Complementary Effects of Dark Septate Endophytes and Trichoderma Strains on Growth and Active Ingredient Accumulation of Astragalus mongholicus under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and Plant Materials
2.2. Inoculation Assay
2.3. Plant Growth Parameters
2.4. Physiological Parameter of Plant Leaves
2.5. Physiological Parameter and Active Ingredients of Plant Roots
2.6. Quantification of DSE Root Colonization
2.7. Quantification of the N and P Contents of Plants
2.8. Soil Physicochemical Properties
2.9. Statistical Analyses
3. Results
3.1. DSE Colonization and Re-Isolation in Roots
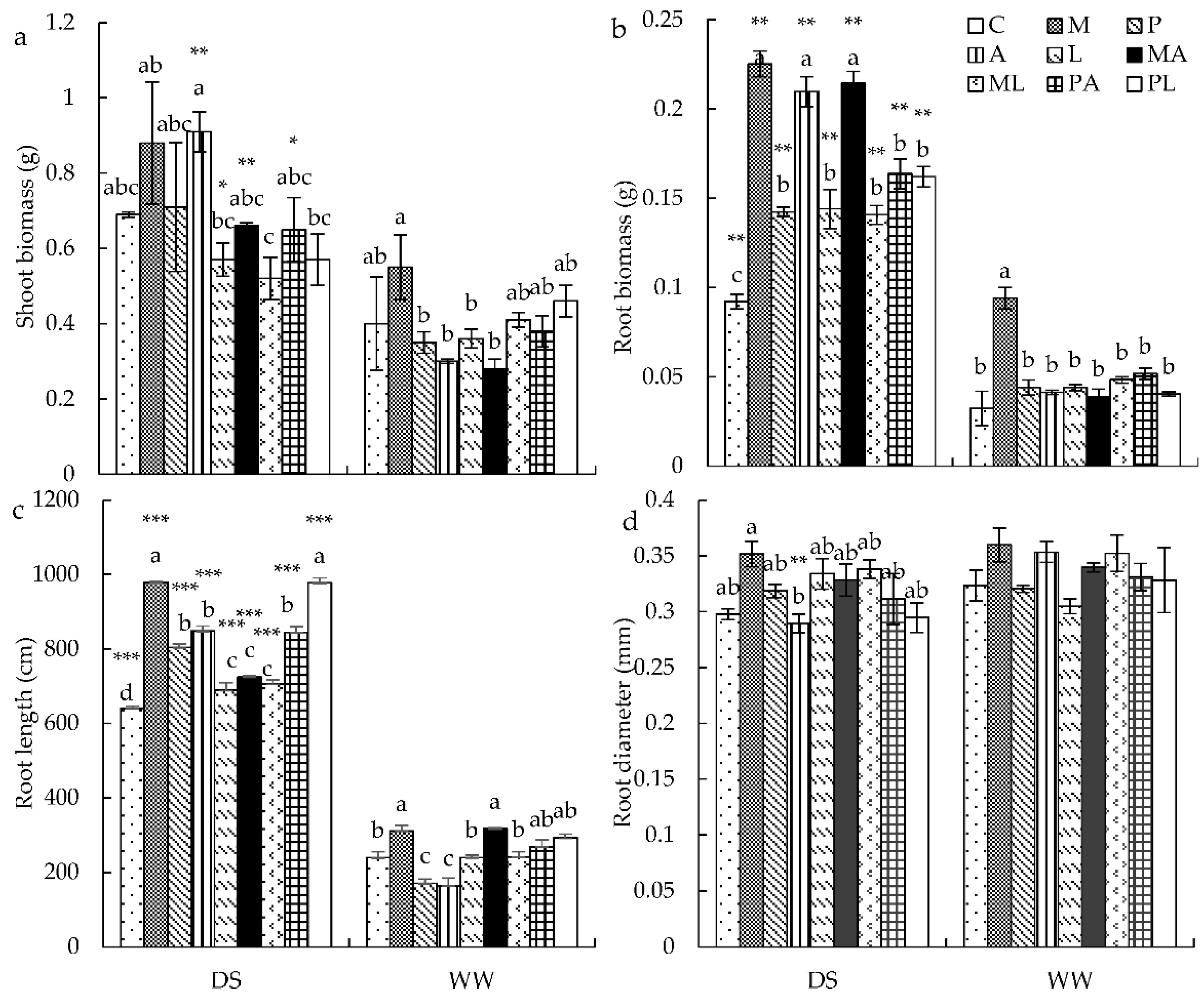
3.2. Plant Growth Parameters
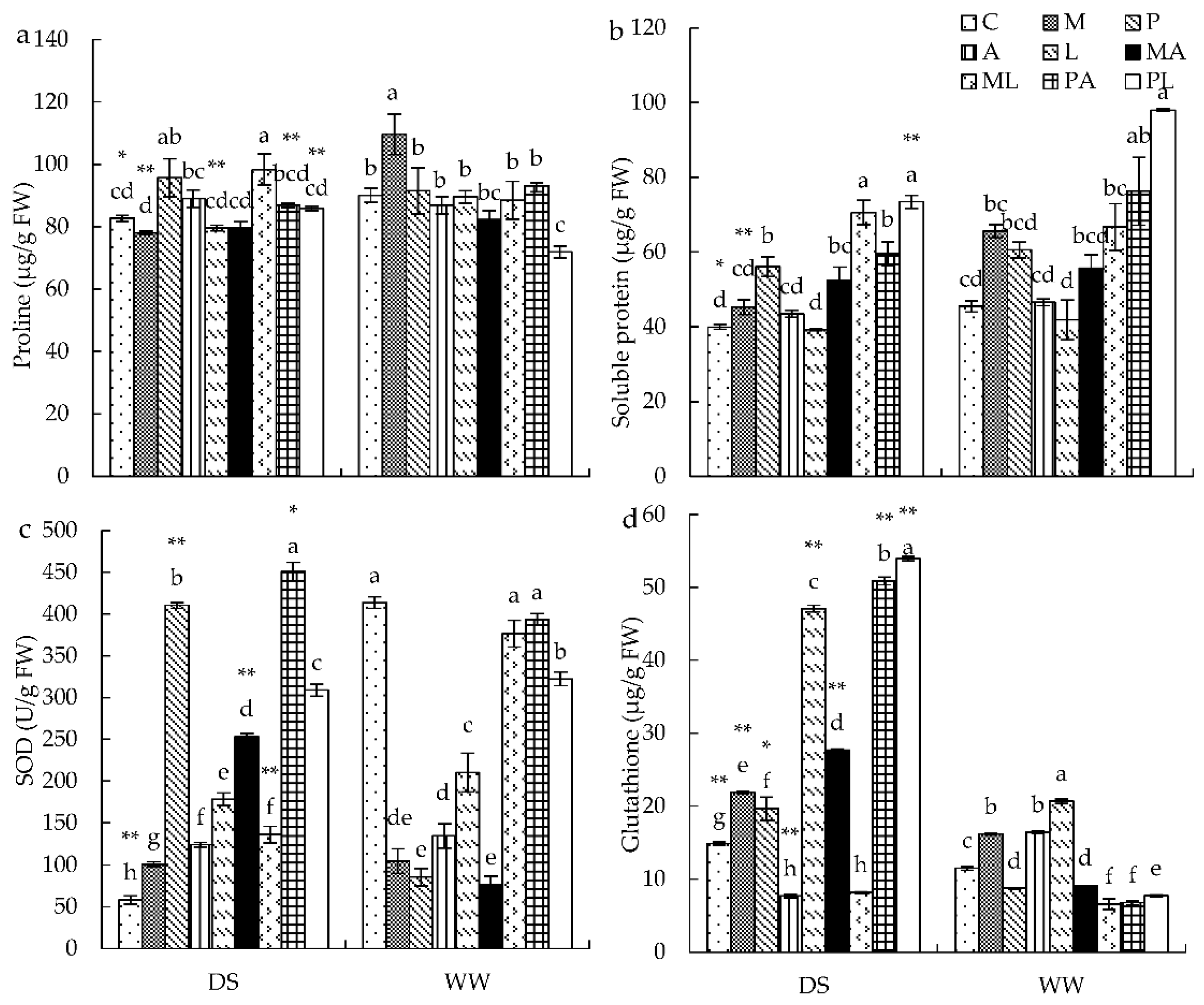
3.3. Osmotic Materials and Antioxidant Enzyme Activities in Leaves
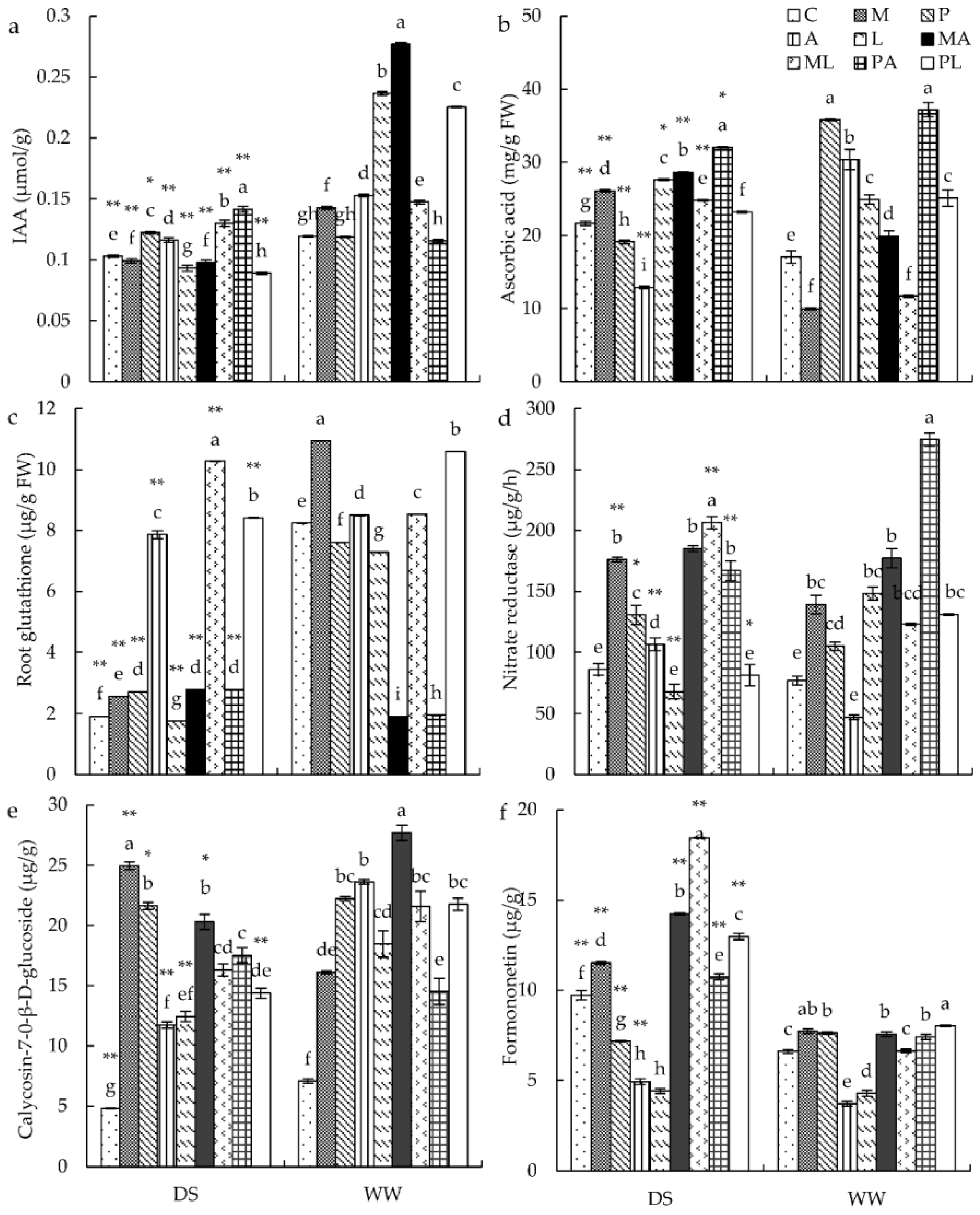
3.4. Antioxidant Parameters and IAA Contents in Roots
3.5. Active Ingredient Contents in the Roots
3.6. N and P Content in the Shoots and Roots
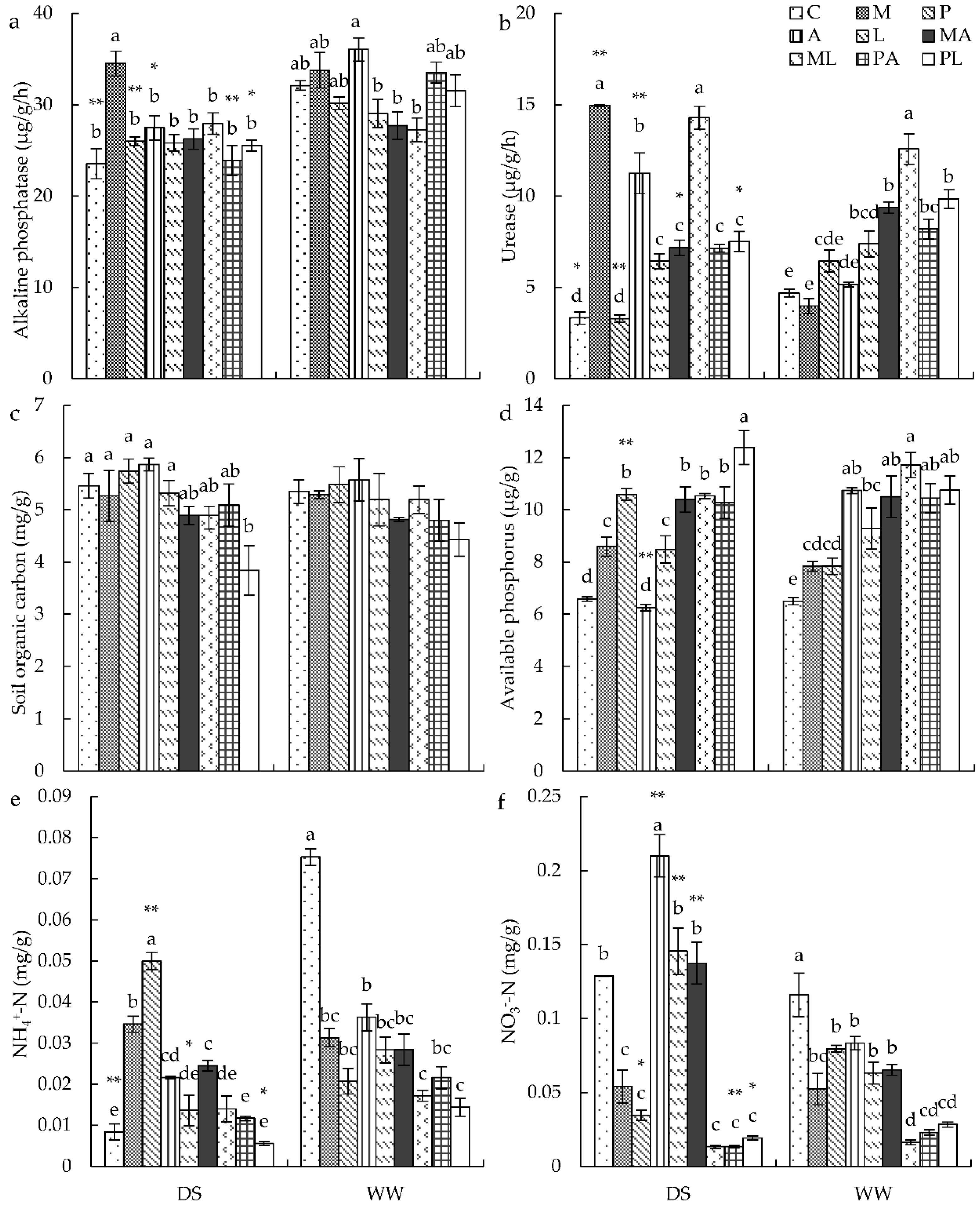
3.7. Soil Factors
3.8. Correlation Analyses of Growth Parameters and Chemical Constituents
3.9. Variation Partitioning Analysis of Growth Parameters and Chemical Constituents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alori, E.T.; Emmanuel, O.C.; Glick, B.R.; Babalola, O.O. Plant-archaea relationships: A potential means to improve crop production in arid and semi-arid regions. World J. Microbiol. Biotechnol. 2020, 36, 133. [Google Scholar]
- Farrell, C.; Szota, C.; Arndt, S.K. Does the turgor loss point characterize drought response in dryland plants? Plant Cell Environ. 2017, 40, 1500–1511. [Google Scholar] [CrossRef]
