De Novo Purine Nucleotide Biosynthesis Pathway Is Required for Development and Pathogenicity in Magnaporthe oryzae
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Deletion and Complementation Assay
2.3. Fungal Growth, Conidiation and Adenine Treated Assay
2.4. Phenotypic Assays on Rice and Barley Leaves
2.5. Fluorescence Observation
2.6. Western Blot Analysis
2.7. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR) Assay
2.8. Yeast Two-Hybrid Assay and Co-Immunoprecipitation Assay
3. Results
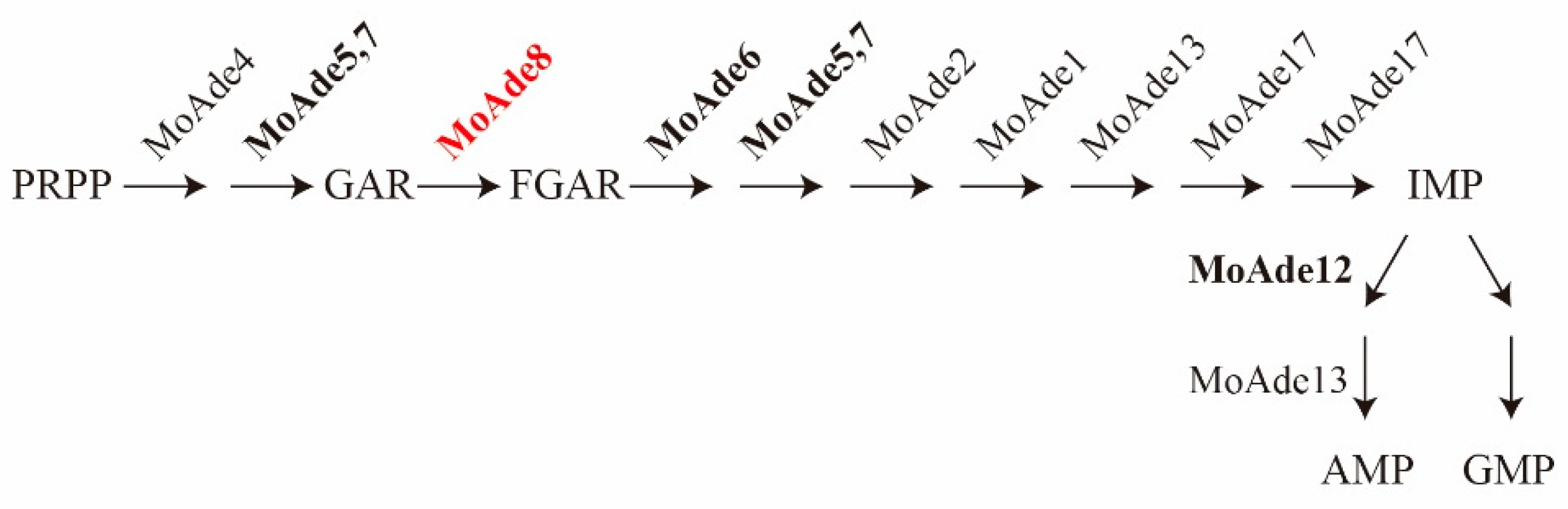
3.1. Identification of MoAde8 in M. oryzae
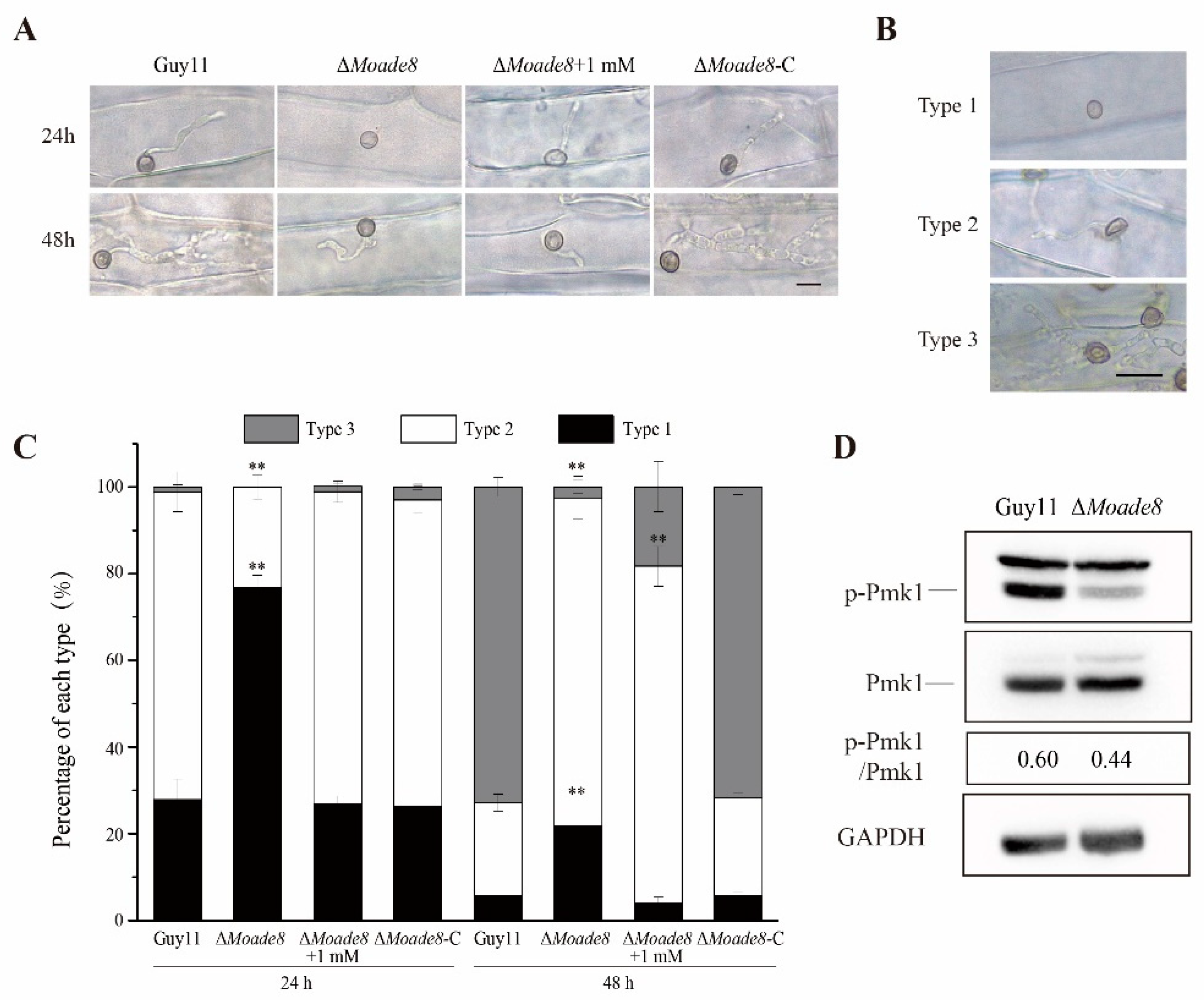
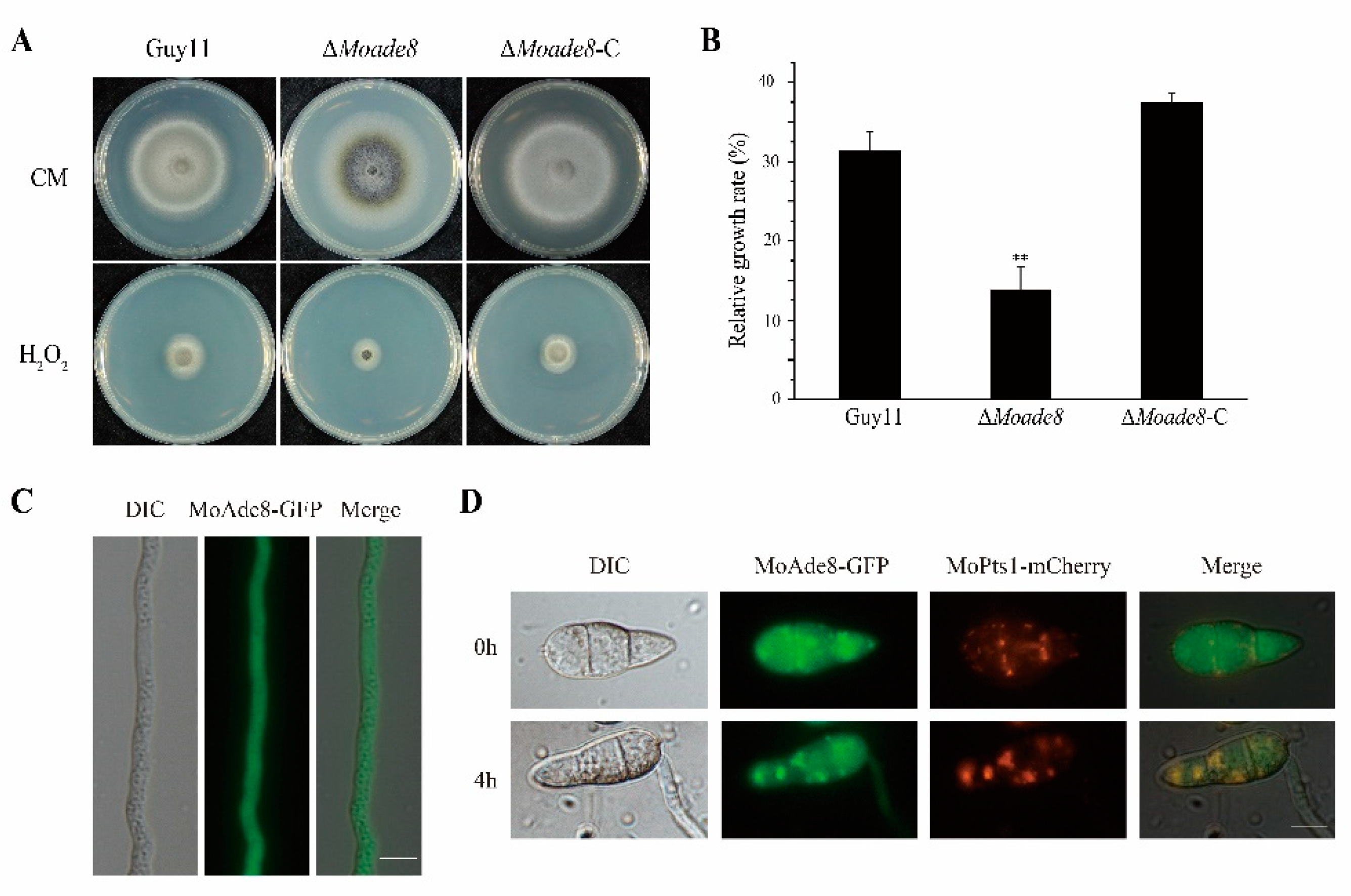
3.2. MoAde8 Is Essential for Conidiation and Virulence
3.3. Exogenous Adenine Rescues the Pathogenicity-Related Phenotypes of ∆Moade8
3.4. MoAde8 Are Associated with Invasive Growth
3.5. Deletion of MoADE8 Reduces Tolerance to Hyperosmotic Stress
3.6. MoAde8 Is Related to Oxidative Stress Response and Co-Localize with Peroxisomes
3.7. Deletion of MoAde8 Negatively Regulates TOR Kinase Activity
3.8. De Novo Purine Nucleotide Biosynthesis Is Required for the Conidiation and Pathogenicity of M. oryzae
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Howard, R.J. Breaking and Entering: Host Penetration by the Fungal Rice Blast Pathogen Magnaporthe grisea. Annu. Rev. Microbiol. 1996, 50, 491–512. [Google Scholar] [CrossRef]
- Huang, P.Y.; Li, Y.; Wang, J.; Wang, Q.; Huang, Z.C.; Liu, X.H.; Lin, F.C.; Lu, J.P. Casein kinase 2 mediates degradation of transcription factor Pcf1 during appressorium formation in the rice blast fungus. J. Fungi 2022, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.X.; Qian, H.; Liu, M.Y.; Wu, M.H.; Wei, Y.Y.; Zhu, X.M.; Lu, J.P.; Lin, F.C.; Liu, X.H. Endosomal sorting complexes required for transport-0 (ESCRT-0) are essential for fungal development, pathogenicity, autophagy and ER-phagy in Magnaporthe oryzae. Environ. Microbiol. 2022, 24, 1076–1092. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Q.; Chen, G.Q.; Liu, X.H.; Dong, B.; Shi, H.B.; Lu, J.P.; Lin, F.C. Crosstalk between SNF1 Pathway and the Peroxisome-Mediated Lipid Metabolism in Magnaporthe oryzae. PLoS ONE 2014, 9, e103124. