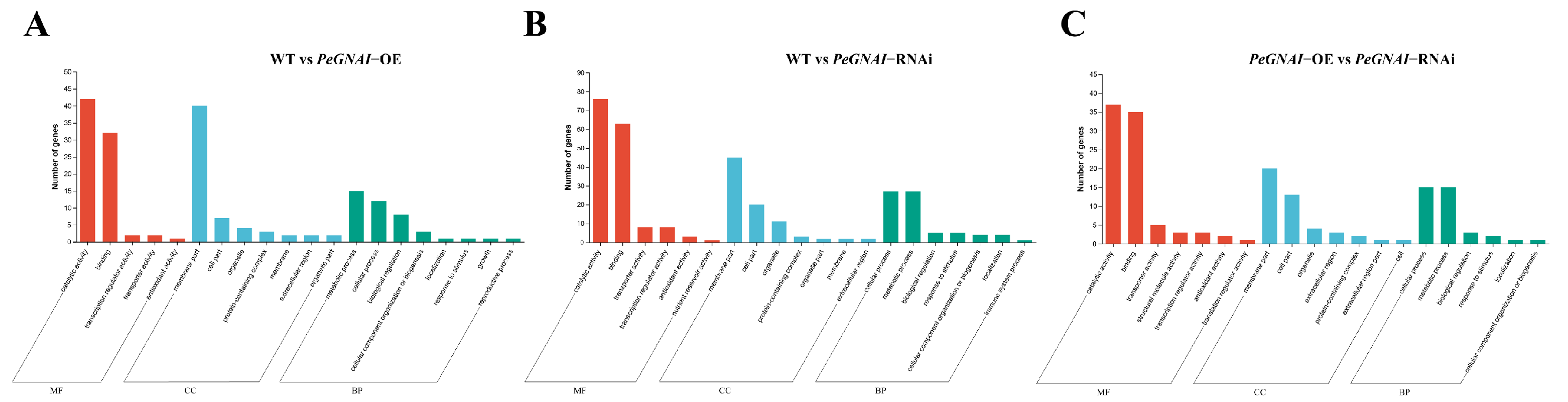
3.5. Functional Annotation of Differentially Expressed Genes
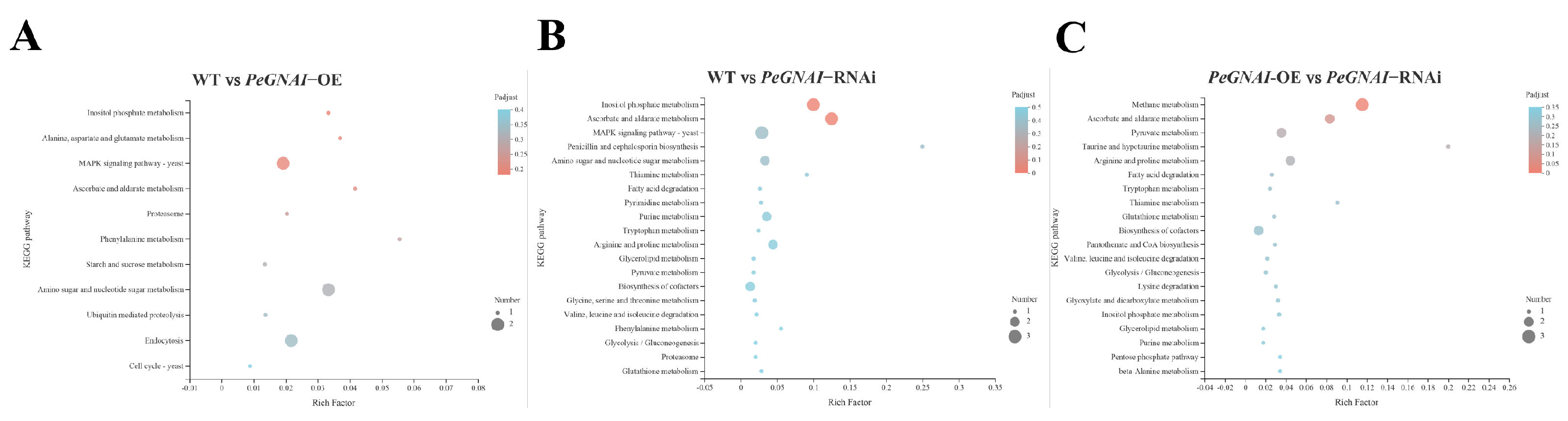
3.6. KEGG Enrichment Analysis for Differentially Expressed Genes
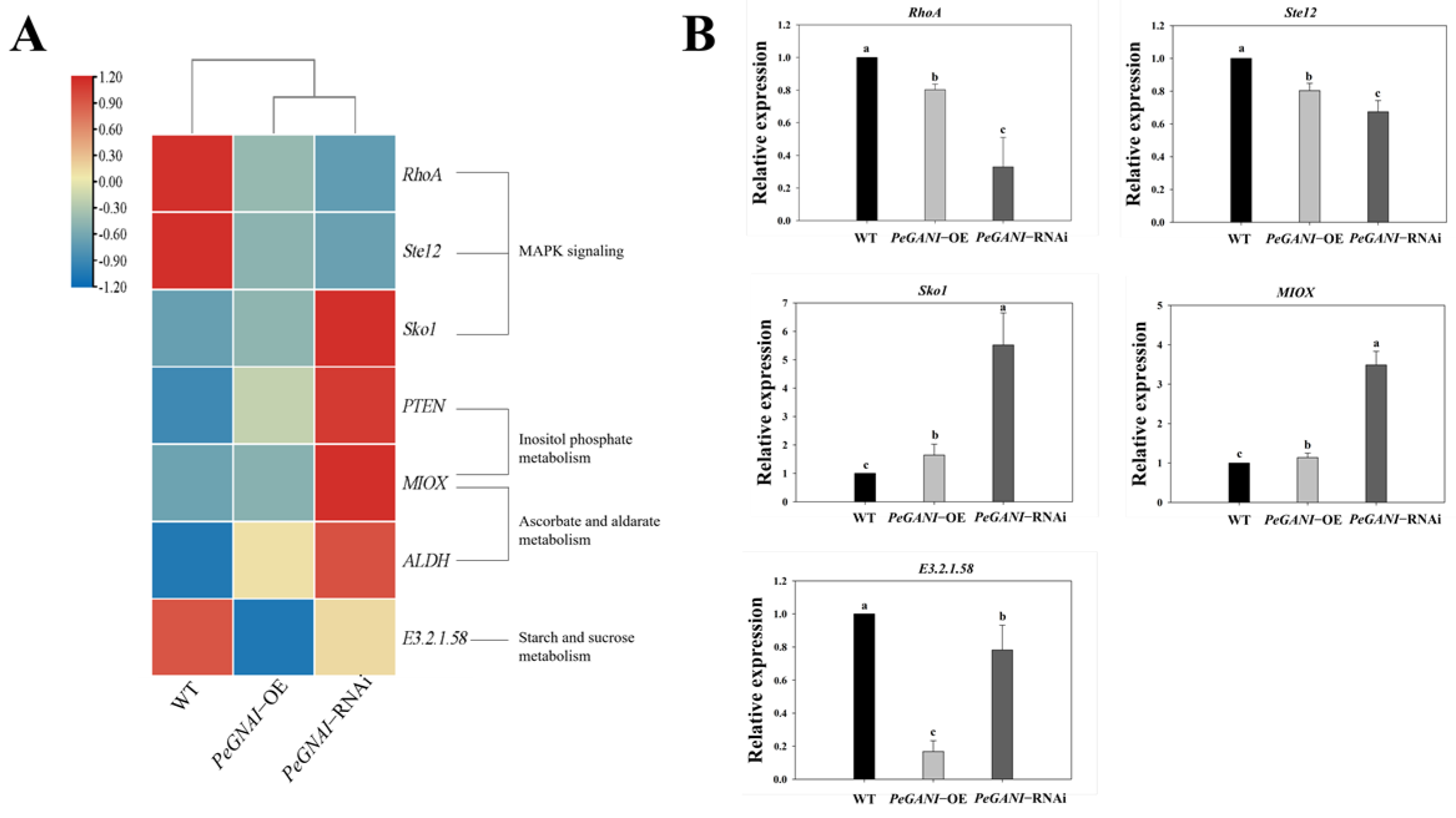
3.7. Expression of DEGs Related to Mycelial Growth and Development in P. eryngii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.; Dai, Y.; Yang, C.; Wei, P.; Song, B.; Yang, Y.; Sun, L.; Zhang, Z.-W.; Li, Y. Comparative Transcriptome Analysis Identified Candidate Genes Related to Bailinggu Mushroom Formation and Genetic Markers for Genetic Analyses and Breeding. Sci. Rep. 2017, 7, 9266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Bao, Z.; Feng, H.; Chen, L.; Li, Q. Nitric oxide enhances resistance of Pleurotus eryngii to cadmium stress by alleviating oxidative damage and regulating of short-chain dehydrogenase/reductase family. Environ. Sci. Pollut. Res. 2022, 29, 53036–53049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Y.; Zhang, F.; Linhardt, R.J.; Zeng, G.; Zhang, A. Extraction, structure and bioactivities of the polysaccharides from Pleurotus eryngii: A review. Int. J. Biol. Macromol. 2020, 150, 1342–1347. [Google Scholar] [CrossRef]
- Zheng, H.-G.; Chen, J.-C.; Weng, M.-J.; Ahmad, I.; Zhou, C.-Q. Structural characterization and bioactivities of a polysaccharide from the stalk residue of Pleurotus eryngii. Food Sci. Technol. 2020, 40, 235–241. [Google Scholar] [CrossRef]
- Ye, D.; Du, F.; Zou, Y.; Hu, Q. Transcriptomics Analysis of Primordium Formation in Pleurotus eryngii. Genes 2021, 12, 1863. [Google Scholar] [CrossRef]
- Kües, U.; Liu, Y. Fruiting body production in basidiomycetes. Appl. Microbiol. Biotechnol. 2000, 54, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Eid, E.M.; Al-Huqail, A.A.; Širić, I.; Adelodun, B.; Fayssal, S.A.; Valadez-Blanco, R.; Goala, M.; Ajibade, F.O.; Choi, K.S.; et al. Kinetic Studies on Delignification and Heavy Metals Uptake by Shiitake (Lentinula edodes) Mushroom Cultivated on Agro-Industrial Wastes. Horticulturae 2022, 8, 316. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.-H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- D’Souza, C.A.; Heitman, J. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 2001, 25, 349–364. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Bian, Z.; Wei, J.; Xu, J.-R. Coregulation of dimorphism and symbiosis by cyclic AMP signaling in the lichenized fungus Umbilicaria muhlenbergii. Proc. Natl. Acad. Sci. USA 2020, 117, 23847–23858. [Google Scholar] [CrossRef]