- Saritha, M.; Kumar, P.; Panwar, N.R.; Burman, U. Intelligent plant-microbe interactions. Arch. Agron. Soil Sci. 2021, 68, 1002–1018. [Google Scholar] [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5, e02952. [Google Scholar]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzalka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Jumpponen, A. Dark septate endophytes—Are they mycorrhizal? Mycorrhiza 2001, 11, 207–211. [Google Scholar] [CrossRef]
- Mandyam, K.; Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef]
- Berthelot, C.; Perrin, Y.; Leyval, C.; Blaudez, D. Melanization and ageing are not drawbacks for successful agro-transformation of dark septate endophytes. Fungal Biol. 2017, 121, 652–663. [Google Scholar] [CrossRef]
- Zhang, H.H.; Tang, M.; Chen, H.; Wang, Y.J. Effects of a dark-septate endophytic isolate LBF-2 on the medicinal plant Lycium barbarum L. J. Microbiol. 2012, 50, 91–96. [Google Scholar] [CrossRef]
- Caldwell, B.; Jumpponen, A.; Trappe, J. Utilization of major detrital substrates by dark-septate, root endophytes. Mycologia 2000, 92, 230–232. [Google Scholar] [CrossRef]
- Hill, P.W.; Broughton, R.; Bougoure, J.; Havelange, W.; Newsham, K.K.; Grant, H.; Murphy, D.V.; Clode, P.; Ramayah, S.; Marsden, K.A.; et al. Angiosperm symbioses with non-mycorrhizal fungal partners enhance N acquisition from ancient organic matter in a warming maritime Antarctic. Ecol. Lett. 2019, 22, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [Green Version]