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 127, 5–19. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Regulation of autophagy by mTOR signaling pathway. Adv. Exp. Med. Biol. 2019, 1206, 67–83. [Google Scholar]
- He, M.; Xu, Y.; Chen, J.; Luo, Y.; Lv, Y.; Su, J.; Kershaw, M.J.; Li, W.; Wang, J.; Yin, J.; et al. MoSnt2-dependent deacetylation of histone H3 mediates MoTor-dependent autophagy and plant infection by the rice blast fungus Magnaporthe oryzae. Autophagy 2018, 14, 1543–1561. [Google Scholar] [CrossRef]
- Zhu, X.M.; Li, L.; Cai, Y.Y.; Wu, X.Y.; Shi, H.B.; Liang, S.; Qu, Y.M.; Naqvi, N.I.; Del Poeta, M.; Dong, B.; et al. A VASt-domain protein regulates autophagy, membrane tension, and sterol homeostasis in rice blast fungus. Autophagy 2021, 17, 2939–2961. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Pedley, A.M.; Benkovic, S.J. Human de novo purine biosynthesis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Adinolfi, E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 2017, 36, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Xiong, L.; Tang, Y.J.; Mehmood, M.A.; Zhao, Z.K.; Bai, F.W.; Zhao, X.Q. Enhanced acetic acid stress tolerance and ethanol production in Saccharomyces cerevisiae by modulating expression of the de novo purine biosynthesis genes. Biotechnol. Biofuels 2019, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Tian, H.; Winograd, N.; Benkovic, S.J. Metabolomics and mass spectrometry imaging reveal channeled de novo purine synthesis in cells. Science 2020, 368, 283–290. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef]
- Rebora, K.; Laloo, B.; Daignan-Fornier, B. Revisiting purine-histidine cross-pathway regulation in Saccharomyces cerevisiae: A central role for a small molecule. Genetics 2005, 170, 61–70. [Google Scholar] [CrossRef]
- Pedley, A.M.; Benkovic, S.J. A new view into the regulation of purine metabolism: The purinosome. Trends Biochem. Sci. 2017, 42, 141–154. [Google Scholar] [CrossRef]
