Toxicological and Biochemical Description of Synergism of Beauveria bassiana and Emamectin Benzoate against Megalurothrips usitatus (Bagrall)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Insects
2.3. Fungus
2.4. Emamectin Benzoate
2.5. Emamectin Benzoate Impact on the Biological Features of Beauveria bassiana
2.6. Bioassay Studies
2.6.1. Bioassay 1: Beauveria bassiana Isolate SB063 Efficacy against Megalurothrips usitatus
2.6.2. Bioassay 2: Efficacy of Emamectin Benzoate against Megalurothrips usitatus
2.6.3. Bioassay 3: Efficacy of Beauveria bassiana and Emamectin Benzoate as Single or Combined Treatment Options against Megalurothrips usitatus
2.7. Megalurothrips Usitatus Enzyme Activity in Response to Single and Combined Treaments with Beauveria bassiana and Emamectin Benzoate
2.7.1. Treatment of Insects and Sample Preparation
2.7.2. Total Protein Assay
2.7.3. Enzyme Activity Assays
2.8. Data Analysis
3. Results
3.1. Effect of Emamectin Benzoate on the Biological Characteristics of Beauveria bassiana
3.2. Bioassay Studies
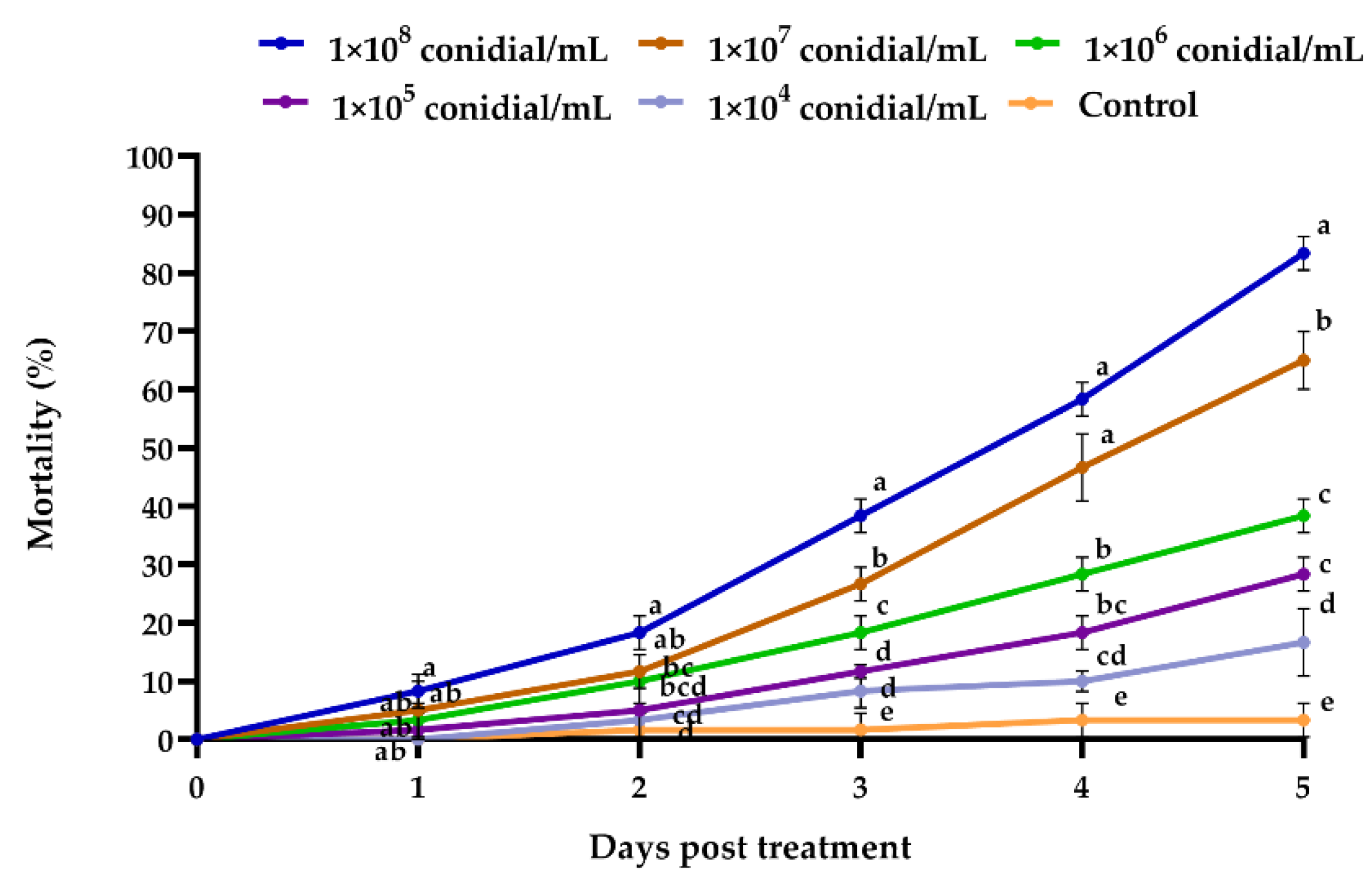
3.2.1. Beauveria bassiana Efficacy against Megalurothrips usitatus
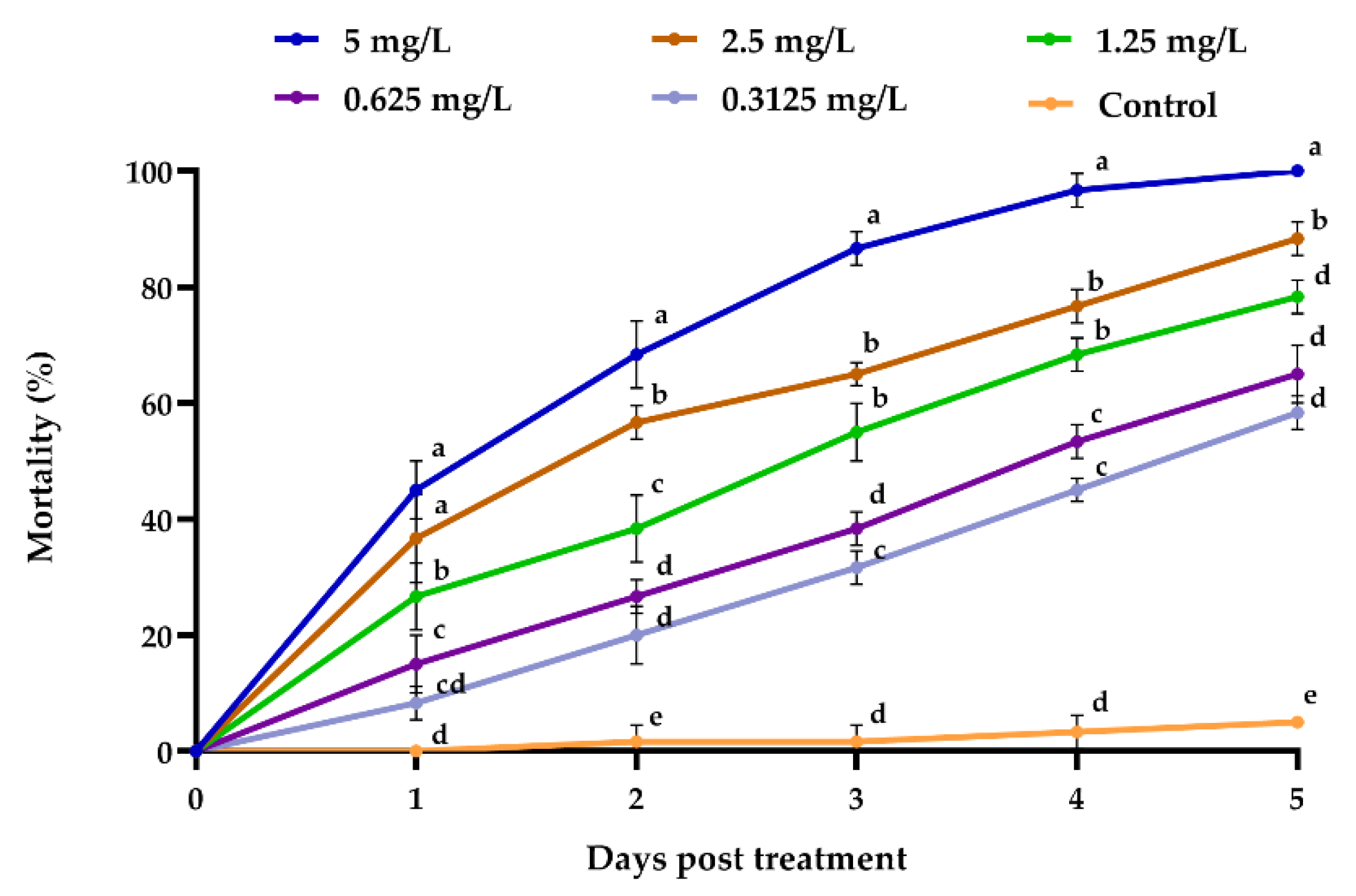
3.2.2. Emamectin Benzoate Efficacy towards Megalurothrips usitatus
3.2.3. Efficacy of Beauveria bassiana and Emamectin Benzoate as Single or Combined Treatments against Megalurothrips usitatus
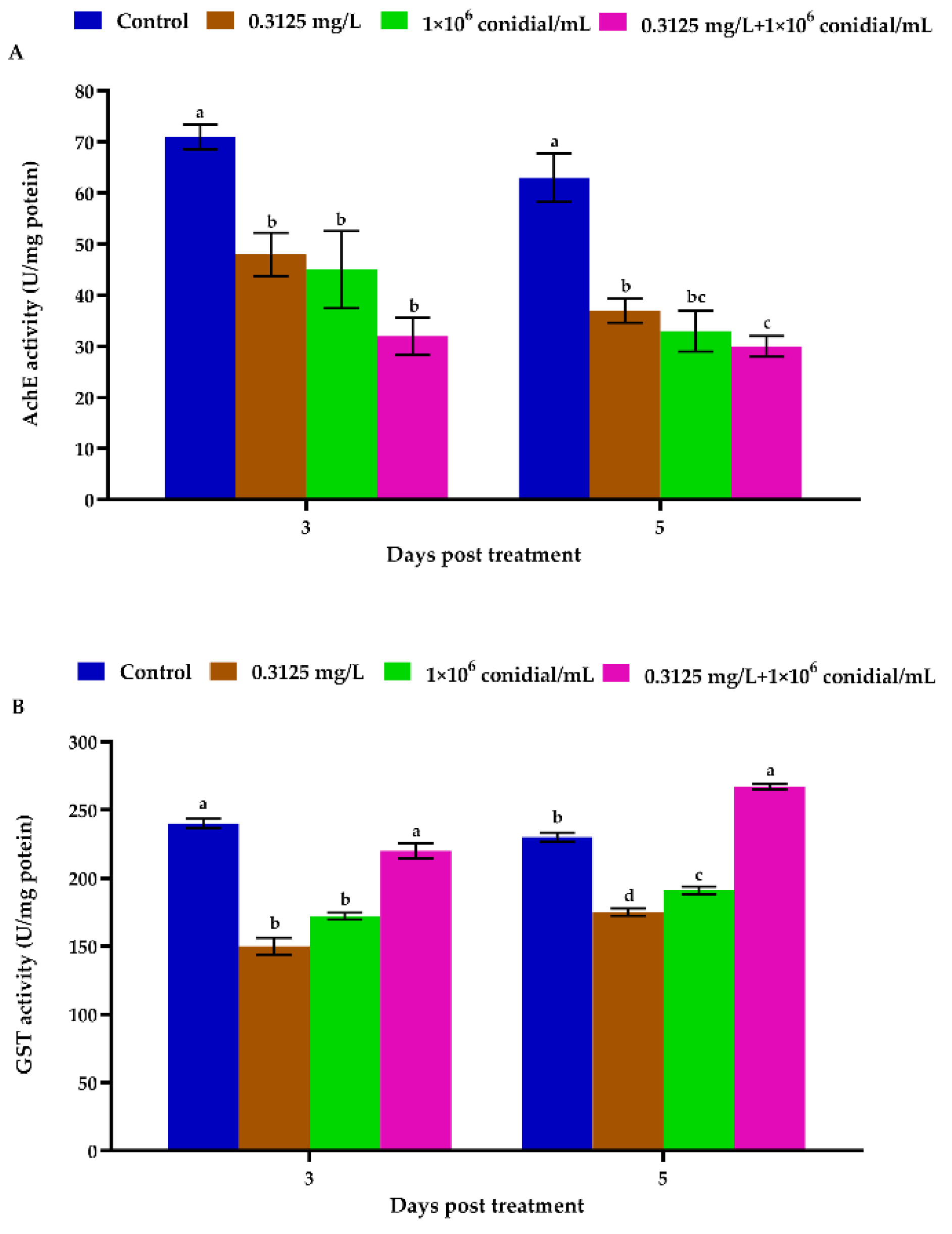
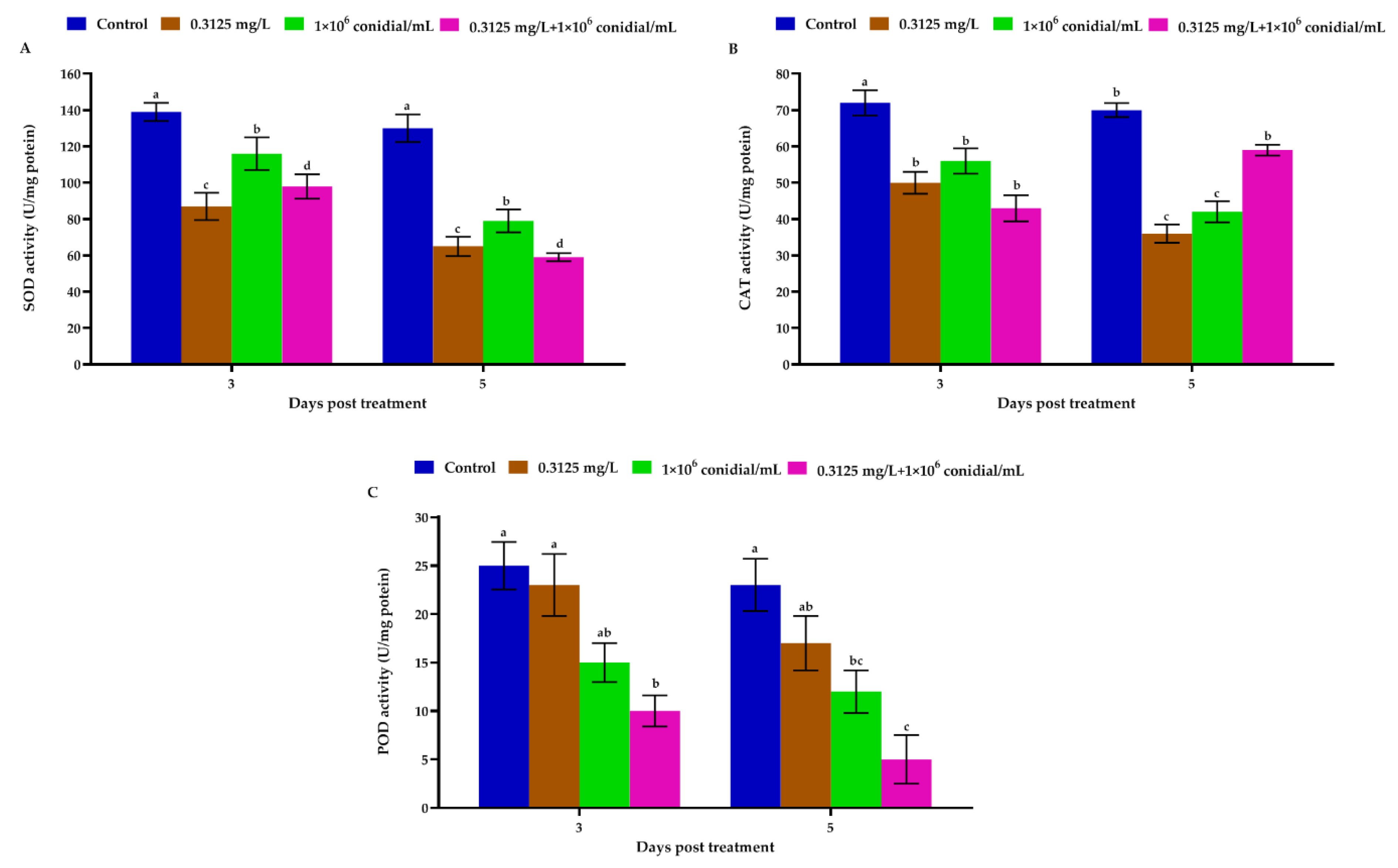
3.3. Megalurothrips Usitatus Enzymatic Response to Individual or Combined Treatments of Beauveria bassiana and Emamectin Benzoate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mound, L.A.; Walker, A.K. Thysanoptera as tropical tramps: New records from New Zealand and the Pacific. N. Z. Entomol. 1987, 9, 70–85. [Google Scholar] [CrossRef]