- Rawal, R.; Scheerens, J.C.; Fenstemaker, S.M.; Francis, D.M.; Miller, S.A.; Benitez, M.S. Novel Trichoderma isolates alleviate water deficit stress in susceptible tomato genotypes. Front. Plant Sci. 2022, 13, 869090. [Google Scholar]
- Pedrero-Méndez, A.; Insuasti, H.C.; Neagu, T.; Illescas, M.; Rubio, M.B.; Monte, E.; Hermosa, R. Why is the correct selection of Trichoderma strains important? The case of wheat endophytic strains of T. Harzianum and T. simmonsii. J. Fungi 2021, 7, 1087. [Google Scholar]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone production profiles in Trichoderma species and their relationship to wheat plant responses to water stress. Pathogens 2021, 10, 991. [Google Scholar]
- Lugo, M.A.; Reinhart, K.O.; Menoyo, E.; Crespo, E.M.; Urcelay, C. Plant functional traits and phylogenetic relatedness explain variation in associations with root fungal endophytes in an extreme arid environment. Mycorrhiza 2015, 25, 85–95. [Google Scholar]
- Zhan, F.D.; He, Y.M.; Li, T.; Yang, Y.Y.; Gurpal, S.T.; Zhao, Z.W. Tolerance and antioxidant response of a dark septate endophyte (DSE), Exophiala pisciphila, to cadmium stress. Bull. Environ. Contam. Toxicol. 2015, 94, 96–102. [Google Scholar] [CrossRef]
- Monica, I.F.D.; Saparrat, M.C.N.; Godeas, A.M.; Scervino, J.M. The co-existence between DSE and AMF symbionts affects plant P pools through P mineralization and solubilization processes. Fungal Ecol. 2015, 17, 10–17. [Google Scholar]
