Sensitivity of Serum Beta-D-Glucan in Candidemia According to Candida Species Epidemiology in Critically Ill Patients Admitted to the Intensive Care Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Microbiology
2.3. Statistical Analysis
2.4. Ethical Considerations
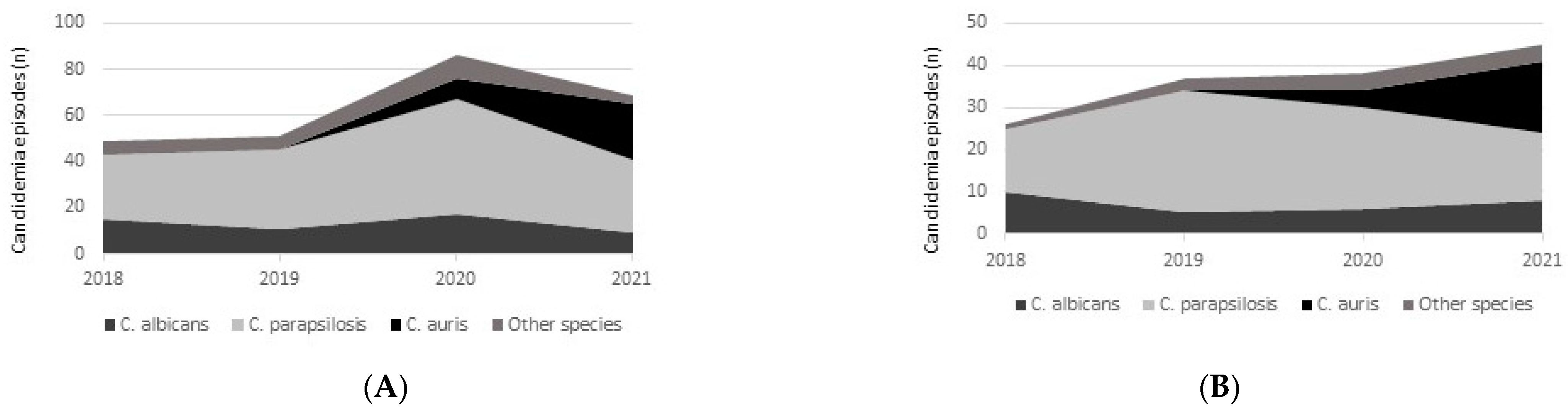
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patterson, T.F.; Donnelly, J.P. New Concepts in Diagnostics for Invasive Mycoses: Non-Culture-Based Methodologies. J. Fungi 2019, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Karageorgopoulos, D.E.; Vouloumanou, E.K.; Ntziora, F.; Michalopoulos, A.; Rafailidis, P.I.; Falagas, M.E. β-D-Glucan Assay for the Diagnosis of Invasive Fungal Infections: A Meta-Analysis. Clin. Infect. Dis. 2011, 52, 750–770. [Google Scholar] [CrossRef] [PubMed]
- Rouzé, A.; Estella, Á.; Timsit, J.-F. Is (1,3)-β-d-Glucan Useless to Guide Antifungal Therapy in ICU? Intensive Care Med. 2022, 48, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Martín-Mazuelos, E.; Loza, A.; Castro, C.; Macías, D.; Zakariya, I.; Saavedra, P.; Ruiz-Santana, S.; Marín, E.; León, C. β-d-Glucan and Candida Albicans Germ Tube Antibody in ICU Patients with Invasive Candidiasis. Intensive Care Med. 2015, 41, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Cruciani, M.; Mengoli, C.; Castagnola, E.; Lortholary, O.; Richardson, M.; Marchetti, O. Third European Conference on Infections in Leukemia (ECIL-3) β-Glucan Antigenemia Assay for the Diagnosis of Invasive Fungal Infections in Patients with Hematological Malignancies: A Systematic Review and Meta-Analysis of Cohort Studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin. Infect. Dis. 2012, 54, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Balletto, E.; Castagnola, E.; Mularoni, A. Beta-D-Glucan in Patients with Haematological Malignancies. J. Fungi 2021, 7, 1046. [Google Scholar] [CrossRef] [PubMed]
- Bloos, F.; Held, J.; Kluge, S.; Simon, P.; Kogelmann, K.; de Heer, G.; Kuhn, S.-O.; Jarczak, D.; Motsch, J.; Hempel, G.; et al. (1 → 3)-β-d-Glucan-Guided Antifungal Therapy in Adults with Sepsis: The CandiSep Randomized Clinical Trial. Intensive Care Med. 2022, 48, 865–875. [Google Scholar] [CrossRef] [PubMed]
- León, C.; Ruiz-Santana, S.; Saavedra, P.; Castro, C.; Loza, A.; Zakariya, I.; Úbeda, A.; Parra, M.; Macías, D.; Tomás, J.I.; et al. Contribution of Candida Biomarkers and DNA Detection for the Diagnosis of Invasive Candidiasis in ICU Patients with Severe Abdominal Conditions. Crit. Care 2016, 20, 149. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* Guideline for the Diagnosis and Management of Candida Diseases 2012: Non-Neutropenic Adult Patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 19–37. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID Task Force on Practical Management of Invasive Candidiasis in Critically Ill Patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Giacobbe, D.R.; Furfaro, E.; Mesini, A.; Marchese, A.; Del Bono, V.; Viscoli, C. Lower Sensitivity of Serum (1,3)-β-d-Glucan for the Diagnosis of Candidaemia Due to Candida Parapsilosis. Clin. Microbiol. Infect. 2016, 22, 646.e5–646.e8. [Google Scholar] [CrossRef] [PubMed]