- Tang, L.D.; Yan, K.L.; Fu, B.L. The life table parameters of Megalurothrips usitatus (Thysanoptera: Thripidae) on four leguminous crops. Fla. Entomol. 2015, 98, 620–625. [Google Scholar] [CrossRef]
- Yang, B.; Du, C.L.; Ali, S.; Wu, J.H. Molecular characterization and virulence of fungal isolates against the bean flower thrips Megalurothrips usitatus Bagnall (Thysanoptera: Thripidae). Egypt. J. Biol. Pest Control 2020, 30, 50. [Google Scholar] [CrossRef]
- Kumar, A.; Nath, P. Evaluation of insecticides and their schedules against infestation of pod bug, Clavigralla gibbosa (Spinola) and flower thrips, Megalurothrips usitatus (Bagnall) on pigeonpea. Pestic. Res. J. 2002, 14, 258–262. [Google Scholar]
- Cuthbertson, A.G.S.; Mathers, J.J.; Croft, P.; Nattriss, N.; Blackburn, L.F.; Luo, W.; Northing, P.; Muari, T.; Jacobson, R.J.; Walters, K.F.A. Prey consumption rates and compatibility with pesticides of four predatory mites from the Family Phytoseiidae attacking Thrips palmi Karny (Thysanoptera: Thripidae). Pest Manag. Sci. 2012, 68, 1289–1295. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Walters, K.F.A.; Northing, P. Susceptibility of Bemisia tabaci immature stages to the entomopathogenic fungus Lecanicillium muscarium on tomato and verbena foliage. Mycopathologia 2005, 159, 23–29. [Google Scholar] [CrossRef]
- Yesica, P.; Cubillos-Salamanca; José, C. Identification of Thrips Species and Resistance of Frankliniella occidentalis (Thysanoptera: Thripidae) to Malathion, Spinosad, and Bifenthrin in Blackberry Crops. Fla. Entomol. 2020, 4, 102. [Google Scholar]
- Ugine, T.A.; Wraight, S.P.; Brownbridge, M. Development of a novel bioassay for estimation of median lethal concentrations (LC50) and doses (LD50) of the entomopathogenic fungus Beauveria bassiana, against western flower thrips, Frankliniella occidentalis. J. Invertebr. Pathol. 2005, 89, 210–218. [Google Scholar] [CrossRef]