- Yuan, S.F.; Li, M.Y.; Fang, Z.Y.; Liu, Y.; Shi, W.; Pan, B.; Wu, K.; Shi, J.X.; Shen, B.; Shen, Q.R. Biological control of tobacco bacterial wilt using Trichoderma harzianum, amended bioorganic fertilizer and the arbuscular mycorrhizal fungi Glomus mosseae. Biol. Control 2016, 92, 164–171. [Google Scholar]
- El-Sharkawy, E.E.S.; Abdelrazik, E. Biocontrol of Fusarium root rot in squash using mycorrhizal fungi and antagonistic microorganisms. Egypt. J. Biol. Pest Control 2022, 32, 13. [Google Scholar] [CrossRef]
- Kaewchai, S. Mycofungicides and fungal biofertilizers. Fungal Divers. 2009, 38, 25–50. [Google Scholar]
- Yin, D.C.; Deng, X.; Song, R.Q. Synergistic effects between Suilllus luteus and Trichoderma virens on growth of Korean spruce seedlings and drought resistance of Scotch pine seedlings. J. For. Res. (Harbin) 2016, 27, 193–201. [Google Scholar]
- Xiong, Y.; Zhao, Q.Y.; Gu, L.Y.; Liu, C.Y.; Wang, C. Shenqi Fuzheng injection reverses cisplatin resistance through mitofusin-2-mediated cell cycle arrest and apoptosis in A549/DDP cells. Evid. Based Complement. Altern. Med. 2018, 2018, 8258246. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, B.Z.; Jia, X.; Sun, S.Y.; Su, Y.L.; Chen, G.L. Differential expression of calycosin-7-O-β-D-glucoside biosynthesis genes and accumulation of related metabolites in different organs of Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao under drought stress. Appl. Biochem. Biotechnol. 2022, 194, 3182–3195. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.P.; Xue, Z.Q.; Yang, Z.Y.; Chai, Z.; Niu, J.P.; Shi, Z.Y. Effects of microbial organic fertilizers on Astragalus membranaceus growth and rhizosphere microbial community. Ann. Microbiol. 2021, 71, 11. [Google Scholar]