- Jinnah, H.A.; Sabina, R.L.; Van Den Berghe, G. Metabolic disorders of purine metabolism affecting the nervous system. Handb. Clin. Neurol. 2013, 113, 1827–1836. [Google Scholar]
- Balasubramaniam, S.; Duley, J.A.; Christodoulou, J. Inborn errors of purine metabolism: Clinical update and therapies. J. Inherit. Metab. Dis. 2014, 37, 669–686. [Google Scholar] [CrossRef]
- Fridley, B.L.; Batzler, A.; Li, L.; Li, F.; Matimba, A.; Jenkins, G.D.; Ji, Y.; Wang, L.; Weinshilboum, R.M. Gene set analysis of purine and pyrimidine antimetabolites cancer therapies. Pharm. Genom. 2011, 21, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ren, W.K.; Huang, X.G.; Deng, J.P.; Li, T.J.; Yin, Y.L. Potential mechanisms connecting purine metabolism and cancer therapy. Front. Immunol. 2018, 9, 1697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Morar, M.; Ealick, S.E. Structural biology of the purine biosynthetic pathway. Cell. Mol. Life Sci. 2008, 65, 3699–3724. [Google Scholar] [CrossRef] [PubMed]
- Welin, M.; Grossmann, J.G.; Flodin, S.; Nyman, T.; Stenmark, P.; Tresaugues, L.; Kotenyova, T.; Johansson, I.; Nordlund, P.; Lehtio, L. Structural studies of tri-functional human GART. Nucleic Acids Res. 2010, 38, 7308–7319. [Google Scholar] [CrossRef]
- Agnese, K.; Juris, K.; Janis, L. Adenine auxotrophy--be aware: Some effects of adenine auxotrophy in Saccharomyces cerevisiae strain W303-1A. FEMS Yeast Res. 2014, 14, 697–707. [Google Scholar]
- Ng, A.; Uribe, R.A.; Yieh, L.; Nuckels, R.; Gross, J.M. Zebrafish mutations in gart and paics identify crucial roles for de novo purine synthesis in vertebrate pigmentation and ocular development. Development 2009, 136, 2601–2611. [Google Scholar] [CrossRef]
- Lu, J.P.; Cao, H.J.; Zhang, L.L.; Huang, P.Y.; Lin, F.C. Systematic analysis of Zn2Cys6 transcription factors required for development and pathogenicity by high-throughput gene knockout in the rice blast fungus. PLoS Pathog. 2014, 10, e1004432. [Google Scholar] [CrossRef]
- Rho, H.S.; Kang, S.; Lee, Y.H. Agrobacterium tumefaciens-mediated transformation of the plant pathogenic fungus, Magnaporthe grisea. Mol. Cells 2001, 12, 407–411. [Google Scholar]