- Gill, S.A.; Ressr, R.; Ranpp, M.J. Battling thrips: Five pesticides put to the test. Grower Talks 1998, 10, 46–48. [Google Scholar]
- Ali, S.; Zhang, C.; Wang, Z.Q.; Wang, X.M.; Wu, J.H.; Cuthbertson, A.G.S.; Shao, Z.F.; Qiu, B.L. Toxicological and biochemical basis of synergism between the entomopathogenic fungus Lecanicillium muscarium and the insecticide matrine against Bemisia tabaci (Gennadius). Sci. Rep. 2017, 7, 46558. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S. Update on the status of Bemisia tabaci in the UK and the use of entomopathogenic fungi within eradication programmes. Insects 2013, 4, 198–205. [Google Scholar]
- Wu, J.H.; Yang, B.; Zhang, X.C.; Cuthbertson, A.G.S.; Ali, S. Synergistic interaction between the entomopathogenic fungus Akanthomyces attenuatus (Zare & Gams) and the botanical insecticide matrine against Megalurothrips usitatus (Bagrall). J. Fungi 2021, 7, 536. [Google Scholar]
- Bitencourt, R.D.O.B.; dos Santos Mallet, J.R.; Mesquita, E.; Golo, P.S.; Fiorotti, J.; Bittencourt, V.R.E.P.; Pontes, E.G.; da Costa Angelo, I. Larvicidal activity, route of interaction and ultrastructural changes in Aedes aegypti exposed to entomopathogenic fungi. Acta Trop. 2021, 213, 105732. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, T.; Takken, W.; Koenraadt, C.J. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Filaria J. 2011, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.Q.; Zou, W.H.; Li, D.L.; Chen, J.T.; Huang, Q.; Zhou, L.J.; Tian, X.X.; Chen, Y.J.; Peng, H.J. Expression of Bacillus thuringiensis toxin Cyt2Ba in the entomopathogenic fungus Beauveria bassiana increases its virulence towards Aedes mosquitoes. PLoS Negl. Trop. Dis. 2019, 13, 1935–2735. [Google Scholar]