- Sun, H.F.; Kong, L.L.; Du, H.Z.; Chai, Z.; Gao, J.P.; Cao, Q.F. Benefits of Pseudomonas poae s61 on Astragalus mongholicus growth and bioactive compound accumulation under drought stress. J. Plant Interact. 2019, 14, 205–212. [Google Scholar] [CrossRef]
- Han, L.; Zuo, Y.L.; He, X.L.; Hou, Y.T.; Li, M.; Li, B.K. Plant identity and soil variables shift the colonisation and species composition of dark septate endophytes associated with medicinal plants in a northern farmland in China. Appl. Soil Ecol. 2021, 167, 104042. [Google Scholar]
- He, C.; Wang, W.Q.; Hou, J.L. Plant performance of enhancing licorice with dual inoculating dark septate endophytes and Trichoderma viride mediated via effects on root development. BMC Plant Biol. 2020, 20, 325. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Meth. Enzymol. 1985, 113, 548–555. [Google Scholar]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. Methods Mol. Biol. 2010, 639, 273–281. [Google Scholar] [PubMed]
- Reis, A.R.; Favarin, J.L.; Gallo, L.A.; Malavolta, E.; Moraes, M.F.; Lavres, J. Nitrate reductase and glutamine synthetase activity in coffee leaves during fruit development. Revista Brasileira Ciência Solo 2009, 33, 315–324. [Google Scholar]
- Kampfenkel, K.; Vanmontagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- National Pharmacopoeia Commission. Chinese Pharmacopoeia; Medical Science and Technology Press: Beijing, China, 2015.