- Stateczny, D.; Oppenheimer, J.; Bommert, P. G protein signaling in plants: Minus times minus equals plus. Curr. Opin. Plant Biol. 2016, 34, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.-Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Xu, J.; Maeda, S.; Duc, N.M.; Ahn, D.; Du, Y.; Chung, K.Y. Structural mechanism underlying primary and secondary coupling between GPCRs and the Gi/o family. Nat. Commun. 2020, 11, 3160. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Li, Y.; Zhang, X.; Xuan, M.; Zhang, B.; Ye, W.; Zheng, X.; Govers, F.; Wang, Y. G protein α subunit suppresses sporangium formation through a serine/threonine protein kinase in Phytophthora sojae. PLoS Pathog. 2020, 16, e1008138. [Google Scholar] [CrossRef]
- McCudden, C.R.; Hains, M.D.; Kimple, R.J.; Siderovski, D.; Willard, F.S. G-protein signaling: Back to the future. Cell Mol. Life Sci. 2005, 62, 551–577. [Google Scholar] [CrossRef]
- Li, L.; Wright, S.J.; Krystofova, S.; Park, G.; Borkovich, K.A. Heterotrimeric G Protein Signaling in Filamentous Fungi. Annu. Rev. Microbiol. 2007, 61, 423–452. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, G.; Li, Z.; Qin, Y.; Qu, Y.; Song, X. G protein-cAMP signaling pathway mediated by PGA3 plays different roles in regulating the expressions of amylases and cellulases in Penicillium decumbens. Fungal Genet. Biol. 2013, 58–59, 62–70. [Google Scholar] [CrossRef]
- Du, M.; Xie, Y.; Wang, M.; Yang, H.; Hu, B.; Mukhtar, I.; Liu, Y.; Tao, Y.; Liu, F.; Xie, B. FFGA1 Protein Is Essential for Regulating Vegetative Growth, Cell Wall Integrity, and Protection against Stress in Flammunina filiformis. J. Fungi 2022, 8, 401. [Google Scholar] [CrossRef]
- Valle-Maldonado, M.I.; Patiño-Medina, J.A.; Pérez-Arques, C.; Reyes-Mares, N.Y.; Jácome-Galarza, I.E.; Ortíz-Alvarado, R.; Vellanki, S.; Ramírez-Díaz, M.I.; Lee, S.C.; Garre, V.; et al. The heterotrimeric G-protein beta subunit Gpb1 controls hyphal growth under low oxygen conditions through the protein kinase A pathway and is essential for virulence in the fungus Mucor circinelloides. Cell Microbiol. 2020, 22, e13236. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, H.; Liu, Z.; Wang, Z.; Huang, B. G-Protein Subunit Gαi in Mitochondria, MrGPA1, Affects Conidiation, Stress Resistance, and Virulence of Entomopathogenic Fungus Metarhizium robertsii. Front. Microbiol. 2020, 11, 1251. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.D.; Ai, L.Y.; Liu, Y.C.; Yan, M.; Sun, S.J. Comparative transcriptomics analyses of Pleurotus eryngii at different developmental stages. Mycosystema 2018, 37, 1586–1597. [Google Scholar] [CrossRef]