- Liu, X.H.; Lu, J.P.; Zhang, L.; Dong, B.; Min, H.; Lin, F.C. Involvement of a Magnaporthe grisea serine/threonine kinase gene, MgATG1, in appressorium turgor and pathogenesis. Eukaryot. Cell 2007, 6, 997–1005. [Google Scholar] [CrossRef]
- Purdue, P.E.; Lazarow, P.B. Pex18p is constitutively degraded during peroxisome biogenesis. J. Biol. Chem. 2001, 276, 47684–47689. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhang, Z.; Wang, Y.L.; Li, L.; Chai, R.Y.; Mao, X.Q.; Jiang, H.; Qiu, H.P.; Du, X.F.; Lin, F.C.; et al. PTS1 peroxisomal import pathway plays shared and distinct roles to PTS2 pathway in development and pathogenicity of Magnaporthe oryzae. PLoS ONE 2013, 8, e55554. [Google Scholar]
- Li, L.; Zhu, X.M.; Shi, H.B.; Feng, X.X.; Liu, X.H.; Lin, F.C. MoFap7, a ribosome assembly factor, is required for fungal development and plant colonization of Magnaporthe oryzae. Virulence 2019, 10, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Ning, G.A.; Huang, L.Y.; Zhao, Y.H.; Dong, B.; Lu, J.P.; Lin, F.C. Calpains are involved in asexual and sexual development, cell wall integrity and pathogenicity of the rice blast fungus. Sci. Rep. 2016, 7, 31204. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, G.; Barnes, T.; Bleskan, J.; Backer, L.; Cox, M.; Patterson, D. The human GARS-AIRS-GART gene encodes two proteins which are differentially expressed during human brain development and temporally overexpressed in cerebellum of individuals with Down syndrome. Hum. Mol. Genet. 1997, 6, 2043–2050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rudolph, J.; Stubbe, J. Investigation of the mechanism of phosphoribosylamine transfer from glutamine phosphoribosylpyrophosphate amidotransferase to glycinamide ribonucleotide synthetase. Biochemistry 1995, 34, 2241–2250. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yan, Y.X.; Qu, Y.M.; Wang, J.; Feng, X.X.; Liu, X.H.; Lin, F.C.; Lu, J.P. Role refinement of melanin synthesis genes by gene knockout reveals their functional diversity in Pyricularia oryzae strains. Microbiol. Res. 2021, 242, 126620. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Yu, Q.; Dong, B.; Zhang, Y.; Liu, X.H.; Lin, F.C.; Liang, S. MoLEU1, MoLEU2, and MoLEU4 regulated by MoLEU3 are involved in leucine biosynthesis, fungal development, and pathogenicity in Magnaporthe oryzae. Environ. Microbiol. Rep. 2019, 11, 784–796. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Yu, Q.; Su, Z.Z.; Dong, B.; Lu, J.P.; Lin, F.C.; Liao, Q.S.; Liu, X.H. PoMet3 and PoMet14 associated with sulfate assimilation are essential for conidiogenesis and pathogenicity in Pyricularia oryzae. Curr. Genet. 2020, 66, 765–774. [Google Scholar] [CrossRef]
- Ljungdahl, P.O.; Daignan-Fornier, B. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics 2012, 190, 885–929. [Google Scholar]
- Li, G.; Zhou, X.; Xu, J.R. Genetic control of infection-related development in Magnaporthe oryzae. Curr. Opin. Microbiol. 2012, 15, 678–684. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, X.; Liu, H.; Xu, J.R. Mitogen-activated protein kinase signaling in plant pathogenic fungi. PLoS Pathog. 2018, 14, e1006875. [Google Scholar]
- Jacob, S.; Foster, A.J.; Yemelin, A.; Thines, E. High osmolarity glycerol (HOG) signalling in Magnaporthe oryzae: Identification of MoYPD1 and its role in osmoregulation, fungicide action, and pathogenicity. Fungal Biol. 2015, 119, 580–594. [Google Scholar] [PubMed]
- Dixon, K.P.; Xu, J.R.; Smirnoff, N.; Talbot, N.J. Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 1999, 11, 2045–2058. [Google Scholar] [PubMed]
- Turra, D.; Segorbe, D.; Di Pietro, A. Protein kinases in plant-pathogenic fungi: Conserved regulators of infection. Annu. Rev. Phytopathol. 2014, 52, 267–288. [Google Scholar]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar]
- Li, P.; Zhao, L.; Qi, F.; Htwe, N.; Li, Q.; Zhang, D.; Lin, F.; Shang-Guan, K.; Liang, Y. The receptor-like cytoplasmic kinase RIPK regulates broad-spectrum ROS signaling in multiple layers of plant immune system. Mol. Plant 2021, 14, 1652–1667. [Google Scholar]