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–163. [Google Scholar]
- Fraser, L.H.; Carty, S.M.; Steer, D. A test of four plant species to reduce total nitrogen and total phosphorus from soil leachate in subsurface wetland microcosms. Bioresour. Technol. 2004, 94, 185–192. [Google Scholar]
- Rawls, W.J.; Pachepsky, Y.A.; Ritchie, J.C.; Sobecki, T.M.; Bloodworth, H. Effect of soil organic carbon on soil water retention. Geoderma 2003, 116, 61–76. [Google Scholar]
- Tarafdar, J.C.; Marschner, H. Phosphatase activity in the rhizosphere and hyphosphere of VA mycorrhizal wheat supplied with inorganic and organic phosphorus. Soil Biol. Biochem. 1994, 26, 387–395. [Google Scholar]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar]
- Bao, S.D. Agrochemical Analysis of Soil; Chinese Agricultural Press: Beijing, China, 2000; pp. 44–49. [Google Scholar]
- Yan, L.; Zhu, J.; Zhao, X.X.; Shi, J.L.; Jiang, C.M.; Shao, D.Y. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biot. 2019, 103, 3327–3340. [Google Scholar]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [PubMed]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Li, X.; He, C.; He, X.L.; Su, F.; Hou, L.F.; Ren, Y.; Hou, Y.T. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil 2019, 439, 259–272. [Google Scholar]
- Harman, G.E. Myths and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzianum T22. Plant Dis. 2000, 84, 377–393. [Google Scholar]
- Basirat, M.; Mousavi, S.M.; Abbaszadeh, S.; Ebrahimi, M.; Zarebanadkouki, M. The rhizosheath: A potential root trait helping plants to tolerate drought stress. Plant Soil 2019, 445, 565–575. [Google Scholar]
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 2004, 55, 2343–2351. [Google Scholar]
- Wang, X.; Ding, T.; Li, Y.; Guo, Y.; Li, Y.; Duan, T. Dual inoculation of alfalfa (Medicago sativa L.) with Funnelliformis mosseae and Sinorhizobium medicae can reduce Fusarium wilt. J. Appl. Microbiol. 2020, 129, 665–679. [Google Scholar]
- Li, M.; Hou, L.F.; Liu, J.Q.; Yang, J.Y.; Zuo, Y.L.; Zhao, L.L.; He, X.L. Growth-promoting effects of dark septate endophytes on the non-mycorrhizal plant Isatis indigotica under different water conditions. Symbiosis 2021, 85, 291–303. [Google Scholar]
- Kushwaha, R.K.; Singh, S.; Pandey, S.S.; Rao, D.K.V.; Nagegowda, D.A.; Kalra, A.; Babu, C.S.V. Compatibility of inherent fungal endophytes of Withania somnifera with Trichoderma viride and its impact on plant growth and withanolide content. J. Plant Growth Regul. 2019, 38, 1228–1242. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Xia, R.X.; Zou, Y.N. Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J. Plant Physiol. 2006, 163, 1101–1110. [Google Scholar] [CrossRef]
- Pandey, V.; Ansari, M.W.; Tula, S.; Yadav, S.; Sahoo, R.K.; Shukla, N.; Bains, G.; Badal, S.; Chandra, S.; Gaur, A.K.; et al. Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta 2016, 243, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Singh, V.; Shukla, R.; Sahu, P.; Prabha, R.; Gupta, A.; Sarma, B.K.; Gupta, V.K. Stage-dependent concomitant microbial fortification improves soil nutrient status, plant growth, antioxidative defense system and gene expression in rice. Microbiol. Res. 2020, 239, 126538. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Tajti, J.; Szalai, G.; Peeva, V.; Vegh, B.; Janda, T. Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 2018, 8, 12839. [Google Scholar] [CrossRef]
- Alwhibi, M.S.; Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar]
- He, C.; Wang, W.Q.; Hou, J.L. Plant growth and soil microbial impacts of enhancing licorice with inoculating dark septate endophytes under drought stress. Front. Microbiol. 2019, 10, 2277. [Google Scholar] [CrossRef]
- Valli, P.P.S.; Muthukumar, T. Dark septate root endophytic fungus Nectria haematococca improves tomato growth under water limiting conditions. Indian J. Microbiol. 2018, 58, 489–495. [Google Scholar] [CrossRef]