- Shi, L.; Fang, X.; Li, M.; Mu, D.; Ren, A.; Tan, Q.; Zhao, M. Development of a simple and efficient transformation system for the basidiomycetous medicinal fungus Ganoderma lucidum. World J. Microbiol. Biotechnol. 2011, 28, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, M.; Qian, X.; Yang, Z.; Xu, Y.; Wang, T.; Cao, J.; Sun, S. Bacterial Community Composition in the Growth Process of Pleurotus eryngii and Growth-Promoting Abilities of Isolated Bacteria. Front. Microbiol. 2022, 13, 787628. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, L.; Wu, X.; Gao, W.; Zhang, J.; Huang, C. Expression patterns of two pal genes of Pleurotus ostreatus across developmental stages and under heat stress. BMC Microbiol. 2019, 19, 231. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, D.; Zheng, L.; Chen, L.; Ma, A. Characterization of a G protein α subunit encoded gene from the dimorphic fungus-Tremella fuciformis. Antonie Leeuwenhoek 2021, 114, 1949–1960. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, B.; Jing, Z.; Lu, Y.; Ye, J.; Chen, Y.; Liu, F.; Li, S.; Xie, B.; Tao, Y. Flammulina filiformis Pkac Gene Complementing in Neurospora crassa Mutant Reveals Its Function in Mycelial Growth and Abiotic Stress Response. Life 2022, 12, 1336. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Peng, Y.; Yang, W.; Shi, J. Antifungal activity of 1-octen-3-ol against Monilinia fructicola and its ability in enhancing disease resistance of peach fruit. Food Control 2022, 135, 108804. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, Y.; Feng, Z.; Tan, Q. Effect of Different Packaging Films on Postharvest Quality and Selected Enzyme Activities of Hypsizygus marmoreus Mushrooms. J. Agric. Food Chem. 2008, 56, 11838–11844. [Google Scholar] [CrossRef]
- Sun, S.-J.; Liu, Y.-C.; Weng, C.-H.; Sun, S.-W.; Li, F.; Li, H.; Zhu, H. Cyclic Dipeptides Mediating Quorum Sensing and Their Biological Effects in Hypsizygus Marmoreus. Biomolecules 2020, 10, 298. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Y.; Xie, J.; Zhao, S.; Qin, W.; Song, Q.; Wang, S.; Rong, C. Bacterial Infection Induces Ultrastructural and Transcriptional Changes in the King Oyster Mushroom (Pleurotus eryngii). Microbiol. Spectr. 2022, 10, e0144522. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Boulaka, A.; Mantellou, P.; Stanc, G.-M.; Souka, E.; Valavanis, C.; Saxami, G.; Mitsou, E.; Koutrotsios, G.; Zervakis, G.I.; Kyriacou, A.; et al. Genoprotective activity of the Pleurotus eryngii mushrooms following their in vitro and in vivo fermentation by fecal microbiota. Front. Nutr. 2022, 9, 988517. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Liu, J.; Fang, Y.; Shao, Y.; Li, L.; Yu, J.-H.; Chen, F. Effects of Different G-Protein α-Subunits on Growth, Development and Secondary Metabolism of Monascus ruber M7. Front. Microbiol. 2019, 10, 1555. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, S.; Li, R.; Liu, C.; Fan, W.; Hu, T.; Zhao, A. Host-Induced Gene Silencing of a G Protein α Subunit Gene CsGpa1 Involved in Pathogen Appressoria Formation and Virulence Improves Tobacco Resistance to Ciboria shiraiana. J. Fungi 2021, 7, 1053. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-H.; Lee, N.-Y.; Kim, S.-S.; Park, H.-S.; Shin, K.-S. Comparative Characterization of G Protein α Subunits in Aspergillus fumigatus. Pathogens 2020, 9, 272. [Google Scholar] [CrossRef]
- Boruah, P.; Sarmah, P.; Das, P.K.; Goswami, T. Exploring the lignolytic potential of a new laccase producing strain Kocuria sp. PBS-1 and its application in bamboo pulp bleaching. Int. Biodeterior. Biodegrad. 2019, 143, 104726. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Hao, X.; Wang, D.; Akhberdi, O.; Xiang, B.; Zhu, X. Regulation of the Gα-cAMP/PKA signaling pathway in cellulose utilization of Chaetomium globosum. Microb. Cell Factories 2018, 17, 160. [Google Scholar] [CrossRef]
- Hu, Y.; Hao, X.; Chen, L.; Akhberdi, O.; Yu, X.; Liu, Y.; Zhu, X. Gα-cAMP/PKA pathway positively regulates pigmentation, chaetoglobosin A biosynthesis and sexual development in Chaetomium globosum. PLoS ONE 2018, 13, e0195553. [Google Scholar] [CrossRef]
- Li, X.; Zhong, K.; Yin, Z.; Hu, J.; Wang, W.; Li, L.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. The seven transmembrane domain protein MoRgs7 functions in surface perception and undergoes coronin MoCrn1-dependent endocytosis in complex with Gα subunit MoMagA to promote cAMP signaling and appressorium formation in Magnaporthe oryzae. PLoS Pathog. 2019, 15, e1007382. [Google Scholar] [CrossRef]
- Miwa, T.; Takagi, Y.; Shinozaki, M.; Yun, C.-W.; Schell, W.A.; Perfect, J.R.; Kumagai, H.; Tamaki, H. Gpr1, a Putative G-Protein-Coupled Receptor, Regulates Morphogenesis and Hypha Formation in the Pathogenic Fungus Candida albicans. Eukaryot. Cell 2004, 3, 919–931. [Google Scholar] [CrossRef]
- Yao, Y.; Sakamoto, T.; Honda, Y.; Kagotani, Y.; Izumitsu, K.; Suzuki, K.; Irie, T. The White-Rot Fungus Pleurotus ostreatus Transformant Overproduced Intracellular cAMP and Laccase. Biosci. Biotechnol. Biochem. 2013, 77, 2309–2311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watts, V.J.; Neve, K.A. Sensitization of adenylate cyclase by Gαi/o-coupled receptors. Pharmacol. Ther. 2005, 106, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Wirth, S.; Kunert, M.; Ahrens, L.-M.; Krause, K.; Broska, S.; Paetz, C.; Kniemeyer, O.; Jung, E.-M.; Boland, W.; Kothe, E. The regulator of G-protein signalling Thn1 links pheromone response to volatile production in Schizophyllum commune. Environ. Microbiol. 2018, 20, 3684–3699. [Google Scholar] [CrossRef]