- Chibabhai, V.; Fadana, V.; Bosman, N.; Nana, T. Comparative Sensitivity of 1,3 Beta-D-Glucan for Common Causes of Candidaemia in South Africa. Mycoses 2019, 62, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, J.; Niamatullah, H.; Irfan, S.; Zafar, A.; Malik, F.; Jabeen, K. Comparison of β-d-Glucan Levels between Candida Auris and Other Candida Species at the Time of Candidaemia: A Retrospective Study. Clin. Microbiol. Infect. 2021, 27, 1519.e1–1519.e5. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Furfaro, E.; Magnasco, L.; Codda, G.; Giacobbe, D.R.; Dentone, C.; Vena, A.; Marchese, A.; Bassetti, M. Levels of Beta-D-Glucan in Candida Auris Supernatants, an in Vitro and in Vivo Preliminary Study. Clin. Microbiol. Infect. 2022, 28, 1154.e1–1154.e3. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, A.; Poissy, J.; Croxatto, A.; Bochud, P.-Y.; Pagani, J.-L.; Lamoth, F. Impact of the Beta-Glucan Test on Management of Intensive Care Unit Patients at Risk for Invasive Candidiasis. J. Clin. Microbiol. 2020, 58, e01996-19. [Google Scholar] [CrossRef]
- Haydour, Q.; Hage, C.A.; Carmona, E.M.; Epelbaum, O.; Evans, S.E.; Gabe, L.M.; Knox, K.S.; Kolls, J.K.; Wengenack, N.L.; Prokop, L.J.; et al. Diagnosis of Fungal Infections. A Systematic Review and Meta-Analysis Supporting American Thoracic Society Practice Guideline. Ann. Am. Thorac. Soc. 2019, 16, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Posteraro, B.; D’Arrigo, S.; Spinazzola, G.; Gaspari, R.; Bello, G.; Montini, L.M.; Cutuli, S.L.; Grieco, D.L.; Di Gravio, V.; et al. (1,3)-β-d-Glucan-Based Empirical Antifungal Interruption in Suspected Invasive Candidiasis: A Randomized Trial. Crit. Care 2020, 24, 550. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Asperges, E.; Cortegiani, A.; Grecchi, C.; Rebuffi, C.; Zuccaro, V.; Scudeller, L.; Bassetti, M.; The FUNDICU investigators. Performance of Existing Clinical Scores and Laboratory Tests for the Diagnosis of Invasive Candidiasis in Critically Ill, Nonneutropenic, Adult Patients: A Systematic Review with Qualitative Evidence Synthesis. Mycoses 2022. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Mikulska, M.; Tumbarello, M.; Furfaro, E.; Spadaro, M.; Losito, A.R.; Mesini, A.; De Pascale, G.; Marchese, A.; Bruzzone, M.; et al. Combined Use of Serum (1,3)-β-d-Glucan and Procalcitonin for the Early Differential Diagnosis between Candidaemia and Bacteraemia in Intensive Care Units. Crit. Care 2017, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jiménez, M.C.; Muñoz, P.; Valerio, M.; Alonso, R.; Martos, C.; Guinea, J.; Bouza, E. Candida Biomarkers in Patients with Candidaemia and Bacteraemia. J. Antimicrob. Chemother. 2015, 70, 2354–2361. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Candida Species (Total Number of Episodes = 146; Total Number of BDG Samples = 187) § | Median BDG Value (IQR), in pg/mL §§ | Median Time from Candidemia Onset to First BDG Determination (IQR), in Days | Sensitivity (95% CI) |