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Alleviation of the adverse effects of salinity stress in wheat (Triticum aestivum L.) by seed biopriming with salinity tolerant isolates of Trichoderma harzianum. Plant Soil 2011, 347, 387–400. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Mahdavikia, H.; Subramanian, S.; Alipour, H.; Siddique, K.H.M.; Smith, D.L. Co-inoculation of phosphate-solubilizing bacteria and mycorrhizal fungi: Effect on seed yield, physiological variables, and fixed oil and essential oil productivity of ajowan (Carum copticum L.) under water deficit. J. Soil Sci. Plant Nutr. 2021, 21, 3159–3179. [Google Scholar] [CrossRef]
- Hernández, L.E.; Sobrino-Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Caceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Samsampour, D.; Seyahooei, M.A.; Bagheri, A.; Soltani, J. Fungal endophytes alleviate drought-induced oxidative stress in mandarin (Citrus reticulata L.): Toward regulating the ascorbate-glutathione cycle. Sci. Hortic. 2020, 261, 108991. [Google Scholar] [CrossRef]
- Mastouri, F.; Bjorkman, T.; Harman, G.E. Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol. Plant Microbe Interact. 2012, 25, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Oelmuller, R.; Zhang, W.Y. Role of phytohormones in Piriformospora indica-induced growth promotion and stress tolerance in plants: More questions than answers. Front. Microbiol. 2018, 9, 1646. [Google Scholar] [CrossRef]
- Wu, F.L.; Li, Y.; Tian, W.; Sun, Y.; Chen, F.; Zhang, Y.; Zhai, Y.; Zhang, J.; Su, H.; Wang, L. A novel dark septate fungal endophyte positively affected blueberry growth and changed the expression of plant genes involved in phytohormone and flavonoid biosynthesis. Tree Physiol. 2020, 40, 1080–1094. [Google Scholar] [CrossRef]
- Han, L.; Zhou, X.; Zhao, Y.T.; Zhu, S.S.; Wu, L.X.; He, Y.L.; Ping, X.R.; Lu, X.Q.; Huang, W.Y.; Qian, J.; et al. Colonization of endophyte Acremonium sp. D212 in Panax notoginseng and rice mediated by auxin and jasmonic acid. J. Integr. Plant Biol. 2020, 62, 1433–1451. [Google Scholar] [CrossRef]
- Qiang, X.J.; Ding, J.J.; Lin, W.; Li, Q.Z.; Xu, C.Y.; Zheng, Q.; Li, Y.Z. Alleviation of the detrimental effect of water deficit on wheat (Triticum aestivum L.) growth by an indole acetic acid-producing endophytic fungus. Plant Soil 2019, 439, 373–391. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.F.; Fang, W.T.; Shin, L.Y.; Wei, J.Y.; Fu, S.F.; Chou, J.Y. Indole-3-acetic acid-producing yeasts in the phyllosphere of the carnivorous plant Drosera indica L. PLoS ONE 2014, 9, e114196. [Google Scholar] [CrossRef] [PubMed]
- Boudsocq, S.; Niboyet, A.; Lata, J.C.; Raynaud, X.; Loeuille, N.; Mathieu, J.; Blouin, M.; Abbadie, L.; Barot, S. Plant preference for ammonium versus nitrate: A neglected determinant of ecosystem functioning? Am. Nat. 2012, 180, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.; Miller, A.J.; Lindsey, K.; Whalley, W.R. Roots, water, and nutrient acquisition: Let’s get physical. Trends Plant Sci. 2012, 17, 701–710. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Upson, R.; Read, D.J.; Newsham, K.K. Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes. Mycorrhiza 2009, 20, 1–11. [Google Scholar] [CrossRef]
- Wu, F.L.; Qu, D.H.; Wei, T.; Wang, M.Y.; Chen, F.Y.; Li, K.K.; Sun, Y.D.; Su, Y.H.; Yang, L.N.; Su, H.Y.; et al. Transcriptome analysis for understanding the mechanism of dark septate endophyte S16 in promoting the growth and nitrate uptake of sweet cherry. J. Integr. Agric. 2021, 20, 1819–1831. [Google Scholar] [CrossRef]
- Govarthanana, M.; Mythilib, R.; Selvankumarb, T.; Kamala-Kannanc, S.; Kim, H. Myco-phytoremediation of arsenic- and lead-contaminated soils by Helianthus annuus and wood rot fungi, Trichoderma sp. isolated from decayed wood. Ecotoxicol. Environ. Saf. 2018, 151, 279–284. [Google Scholar] [CrossRef]
- Xie, L.L.; He, X.L.; Wang, K.; Hou, L.F.; Sun, Q. Spatial dynamics of dark septate endophytes in the roots and rhizospheres of Hedysarum scoparium in northwest China and the influence of edaphic variables. Fungal Ecol. 2017, 26, 135–143. [Google Scholar] [CrossRef]
- Luo, Z.B.; Luo, J. Uncovering the physiological mechanisms that allow nitrogen availability to affect drought acclimation in Catalpa bungei. Tree Physiol. 2017, 37, 1453–1456. [Google Scholar] [CrossRef]
- Dezam, A.P.G.; Vasconcellos, V.M.; Lacava, P.T.; Farinas, C.S. Microbial production of organic acids by endophytic fungi. Biocatal. Agric. Biotechnol. 2017, 11, 282–287. [Google Scholar] [CrossRef]