- Wang, J.Y.; Li, L.; Chai, R.Y.; Qiu, H.P.; Zhang, Z.; Wang, Y.L.; Liu, X.H.; Lin, F.C.; Sun, G.C. Pex13 and Pex14, the key components of the peroxisomal docking complex, are required for peroxisome formation, host infection and pathogenicity-related morphogenesis in Magnaporthe oryzae. Virulence 2019, 10, 292–314. [Google Scholar]
- Ben-Sahra, I.; Howell, J.J.; Asara, J.M.; Manning, B.D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 2013, 339, 1323–1328. [Google Scholar]
- Ben-Sahra, I.; Hoxhaj, G.; Ricoult, S.J.H.; Asara, J.M.; Manning, B.D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 2016, 351, 728–733. [Google Scholar]
- Qian, B.; Liu, X.Y.; Jia, J.; Cai, Y.C.; Chen, C.; Zhang, H.F.; Zheng, X.B.; Wang, P.; Zhang, Z.G. MoPpe1 partners with MoSap1 to mediate TOR and cell wall integrity signalling in growth and pathogenicity of the rice blast fungus Magnaporthe oryzae. Environ. Microbiol. 2018, 20, 3964–3979. [Google Scholar]
- Scott, R.C.; Schuldiner, O.; Neufeld, T.P. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 2004, 7, 167–178. [Google Scholar] [PubMed]
- Pu, Y.T.; Luo, X.J.; Bassham, D.C. TOR-dependent and -independent pathways regulate autophagy in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1204. [Google Scholar] [PubMed]
- Noda, T.; Ohsumi, Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 1998, 273, 3963–3966. [Google Scholar] [PubMed]
- Cai, Y.Y.; Wang, J.Y.; Wu, X.Y.; Liang, S.; Zhu, X.M.; Li, L.; Lu, J.P.; Liu, X.H.; Lin, F.C. MoOpy2 is essential for fungal development, pathogenicity, and autophagy in Magnaporthe oryzae. Environ. Microbiol. 2022, 24, 1653–1671. [Google Scholar]
- Zhu, X.M.; Liang, S.; Shi, H.B.; Lu, J.P.; Dong, B.; Liao, Q.S.; Lin, F.C.; Liu, X.H. VPS9 domain-containing proteins are essential for autophagy and endocytosis in Pyricularia oryzae. Environ. Microbiol. 2018, 20, 1516–1530. [Google Scholar]
- Daignan-Fornier, B.; Pinson, B. Yeast to Study Human Purine Metabolism Diseases. Cells 2019, 8, 67. [Google Scholar]
- Berman, E.M.; Werbel, L.M. The Renewed Potential for Folate Antagonists in Contemporary Cancer-Chemotherapy. J. Med. Chem. 1991, 34, 479–485. [Google Scholar]