- Yang, D.S.; Cui, B.; Wang, C.X.; Zhao, X.; Zeng, Z.H.; Wang, Y.; Sun, C.J.; Liu, G.Q.; Cui, H.X. Preparation and characterization of Emamectin Benzoate solid nanodispersion. J. Nanomater. 2017, 2017, 6560780. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Luo, F.; Zhang, X.; Jiang, Y.; Lou, Z.; Chen, Z. Dissipation, transfer and safety evaluation of emamectin benzoate in tea. Food Chem. 2016, 202, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Han, P.J.; Fan, R.J.; Feng, Y.T.; Du, E.Q.; Zhao, R.H. Control efficacy of 2.2% emamectin benzoate ME against rice stem borer (Chilo suppressalis). J. South. Agric. 2011, 42, 403–405. [Google Scholar]
- Ni, J.P.; Hou, H.M.; Zeng, X.; Huang, C.X. Bioactivity comparison on emamectin-benzoate and avermectin, modern agrochemicals. Mod. Agrochem. 2003, 2, 38–40. [Google Scholar]
- Liu, S.; Zhang, F.; Wang, L. Dissipation and Residues of Emamectin Benzoate in Cabbage. Bull. Environ. Contam. Toxicol. 2012, 89, 654–657. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Slyamova, N.D.; Kryukovi, N.D.; Yaroslavtseva, O.N.; Levchenko, M.V.; Belgibaeva, A.B.; Adilkhanzy, A.; Glupov, V.V. The activity of nonspecific esterases and glutathione-S-transferase in Locusta migratoria larvae infected with the fungus Metarhizium anisopliae (Ascomycota, Hypocreales). Entomol. Rev. 2012, 92, 27–31. [Google Scholar]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [PubMed]
- Jia, M.; Cao, G.C.; Li, Y.; Tu, X.B.; Wang, G.J.; Nong, X.Q.; Whitman, D.W.; Zhang, Z. Biochemical basis of synergism between pathogenic fungus Metarhizium anisopliae and insecticide chlorantraniliprole in Locusta migratoria (Meyen). Sci. Rep. 2016, 6, 28424. [Google Scholar] [PubMed]