- Murry, R.; Traxler, L.; Pötschner, J.; Krüger, T.; Kniemeyer, O.; Krause, K.; Kothe, E. Inositol Signaling in the Basidiomycete Fungus Schizophyllum commune. J. Fungi 2021, 7, 470. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, P.; Ma, Y.; Jiang, R.; Jiang, C.; Wang, G. Insights into intracellular signaling network in Fusarium species. Int. J. Biol. Macromol. 2022, 222, 1007–1014. [Google Scholar] [CrossRef]
- Martínez-Soto, D.; Ruiz-Herrera, J. Functional analysis of the MAPK pathways in fungi. Rev. Iberoam. Micol. 2017, 34, 192–202. [Google Scholar] [CrossRef]
- Kwon, M.J.; Arentshorst, M.; Roos, E.D.; Hondel, C.A.M.J.J.V.D.; Meyer, V.; Ram, A.F.J. Functional characterization of Rho GTPases in Aspergillus niger uncovers conserved and diverged roles of Rho proteins within filamentous fungi. Mol. Microbiol. 2010, 79, 1151–1167. [Google Scholar] [CrossRef]
- Heasman, S.J.; Ridley, A.J. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008, 9, 690–701. [Google Scholar] [CrossRef]
- Nozaki, S.; Furuya, K.; Niki, H. The Ras1-Cdc42 pathway is involved in hyphal development of Schizosaccharomyces japonicus. FEMS Yeast Res. 2018, 18, foy031. [Google Scholar] [CrossRef]
- Fortwendel, J.R. Orchestration of morphogenesis in filamentous fungi: Conserved roles for Ras signaling networks. Fungal Biol. Rev. 2015, 29, 54–62. [Google Scholar] [CrossRef]
- Manglekar, R.R.; Geng, A. CRISPR-Cas9-mediated seb1 disruption in Talaromyces pinophilus EMU for its enhanced cellulase production. Enzym. Microb. Technol. 2020, 140, 109646. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Du, Y.; Jin, K.; Xia, Y. The Ste12-like transcription factor MaSte12 is involved in pathogenicity by regulating the appressorium formation in the entomopathogenic fungus, Metarhizium acridum. Appl. Microbiol. Biotechnol. 2017, 101, 8571–8584. [Google Scholar] [CrossRef] [PubMed]