- Henikoff, S. The Saccharomyces-Cerevisiae Ade5,7 Protein Is Homologous To Overlapping Drosophila-Melanogaster-Gart Polypeptides. J. Mol. Biol. 1986, 190, 519–528. [Google Scholar]
- Tang, W.; Jiang, H.L.; Zheng, Q.J.; Chen, X.H.; Wang, R.F.; Yang, S.; Zhao, G.Y.; Liu, J.; Norvienyeku, J.; Wang, Z.H. Isopropylmalate isomerase MoLeu1 orchestrates leucine biosynthesis, fungal development, and pathogenicity in Magnaporthe oryzae. Appl. Microbiol. Biot. 2019, 103, 327–337. [Google Scholar]
- Zhang, Y.; Shi, H.B.; Liang, S.; Ning, G.A.; Xu, N.C.; Lu, J.P.; Liu, X.H.; Lin, F.C. MoARG1, MoARG5,6 and MoARG7 involved in arginine biosynthesis are essential for growth, conidiogenesis, sexual reproduction, and pathogenicity in Magnaporthe oryzae. Microbiol. Res. 2015, 180, 11–22. [Google Scholar]
- Fernandez, J.; Yang, K.T.; Cornwell, K.M.; Wright, J.D.; Wilson, R.A. Growth in rice cells requires de novo purine biosynthesis by the blast fungus Magnaporthe oryzae. Sci. Rep. 2013, 3, 2398. [Google Scholar] [PubMed]
- Zhang, Z.; Hao, Z.; Chai, R.; Qiu, H.; Wang, Y.; Wang, J.; Sun, G. Adenylsuccinate Synthetase MoADE12 Plays Important Roles in the Development and Pathogenicity of the Rice Blast Fungus. J. Fungi 2022, 8, 780. [Google Scholar]
- Sun, M.L.; Bian, Z.Y.; Luan, Q.Q.; Chen, Y.T.; Wang, W.; Dong, Y.R.; Chen, L.F.; Hao, C.F.; Xu, J.R.; Liu, H.Q. Stage-specific regulation of purine metabolism during infectious growth and sexual reproduction in Fusarium graminearum. New Phytol. 2021, 230, 757–773. [Google Scholar] [PubMed]
- Qu, Y.M.; Cao, H.J.; Huang, P.Y.; Wang, J.; Liu, X.H.; Lu, J.P.; Lin, F.C. A kelch domain cell end protein, PoTea1, mediates cell polarization during appressorium morphogenesis in Pyricularia oryzae. Microbiol. Res. 2022, 259, 126999. [Google Scholar] [PubMed]
- Bai, J.A.; Jie, H.; Wei, S.; Wang, S.; Guo, H.; Tang, Q. GART mediates the renewal of intestinal epithelial barrier via p38/p53/PUMA cascade in colitis. Apoptosis 2016, 21, 1386–1397. [Google Scholar]
- Cao, H.; Huang, P.; Zhang, L.; Shi, Y.; Sun, D.; Yan, Y.; Liu, X.; Dong, B.; Chen, G.; Snyder, J.H.; et al. Characterization of 47 Cys2 -His2 zinc finger proteins required for the development and pathogenicity of the rice blast fungus Magnaporthe oryzae. New Phytol. 2016, 211, 1035–1051. [Google Scholar]