- Lumjuan, N.; McCarroll, L.; Prapanthadara, L.; Hemingway, J.; Ranson, H. Elevated activity of an Epsilon class glutathione transferase confers DDT resistance in the dengue vector, Aedes aegypti. Insect Biochem. Mol. Biol. 2005, 35, 861–871. [Google Scholar] [PubMed]
- Oehmichen, M.; Besserer, K. Forensic significance of acetylcholine esterase histochemistry in organophosphate intoxication. Int. J. Legal Med. 1982, 89, 149–165. [Google Scholar]
- Gopalakrishnan, S.; Chen, F.Y.; Thilagam, H.; Xu, W.F.; Wang, K.J. Modulation and interaction of immune-associated parameters with antioxidant in the immunocytes of Crab Scylla paramamosain Challenged with lipopolysaccharides. Evid.-Based Complementary Altern. Med. 2001, 1, 824962. [Google Scholar]
- Cerenius, L.; Söderhäll, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar]
- Zibaee, A.; Bandani, A.R.; Tork, M. Effect of the entomopathogenic fungus, Beauveria bassiana, and its secondary metabolite on detoxifying enzyme activities and acetylcholinesterase (AChE) of the Sunn pest, Eurygaster integriceps (Heteroptera: Scutellaridae). Biocontrol Sci. Technol. 2009, 19, 485–498. [Google Scholar]
- Espinosa, P.J.; Contreras, J.; Quinto, V.; Grávalos, C.; Fernández, E.; Bielza, P. Metabolic mechanisms of insecticide resistance in the western flower thrips, Frankliniella occidentalis (Pergande). Pest Manag. Sci. 2005, 61, 1009–1015. [Google Scholar]
- Du, C.; Yang, B.; Wu, J.H.; Ali, S. Identification and virulence characterization of two Akanthomyces attenuatus isolates against Megalurothrips usitatus (Thysanoptera: Thripidae). Insects 2019, 10, 168. [Google Scholar]
- Wu, J.H.; Yu, X.; Wang, X.S.; Tang, L.D.; Ali, S. Matrine enhances the pathogenicity of Beauveria brongniartii against Spodoptera litura (Lepidoptera: Noctuidae). Front. Microbiol. 2019, 10, 1812. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [PubMed]