|---|---|---|---|
| For all samples (n = 187) | 84 (21–314) | −0.06 (−1.28, 0.83) | 51.3% (44.1–58.5%) |
| For all episodes (n = 146) | 47.3% (39.0–55.0%) | ||
| For C. albicans (n = 40) samples | 182 (30.5–523) | 0 (−1.16–0.78) | 65.0% (48.7–78.4%) |
| For C. albicans (n = 29) episodes | 62.1% (42.8–78.2%) | ||
| For C. parapsilosis (n = 105) samples | 78 (19–290) | −0.08 (−1.29–1.00) | 48.6% (39.1–58.2%) |
| For C. parapsilosis (n = 84) episodes | 44.0% (33.7–54.9%) | ||
| For C. auris (n = 26) samples | 48 (15–159) | 0 (−1.99–0.83) | 42.3% (24.5–62.4%) |
| For C. auris (n = 21) episodes | 42.9% (23.0–65.3%) | ||
| For other species (n = 16) samples * | 81 (29–195) | −0.62 (−1.99–0.53) | 50.0% (25.6–74.4%) |
| For other species (n = 12) episodes ** | 41.7% (16.4–72.2%) |
| BDG Values * | All (n = 37) | C. albicans (n = 10) | C. auris (n = 5) | C. parapsilosis (n = 18) | Other Species (n = 4) |
|---|---|---|---|---|---|
| Discordant values | 7 (18.9) | 1 (10) | 2 (40) | 3 (16.7) | 1 (25) |
| Concordant values | 30 (81.1) | 9 (90) | 3 (60) | 15 (83.3) | 3 (75) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikulska, M.; Magnasco, L.; Signori, A.; Sepulcri, C.; Dettori, S.; Tutino, S.; Vena, A.; Miletich, F.; Ullah, N.; Morici, P.; et al. Sensitivity of Serum Beta-D-Glucan in Candidemia According to Candida Species Epidemiology in Critically Ill Patients Admitted to the Intensive Care Unit. J. Fungi 2022, 8, 921. https://doi.org/10.3390/jof8090921
Mikulska M, Magnasco L, Signori A, Sepulcri C, Dettori S, Tutino S, Vena A, Miletich F, Ullah N, Morici P, et al. Sensitivity of Serum Beta-D-Glucan in Candidemia According to Candida Species Epidemiology in Critically Ill Patients Admitted to the Intensive Care Unit. Journal of Fungi. 2022; 8(9):921. https://doi.org/10.3390/jof8090921
Chicago/Turabian StyleMikulska, Malgorzata, Laura Magnasco, Alessio Signori, Chiara Sepulcri, Silvia Dettori, Stefania Tutino, Antonio Vena, Franca Miletich, Nadir Ullah, Paola Morici, and et al. 2022. "Sensitivity of Serum Beta-D-Glucan in Candidemia According to Candida Species Epidemiology in Critically Ill Patients Admitted to the Intensive Care Unit" Journal of Fungi 8, no. 9: 921. https://doi.org/10.3390/jof8090921
APA StyleMikulska, M., Magnasco, L., Signori, A., Sepulcri, C., Dettori, S., Tutino, S., Vena, A., Miletich, F., Ullah, N., Morici, P., Ball, L., Pelosi, P., Marchese, A., Giacobbe, D. R., & Bassetti, M. (2022). Sensitivity of Serum Beta-D-Glucan in Candidemia According to Candida Species Epidemiology in Critically Ill Patients Admitted to the Intensive Care Unit. Journal of Fungi, 8(9), 921. https://doi.org/10.3390/jof8090921