- Srere, P.A. The Metabolon. Trends Biochem. Sci. 1985, 10, 109–110. [Google Scholar]
- An, S.G.; Kumar, R.; Sheets, E.D.; Benkovic, S.J. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science 2008, 320, 103–106. [Google Scholar]
- Miura, N. Condensate Formation by Metabolic Enzymes in Saccharomyces cerevisiae. Microorganisms 2022, 10, 232. [Google Scholar]
- Emmanuel, N.; Ragunathan, S.; Shan, Q.; Wang, F.; Giannakou, A.; Huser, N.; Jin, G.X.; Myers, J.; Abraham, R.T.; Unsal-Kacmaz, K. Purine Nucleotide Availability Regulates mTORC1 Activity through the Rheb GTPase. Cell Rep. 2017, 19, 2665–2680. [Google Scholar]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One Drug, Many Effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.-Y.; Sun, L.-X.; Qian, H.; Zhang, Y.-R.; Zhu, X.-M.; Li, L.; Liang, S.; Lu, J.-P.; Lin, F.-C.; Liu, X.-H. De Novo Purine Nucleotide Biosynthesis Pathway Is Required for Development and Pathogenicity in Magnaporthe oryzae. J. Fungi 2022, 8, 915. https://doi.org/10.3390/jof8090915
Liu M-Y, Sun L-X, Qian H, Zhang Y-R, Zhu X-M, Li L, Liang S, Lu J-P, Lin F-C, Liu X-H. De Novo Purine Nucleotide Biosynthesis Pathway Is Required for Development and Pathogenicity in Magnaporthe oryzae. Journal of Fungi. 2022; 8(9):915. https://doi.org/10.3390/jof8090915
Chicago/Turabian StyleLiu, Meng-Yu, Li-Xiao Sun, Hui Qian, Yun-Ran Zhang, Xue-Ming Zhu, Lin Li, Shuang Liang, Jian-Ping Lu, Fu-Cheng Lin, and Xiao-Hong Liu. 2022. "De Novo Purine Nucleotide Biosynthesis Pathway Is Required for Development and Pathogenicity in Magnaporthe oryzae" Journal of Fungi 8, no. 9: 915. https://doi.org/10.3390/jof8090915
APA StyleLiu, M.-Y., Sun, L.-X., Qian, H., Zhang, Y.-R., Zhu, X.-M., Li, L., Liang, S., Lu, J.-P., Lin, F.-C., & Liu, X.-H. (2022). De Novo Purine Nucleotide Biosynthesis Pathway Is Required for Development and Pathogenicity in Magnaporthe oryzae. Journal of Fungi, 8(9), 915. https://doi.org/10.3390/jof8090915