- Ellman, G.L.; Coutney, K.D.; Andre, V.; Featherstone, R.M. A new and rapid calorimetric determination of acetylcholinestrase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [PubMed]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-Transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [PubMed]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar]
- Shannon, L.M.; Kay, E.; Lew, J.Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J. Biol. Chem. 1966, 241, 2166–2172. [Google Scholar] [CrossRef]
- IBM. SPSS Statistics for Windows, version 19.0; IBM Corp: Armonk, NY, USA, 2010. [Google Scholar]
- Wu, J.; Li, J.; Zhang, C.; Yu, X.; Cuthbertson, A.G.S.; Ali, S. Biological impact and enzyme activities of Spodoptera litura (Lepidoptera: Noctuidae) in response to synergistic action of matrine and Beauveria brongniartii. Front. Physiol. 2020, 11, 584405. [Google Scholar]
- Cruces, L.; Pena, E.L.; Clercq, P. Field evaluation of cypermethrin, imidacloprid, teflubenzuron and emamectin benzoate against pests of quinoa (Chenopodium quinoa Willd.) and their side effects on non-target species. Plants 2021, 10, 1788. [Google Scholar]
- Grafton-Cardwell, E.E.; Godfrey, L.D.; Chaney, W.E.; Bentley, W.J. Various novel insecticides are less toxic to humans, more specific to key pests. Calif. Agric. 2005, 59, 29–34. [Google Scholar]
- Castinerias, A.; Calderon, A.; Lopez, M. Effect of biocides and fertilizers used in banana cultivation in Cuba on entomopathogenic fungi, Metarhizium anisopliae. Prot. Plantas 1991, 1, 33–42. [Google Scholar]
- Loria, R.; Galaini, S.; Roberts, D.W. Survival of inoculum of the entomopathogenic fungus Beauveria bassiana as influenced by fungicides. Environ. Entomol. 1983, 12, 1724–1726. [Google Scholar] [CrossRef]