- Canada-Coyote, E.; Ramirez-Pimentel, J.G.; Aguirre-Manilla, C.L.; Raya-Perez, J.C.; Acosta-Garcia, G.; Iturriaga, G. Trichoderma harzianum mutants enhance antagonism against phytopathogenic fungi, phosphorus assimilation and drought tolerance in Jalapeno pepper plants. Chil. J. Agric. Res. 2021, 81, 270–280. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.A. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015, 14, 1588–1597. [Google Scholar] [CrossRef]
- Deng, B.; Li, Y.Y.; Xu, D.D.; Ye, Q.D.; Liu, G.H. Nitrogen availability alters flavonoid accumulation in Cyclocarya paliurus via the effects on the internal carbon/nitrogen balance. Sci. Rep. 2019, 9, 2370. [Google Scholar] [CrossRef] [PubMed]
- Alemneh, A.A.; Cawthray, G.R.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Ability to produce indole acetic acid is associated with improved phosphate solubilising activity of rhizobacteria. Arch. Microbiol. 2021, 203, 3825–3837. [Google Scholar] [CrossRef]
- Xu, Y.J.; Xu, C.Y.; Zhang, D.J.; Deng, X.Z. Phosphorus -induced change in root hair growth is associated with IAA accumulation in walnut. Not. Bot. Horti. Agrobo. 2021, 49, 12504. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Z.G.; Mo, S.R.; Liu, J.; Xing, Y.; Wang, Y.P.; Ge, C.L.; Wang, Y.L. Mechanism of low phosphorus inducing the main root lengthening of rice. J. Plant Growth Regul. 2021, 40, 1032–1043. [Google Scholar] [CrossRef]

| SAP | ALP | U | NH4+-N | NO3−-N | ||
|---|---|---|---|---|---|---|
| DSE | Shoot biomass | 0.008 | 0.341 | 0.358 | −0.083 | −0.023 |
| Root biomass | 0.366 | 0.915 ** | 0.924 ** | 0.117 | −0.643 * | |
| Root length | 0.391 | 0.734 * | 0.786 ** | 0.210 | −0.639 * | |
| Calycosin-7-O-glucoside | 0.808 ** | 0.664 * | 0.542 | 0.656 * | −0.937 ** | |
| Formononetin | −0.564 | 0.598 * | 0.808 ** | −0.769 ** | 0.276 | |
| IAA | 0.715 * | −0.444 | −0.662 * | 0.891 ** | −0.470 | |
| Nitrate reductase | 0.471 | 0.842 ** | 0.843 ** | 0.240 | −0.694 * | |
| Trichoderma | Shoot biomass | −0.799 ** | 0.362 | 0.717 * | 0.592 * | 0.729 * |
| Root biomass | −0.084 | 0.498 | 0.950 ** | 0.887 ** | 0.842 ** | |
| Root length | −0.304 | 0.307 | 0.761 ** | 0.603 * | 0.549 | |
| Calycosin-7-O-glucoside | 0.422 | 0.465 | 0.703 * | 0.600 * | 0.528 | |
| Formononetin | −0.404 | −0.498 | −0.702 * | −0.610 * | −0.543 | |
| IAA | −0.683 * | 0.269 | 0.706 * | 0.633 * | 0.716 * | |
| Nitrate reductase | −0.658 * | 0.006 | 0.544 | 0.558 | 0.708 * | |
| Co-inoculation | Shoot biomass | −0.284 | −0.074 | −0.546 * | 0.019 | 0.438 |
| Root biomass | 0.612 ** | 0.260 | 0.201 | 0.656 ** | 0.026 | |
| Root length | 0.567 * | −0.103 | 0.036 | −0.251 | −0.486 * | |
| Calycosin-7-O-glucoside | 0.646 ** | 0.379 | 0.504 * | 0.604 ** | −0.288 | |
| Formononetin | 0.416 | 0.623 ** | 0.913 ** | 0.343 | −0.313 | |
| IAA | −0.041 | 0.020 | 0.454 * | 0.036 | −0.535 * | |
| Nitrate reductase | 0.144 | 0.349 | 0.677 ** | 0.658 ** | −0.140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Ren, Y.; He, C.; Yao, J.; Wei, M.; He, X. Complementary Effects of Dark Septate Endophytes and Trichoderma Strains on Growth and Active Ingredient Accumulation of Astragalus mongholicus under Drought Stress. J. Fungi 2022, 8, 920. https://doi.org/10.3390/jof8090920
Li M, Ren Y, He C, Yao J, Wei M, He X. Complementary Effects of Dark Septate Endophytes and Trichoderma Strains on Growth and Active Ingredient Accumulation of Astragalus mongholicus under Drought Stress. Journal of Fungi. 2022; 8(9):920. https://doi.org/10.3390/jof8090920
Chicago/Turabian StyleLi, Min, Yanfang Ren, Chao He, Jiaojie Yao, Miao Wei, and Xueli He. 2022. "Complementary Effects of Dark Septate Endophytes and Trichoderma Strains on Growth and Active Ingredient Accumulation of Astragalus mongholicus under Drought Stress" Journal of Fungi 8, no. 9: 920. https://doi.org/10.3390/jof8090920
APA StyleLi, M., Ren, Y., He, C., Yao, J., Wei, M., & He, X. (2022). Complementary Effects of Dark Septate Endophytes and Trichoderma Strains on Growth and Active Ingredient Accumulation of Astragalus mongholicus under Drought Stress. Journal of Fungi, 8(9), 920. https://doi.org/10.3390/jof8090920