- Dolan, J.W.; Kirkman, C.; Fields, S. The yeast STE12 protein binds to the DNA sequence mediating pheromone induction. Proc. Natl. Acad. Sci. USA 1989, 86, 5703–5707. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-Q.; Li, P.; Wu, M.; Hao, Z.-M.; Gong, X.-D.; Zhang, X.-Y.; Tian, L.; Zhang, P.; Wang, Y.; Cao, Z.-Y.; et al. StSTE12 is required for the pathogenicity of Setosphaeria turcica by regulating appressorium development and penetration. Microbiol. Res. 2014, 169, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Monge, R.; Román, E.; Arana, D.M.; Prieto, D.; Urrialde, V.; Nombela, C.; Pla, J. The Sko1 protein represses the yeast-to-hypha transition and regulates the oxidative stress response in Candida albicans. Fungal Genet. Biol. 2010, 47, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fan, F.; Yang, J.; Wang, X.; Qiu, D.; Jiang, L. FgTep1p is linked to the phosphatidylinositol-3 kinase signalling pathway and plays a role in the virulence of Fusarium graminearum on wheat. Mol. Plant Pathol. 2010, 11, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Heymont, J.; Berenfeld, L.; Collins, J.; Kaganovich, A.; Maynes, B.; Moulin, A.; Ratskovskaya, I.; Poon, P.P.; Johnston, G.C.; Kamenetsky, M.; et al. TEP1, the yeast homolog of the human tumor suppressor gene PTEN/MMAC1/TEP1, is linked to the phosphatidylinositol pathway and plays a role in the developmental process of sporulation. Proc. Natl. Acad. Sci. USA 2000, 97, 12672–12677. [Google Scholar] [CrossRef]
- Li, J.; Garavaglia, S.; Ye, Z.; Moretti, A.; Belyaeva, O.V.; Beiser, A.; Ibrahim; Wilk, A.; McClellan, S.; Klyuyeva, A.V.; et al. A specific inhibitor of ALDH1A3 regulates retinoic acid biosynthesis in glioma stem cells. Commun. Biol. 2021, 4, 1420. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Sun, M.; Yu, M.; Xu, Y.; Xie, J.; Zhang, H.; Chen, J.; Xu, T.; Qian, X.; Sun, S. Transcriptome Analysis Reveals the Function of a G-Protein α Subunit Gene in the Growth and Development of Pleurotus eryngii. J. Fungi 2023, 9, 69. https://doi.org/10.3390/jof9010069
Cao J, Sun M, Yu M, Xu Y, Xie J, Zhang H, Chen J, Xu T, Qian X, Sun S. Transcriptome Analysis Reveals the Function of a G-Protein α Subunit Gene in the Growth and Development of Pleurotus eryngii. Journal of Fungi. 2023; 9(1):69. https://doi.org/10.3390/jof9010069
Chicago/Turabian StyleCao, Jixuan, Meijing Sun, Mingming Yu, Yanfei Xu, Jiacheng Xie, Huangru Zhang, Jiayi Chen, Tao Xu, Xin Qian, and Shujing Sun. 2023. "Transcriptome Analysis Reveals the Function of a G-Protein α Subunit Gene in the Growth and Development of Pleurotus eryngii" Journal of Fungi 9, no. 1: 69. https://doi.org/10.3390/jof9010069
APA StyleCao, J., Sun, M., Yu, M., Xu, Y., Xie, J., Zhang, H., Chen, J., Xu, T., Qian, X., & Sun, S. (2023). Transcriptome Analysis Reveals the Function of a G-Protein α Subunit Gene in the Growth and Development of Pleurotus eryngii. Journal of Fungi, 9(1), 69. https://doi.org/10.3390/jof9010069