- Xu, D.; Ali, S.; Huang, Z. Insecticidal activity influence of 20-Hydroxyecdysone on the pathogenicity of Isaria fumosorosea against Plutella xylostella. Biol. Control 2011, 56, 239–244. [Google Scholar]
- Ishaaya, I.; Kontsedalov, S.; Horowitz, A.R. Emamectin, a novel insecticide for controlling field crop pests. Pest Manag. Sci. 2002, 58, 1091–1095. [Google Scholar]
- Xu, Y.Q.; Orozco, R.; Wijerante, E.M.K.; Espinosa-Artiles, P.; Gunatilaka, A.A.L.; Stock, S.P.; Molnar, I. Biosynthesis of the cyclooligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveria bassiana. Fungal Gen. Biol. 2009, 46, 353–364. [Google Scholar]
- Ding, S.Y.; Li, H.Y.; Li, X.F.; Zhang, Z.Y. Effects of two kinds of transgenic poplar on protective enzymes system in the midgut of larvae of American white moth. J. For. Res. Jpn. 2001, 12, 119–122. [Google Scholar]

| Treatments | Megalurothrips usitatus Mortality (%) at Different Time Intervals (d) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Emamectin benzoate (mg/L) | 0.315 | 8.33 ± 1.67 de | 20 cd | 31.67 ± 1.67 cd | 45 ± 2.89 c | 58.33 ± 4.41 c |
| 0.625 | 15 cd | 26.67 ± 1.67 c | 38.33 ± 1.67 c | 53.33 ± 3.33 bc | 65 ± 2.89 c | |
| Beauveria bassiana (conidia/mL) | 1 × 107 | 5 ± 2.89 e | 11.67 ± 1.67 de | 26.67 ± 1.67 de | 46.67 ± 1.67 c | 65 ± 2.89 c |
| 1 × 106 | 3.33 ± 1.67 e | 10 ± 2.89 de | 18.33 ± 1.67 e | 28.33 ± 3.33 d | 38.33 ± 1.67 d | |
| Beauveria bassiana (conidia/mL) + Emamectin benzoate (mg/L) | 1 × 107 + 0.625 | 38.33 * ± 1.67 a | 61.67 * ± 1.67 a | 81.67 * ± 1.67 a | 100 a * | 100 a |
| (19.25; 18.91) | (35.23; 19.85) | (54.78; 13.20) | (75.11;8.25) | (87.75; 1.71) | ||
| 1 × 106 + 0.625 | 26.67 * ± 1.67 b | 53.33 * ± 3.33 a | 70 * ± 2.89 b | 100 a * | 100 a * | |
| (17.83; 4.38) | (34.00; 10.99) | (49.63; 8.36) | (66.55; 16.81) | (78.42; 5.94) | ||
| 1 × 107 + 0.3125 | 30 * ± 2.89 ab | 53.33 * ± 3.33 a | 71.67 * ± 1.67 b | 98.33 * ± 1.67 a | 100a | |
| (12.91; 22.60) | (29.34; 19.62) | (49.89;9.50) | (70.67; 10.83) | (85.41; 2.49) | ||
| 1 × 106 + 0.3125 | 23.33 * ± 1.67 bc | 41.67 * ± 3.33 b | 63.33 * ± 1.67 b | 93.33 * ± 1.67 a | 100 a * | |
| (11.38; 12.54) | (28.00; 6.67) | (44.19; 8.28) | (60.58; 17.70) | (74.30; 8.89) | ||
| Control | 0 | 1.67 ± 1.67 e | 3.33 ± 1.67 e | 3.33 ± 1.67 f | 5 e | 6.67 ± 1.67 e |
| F; df; p | 50.40; 8, 18 <0.001 | 82.42; 8, 18 <0.001 | 221.73; 8, 18 <0.001 | 293.79; 8, 18 <0.001 | 237.57; 8, 18 <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, X.; Tian, Q.; Ali, S.; Tang, L.; Wu, J. Toxicological and Biochemical Description of Synergism of Beauveria bassiana and Emamectin Benzoate against Megalurothrips usitatus (Bagrall). J. Fungi 2022, 8, 916. https://doi.org/10.3390/jof8090916
Zhang Y, Zhang X, Tian Q, Ali S, Tang L, Wu J. Toxicological and Biochemical Description of Synergism of Beauveria bassiana and Emamectin Benzoate against Megalurothrips usitatus (Bagrall). Journal of Fungi. 2022; 8(9):916. https://doi.org/10.3390/jof8090916
Chicago/Turabian StyleZhang, Youdan, Xiaochen Zhang, Qingheng Tian, Shaukat Ali, Liangde Tang, and Jianhui Wu. 2022. "Toxicological and Biochemical Description of Synergism of Beauveria bassiana and Emamectin Benzoate against Megalurothrips usitatus (Bagrall)" Journal of Fungi 8, no. 9: 916. https://doi.org/10.3390/jof8090916
APA StyleZhang, Y., Zhang, X., Tian, Q., Ali, S., Tang, L., & Wu, J. (2022). Toxicological and Biochemical Description of Synergism of Beauveria bassiana and Emamectin Benzoate against Megalurothrips usitatus (Bagrall). Journal of Fungi, 8(9), 916. https://doi.org/10.3390/jof8090916






